Abstract
Background:
Vascular dysfunction due to hyperglycemia in individuals with diabetes is a factor contributing to distal symmetric polyneuropathy (DSPN). Reactive oxygen species reduce the bioavailability of nitric oxide (NO), a powerful vasodilator, resulting in reduced circulation and nerve ischemia. Increases in blood NO concentrations and circulation have been attributed to whole body vibration (WBV). The purpose of this study was to the determine the effects of low-frequency, low-amplitude WBV on whole blood NO concentrations and skin blood flow (SBF) in individuals with symptoms of DSPN.
Methods:
Ten patients with diabetes and impaired sensory perception in the lower limbs participated in this crossover study. Each submitted to 2 treatment conditions, WBV and sham, with a 1-week washout period between. Blood draws for NO analysis and laser Doppler imager scans of SBF were performed before, immediately after, and following a 5-minute recovery of each of the treatments.
Results:
Low-frequency, low-amplitude WBV significantly increased SBF compared to the sham condition (F2,18 = 5.82, P = .0115). Whole blood NO concentrations did not differ between the WBV and sham conditions immediately or 5 minutes after treatment (F2,18 = 1.88, P = .1813).
Conclusions:
These findings demonstrate that patients with diabetes respond to WBV with increased SBF compared to the sham condition. The implication is that WBV is a potential nonpharmacological therapy for neurovascular complications of diabetes.
Keywords: diabetes, neuropathy, nitric oxide, skin blood flow, whole body vibration
Neuropathies are among the most common complications of diabetes mellitus1 and are the cause of more than 60% of all nontraumatic amputations in the United States.2 Neuropathy is a set of syndromes, each with a wide range of clinical and subclinical manifestations, the most common of which is distal symmetric polyneuropathy (DSPN).1,3 Distal symmetric polyneuropathy occurs in both type I and type II diabetes, and the symptoms range considerably. Some patients experience no symptoms but show deficits during neurological examinations, while others experience negative symptoms such as loss of thermal and tactile sensations, especially in the lower limbs.1,3 Still others may experience dysesthesia, a painful prickling or electric shock–like sensation in the legs and/or feet, especially at night.4
The etiology of diabetic neuropathy is multifactorial,3 making it difficult to identify the treatment. However, a common factor in each of the proposed underlying mechanisms of the pathogenesis is reactive oxygen species, which are the products of metabolic dysfunctions that result from hyperglycemia.5-7 Herein lies some insight into addressing the problem of DSPN. Oxidative stress from these free radicals is implicated in vascular dysfunction,7 including a change in the expression of endothelial nitric oxide (NO) synthase,8 resulting in reduced bioavailability of NO. Reduced bioavailability of NO is a factor in nerve ischemia9; therefore, therapies to increase NO may result in increased blood flow and a decrease in symptoms of DSPN.
Nitric oxide production is induced by laminar shear stress, resulting from the frictional forces between the vascular endothelium and moving blood.10 An example of this type of shear stress can be found when blood flows in the vessels during moderate exercise.11,12 Externally applied, low-frequency vibration also results in endothelial shear stress sufficient to produce NO and improve blood flow.13-18 This type of vibration is typically applied to the whole body by having the user stand on a vibration platform.
Prior research has shown that whole body vibration (WBV) improves skin blood flow (SBF)16 in the lower legs and increases NO17 when applied to healthy individuals. Maloney-Hinds et al18 showed that NO production and SBF also increase in individuals with diabetes. However, in their study, vibration was applied locally to the arm only.
The purpose of this investigation was to determine if WBV augments whole blood NO concentrations and SBF in the feet of individuals with diabetic peripheral neuropathy. If so, then WBV may be a noninvasive, nonpharmaceutical means to address one of the underlying complications of diabetes and alleviate the symptoms associated with diabetic neuropathy.
Methods
Study Design
This was a repeated-measures crossover study consisting of 2 treatment conditions: WBV and sham. The outcome measures were SBF and whole blood NO concentrations, taken at 3 times: before treatment, immediately after treatment, and following a 5-minute recovery.
Participants
Twelve individuals, 4 males and 8 females with physician-diagnosed diabetes and/or peripheral neuropathy, were recruited for this study. All candidates were screened for prescription medication that included NO donors, known cardiovascular disease, indications of deep vein thrombosis (DVT), or other health issues that may preclude them from safely participating in vibration treatments, such as orthopedic impairments. In addition to self-reported loss of sensation, all research participants also submitted to quantitative sensory testing (QST) using the TSAII Neurosensory Analyzer (Medoc Ltd, Ramat Yishai, Israel) to confirm that thermal sensory thresholds were outside of age-matched normative data (Table 1). All research participants underwent 2 treatment conditions: a sham timed condition and a WBV treatment in a randomized block order. Participants made 2 visits, 1 for each treatment, with a 1-week washout period between each visit to ensure no lasting effects of vibration. Two candidates were excluded; one failed the DVT screen, and another was not able to complete the study. In all, 10 older participants (mean age, 71 ± 8.27 years), 3 males and 7 females diagnosed with diabetes and/or loss of sensation, completed this research study (Table 2). The institutional review board of Brigham Young University approved all procedures, and all participants provided written informed consent.
Table 1.
Participant Sensory Profile.
| Participant # | Sex | Age, y | Self-reported loss of sensation | QST: cold threshold, °C | Cold threshold within norm | QST: hot threshold, °C | Hot threshold within norm |
|---|---|---|---|---|---|---|---|
| 1 | M | 65 | Y | 28.3 | N | 45.2 | N |
| 2 | M | 83 | Y | 3.4 | Na | 48.8 | Na |
| 3 | M | 73 | Y | 8.8 | N | 48.2 | N |
| 4 | F | 69 | Y | 10.6 | N | 45.8 | N |
| 5 | F | 77 | Y | 0.0 | N | 50.0 | N |
| 6 | F | 80 | Y | 24.8 | Na | 46.6 | Na |
| 7 | F | 79 | Y | 12.3 | N | 49.7 | N |
| 8 | F | 66 | Y | 20.6 | N | 46.9 | N |
| 9 | F | 58 | Y | 22.3 | N | 46.0 | N |
| 10 | F | 60 | Y | 28.8 | Y | 37.5 | Y |
F, female; M, male; N, no; QST, quantitative sensory testing; Y, yes.
Normative data were not available for this age and sex, so the range for one younger age group was used for comparison.
Table 2.
Participant Demographics.
| Characteristics | Values |
|---|---|
| Sex, male/female, n | 3/7 |
| Age, y | 71 ± 8.27 |
| Body mass index, kg/m2 | 30.1 ± 5.12 |
| Duration of diabetes, y | 12.5 ± 6.9 |
Data are presented as mean ± standard error unless otherwise indicated.
WBV Treatment
Whole body vibration was performed on a Galileo 2000 (Orthometrix, White Plains, NY, USA), which is an alternating or reciprocating vibration platform. The frequency was set at 26 Hz. The amplitude, which was approximately 2 mm, was determined by the width of the participants’ stance on the platform. In the sham condition, participants completed the same protocol as in the WBV condition; however, vibration was not imposed.
SBF Measurements
Skin blood flow was measured using a laser Doppler imager (LDI) (Moor Instruments Inc, Oxford, UK). The LDI enables rapid, noninvasive analysis of changes in blood flow in the skin and correlates well with other methods for measuring skin perfusion.19,20
When performing the LDI scan, the laser source was placed 35 cm from the middle of the scan area on the foot. The scan area was set at 5 × 5 cm, and the resolution of the scan was set at 100 × 100 pixels. The scan rate was 4 ms/pixel, and a complete scan required 1 minute and 15 seconds. Markers were placed on the skin just outside of the scan area to allow for repeat measurements in the same region of interest (ROI). The resulting images were evaluated in Moor LDI Processing software v3.1 (Moor Instruments Inc). The mean flux was determined for a 15-cm2 area centered over the ROI.
Blood NO Measurements
At each appointed time, three 2-mL samples of venous blood were drawn from the brachial vein at the cubital fossa. The blood was placed in a tube containing 5 µL of 1000 U/mL heparin and immediately treated with nitrite preservation solution in a 4:1 ratio (vol/vol; whole blood/preservation solution). The preservation solution consisted of 0.8 M ferricyanide and 0.1 M N-ethylmaleimide gently mixed with IGEPAL CA-630, equaling 10:1 vol/vol (total solution/IGEPAL CA-630). Each sample was placed in dry ice and then stored at –80°C until analysis.21
Upon completion of data collection, all the blood samples were processed for nitrite concentration analysis using an ozone-based chemiluminescence detector (CLD) (GE Analytical Instruments, Boulder, CO, USA). The blood was thawed in a 37°C water bath, deproteinated with 100% methanol (1:1 vol:vol), and centrifuged for 3 minutes at 15,000 rpm, and the supernatant fraction was transferred to a clean tube. We repeated the centrifuge and transfer processes twice to remove any residual precipitate. Duplicate aliquots of 100 µL of the nitrite-containing blood samples were injected with a Hamilton syringe (Hamilton Co, Reno, NV, USA) into a glass purge vessel connected to the CLD. The purge vessel contained an acidified triiodide solution consisting of potassium iodide, ultrapure water, and glacial acetic acid. One hundred microliters of dilute solution of antifoaming agent were also added to the purge vessel and changed after every 4 to 6 injections. In the purge vessel, the nitrites in the sample underwent conversion to NO in a chemiluminescent reaction with ozone. The emitted light was detected by a photomultiplier tube in the CLD and recorded as a voltage signal in data analysis software (Liquid Program v3.21, GE Analytical Instruments, Boulder, CO, USA). After all the samples had been injected, we used selection tools in the data analysis program to identify the baseline and peaks of the voltage signals. Finally, the peak tracings were integrated, generating mean values for the NO concentrations in each sample.
Procedure
Upon meeting the inclusion criteria and randomly determining the order of treatment (WBV or sham), the research participant was seated in a dental chair with knees bent at 90° and bare feet resting on an adjustable platform. A 25-cm2 ROI was marked on the center of the dorsum of the participant’s right foot with a 5 × 5–cm cardboard square and a highlighter, allowing for consistent alignment with the LDI. After a 10- to 15-minute acclimatization period, the first LDI scan was performed. Following the scan, a venous catheter was inserted into a large antecubital vein for the first and subsequent blood draws.
After putting shoes on, the participant moved to the vibration platform (5 ft from the chair) and then underwent the randomly assigned WBV or sham treatment. The participant stood on the vibration platform with knees flexed 30° to 40° to attenuate vibration at the pelvis (Figure 1). Ten 30-second bouts of WBV were performed with a 1-minute rest between each bout. During the WBV condition, the platform was powered on so that it vibrated. During the sham condition, the platform was powered off so that the participant stood with knees flexed for ten 30-second bouts on the nonvibrating platform. Following the last bout, the participant immediately returned to the chair and removed the right shoe and sock, and a second LDI scan was performed, followed by a second blood draw. The participant remained seated for an additional 5 minutes from the end of the vibration treatment, after which the third LDI scan was performed and the final blood sample drawn. One week later, the participant completed the protocol for the second treatment condition.
Figure 1.

During both whole body vibration (WBV) and sham conditions, the participants stood in a semisquat position on the platform with feet at a width to define a 2-mm amplitude. During WBV, the platform vibrated. During the sham condition, the machine was turned off.
The LDI scans were evaluated for the mean flux in image processing software. We defined a 15-cm2 ROI and applied it to the same location of each scanned image.
Statistical Analysis
To determine differences in SBF and NO production between treatment conditions, we used a 2 × 3 mixed-model ANCOVA with baseline values as the covariate. We further used Tukey-Kramer post hoc tests to determine differences between the conditions at each time point. We used JMP 9 Pro (SAS Inc, Cary, NC, USA) for all statistical analyses, with an α level of .05.
Results
The WBV and sham conditions had similar SBF (P = .999) and whole blood NO concentrations (P = 1.0) at baseline. Immediately following WBV treatment, SBF significantly increased 15% compared to the sham condition (F2,18 = 5.82, P = .01). However, there was no difference 5 minutes after treatment between the conditions (P = .25) (Figure 2). Whole blood NO concentrations did not differ between the WBV and sham conditions immediately after or 5 minutes after treatment (F2,18 = 1.88, P = .18) (Figure 3).
Figure 2.
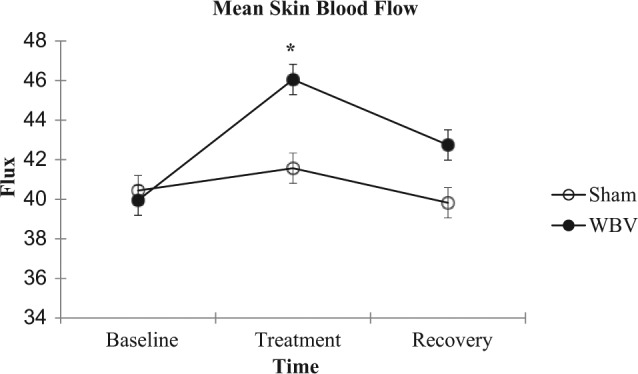
Mean (±standard error) skin blood flow for each group (n = 10) at baseline, immediately after the treatment condition, and after 5 minutes of recovery. *P < .05 different from sham condition.
Figure 3.
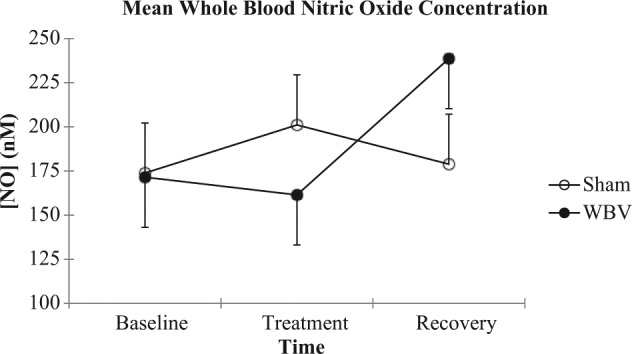
Mean (±standard error) whole blood nitric oxide concentration for each group (n = 10) at baseline, immediately after the treatment condition, and after 5 minutes of recovery.
Discussion
The purpose of this study was to evaluate the effect of WBV on SBF and blood NO concentrations in individuals with DSPN. The results of this study indicate that low-frequency, low-amplitude WBV increases SBF in the feet of individuals who suffer from neuropathy. These results are consistent with findings from other authors who measured the effects of WBV on SBF.16,18
Although no other WBV studies explore any connection between increases in SBF and improvements in sensory nerve impairment, there is evidence that WBV improves sensory symptoms of neuropathy. In several case studies on patients with diabetic peripheral neuropathy, vibration treatment reduced neuropathic pain ratings from above 6 (of 10) to below 2 on a visual analog scale.22-24 Yoosefinejad et al23 did not attempt to provide an explanation for the mechanism by which WBV reduced pain; however, Hong et al24 attributed the reduction of pain to the modulation of pain pathways in the central nervous system due to vibration. The authors did not consider the theory of increased SBF.
While no WBV studies link increases in SBF to peripheral sensory changes, research of other therapeutic modalities that address peripheral neuropathy has shown an association. For example, in a study that tested the effectiveness of the PKC-β inhibitor ruboxistaurin, Casellini et al25 showed that after daily intake of the medication for 6 months, SBF at the distal calf significantly increased and that results on both the Neuropathy Total Symptom Score–6 (NTSS-6) questionnaire and the Norfolk Quality of Life Questionnaire–Diabetic Neuropathy (QOL-DN) improved. Rendell and Bamisedun26 showed that after 6 months of treatment with the antioxidant pentoxifylline, patients with neuropathy exhibited concurrent increases in SBF in the lower extremities, lower threshold in current perception, and improvement in neuropathic symptoms. Ono et al27 not only showed that sensory nerve improvements accompanied increases in SBF but also that the degree of vibration thresholds in the lower extremities was positively correlated with the degree of SBF in the toes of patients with type II diabetes after a 4-week daily dosage of KDI-792, a thromboxane A2 antagonist and prostaglandin I2 promoter. In a nonpharmacological study, twelve 30-minute treatments of monochromatic near-infrared light therapy on the lower leg and foot improved sensitivity in the feet of patients with diabetes.28 The authors attributed the improvement to NO-induced increases in SBF. Each of these modalities is a very different path to the same end result of increased blood flow and improved symptoms of DSPN.
Based on evidence from other studies,18,28 we hypothesized that increases in SBF during WBV could be attributed to the action of NO on vascular tone. However, there were no significant differences in blood NO concentrations between the sham and WBV conditions following the treatment. There are several possible explanations for why there was an increase in SBF without accompanying increases in blood NO concentrations.
The increase in SBF that we measured could have been due to a local effect of NO induced by shear stress in the feet. This is supported by studies in which NO increases were found concurrently with increases in SBF after vibration, and blood for the NO sample was drawn at the same location as the applied vibration.18,29 However, in our study, blood samples for NO concentration measurements were drawn from the antecubital fossa due to inherent problems associated with lower extremity wounds in patients with diabetes, whereas the SBF measurements were made at the dorsum of the foot. In addition, the participant was in a squatting position to attenuate vibration; therefore, shear stress–induced NO production may not have occurred in the arm. An additional study on healthy individuals wherein the blood samples are collected in the lower limb following WBV will provide a good test of whether NO is acting locally to increase SBF.
Another consideration is that factors other than NO may have caused the increase in SBF. For example, SBF may have increased as part of a histamine response to WBV. Indeed, in addition to the increase in blood flow, 2 of the participants observed additional warmth and itchiness in their feet and legs following WBV. However, 3 of the participants who indicated that they were taking an antihistamine showed an increase in SBF after WBV. While the mechanism by which SBF increases with WBV is not certain, the implication remains that WBV has potential to be a nonpharmacological alternative to address DSPN.
Limitations to this study provide opportunity for additional research. Because of the time requirements of our study, 7 of our 10 participants were older females with fewer commitments to work and family than male or younger would-be participants. More exploration needs to be conducted to determine if sex differences in central and local controls of cutaneous blood flow are involved in the response to WBV. However, given that the differences have been attributed to estrogen levels,30 the age of our sample population may have prevented skewed results. Our sample population consisted of individuals diagnosed with diabetes and self-reported neuropathy, confirmed with QST. We did not confirm that our participants’ neuropathy was the result of diabetes. Neuropathy may also be associated with exposure to heavy metal toxins, infections, and poor dietary habits including B12 deficiency. If the mechanism for neuropathy is different than the hyperglycemia-induced lack of NO bioavailability as we hypothesized, then the response to WBV may not be as we predicted. Investigations on the effect of WBV on research participants without diabetes but with neuropathy can provide additional insights.
Conclusions
We demonstrated that immediately following ten 1-minute bouts of WBV at a frequency of 26 Hz and amplitude of 2 mm, SBF on the dorsum of the foot was significantly elevated but decreased towards baseline by 5 minutes into the recovery time. We suspect that this return is due to changes in centrally controlled SBF based on evidence that activation of the sympathetic nervous system is dependent on vibration frequency.31 Blood NO concentrations at the antecubital fossa are not affected perhaps because of the attenuation of vibration at that location or perhaps because an increase in NO due to shear stress is only a local affect. Although there is evidence that neuropathic pain is subdued by WBV, the relationship between increased SBF and reduced neuropathic pain is yet to be clearly established. Pharmacological and near-infrared light studies imply that decreased pain may be attributed to increased SBF and that increased SBF may be attributed to NO-induced vessel dilation. Additional research involving WBV as a therapy for DSPN should include a sensory component and measurements for NO production at the same location as the blood draw.
Footnotes
Abbreviations: CLD, chemiluminescence detector; DSPN, distal symmetric polyneuropathy; DVT, deep vein thrombosis; LDI, laser Doppler imager; NO, nitric oxide; QST, quantitative sensory testing; ROI, region of interest; SBF, skin blood flow; WBV, whole body vibration.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Boulton AJ, Vinik AI, Arezzo JC, et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28:956-962. [DOI] [PubMed] [Google Scholar]
- 2. Li YF, Burrows N, Gregg E, Geiss L. Declining trends in hospitalizations for non-traumatic lower extremity amputation in the diabetic population: United States, 1988-2006. Diabetes. 2010;59:A50. [Google Scholar]
- 3. Vinik AI, Park TS, Stansberry KB, Pittenger GL. Diabetic neuropathies. Diabetologia. 2000;43:957-973. [DOI] [PubMed] [Google Scholar]
- 4. Boulton AJ, Malik RA, Arezzo JC, Sosenko JM. Diabetic somatic neuropathies. Diabetes Care. 2004;27:1458-1486. [DOI] [PubMed] [Google Scholar]
- 5. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813-820. [DOI] [PubMed] [Google Scholar]
- 6. Cameron NE, Eaton SEM, Cotter MA, Tesfaye S. Vascular factors and metabolic interactions in the pathogenesis of diabetic neuropathy. Diabetologia. 2001;44:1973-1988. [DOI] [PubMed] [Google Scholar]
- 7. Dickinson PJ, Carrington AL, Frost GS, Boulton AJM. Neurovascular disease, antioxidants and glycation in diabetes. Diabetes Metab Res Rev. 2002;18:260-272. [DOI] [PubMed] [Google Scholar]
- 8. Zou MH, Shi C, Cohen RA. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J Clin Invest. 2002;109:817-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cameron NE, Cotter MA. The relationship of vascular changes to metabolic factors in diabetes-mellitus and their role in the development of peripheral-nerve complications. Diabetes Metab Rev. 1994;10:189-224. [DOI] [PubMed] [Google Scholar]
- 10. Ulker P, Yaras N, Yalcin O, et al. Shear stress activation of nitric oxide synthase and increased nitric oxide levels in human red blood cells. Nitric Oxide. 2011;24:184-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sackner MA, Gummels E, Adams JA. Effect of moderate-intensity exercise, whole-body periodic acceleration, and passive cycling on nitric oxide release into circulation. Chest. 2005;128:2794-2803. [DOI] [PubMed] [Google Scholar]
- 12. Gielen S, Sandri M, Erbs S, Adams V. Exercise-induced modulation of endothelial nitric oxide production. Curr Pharm Biotechnol. 2011;12:1375-1384. [DOI] [PubMed] [Google Scholar]
- 13. Yue Z, Mester J. On the cardiovascular effects of whole-body vibration, part I. Longitudinal effects: hydrodynamic analysis. Stud Appl Math. 2007;119:95-109. [Google Scholar]
- 14. Mester J, Kleinoder H, Yue Z. Vibration training: benefits and risks. J Biomech. 2006;39:1056-1065. [DOI] [PubMed] [Google Scholar]
- 15. Lythgo N, Eser P, de Groot P, Galea M. Whole-body vibration dosage alters leg blood flow. Clin Physiol Funct Imaging. 2009;29:53-59. [DOI] [PubMed] [Google Scholar]
- 16. Lohman EB, 3rd, Petrofsky JS, Maloney-Hinds C, Betts-Schwab H, Thorpe D. The effect of whole body vibration on lower extremity skin blood flow in normal subjects. Med Sci Monit. 2007;13:CR71-CR76. [PubMed] [Google Scholar]
- 17. Sackner MA, Gummels E, Adams JA. Nitric oxide is released into circulation with whole-body, periodic acceleration. Chest. 2005;127:30-39. [DOI] [PubMed] [Google Scholar]
- 18. Maloney-Hinds C, Petrofsky JS, Zimmerman G, Hessinger DA. The role of nitric oxide in skin blood flow increases due to vibration in healthy adults and adults with type 2 diabetes. Diabetes Technol Ther. 2009;11:39-43. [DOI] [PubMed] [Google Scholar]
- 19. Millet C, Roustit M, Blaise S, Cracowski JL. Comparison between laser speckle contrast imaging and laser doppler imaging to assess skin blood flow in humans. Microvasc Res. 2011;82:147-151. [DOI] [PubMed] [Google Scholar]
- 20. Essex TJ, Byrne PO. A laser doppler scanner for imaging blood flow in skin. J Biomed Eng. 1991;13:189-194. [DOI] [PubMed] [Google Scholar]
- 21. Pelletier MM, Kleinbongard P, Ringwood L, et al. The measurement of blood and plasma nitrite by chemiluminescence: pitfalls and solutions. Free Radic Biol Med. 2006;41:541-548. [DOI] [PubMed] [Google Scholar]
- 22. Hong J. Whole body vibration therapy for diabetic peripheral neuropathic pain: a case report. Health Science Journal. 2011;5:66-71. [Google Scholar]
- 23. Yoosefinejad AK, Shadmehr A, Olyaei G, Talebian S, Bagheri H, Mohajeri-Tehrani MR. Effects of whole-body vibration on a diabetic type 2 patient with peripheral neuropathy. Health Science Journal. 2012;6:576-583. [Google Scholar]
- 24. Hong J, Barnes M, Kessler N. Case study: use of vibration therapy in the treatment of diabetic peripheral small fiber neuropathy. J Bodyw Mov Ther. 2013;17:235-238. [DOI] [PubMed] [Google Scholar]
- 25. Casellini CM, Barlow PM, Rice AL, et al. A 6-month, randomized, double-masked, placebo-controlled study evaluating the effects of the protein kinase c-beta inhibitor ruboxistaurin on skin microvascular blood flow and other measures of diabetic peripheral neuropathy. Diabetes Care. 2007;30:896-902. [DOI] [PubMed] [Google Scholar]
- 26. Rendell M, Bamisedun O. Skin blood flow and current perception in pentoxifylline-treated diabetic neuropathy. Angiology. 1992;43:843-851. [DOI] [PubMed] [Google Scholar]
- 27. Ono Y, Katoh M, Hirayama A, Koike T. Improvement in blood flow and diabetic neuropathy by thromboxane A2 dual blocker KDI-792. Prostaglandins Leukot Essent Fatty Acids. 1995;53:139-145. [DOI] [PubMed] [Google Scholar]
- 28. Kochman AB, Carnegie DH, Burke TJ. Symptomatic reversal of peripheral neuropathy in patients with diabetes. J Am Podiatr Med Assoc. 2002;92:125-130. [DOI] [PubMed] [Google Scholar]
- 29. Ichioka S, Yokogawa H, Nakagami G, Sekiya N, Sanada H. In vivo analysis of skin microcirculation and the role of nitric oxide during vibration. Ostomy Wound Manage. 2011;57:40-47. [PubMed] [Google Scholar]
- 30. Cooke JP, Creager MA, Osmundson PJ, Shepherd JT. Sex differences in control of cutaneous blood flow. Circulation. 1990;82:1607-1615. [DOI] [PubMed] [Google Scholar]
- 31. Ando H, Noguchi R. Dependence of palmar sweating response and central nervous system activity on the frequency of whole-body vibration. Scand J Work Environ Health. 2003;29:216-219. [DOI] [PubMed] [Google Scholar]


