Abstract
Background:
The purpose of this study was to investigate the effect of using a 1-point calibration approach instead of a 2-point calibration approach on the accuracy of a continuous glucose monitoring (CGM) algorithm.
Method:
A previously published real-time CGM algorithm was compared with its updated version, which used a 1-point calibration instead of a 2-point calibration. In addition, the contribution of the corrective intercept (CI) to the calibration performance was assessed. Finally, the sensor background current was estimated real-time and retrospectively. The study was performed on 132 type 1 diabetes patients.
Results:
Replacing the 2-point calibration with the 1-point calibration improved the CGM accuracy, with the greatest improvement achieved in hypoglycemia (18.4% median absolute relative differences [MARD] in hypoglycemia for the 2-point calibration, and 12.1% MARD in hypoglycemia for the 1-point calibration). Using 1-point calibration increased the percentage of sensor readings in zone A+B of the Clarke error grid analysis (EGA) in the full glycemic range, and also enhanced hypoglycemia sensitivity. Exclusion of CI from calibration reduced hypoglycemia accuracy, while slightly increased euglycemia accuracy. Both real-time and retrospective estimation of the sensor background current suggest that the background current can be considered zero in the calibration of the SCGM1 sensor.
Conclusions:
The sensor readings calibrated with the 1-point calibration approach indicated to have higher accuracy than those calibrated with the 2-point calibration approach.
Keywords: continuous glucose monitoring algorithm, hypoglycemia, 1-point calibration, 2-point calibration, type 1 diabetes
Continuous glucose monitoring (CGM) has facilitated the management of diabetes mellitus in recent years. However, due to substantial deviation of CGM data from blood glucose (BG) values, CGM systems require sophisticated signal processing to provide patients and health care professionals with the useful information they carry.1-3 Improved CGM algorithms have been developed to reduce BG-CGM deviation. Examples are the model-based optimal filtering approaches (Kalman filtering and Wiener filtering) presented by Guerra et al,4 Bequette,5 and Barry Keenan et al,6 in which BG is inferred from CGM using the BG-to-interstitial glucose (IG) kinetic model. However, the quality of BG estimation from CGM data using the optimal filtering is strongly affected by the quality of CGM calibration.5
Calibration is one of the main factors affecting the accuracy of CGM, and a large portion of the sensor glucose deviation from reference BG level is caused by the calibration error.2,7-12 Facchinetti et al12 dissected the sensor error into 3 principal components: error due to the BG-to-IG kinetics, the calibration error, and the error caused by the measurement noise. Their study indicated that the calibration-related error due to the insufficient estimation of the calibration coefficients causes the biggest mean absolute relative deviation (ARD) of CGM from BG, among the 3 sources of error (12.8% mean ARD caused by the calibration error compared to 3.5% mean ARD due to the BG-to-IG kinetics, and 5.6% mean ARD due to the measurement noise).
An important cause of the insufficient CGM calibration is the inaccurate estimation of the sensor background current in calibration,7-10 which causes large CGM inaccuracy particularly in hypoglycemia, and as a result, the hypoglycemic BG values are overestimated by CGM readings.13-15 This can lead to failure in detection of hypoglycemia, and consequently causes critical situations for the patient.9,10,16 The in-vivo sources of the sensor background current, which is a glucose-nonspecific current, are 3 main interfering substances in interstitial fluid (ISF) including ascorbic acid, acetaminophen, and uric acid.7,10,11,17 These 3 components are oxidized and generate current, in the presence of the voltage applied between the sensor electrodes7,11
| (1) |
where ISIG (interstitial signal) is the current measured by the sensor, is the true glucose current, and is the sensor background current.
It is difficult to directly measure the sensor background current in vivo;7 therefore, if the current is not negligible, it has to be estimated mathematically by means of a 2-point calibration algorithm where the background current is estimated by measuring the sensor output at 2 or more different BG concentrations and finding the y-intercept at zero glucose level.3,7,17-21 Although theoretically attractive, estimation of the sensor background current with an acceptable accuracy is difficult to achieve through a 2-point calibration, mainly because of the physiological time lag between BG and IG.3,11,22 The time lag causes a variable gradient between BG and IG.3,6,22-25 Based on the fact that CGM sensor samples IG, there is also a variable gradient between BG and ISIG. Because of the variable gradient between BG and ISIG, and also considering the fact that ISIG values are calibrated against BG values, a linear calibration with fixed parameters (slope and intercept) has a substantial error in the estimation of the sensor background current. Therefore, the time lag is a major cause of inaccurate estimation of the sensor background current especially if the calibration points are taken at the non–steady state between BG and IG (eg, dropping BG to hypoglycemia, and after a meal), which the effect of lag is maximized.4,26-29
Rebrin et al13 demonstrated that any mismatch between the true background current and the estimated current by the calibration results in a CGM sensor that underestimates BG values above the calibration point and overestimates the values below the calibration point. Therefore, although theoretically the sensor background current can be estimated by a 2-point calibration, the error of the estimation itself affects the accuracy of the calibrated sensor readings to a large extent. Choleau et al8 demonstrated that considering zero value for the background current by using a 1-point calibration produces more accurate sensor readings than estimating the current by a 2-point calibration.
In a previous study,30 we developed a CGM algorithm with the aim of shortening filtering delay, and reducing the BG-CGM deviation in hypoglycemia. We used a 2-point calibration approach to estimate the sensor sensitivity and the sensor background current. We compared our algorithm with an alternative CGM algorithm, using data from 16 type 1 diabetes patients. The results were promising and indicated the potential of our algorithm to improve CGM accuracy. The aim of the current study is updating the algorithm by using a 1-point calibration approach instead of a 2-point calibration approach, and investigating the effect of this algorithm modification on the CGM accuracy by evaluating the algorithm in a larger number of type 1 diabetes patients.
Subjects and Methods
Subjects
CGM data were collected using the SCGM1 (Roche Diagnostics, Mannheim, Germany), a microdialysis-based CGM system which allows up to 120 hours of 1-minute dialysate glucose measurements. Two centers contributed to the data collection that was part of the clinical in vivo development phase of the SCGM1 system: Medical Department M, Aarhus University Hospital, Denmark, and Profil Institute for Metabolic Research, Neuss, Germany.31 The data included more than 200 data sets from type 1 and type 2 diabetic patients. In addition to the CGM data, reference values in the form of capillary BG measurements were obtained by nurses up to 20 times per day using a built-in BG meter; each measurement was performed twice for confirmation of the value. Data capture lasted for up to 5 days per patient. The data for the current study were selected from this population using the following criteria:31 (1) data sets from patients with type 1 diabetes and (2) sufficient technical quality of the data. Applying these inclusion criteria, we retained data sets from 132 type 1 diabetic patients with a total of 14 571 hours of 1-minute CGM data and 18 056 reference-sensor pairs. Reference BG measurements were used for calibration and evaluation of the algorithm. Only the reference BG values within the measurement range of CGM systems (40 ≤ BG ≤ 400) were considered valid for calibration and evaluation of the algorithm. The 2 values at each reference BG measurement instance were averaged before they were used in the algorithm. Table 1 gives the characteristics of the patients enrolled in the study.
Table 1.
Patient Characteristics.
| N | 132 |
|---|---|
| Age (years) | 35.0 (10.9)a |
| Gender | 83 male, 49 female |
| Body mass index (kg/m2) | 24.4 (4.1)a |
| Glycosylated hemoglobin % (HbA1c%) | 8.0 (1.6)a |
Data are mean (SD).
Continuous Glucose Monitoring Algorithm
The algorithm includes the main elements in Figure 1. It operates on the ISIG which is the raw current measured by the CGM sensor with a sampling frequency of 1 per minute. The newly developed algorithm is described in detail in our previous work.30 Therefore, to keep this section concise, only a brief description of the algorithm is given.
Figure 1.
Overall diagram of the algorithm for processing of continuous glucose monitoring data.
The rate-limiting filter in the first block applies a limit on the signal rate of change, set on a physiological threshold of 4 mg/dl/min.21,32 If the rate of change between 2 successive ISIG values exceeds the defined limit, the most recent ISIG value is replaced by a weighted local polynomial regression estimate.30 In the second block, first, the number of zero crossings (ZCs) of the signal first-order differences is calculated in 5-minute segments of the signal. The signal segment is considered noisy if the number of ZCs is larger than 1. Second, the noisy segments of the signal are filtered by a weighted moving average of order 50. Finally, in the third block, the current measured by the sensor, which is processed in the preceding blocks, is converted into a glucose level (mg/dl). The signal is calibrated by a 2-point calibration approach, coefficients being estimated using robust regression with a bi-square weight function. A maximum of 4 BG-ISIG pairs and a minimum of 2 pairs per day are utilized for calibration, forming the calibration set. The calibration set is corrected for any existing low correlation coefficients between reference BG measurements and ISIG values and also for a low relative standard deviation (RSD) of the BG values. A corrective intercept (CI) as described by Mahmoudi et al30 is subtracted from the calibrated sensor glucose (SG) to correct the overestimation of BG by CGM in the hypoglycemic range of BG (BG ≤ 70 mg/dl).
| (2) |
CI is a 2-order polynomial which its value is adjusted with the value of the SG.
| (3) |
For other values of SG, CI is zero.
The coefficients , , and are estimated by fitting the 3 following points to equation 3
| (4) |
Calibration
Basically, 2 types of calibration procedures have been used in the commercial CGM systems.5,8,11,33 1-point calibration, which essentially requires only 1 sensor signal ()- BG () pair for calibration and is applicable when the background current of the sensor () is known and remains constant or it is zero. Then the sensor sensitivity is obtained by
| (5) |
A 2-point calibration is used when is not known and need to be estimated, and is based on the 2 sensor signal–BG pairs, where the subscripts 1 and 2 represent the first and the second calibration data points, respectively
| (6) |
The sensor sensitivity and the background current are estimated from
| (7) |
When multiple data points are available, a linear regression can be used to fit slope and intercept to the data. Standard linear regression techniques find the and that minimize the sum of the squares of the errors (differences between measurements and model predictions).
To upgrade the algorithm, we replaced the 2-point calibration approach in the algorithm with a 1-point calibration approach, and compared the accuracy of this new version of the algorithm with the old version. The other parts of the algorithm remained the same. In the calibration regression, we used the ISIG signal as the independent variable in the regression analysis of the calibration line. The 2 calibration approaches which we compared are given in Table 2, where is the calibrated SG, is the ISIG signal, and are the slope and the intercept of the calibration, respectively, and CI is the corrective intercept.
Table 2.
The Two Calibration Versions Being Compared in the Study.
| 2-point calibration | (8) | |
| 1-point calibration | (9) |
Performance Measures
Three performance metrics were used to evaluate the performance of the 2 algorithm versions.
Absolute Relative Deviation (ARD)
The ARD was calculated for each patient as the relative deviation of the processed SG values from the paired reference BG measurements.
| (10) |
Clarke Error Grid Analysis (EGA)
The often used Clarke EGA introduced by Clarke et al34 was applied to classify BG-SG pairs into different clinically interpretable categories.
Sensitivity and Specificity of Hypo- and Hyperglycemia
The sensitivity and specificity of the 2 algorithms for hypoglycemia and hyperglycemia detection were measured using the single measurements of reference BG values and their paired SGs. Hypoglycemia and hyperglycemia were defined as single reference BG ≤ 70 mg.dl−1 and > 180 mg.dl−1 respectively.
| (11) |
| (12) |
Sensitivity and Specificity of CI, and Contribution of CI to the Calibration
The sensitivity of CI is the percentage of the non-hypoglycemic SG values () which have been affected by CI and transferred to the hypoglycemic range (), while they have concurrent hypoglycemic BG level. The specificity of the corrective intercept is the percentage of the non-hypoglycemic SG values which have been affected by CI and not transferred to the hypoglycemic range, and they have concurrent non-hypoglycemic BG level.
We further excluded CI from the 1-point and 2-point calibrations and evaluated the effect on the CGM accuracy, to assess the contribution of CI to the calibration improvement.
Retrospective and Real-Time Estimation of the Sensor Background Current
For retrospective estimation of I0, using the complete data set, we fitted the line
| (13) |
to all BG-ISIG pairs, and estimated m and I0.
For real-time estimation of I0, we rearranged equation 8 excluding CI, where x values are the reference BG measurements and y values are their paired ISIG readings in the calibration.
| (14) |
Equation 14 gives . In each calibration update, I0 was calculated, and finally averaged throughout the calibration updates in the whole data set. The averaged I0 was then compared with the retrospectively estimated I0
Statistical Analysis
Statistical analyses were performed in MATLAB (version 8.1.0. 604 [R2013a]). The Wilcoxon signed rank test was used to compare the median ARD of the 2 algorithm versions. A value of P < .05 was considered statistically significant for the test.
Results
For evaluation, the BG measurements were paired with the SG values from the same time. The pairs that were used for calibration were not used for evaluation. The evaluation results are reported pooling all BG-SG pairs.
The SCGM1 system has around 31 minutes (SD ± 2 minutes) of micro-dialysis transport lag.31,35 We considered this delay fixed throughout the data and corrected for it by shifting the data 31 minutes backward before applying the algorithms.
Table 3 demonstrates the median and mean ARD for BG-SG pairs.
Table 3.
Median (Mean) Absolute Relative Deviation Percentage.
| Glycemic range | Hypoglycemia | Euglycemia | Hyperglycemia | Full glycemic range |
|---|---|---|---|---|
| Number of readings (percentage of the total number) | 604 (8%) | 4546 (63%) | 2077 (29%) | 7227 |
| 2-point calibration | 18.4 (30.1) | 11.4 (16.2) | 9.2 (13.0) | 11.0 (16.5) |
| 1-point calibration | 12.1 (20.4) | 11.5 (16.3) | 8.8 (12.8) | 10.7 (15.6) |
There was a statistically significant difference between the median ARD of the algorithm with the 1-point calibration approach and the median ARD of the algorithm with the 2-point calibration approach, in hypoglycemia (P < .0001), hyperglycemia (P < .01), and the full glycemic range (P < .001). No statistically significant difference was perceived in euglycemia (P > .05).
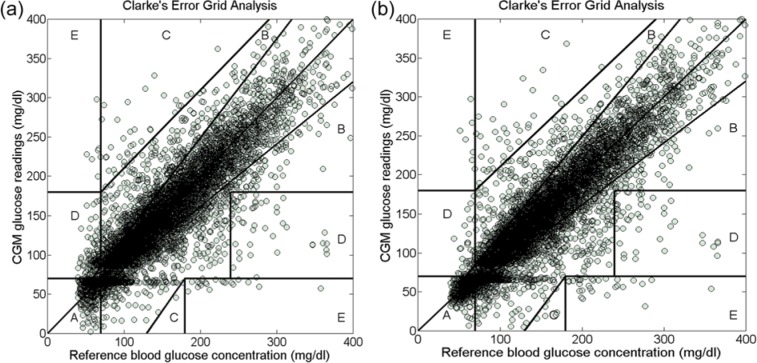
Table 4 indicates the results of Clarke EGA obtained from the 2 versions of the algorithm, in hypoglycemia and in all glycemic ranges.
Table 4.
Paired CGM sensor readings in the Clarke error grid zones in hypoglycemia.
| Hypoglycemia (%) |
Euglycemia (%) |
Hyperglycemia (%) |
Full glycemic range (%) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Calibration algorithm | Zone A | Zone D | Zone E | Zone A | Zone B | Zone C | Zone A | Zone B | Zone C | Zone D | Zone E | Zone A+B |
| 2-point calibration | 77.5 | 21.3 | 1.2 | 73.1 | 26.1 | 0.8 | 79.9 | 16.4 | 0.1 | 2.5 | 1.1 | 96.6 |
| 1-point calibration | 91.6 | 7.3 | 1.1 | 73.4 | 25.8 | 0.8 | 83.2 | 13.2 | 0.1 | 2.1 | 1.4 | 97.7 |
Figure 2 depicts the distribution of the BG-SG pairs in the Clarke EGA zones for the 2 algorithms.
Figure 2.
Clarke error grid analysis for the continuous glucose monitoring readings in the full glycemic range. (a) The algorithm with the 2-point calibration approach. (b) The algorithm with the 1-point calibration approach.
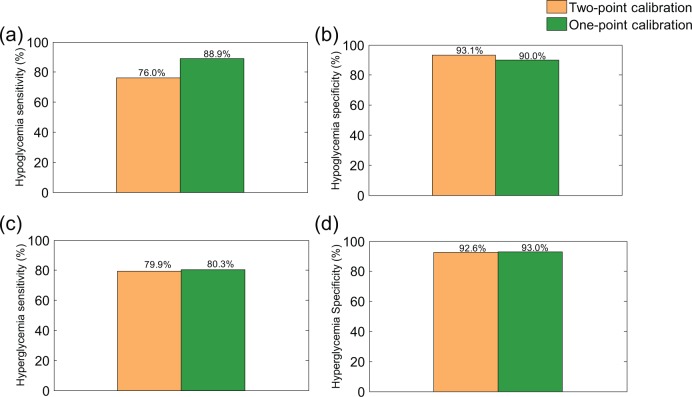
Figure 3 demonstrates the sensitivity and specificity of hypoglycemia and hyperglycemia detection for the 2 versions of the calibration algorithm.
Figure 3.
Hypoglycemia and hyperglycemia sensitivity and specificity, for the 2-point and the 1-point calibration approaches. (a). Hypoglycemia sensitivity; (b) Hypoglycemia specificity; (c) Hyperglycemia sensitivity; (d) Hyperglycemia specificity.
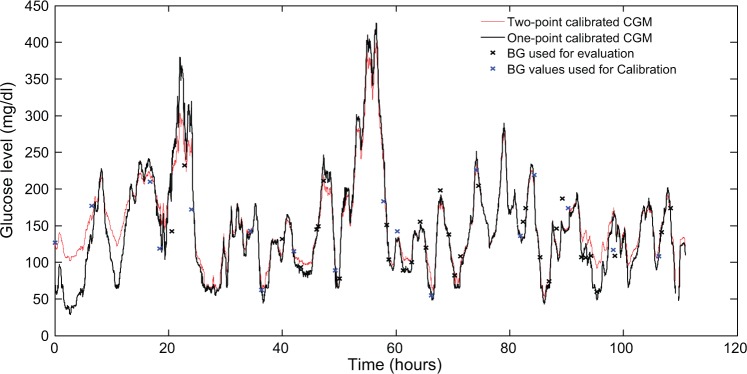
Figure 4.
The continuous glucose monitoring graphs calibrated by the 2 algorithms, along with the blood glucose measurements, used for calibration and evaluation.
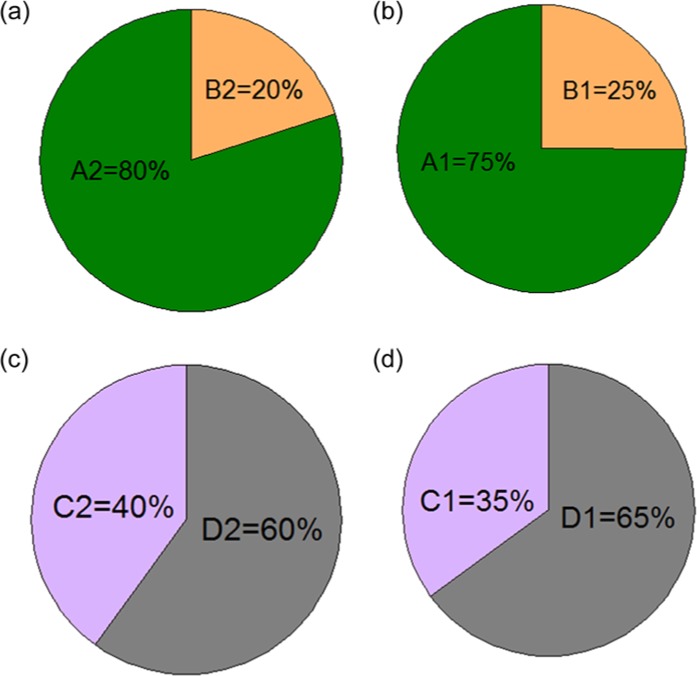
Figure 5 demonstrates the sensitivity and specificity of CI for correction of undetected hypoglycemia.
Figure 5.
Sensitivity and specificity of the corrective intercept. (a) Sensitivity of the corrective intercept for the 2-point calibration, indicated by A1. B1 is the percentage of the SG values that are not transferred to the hypoglycemic range by the corrective intercept, while they have concurrent hypoglycemic BG values. (b) Sensitivity of the corrective intercept for the 1-point calibration, indicated by A1. B2 is the percentage of the sensor glucose values that are not transferred to the hypoglycemic range by the corrective intercept, while they have concurrent hypoglycemic blood glucose values. (c) Specificity of the corrective intercept for the 2-point calibration, indicated by C2. D2 is the percentage of the sensor glucose values that are transferred to the hypoglycemic range by the corrective intercept, while they have concurrent non-hypoglycemic blood glucose values. (d) Specificity of the corrective intercept for the 1-point calibration, indicated by C1. D1 is the percentage of the sensor glucose values that are transferred to the hypoglycemic range by the corrective intercept, while they have concurrent non-hypoglycemic blood glucose values.
After exclusion of CI from the 2-point calibration, the median (mean) ARD were 21.2% (32.6%), 10.8% (15.6%), 9.2% (13.0%), and 10.8% (16.3%) in hypoglycemia, euglycemia, hyperglycemia, and the full glycemic range, retrospectively. For the 1-point calibration, exclusion of CI gave the median (mean) ARD of 14.0% (22.1%), 10.7% (15.5%), 8.8% (12.8%), and 10.3% (15.3%), in hypoglycemia, euglycemia, hyperglycemia, and the full glycemic range, respectively.
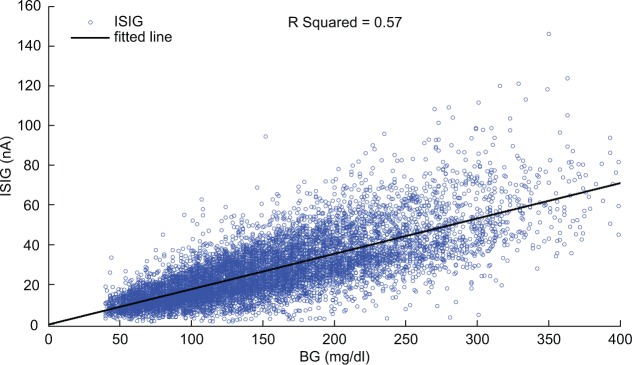
Figure 6 indicates the scatter plot of all ISIG values versus their paired BG measurements, in the complete data set. In total, 9000 BG-ISIG pairs were used for retrospective estimation of I0. Fitting all BG-ISIG pairs to the line in equation 13 gave m = 0.178 nA/mg/dl, with 95% confidence interval of 0.175, 0.181, and I0 = −0.246 nA, with 95% confidence interval of −0.769, 0.277. The 95% confidence interval of I0 contains zero, suggesting that the retrospectively estimated I0 is not statistically greater than zero.
Figure 6.
Scatter plot of all BG-ISIG pairs in the data set, and the line fitted to them ().
The mean of the real-time estimated I0 is -3.5 nA (SD ± 12.5), and 95% confidence interval of −27.9, 21.0. The 95% confidence interval contains zero, suggesting that the real-time estimated I0 is not statistically greater than zero.
Discussion
We compared the accuracy of the 1-point calibration approach with the 2-point calibration approach in our previously published algorithm, by using data from 132 type 1 diabetes patients.
Using the 1-point calibration approach reduced the median and mean ARD in hypoglycemia and hyperglycemia, but did not change ARD in euglycemia. Because the sensor background current exacerbates the CGM inaccuracy mainly in hypoglycemia and hyperglycemia, it seems reasonable that any modification in a calibration algorithm, dealing with the estimation of the background current, affects the CGM accuracy mainly in hypoglycemia and hyperglycemia rather than euglycemia. That explains the obtained results in which the 1-point calibration altered the accuracy in hypoglycemia and hyperglycemia, but not in euglycemia.
Kovatchev et al36 evaluated the accuracy of 4 commercially available CGM devices and indicated that the overall mean ARDs of Guardian, Dexcom, Navigator, and Glucoday are 15.2%, 21.2%, 15.3%, and 15.6%, respectively. Although the overall mean ARD of 15.6% in the 1-point calibration achieved in our study is comparable with those results, a direct comparison is hardly possible due to the different study designs. For example, Kovatchev et al36 reported the use of arterial BG measured by YSI BG analyzer, but we used BG values measured by SMBG meters. A fair comparison of calibration algorithms can be achieved by implementing the algorithms on identical CGM systems worn simultaneously by all subjects.
The overall reduction in the median absolute relative differences (MARD) from 11% in the 2-point calibration to 10.7% in the 1-point calibration may have little impact on a clinician’s confidence in the device; however, the large reduction of the hypoglycemia inaccuracy would likely change clinical perception and can influence the CGM-based clinical decision making in hypoglycemia treatment.
The percentage of the CGM readings in zone A of the Clarke EGA in hypoglycemia and in zone A+B in the full glycemic range increased by using a 1-point calibration instead of a 2-point calibration approach. Because the percentage of the sensor readings in the clinically acceptable zones (zone A in hypoglycemia and zone A+B in the full glycemic range) is an indicator of the CGM clinical safety, the results imply that the use of a 1-point calibration approach may enhance the usefulness and the clinical reliability of the real-time CGM, and increase the patients’ confidence in the glycemic values measured by the sensor.
Using 1-point calibration increased hypoglycemia sensitivity with 12.9%, and had little impact on hyperglycemia sensitivity. This may originate from the larger effect of the 1-point calibration on ARD in the hypoglycemic range rather than that in the hyperglycemic range. The specificity of both hypoglycemia and hyperglycemia slightly decreased, by replacing the 2-point calibration with the 1-point calibration approach.
Results indicate that, in our algorithm, the 1-point calibration approach has generally higher accuracy than the 2-point calibration approach. This is aligned with the results obtained by Choleau et al8 who demonstrated that a 1-point calibration approach produces more accurate CGM readings than a 2-point calibration. It is worth pointing out that the slope and the intercept in the calibration algorithm are not solely identified by the sensor sensitivity and the glucose-nonspecific current due to the interfering substances. However, they are mathematical operators, and according to the statistics of a straight line, their precision is dictated by the precision of both the measurement of the sensor output and the SMBG values. It is therefore expected that the precision of the estimated slope and intercept be poorer in the case of a 2-point calibration compared to a 1-point calibration, because of the greater measurement uncertainty in the 2-point calibration.7,37 Furthermore, if the calibration points are not taken in steady-state condition, the estimated intercept significantly deviates from the true glucose-nonspecific interfering current.37 The accuracy of a 2-point calibration may improve by increasing the number of calibration points used to estimate the slope and the intercept in the calibration regression;38 however, this may not be practical, because the CGM manufacturers strive to keep the number of calibration points as limited as possible.
In the 1-point calibration used in our CGM algorithm, the sensor background current is not considered zero when the calibrated sensor readings are between 70 mg/dl and 85 mg/dl, and we tried to compensate for the effect of background current by inclusion of CI in the calibration. Correction of the sensor background current in the CGM calibration is also suggested by other researchers. Youssef et al7 improved the calibration accuracy by correction for the background current in amperiometric glucose sensors. They demonstrated that the use of a background current of 4 nA in the calibration improves the general accuracy in the Guardian® REAL-Time CGM and also reduces the hypoglycemia overestimation and therefore, leads to better hypoglycemia detection. Also in a published patent from the Medtronic,39 correction for the sensor background current is suggested by inclusion of an offset in the calibration with the 2 levels of zero and 3 nA, depending on the sensor sensitivity; however, the amplitude of this intercept cannot adapt to the glucose level, while CI in our calibration algorithm varies depending on the sensor glucose levels.
Comparing the ARD after excluding CI with the results in Table 3 indicated that for the 2-point calibration, removing CI exacerbates hypoglycemia inaccuracy by 15% increasing of the MARD, and improves euglycemia accuracy by 5% reducing of the MARD. For the 1-point calibration, removing CI exacerbates hypoglycemia inaccuracy by 16% increasing of the MARD, and slightly improves euglycemia accuracy by 7% reducing of the MARD. Because CI acts on the normal SG range (), and due to the moderately low specificity of CI (Figure 5), some of the non-hypoglycemic SG values which are transferred to hypoglycemic range by CI may not have concurrent BG values. That is the reason for the slight reduction in euglycemia accuracy by inclusion of CI. However, comparison between the percentage of the MARD increase in hypoglycemia with the percentage of the MARD reduction in euglycemia, after exclusion of CI from the 2 calibration algorithms, suggests that the presence of CI in the calibration may be yet advantageous despite reducing the euglycemia accuracy. The retrospective and real-time estimations of the sensor background current indicated that the 95% confidence intervals contain zero for both estimation methods. This may suggest that the background current can be considered zero, and a simple 1-point approach suffices for calibration. However, this may not apply to other types of CGM sensors.
It should be noted that in addition to the value of the background current and the way it is estimated in calibration, a host of other factors also affects the quality of a 2-point calibration, and consequently the CGM accuracy. These factors concern the reference BG values used for calibration, including calibration timing and the number of reference BG measurements used in the calibration, the glycemic range in which reference BG values fall, the rate of change of reference BG values at the calibration time, and the difference between the 2 reference BG values.22,28,38,40,41 The DirectNet study group38 indicated that the timing of calibration is more influential on the CGM accuracy than the number of BG points used for calibration. It was also demonstrated that CGM accuracy worsens when calibration is performed in the rate-of-change of ±15 mg/ dl/min, suggesting for calibrating in the periods of glucose stability. Aussedat et al41 introduced a method to identify the 4-min windows of glucose stability in which the sensor signal does not change more than 1%, and also to update calibration when the difference between the two sensor signal values is greater than 2 nA. King et al40 indicated that if the two reference glucose values in the 2-point calibration differ by > 30 mg/dl, the quality of calibration improves. Zueger et al42 demonstrated that preprandial calibration compared to postprandial calibration produces more accurate CGM data in euglycemic and hyperglycemic ranges of reference glucose values. based on preprandial reference glucose values results in significantly higher sensor accuracy in hyperglycemia and euglycemia. Iscoe et al28 indicated that having one of the reference glucose measurements in hypoglycemia reduces the overestimation of hypoglycemic glucose values by sensor.
The reference glucose meters have error compared to gold standard BG assays, for example, laboratory measurements, which also affects the calibration accuracy. In the current study, the accuracy of the BG measurements was not assessed by comparing with a gold standard measurement; however, the authors performed an analysis to indicate the precision of the BG values, by measuring the absolute difference between the 2 capillary BG values taken at each instance. The mean of the absolute differences across the data was 7.7 mg/dl (SD ± 14.3 mg/dl). This may imply that the 2 reference BG values taken at each instance were in reasonable agreement, and therefore a third confirmatory measurement seems unnecessary.
Conclusions
A previously published CGM algorithm was modified by replacing the 2-point calibration approach with the 1-point calibration approach. The results demonstrate that using a 1-point calibration may be superior to a 2-point calibration, in mitigating the insufficiency of the CGM calibration.
Footnotes
Abbreviations: BG, blood glucose; CGM, continuous glucose monitoring; CI, corrective intercept; EGA, error grid analysis; IG, interstitial glucose; ISF, interstitial fluid; ISIG, interstitial signal; MARD, median absolute relative differences; RSD, relative standard deviation; SG, sensor glucose; SMBG, self-monitoring of blood glucose; ZCs, zero crossings.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Roche Diagnostics, Aalborg University.
References
- 1. Rossetti P, Bondia J, Vehi J, Fanelli CG. Estimating plasma glucose from interstitial glucose: the issue of calibration algorithms in commercial continuous glucose monitoring devices. Sensors (Basel). 2010;10(12):10936-10952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barceló Rico F. A multiple local models approach to accuracy improvement in continuous glucose monitoring. Diabetes Technol Ther. 2012;14(1):74-82. [DOI] [PubMed] [Google Scholar]
- 3. Facchinetti A, Sparacino G, Cobelli C. Enhanced accuracy of continuous glucose monitoring by online extended Kalman filtering. Diabetes Technol Ther. 2010;12(5):353-363. [DOI] [PubMed] [Google Scholar]
- 4. Guerra S, Facchinetti A, Sparacino G, Nicolao GD, Cobelli C. Enhancing the accuracy of subcutaneous glucose sensors: a real-time deconvolution-based approach. IEEE Trans Biomed Eng. 2012;59(6):1658-1669. [DOI] [PubMed] [Google Scholar]
- 5. Bequette BW. Continuous glucose monitoring: real-time algorithms for calibration, filtering, and alarms. Diabetes Sci Technol. 2010;4(2):404-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barry Keenan D, Mastrototaro JJ, Weinzimer SA, Steil GM. Interstitial fluid glucose time-lag correction for real-time continuous glucose monitoring. Biomedical Signal Processing and Control. 2013;8(1):81-89. [Google Scholar]
- 7. El Youssef J, Castle JR, Engle JM, Massoud RG, Ward WK. Continuous glucose monitoring in subjects with type 1 diabetes: improvement in accuracy by correcting for background current. Diabetes Technol Ther. 2010;12(11):921-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Choleau C, Klein JC, Reach G, et al. Calibration of a subcutaneous amperometric glucose sensor implanted for 7 days in diabetic patients. Part 2. Superiority of the one-point calibration method. Biosens Bioelectron. 2002;17(8):647-654. [DOI] [PubMed] [Google Scholar]
- 9. Signal M, Le Compte A, Harris DL, et al. Impact of retrospective calibration algorithms on hypoglycemia detection in newborn infants using continuous glucose monitoring. Diabetes Technol Ther. 2012;14(10):883-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rebrin K. Use of subcutaneous interstitial fluid glucose to estimate blood glucose: revisiting delay and sensor offset. Diabetes Sci Technol. 2010;4(5):1087-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vaddiraju S, Burgess DJ, Tomazos I, Jain FC, Papadimitrakopoulos F. Technologies for continuous glucose monitoring: current problems and future promises. Diabetes Sci Technol. 2010;4(6):1540-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Facchinetti A, Favero SD, Sparacino G, Castle JR, Ward WK, Cobelli C. Modeling the glucose sensor error. IEEE Trans Biomed Eng. 2014;6(3):620-629. [DOI] [PubMed] [Google Scholar]
- 13. Rebrin K, Sheppard NF, Jr, Steil GM. Use of subcutaneous interstitial fluid glucose to estimate blood glucose: revisiting delay and sensor offset. Diabetes Sci Technol. 2010;4(5):1087-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kovatchev B, Anderson S, Heinemann L, Clarke W. Comparison of the numerical and clinical accuracy of four continuous glucose monitors. Diabetes Care. 2008;31(6):1160-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bay C, Kristensen PL, Pedersen-Bjergaard U, Tarnow L, Thorsteinsson B. Nocturnal continuous glucose monitoring: accuracy and reliability of hypoglycemia detection in patients with type 1 diabetes at high risk of severe hypoglycemia. Diabetes Technol Ther. 2013;15(5):371-377. [DOI] [PubMed] [Google Scholar]
- 16. Facchinetti A, Sparacino G, Guerra S, et al. Real-time improvement of continuous glucose monitoring accuracy: the smart sensor concept. Diabetes Care. 2013;36(4):793-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Choleau C, Klein J, Reach G, et al. Calibration of a subcutaneous amperometric glucose sensor implanted for 7 days in diabetic patients. Part 2. Superiority of the one-point calibration method. Biosens Bioelectron. 2002;17:647-654. [DOI] [PubMed] [Google Scholar]
- 18. Bequette B. Continuous glucose monitoring: real-time algorithms for calibration, filtering, and alarms. J Diabetes Sci Technol. 2010;4(2):404-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Knobbe EJ, Buckingham B. The extended Kalman filter for continuous glucose monitoring. Diabetes Technol Ther. 2005;7(1):15-27. [DOI] [PubMed] [Google Scholar]
- 20. Facchinetti A, Sparacino G, Cobelli C. Online denoising method to handle intraindividual variability of signal-to-noise ratio in continuous glucose monitoring. IEEE Trans Biomed Eng. 2011;58(9):2664-2671. [DOI] [PubMed] [Google Scholar]
- 21. Facchinetti A, Sparacino G, Cobelli C. An online self-tunable method to denoise CGM sensor data. IEEE Trans Biomed Eng. 2010;57(3):634-641. [DOI] [PubMed] [Google Scholar]
- 22. Kovatchev BP, King C, Breton M, Anderson S, Clarke W. Clinical assessment and mathematical modeling of the accuracy of continuous glucose sensors (CGS). Conf Proc IEEE Eng Med Biol Soc. 2006;1:71-74. [DOI] [PubMed] [Google Scholar]
- 23. Roe JN, Smoller BR. Bloodless glucose measurements. Crit Rev Ther Drug Carrier Syst. 1998;15(3):199-241. [PubMed] [Google Scholar]
- 24. Facchinetti A, Sparacino G, Cobelli C. Modeling the error of continuous glucose monitoring sensor data: critical aspects discussed through simulation studies. Diabetes Sci Technol. 2010;4(1):4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sparacino G, Facchinetti A, Cobelli C. “Smart” continuous glucose monitoring sensors: on-line signal processing issues. Sensors (Basel). 2010;10(7):6751-6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kulcu E. Physiological differences between interstitial glucose and blood glucose measured in human subjects. Diabetes Care. 2003;26(8):2405-2409. [DOI] [PubMed] [Google Scholar]
- 27. Breton M, Kovatchev B. Analysis, modeling, and simulation of the accuracy of continuous glucose sensors. Diabetes Technol Ther. 2008;2(5):853-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Iscoe KE, Davey RJ, Fournier PA. Do different glucose levels at calibration influence accuracy of continuous glucose monitoring readings in vitro? Diabetes Sci Technol. 2012;6(2):477-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Klonoff DC. Continuous glucose monitoring: an endocrine society clinical practice guideline. Clin Endocrinol Metab. 2011;96(10):2968-2979. [DOI] [PubMed] [Google Scholar]
- 30. Mahmoudi Z, Johansen MD, Christiansen JS, Hejlesen OK. A multi-step algorithm for processing and calibration of micro-dialysis continuous glucose monitoring data. Diabetes Technol Ther. 2013; 15(10):825-835. [DOI] [PubMed] [Google Scholar]
- 31. Nielsen JK, Gravholt CH, Djurhuus CB, et al. Continuous subcutaneous glucose monitoring shows a close correlation between mean glucose and time spent in hyperglycemia and hemoglobin A1c. Diabetes Technol Ther. 2007;1(6):857-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kovatchev BP, Clarke WL, Breton M, Brayman K, McCall A. Quantifying temporal glucose variability in diabetes via continuous glucose monitoring: mathematical methods and clinical application. Diabetes Technol Ther. 2005;7(6):849-862. [DOI] [PubMed] [Google Scholar]
- 33. Heller A. Implanted electrochemical glucose sensors. Annu Rev Biomed Eng. 1999;1:153-175. [DOI] [PubMed] [Google Scholar]
- 34. Clarke WL. Evaluating clinical accuracy of systems for self-monitoring of blood glucose. Diabetes Care. 1987;10(5):622. [DOI] [PubMed] [Google Scholar]
- 35. Keenan DB, Mastrototaro JJ, Voskanyan G, Steil GM. Delays in minimally invasive continuous glucose monitoring devices: a review of current technology. Diabetes Sci Technol. 2009;3(5):1207-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kovatchev B, Anderson S, Heinemann L, Clarke W. Comparison of the numerical and clinical accuracy of four continuous glucose monitors. Diabetes Care. 2008;31(6):1160-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Choleau C, Klein JC, Reach G, et al. Calibration of a subcutaneous amperometric glucose sensor implanted for 7 days in diabetic patients. Part 2. Superiority of the one-point calibration method. Biosens Bioelectron. 2002;17(8):647-654. [DOI] [PubMed] [Google Scholar]
- 38. Diabetes Research in Children Network (Direcnet) Study Group, Buckingham BA, Kollman C, et al. Evaluation of factors affecting CGMS calibration. Diabetes Technol Ther. 2006;8(3):318-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mueller JC, Keenan DB, Wang L, Mastrototaro JJ, inventors. Systems and methods for calibrating physiological characteristic sensors. US patent; 2010. [Google Scholar]
- 40. King C, Anderson SM, Breton M, Clarke WL, Kovatchev BP. Modeling of calibration effectiveness and blood-to-interstitial glucose dynamics as potential confounders of the accuracy of continuous glucose sensors during hyperinsulinemic clamp. Diabetes Sci Technol. 2007;1(3):317-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Aussedat B, Thome-Duret V, Reach G, et al. A user-friendly method for calibrating a subcutaneous glucose sensor-based hypoglycemic alarm. Biosens Bioelectron. 1997;12(11):1061-1071. [DOI] [PubMed] [Google Scholar]
- 42. Zueger T, Diem P, Mougiakakou S, Stettler C. Influence of time point of calibration on accuracy of continuous glucose monitoring in individuals with type 1 diabetes. Diabetes Technol Ther. 2012;14(7):583-588. [DOI] [PubMed] [Google Scholar]