Abstract
Background:
The Hypoglycemia-Hyperglycemia Minimizer (HHM) System aims to mitigate glucose excursions by preemptively modulating insulin delivery based on continuous glucose monitor (CGM) measurements. The “aggressiveness factor” is a key parameter in the HHM System algorithm, affecting how readily the system adjusts insulin infusion in response to changing CGM levels.
Methods:
Twenty adults with type 1 diabetes were studied in closed-loop in a clinical research center for approximately 26 hours. This analysis focused on the effect of the aggressiveness factor on the insulin dosing characteristics of the algorithm and, to a lesser extent, on the glucose control results observed.
Results:
As the aggressiveness factor increased from conservative to medium to aggressive: the maximum observed insulin dose delivered by the algorithm—which is designed to give doses that are corrective in nature every 5 minutes—increased (1.00 vs 1.15 vs 2.20 U, respectively); tendency to adhere to the subject’s nominal basal dose decreased (61.9% vs 56.6% vs 53.4%); and readiness to decrease insulin below basal also increased (18.4% vs 19.4% vs 25.2%). Glucose analyses by both CGM and Yellow Springs Instruments (YSI) indicated that the aggressive setting of the algorithm resulted in the least time spent at levels >180 mg/dL, and the most time spent between 70-180 mg/dL. There was no severe hyperglycemia, diabetic ketoacidosis, or severe hypoglycemia for any of the aggressiveness values investigated.
Conclusions:
These analyses underscore the importance of investigating the sensitivity of the HHM System to its key parameters, such as the aggressiveness factor, to guide future development decisions.
Keywords: aggressiveness factor, algorithm, artificial pancreas, closed loop, tuning parameter
Developing a closed-loop insulin delivery system has the potential to transform diabetes management and improve the lives of those living with the disease. Since the JDRF launched the Artificial Pancreas Project in 2006,1 various studies have reported the feasibility of different closed-loop algorithms to control blood glucose in patients with type 1 diabetes.2-7
We previously reported the performance of the Hypoglycemia-Hyperglycemia Minimizer (HHM) System in a clinical feasibility study of 13 participants with type 1 diabetes.8 The control algorithm of the HHM System automatically adjusted insulin delivery in response to glucose predictions constructed from measurements by a continuous glucose monitor (CGM). Thus, in contrast to reactive threshold-based low glucose suspend systems, which abruptly suspend insulin infusion when the CGM breaches a low threshold (eg, 70 mg/dL), the control algorithm of the HHM System uses a predictive algorithm to mitigate such excursions in advance—if not avoid them altogether. A key variable of the system is the “aggressiveness factor,” which affects how readily the algorithm adjusts insulin infusion in response to changing CGM levels and associated predictions.
While simulations can facilitate an efficient, robust sensitivity analysis of key variables in an algorithm, they cannot be a surrogate for actual clinical evaluations. Hence, the current study prospectively investigated the effect of 3 values of the aggressiveness factor (conservative, medium, and aggressive) on the quantitative insulin-dosing characteristics of the HHM System. In addition, glucose-outcome data were investigated based on both CGM and YSI, as a function of algorithm aggressiveness.
Methods
This nonrandomized, uncontrolled feasibility study enrolled 20 adults with type 1 diabetes at 2 clinical research centers (CRCs; Sansum Diabetes Research Institute [SDRI], Santa Barbara, CA; and the Center for Diabetes Technology at the University of Virginia, Charlottesville, VA) from July 16 to August 22, 2012. Participants were studied in closed-loop for approximately 26 hours, receiving 3 meals of 30-70 g carbohydrates (CHO) each with matched insulin boluses.
This study abided by the principles of the Declaration of Helsinki, received approval from the relevant Institutional Review Board (IRB) at each CRC (Compass Independent Review Board and University of Virginia IRB for Health Sciences Research), and all patients provided written informed consent prior to study initiation.
Study Objectives
The primary objective was to evaluate the effect of the aggressiveness factor on the quantitative insulin-dosing characteristics of the HHM System. Secondary objectives were to evaluate the impact of varying the aggressiveness factor on the ability of the HHM System to control glucose to an approximately normoglycemic zone.
Participants and Study Design
Inclusion and exclusion criteria for participants and study design were previously described by Finan and colleagues.8 In brief, adults with type 1 diabetes using an insulin infusion pump along with rapid-acting insulin, and with a hemoglobin A1c level < 10% were enrolled. Prior to the CRC visit, individual participant pump settings (including basal profiles and insulin-to-CHO ratios) were assessed and a CGM sensor inserted. Assessment of the HHM System occurred during the CRC visit and lasted approximately 26 hours for each participant. All diabetes care in the CRC was performed by study staff. Close clinical supervision and regular YSI monitoring were performed. The CGM was calibrated per manufacturer’s instructions. A follow-up telephone call was conducted 24 hours after discharge.
Treatment Interventions
Treatment interventions included algorithm-initiated treatment for hypoglycemia, and investigator-initiated treatment for hypoglycemia and hyperglycemia. For algorithm-initiated treatment for hypoglycemia, the algorithm of the HHM System includes warnings for imminent hypoglycemia, which advise to consider ingesting 16 g of supplemental CHO. In this study, per protocol the investigator administered the supplemental CHO at the time of the warning.
For investigator-initiated treatment for hypoglycemia, treatment interventions were given in the form of supplemental CHO in the absence of an HHM System warning when the YSI indicated a glucose concentration <60 mg/dL. Likewise, for investigator-initiated treatment for hyperglycemia, treatment interventions were (intended to be) given in the form of additional insulin when the YSI indicated a glucose concentration >300 mg/dL for 1 hour.
Investigational Device
The HHM System comprised a continuous subcutaneous insulin infusion pump (OneTouch® Ping® Glucose Management System; Animas Corporation, Wayne, PA), a CGM system (Dexcom® SEVEN® PLUS Continuous Glucose Monitor; Dexcom, Inc, San Diego, CA), and the HHM algorithm (Zone Model Predictive Controller and Safety Supervision Module) run on the University of California, Santa Barbara/SDRI Artificial Pancreas System (APS©) platform.9 The HHM algorithm included an insulin delivery controller and a safety module with built-in warnings for imminent hypoglycemia.
In brief, the control algorithm operated as follows. The algorithm received a new CGM measurement nominally every 5 minutes. Based on this new glucose datum, glucose and insulin data from the recent past, and internal model states, a prediction describing the optimal glucose outcome for the near future was made by manipulating near-future insulin delivery amounts. The insulin dose determined to result in the best glucose outcome was delivered immediately, realized as a small “micro-bolus.” After 5 minutes had elapsed the process was repeated.
Aggressiveness Factor
The primary variable parameter evaluated during this study was the aggressiveness factor, which affects how quickly the controller responds to changes in measured glucose. Three values of the aggressiveness factor were investigated: a “conservative” value, a “medium” value, and an “aggressive” value. The first 15 participants were randomly assigned to 1 of the 3 values, while ensuring that there were 5 participants studied at each value. Based on the results obtained from the first 15 participants, it was determined to study the last 5 participants at the medium value. Thus, in total, 5 participants used the conservative setting, 5 used the aggressive setting, and 10 used the medium setting.
In brief, the aggressiveness factor in certain model predictive control frameworks is an adjustable tuning parameter that gives “weight” to how undesirable it is to change the value of the so-called manipulated variable away from a set point. In the case of the algorithm of the HHM System, the manipulated variable is the insulin infusion amount and its set point is the subject’s basal profile. A conservative value results in an algorithm that tends to adhere to the basal insulin delivery amount, unless the CGM indicates a severe excursion into hypo- or hyperglycemia. However, an aggressive value results in an algorithm that is quicker to react to changing CGM levels, readily adjusting insulin delivery away from basal to prevent more modest glucose excursions.
In the context of the aggressiveness factor, it is worth stating that “aggressive” does not necessarily imply the delivery of more insulin. It is true that an “aggressive” controller will mitigate predicted hyperglycemia with more insulin more quickly, but on the other end it will mitigate predicted hypoglycemia with a more pronounced and quicker reduction/suspension of insulin. This competing effect was investigated in the study.
The controller is designed to deliver only doses that are corrective in nature, applicable to both above-basal as well as below-basal doses. It is not designed to dose insulin that is directly related to CHO meals. As such, all “controller doses” reported in this study should be construed as corrective doses, and considered accordingly.
Results
Participant Characteristics
A total of 20 adults with type 1 diabetes completed the study, 10 per CRC. Characteristics of the participants are shown in Table 1.
Table 1.
Participant Characteristics.
| Characteristic | Participants (N = 20) |
|---|---|
| Age (years) | |
| Mean ± SD | 42.2 ± 11.7 |
| Range | |
| Gender, n (%) | 21.8-61.3 |
| Female | 11 (55) |
| Male | 9 (45) |
| Ethnicity, n (%) | |
| Hispanic | 2 (10) |
| Non-Hispanic | 18 (90) |
| Body mass index (kg/m2) | |
| Mean ± SD | 26.9 ± 7.4 |
| Range | 20.5-55.0 |
| Duration of type 1 diabetes (years) | |
| Mean ± SD | 25.9 ± 13.1 |
| Range | 5.9-60.0 |
| Duration of pump use (years) | |
| Mean ± SD | 13.2 ± 8.2 |
| Range | 2.1-31.6 |
| Hemoglobin A1c (%) | |
| Mean ± SD | 7.5 ± 0.9 |
| Range | 6.2-9.3 |
Abbreviation: SD, standard deviation.
Effect of the Aggressiveness Factor on Insulin Delivery
The effect of the algorithm aggressiveness factor on insulin delivery characteristics is shown in Table 2. In general, with increasing aggressiveness, the maximum controller doses—which were given every 5 minutes—were larger (up to 2.20 U with the aggressive controller) and controller doses > 0.5 U were more frequent (6.4% of controller doses were >0.5 U using the aggressive setting). The tendency to adhere to basal doses decreased, and the readiness to decrease the insulin dose below basal increased.
Table 2.
Effects of the Algorithm Aggressiveness Factor on Insulin Infusion Characteristics.
| Insulin infusion metric (5-minute dosing intervals) | Conservative (n = 5) | Medium (n = 10) | Aggressive (n = 5) |
|---|---|---|---|
| Maximum controller dose (U) | 1.00 | 1.15 | 2.20 |
| Percentage of controller doses > 0.5 U | 1.2 | 2.2 | 6.4 |
| Percentage of controller doses > basal | 19.7 | 24.0 | 21.4 |
| Percentage of controller doses = basal | 61.9 | 56.6 | 53.4 |
| Percentage of controller doses < basal | 18.4 | 19.4 | 25.2 |
A greater degree of granularity in the effect of the aggressiveness factor on insulin delivery characteristics of the algorithm can be seen when the results are separated by glucose range (Figure 1). The controller using the conservative setting delivered on average 43% less insulin than the subject’s corresponding basal rate at low glucose levels (CGM < 70 mg/dL). By contrast, the controllers using the medium and aggressive settings delivered virtually no insulin in this glucose range (average deliveries, as changes from the corresponding basal, were −97% and −100%, respectively).
Figure 1.
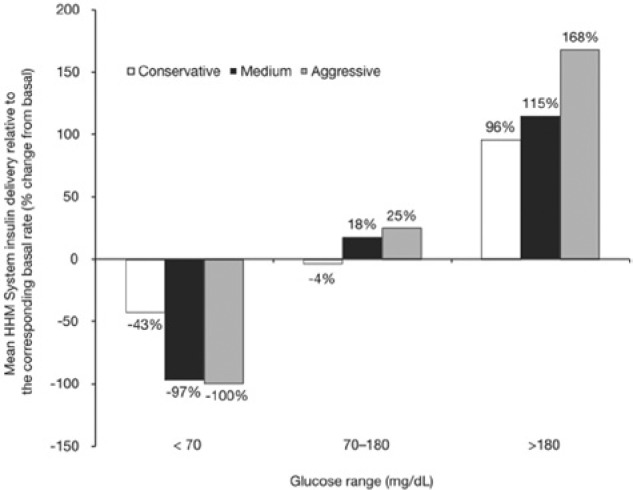
Effect of aggressiveness factor as a function of glucose range. Mean HHM System insulin delivery relative to basal (shown as percentage change from the corresponding basal rate) is shown for each rate of the 3 aggressiveness factors studied and calculated for each of the 3 glucose ranges.
As shown in Figure 1, while in the approximately normoglycemic range (CGM 70-180 mg/dL) the conservative controller adhered largely to basal delivery, with its average dose in this range equal to −4% of the corresponding basal amount. The medium and aggressive controllers’ average deliveries in this range were +18% and +25% of the corresponding basal amount, respectively.
In the hyperglycemic range (CGM >180 mg/dL), the average dose of the HHM System relative to basal increased as controller aggressiveness increased (conservative +96%, medium +115%, and aggressive +168%).
Effect of the Aggressiveness Factor on Glucose Metrics
Irrespective of the aggressiveness factor, participants spent 80-90% of closed-loop time at approximately normoglycemic levels of 70-180 mg/dL. Based on CGM, time spent in this range (70-180 mg/dL) was the highest with the controller using the aggressive setting (89.2% vs 79.7-81.1%) (Table 3). In addition, time spent at glucose levels > 180 mg/dL was the lowest with the aggressive controller (6.2% vs 15.9-17.1%). Time spent at glucose levels < 70 mg/dL was (marginally) the lowest with the controller using the medium setting (3.0% vs 3.2-4.5%).
Table 3.
Glucose Control Metrics by Algorithm Aggressiveness Factor Based on CGM and YSI.
| Closed-loop time spent in glucose ranges, % |
|||
|---|---|---|---|
| Range | Conservative (n = 5) | Medium (n = 10) | Aggressive (n = 5) |
| CGM | |||
| >180 mg/dL | 17.1 ± 12.2 | 15.9 ± 25.4 | 6.2 ± 4.3 |
| 70-180 mg/dL | 79.7 ± 11.8 | 81.1 ± 24.0 | 89.2 ± 9.6 |
| <70 mg/dL | 3.2 ± 2.5 | 3.0 ± 3.0 | 4.5 ± 6.3 |
| <55 mg/dL | 0.3 ± 0.4 | 0.5 ± 1.2 | 1.3 ± 1.8 |
| YSI | |||
| >180 mg/dL | 7.3 ± 9.7 | 15.4 ± 17.3 | 4.7 ± 5.6 |
| 70-180 mg/dL | 81.6 ± 13.9 | 81.5 ± 15.3 | 90.3 ± 9.5 |
| <70 mg/dL | 11.2 ± 16.4 | 3.1 ± 4.3 | 5.0 ± 9.7 |
| <55 mg/dL | 1.7 ± 3.8 | 0.1 ± 0.2 | 0.3 ± 0.6 |
All values mean ± standard deviation. CGM, continuous glucose monitor.
Similar results were obtained with YSI. Time spent in the approximately normoglycemic range (CGM 70-180 mg/dL) was the highest with the controller using the aggressive setting (90.3% vs 81.5-81.6%), and time spent in the hyperglycemic range (CGM >180 mg/dL) was the lowest with the aggressive controller (4.7% vs 7.3-15.4%). Also, time spent at glucose levels <70 mg/dL was the lowest with the medium controller (3.1% vs 5.0-11.2%).
Figure 2 shows the graphical results of the 20 participants in the study. Shown is the mean tracing for all 20 subjects inclusive, plus and minus its 1 standard deviation (SD) envelope. Superimposed, for comparison, are the mean tracings for the 3 aggressiveness factor subcohorts. Only small portions of the ± 1 SD envelope of all participants breached the approximately normoglycemic range (70-180 mg/dL). The mean CGM tracings for all participants and for the medium-setting group were entirely contained in the 70-180 mg/dL range, whereas those of the conservative- and aggressive-setting groups were mostly contained within this range (99% of the conservative tracing was contained, and 95% of the aggressive tracing was contained).
Figure 2.
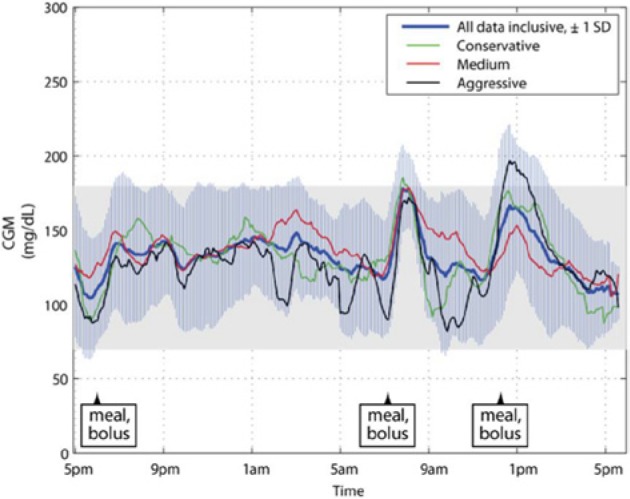
Mean CGM tracings of all patient clinic visits during closed-loop control, plotted inclusively and by aggressiveness factor subcohort. Also shown is the ±1 SD envelope around the mean tracing for the entire cohort. CGM, continuous glucose monitor; SD, standard deviation.
Safety
Protocol-defined glucose-related safety events (severe diabetic ketoacidosis or severe hypoglycemia) did not occur in any study participants. One participant experienced an adverse device effect of nausea, emesis and elevated ketones (0.9 mmol/L), probably related to telemetry issues in the investigational device which caused nondelivery of insulin for about 3 hours. The effect was mild and resolved with treatment by the investigator.
Table 4 summarizes additional safety information related to administrations of supplemental CHO. Sixteen participants required at least 1 algorithm-initiated treatment for hypoglycemia. Two of these participants each required 12 administrations. Excluding these 2 participants, there were only 2.4 such administrations per participant per CRC visit. The HHM System-generated hypoglycemia warnings that initiated these CHO administrations were found to be timely based on investigator concurrence; they were heeded 85% of the time. Two of the 20 participants required protocol-mandated, investigator-initiated treatment for hypoglycemia, based on YSI readings <60 mg/dL in the absence of HHM System-generated warnings. No participants required treatment for hyperglycemia (including the participant with elevated ketones).
Table 4.
Summary of Safety Events.
| Metric | Value |
|---|---|
| Algorithm-initiated treatment for hypoglycemia | |
| Number of participants requiring CHO administration | 16 |
| Average number of CHO administrations per participant, per CRC visit | 3.4 |
| Investigator-initiated treatment for hypoglycemia | |
| Number of participants requiring CHO administration | 2 |
| Average number of CHO administrations per participant, per CRC visit | 0.4 |
| Treatment for hyperglycemia | |
| Number of participants requiring CHO administration | 0 |
CHO, carbohydrates; CRC, clinical research center.
CGM-Related Issues
Two device malfunctions were reported. For 1 participant, there were communication problems between the sensor and the artificial pancreas system that remained after recalibrating the CGM and restarting the system; the study was aborted for that participant. For another participant, both CGM sensors failed and the closed-loop session had to be aborted and rescheduled.
Discussion
This feasibility study demonstrated that the algorithm aggressiveness factor influenced the tendency of the HHM System to modify the insulin dose away from basal, and that the best overall glucose results based on time in the 70-180 mg/dL range were obtained with the most aggressive setting. There were no instances of severe ketoacidosis or severe hypoglycemia.
The primary study objective was to evaluate the effect of the aggressiveness factor on the quantitative insulin-dosing characteristics of the HHM System. Differences in the algorithm’s insulin infusion characteristics were observed among the 3 aggressiveness factors. In general, with increasing aggressiveness, the “larger” doses were larger and more frequent, the tendency to adhere to basal decreased, and the readiness to decrease insulin below basal increased.
Preemptive mitigating action was taken prior to below- and above-zone excursions for all 3 aggressiveness factors. With increasing aggressiveness, however, insulin delivery was reduced to a greater degree during glucose excursions <70 mg/dL (−43% to −100% relative to basal rate), and increased to a greater degree during glucose excursions >180 mg/dL (+96% to +168% relative to basal rate). In summary, the tendency of the algorithm to modify the insulin dose increased as the aggressiveness factor increased. While this result was qualitatively expected from control theory, the quantitative findings from the study serve as an important validation in human subjects.
When analyzing the effect of the aggressiveness factor on glucose metrics, the best overall results in terms of time in the 70-180 mg/dL range were observed using the most aggressive value, by both CGM and YSI. A similar analysis on the effect of the aggressiveness factor in terms of time spent in the low range (<70 mg/dL) yields a different conclusion: the medium aggressiveness factor resulted in the best performance, by both CGM and YSI. Extracting such directional guidance regarding the effect of this parameter on algorithm response and associated outcome is the reason for conducting this type of feasibility study.10
We note that this study is primarily limited by the small sample size, relatively short observation period, and the artificial, sedentary CRC-based environment. Also, because of the small sample size and exploratory analysis of this study, no statistical analyses were performed.
Conclusions
This feasibility study demonstrates that the aggressiveness factor affects the tendency of the algorithm to modify the insulin dose, which underscores the importance of investigating the sensitivity of the HHM System to its key parameters, thereby guiding future development decisions. This study also confirms the potential of the HHM System to maintain good glucose control in individuals with type 1 diabetes, providing further evidence to continue clinical development of the HHM System.
Acknowledgments
The authors would like to thank JDRF for their guidance and support, and the staffs of Sansum Diabetes Research Institute and the UVa Center for Diabetes Technology for their participation in this study. The authors received editorial support from Excerpta Medica for preparation of this manuscript. Parts of the study were presented at the 6th International Conference on Advanced Technologies & Treatments for Diabetes (ATTD), Paris, France, February 27-March 2, 2013; and at the 73rd Scientific Sessions of the American Diabetes Association (ADA), Chicago, IL, June 21-25, 2013.
Footnotes
Abbreviations: CGM, continuous glucose monitoring; CHO, carbohydrates; CRC, clinical research center; HHM, Hypoglycemia-Hyperglycemia Minimizer; IRB, institutional review board; SDRI, Sansum Diabetes Research Institute; YSI, Yellow Springs Instruments.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: DAF and RV are employees of Animas Corporation. TWM, KR, and HA were employees of Animas Corporation at the time of execution of the study. TWM is currently employed by DePuy Synthes. KR is currently employed by Incyte Corporation. HA is currently employed by Sanofi. ED has received product support from Animas Corporation, Dexcom Inc, and Insulet Corporation. MDB has received research grants and product support from Animas, Insulet, Dexcom Inc, Roche, Sanofi, Abbott, BD, Lilly, and Tandem Diabetes Care. SDP and FJD have no conflicts of interest. BPK has acted on the advisory board/consulted for Animas Corporation and Dexcom Inc, and has received research grant and product support from Animas Corporation, Insulet Corporation, and Tandem Diabetes Care. SMA has received research grant and product support from Animas Corporation and Medtronic Inc, product support from Becton Dickenson and Company and consulting fees from Senseonics. HZ has received honoraria for scientific lectures and travel reimbursement from Animas Corporation, Cellnovo, Insulet Corporation, MannKind Corporation, and Roche; and research grant and product support from Animas Corporation, Abbott Laboratories, Dexcom Inc, Eli Lilly and Co, GluMetrics Inc, Insulet Corporation, LifeScan Inc, Medtronic Inc, Novo Nordisk, Roche, and Sanofi; and is a board member of Artificial Pancreas Technologies.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by JDRF and Animas Corporation.
References
- 1. Kowalski AJ. Can we really close the loop and how soon? Accelerating the availability of an artificial pancreas: a roadmap to better diabetes outcomes. Diabetes Technol Ther. 2009;11(suppl 1):S113-S119. [DOI] [PubMed] [Google Scholar]
- 2. Breton M, Farret A, Bruttomesso D, et al. Fully integrated artificial pancreas in type 1 diabetes: modular closed-loop glucose control maintains near normoglycemia. Diabetes. 2012;61(9):2230-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dassau E, Zisser H, Harvey RA, et al. Clinical evaluation of a personalized artificial pancreas. Diabetes Care. 2013;36(4):801-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hovorka R, Allen JM, Elleri D, et al. Manual closed-loop insulin delivery in children and adolescents with type 1 diabetes: a phase 2 randomised crossover trial. Lancet. 2010;375(9716):743-751. [DOI] [PubMed] [Google Scholar]
- 5. Russell SJ, El-Khatib FH, Nathan DM, Magyar KL, Jiang J, Damiano ER. Blood glucose control in type 1 diabetes with bihormonal bionic endocrine pancreas. Diabetes Care. 2012;35(11):2148-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Philip M, Battelino T, Atlas E, et al. Nocturnal glucose control with an artificial pancreas at a diabetes camp. N Engl J Med. 2013;368(9):824-833. [DOI] [PubMed] [Google Scholar]
- 7. Kovatchev BP, Renard E, Cobelli C, et al. Feasibility of outpatient fully integrated closed-loop control: first studies of a wearable artificial pancreas. Diabetes Care. 2013;36(7):1851-1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Finan DA, McCann TW, Jr, Mackowiak L, et al. Closed-loop control performance of the Hypoglycemia-Hyperglycemia Minimizer (HHM) System in a feasibility study. J Diabetes Sci Technol. 2014;8(1):35-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dassau E, Zisser H, Palerm CC, Buckingham BA, Jovanovic L, Doyle FJ., III Modular artificial beta-cell system: a prototype for clinical research. J Diabetes Sci Technol. 2008;2(5):863-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. U.S. Food and Drug Administration/Center for Devices and Radiological Health. The content of investigational device exemption (IDE) and premarket approval (PMA) applications for artificial pancreas device systems. 2012. [Google Scholar]


