Abstract
Background:
In 2008 a Nordic collaboration was established between the quality registries in Denmark, Iceland, Norway, and Sweden to improve quality of care for children with diabetes. This study aimed to describe those registries and confirm that the registry variables are comparable. Selected variables were used to demonstrate outcome measurements.
Methods:
The organization of the registries and methodology are described. Cross-sectional data for patients between birth and 14.9 years with type 1 diabetes mellitus in 2009 (n = 6523) from 89 centers were analyzed. Variables were age, gender, and diabetic ketoacidosis at onset, together with age, gender, HbA1c, insulin regimen, and severe hypoglycemia at follow-up in 2009.
Results:
All 4 registries use a standardized registration at the onset of diabetes and at follow-up, conducted at the local pediatric diabetes centers. Methods for measuring HbA1c varied as did methods of registration for factors such as hypoglycemia. No differences were found between the outcomes of the clinical variables at onset. Significant variations were found at follow-up for mean HbA1c, the proportion of children with HbA1c < 57 mmol/mol (NGSP/DCCT 7.4%), (range 15-31%), the proportion with insulin pumps (range 34-55%), and the numbers with severe hypoglycemia (range 5.6-8.3/100 patient years).
Conclusions:
In this large unselected population from 4 Nordic countries, a high proportion did not reach their treatment target, indicating a need to improve the quality of pediatric diabetes care. International collaboration is needed to develop and harmonize quality indicators and offers possibilities to study large geographic populations, identify problems, and share knowledge.
Keywords: Diabetes Mellitus Type 1, Quality of Health Care, Registries, pediatrics, hemoglobin A1c, glycosylated
Introduction
The worldwide incidence of childhood onset type 1 diabetes mellitus is increasing.1 In 2009, it was predicted that cases in people younger than 15 years of age would increase by 70% between 2005 and 2020.2
Type 1 diabetes is a chronic disease with no cure and high quality of care is essential to avoid acute and long-term complications. Studies, including the Diabetes Control and Complications Trial (DCCT),3,4 have shown that improved metabolic control is important in preventing, delaying or slowing down the progression of long-term complications.
The treatment targets for glycemic control are difficult to achieve,3,5-7 and we need to improve the quality of pediatric diabetes care and to increase our knowledge of the factors associated with glycemic control to improve these outcomes.
Diabetes quality registries enable us to study clinical data and care outcomes. International collaboration is both necessary and desirable if we are to compare incidence, treatment and quality indicators. Quality registry in the Nordic countries provide nationwide population-based data with high ascertainment rates, which are a prerequisite for collaboration. A quality registry allows each country to continuously follow its quality indicators and results, to benchmark its results within the country and with other countries, and to identify areas that need improving. Studies have shown how registries have been used to assess differences in quality of care, treatment patterns, and environmental and behavioral risk factors for cancer and other chronic conditions.1,8,9 The Clinical Practice Consensus Guidelines published by the International Society of Pediatric and Adolescent Diabetes (ISPAD)9 have been implemented in the Nordic countries of Denmark Iceland, Norway, and Sweden. Finland does not have a quality registry that enable us to follow-up results and was not included in this study. The diabetes quality registries include standardized clinical data on diabetes care, including blood samples results, that are prospectively collected each year, based on The Basic Information Sheet for Children & Adolescents, implemented from the St Vincent Declaration.9 The data are then reported to the national quality registry in the respective country.10-12 The quality indicators are used to benchmark the local pediatric diabetes centers in each country.
The quality registries in Denmark and Iceland were established, with the current organizations, in 1996, followed by Sweden in 2000 and Norway in 2006. The Nordic Childhood Diabetes Registry Study Group (NordicDiabKids) comprises members of the steering committees of the four registries and was established in 2008. NordicDiabKids has annual meetings where guidelines and results are presented and areas of improvement and new treatment strategies are discussed. All the registries are based in countries with relatively healthy finances, equal-opportunity health care for all citizens, and no restrictions on insulin or blood glucose devices. The total population of the 4 countries in 2009 was about 20 million, with 9.4 million citizens in Sweden, 5.6 million in Denmark, 4.9 million in Norway, and 0.32 million in Iceland. To our knowledge, no previous comparison has been carried out on national quality registers, diabetes treatment, and care outcomes in different countries.
This study aims to describe the registries in the four Nordic countries, and to confirm that the registry variables were comparable. Selected outcome variables were used to exemplify outcome measurements.
Methods
A cross-sectional design was used to analyze data from 4 nationwide pediatric diabetes quality registries in 2009 and describe the variables. The registries included all pediatric diabetes centers in Denmark (n = 18), Iceland (n = 1), Norway (n = 27), and Sweden (n = 43), which treat all children and adolescents in their catchment areas up to the age of 18, with the exception of Denmark, which goes up to 16. Our study included nationwide, population-based cohorts of patients from birth to 14.9 years with type 1 diabetes from all 4 countries, using data registered in 2009, but excluding patients with onset in 2009.
The variables presented and described at onset were age, gender, and diabetic ketoacidosis (DKA). At follow-up they were age, gender, HbA1c, insulin dose, use of continuous subcutaneous insulin infusion (CSII), and severe hypoglycemia (SH).
For HbA1c at follow-up and insulin regimen, the first registered value for each patient in 2009 (Norway, Sweden) or the value closest to their birthday that year (Denmark, Iceland) was used. The International Federation for Clinical Chemistry and Laboratory Medicine (IFCC) reference method has been adopted in Sweden whereas the National Glycohemoglobin Standardization Program (NGSP)/DCCT values are used in the other 3 countries.13,14 The treatment target of IFCC value 57 mmol/mol corresponds to a 7.4% NGSP/DCCT value and 10 mmol/mol is about 0.9%.13,14 HbA1c values are presented as IFCC units (mmol/mol) followed by NGSP/DCCT (%).
Statistical Methods
PASW Statistics 18 was used for statistical analysis with descriptive statistics presented as means and proportions. SH is presented as number per 100 patient years. ANOVA and chi-square test were used for comparison between countries. P values < .05 were regarded as significant.
Results
Financial Support
The registries in Denmark, Norway, and Sweden are financially supported by governmental or health authorities, and the Icelandic registry is supported by the pediatric hospital where it is based.
Methodology in the Registries
All 4 registries have standardized registration at the onset of diabetes and at follow-up, which is annually in Denmark and Norway and at every visit in Iceland and Sweden. Registration is conducted at the local pediatric diabetes centers and is either paper-based (Norway) or electronic (Sweden, Iceland, Denmark). HbA1c standardization was performed in all countries and locally analyzed (Iceland, Sweden) or centrally measured once a year (Denmark, Norway). Data on DKA were collected from patient records. Registration of SH was based on patients’ self-reported data and insulin doses were self-reported or data were downloaded from insulin pumps. In Denmark and Norway, which have annual registration, adverse events (SH, DKA) were totaled for the previous year or since last registration. In the other 2 countries they were registered at each visit and then totaled, leading to some differences in calculations of risk time.
Variables Registered
Quality indicators were registered and data available from the registries are described in Table 1. All countries used the same method of registering diabetes classification.15 After the onset of diabetes, information on HbA1c value, use of CSII/insulin pen, episodes of DKA and SH were registered in all countries annually or at every visit to the outpatient clinic. Insulin dose (U/kg/24h) was registered in Denmark, Norway, and Sweden. All countries registered blood pressure (BP) and puberty state, with BP at every Swedish visit. Fundus photography, screening for albuminuria, screening for celiac disease, and thyroid morbidity were registered in all countries, but the care routines and examination intervals differed. Some countries registered the number of insulin doses, body mass index standard deviation score (BMI-SDS), blood lipid screening, physical activity, and smoking habits. Ethnicity was documented in a similar manner in all countries, but health-related quality of life was not routinely registered in any.
Table 1.
Variables Registered in the Diabetes Pediatric Registries in the 4 Nordic Countries.
| Denmark |
Iceland |
Norway |
Sweden |
|||||
|---|---|---|---|---|---|---|---|---|
| Variable | Included in the registry, yes/no | Time of registration and frequency | Included in the registry, yes/no | Time of registration and frequency | Included in the registry, yes/no | Time of registration and frequency | Included in the registry, yes/no | Time of registration and frequency |
| Type of diabetes | Yes | At onset | Yes | At onset | Yes | At onset | Yes | At onset |
| HbA1c | Yes | Annually | Yes | Every visit | Yes | Annually | Yes | Every visit |
| Insulin dose, U/kg | Yes | Annually | No | Yes | Annually | Yes | Every visit | |
| CSII | Yes | Annually | Yes | Every visit | Yes | Annually | Yes | Every visit |
| Number of insulin doses | Yes | Annually | No | Yes | Annually | Yes | Every visit | |
| DKA | Yes | Annually | Yes | Every visit | Yes | Annually | Yes | Every visit |
| Severe hypoglycemia | Yes | Annually | Yes | Every visit | Yes | Annually | Yes | Every visit |
| BMI-SDS | Yes | Annually | No | Can be calculated by Swedish standards | Yes | Annually | Yes | Every visit |
| BP | Yes | Annually | Annually | Yes | Annually | Yes | Every visit | |
| Fundus photography | Yes | Every third year from the 12th year | Yes | Every second year above 15 years of age | No | Annually: above 11 or 9 years of age, after 2 and 5 years of diabetes duration resp. | Yes | Every second year above 10 years of age |
| U-Albumin | Yes | Annually after 3 years of duration | Yes | Annually after 18 months of duration | Yes | Annually | Yes | Annually after 18 months of duration |
| Lipids | No | No | Not regularly | Yes | Annually | Yes | Annually | |
| Puberty | Yes | Annually >7 years of age | Yes | Annually ≥10 years of age | Yes | Annually | Yes | Annually ≥10 years of age |
| Ethnicity | Yes | At onset | Yes | At onset or at the first annual examination | Yes | At onset or at the first annual examination | Yes | At onset |
| Physical activity | No | No | No | Yes | Every visit | |||
| Smoking | Yes | Annually | No | Yes | Annually | Yes | Every visit | |
| Coeliac disease morbidity | Yes | Annually | Yes | At onset | Yes | Annually | Yes | Every second year |
| Thyroid morbidity | Yes | Ad hoc | Yes | Yearly | Yes | Annually | Yes | Every second year |
| HRQOL | No | No | No | No | ||||
BMI-SDS, body mass index standard deviation score; BP, blood pressure; DKA, diabetic ketoacidosis; HRQOL, health-related quality of life; CSII, subcutaneous continuous insulin infusion.
Ascertainment and Data Completeness
Ascertainment of incidence in Denmark was tested against the National Discharge Register, with no structured test in the other 3 countries. Through iterative contact with the diabetes centers, the data completeness was considered to be very high. In Norway, information was collected from each pediatric unit. The ascertainment of incident cases was 92% in 2008, and 93% of annual examinations were complete in 2009.16
Definitions Used in the Registries
All countries defined DKA as pH < 7.3 or bicarbonate < 15 mmol/L,17 and SH was defined as hypoglycemia with unconsciousness or seizure.18
Annual Reports
Denmark, Norway, and Sweden produce annual reports that are available on the Internet and include data for each center. The Swedish and Danish reports include the center names, while the Norwegian ones are coded.
Registry Organization
All 4 registries have a steering committee, including a registry keeper and clinicians with research experience. Iceland and Sweden also include physicians and nurses representing different centers. The steering committees vary in size: 3 people in Iceland, 5 in Denmark, 7 in Sweden, and 8 in Denmark. The Norwegian registry has a statistician on the committee and the other committees have access to this expertise.
Ethics
The registries in Denmark, Norway, and Sweden have national quality registry status. In Denmark, patient consent is not required before registration. In Norway, patients, or their parents if they are under 16, have to provide written, informed consent to be registered. Patients in Sweden are informed about the registry before agreeing to be included.
The Icelandic registry has been approved by the National Bioethics Committee and the Data Protection Authority.
Descriptive Data
Sweden has the highest incidence of childhood-onset type 1 diabetes and Iceland has the lowest (Figure 1). Mean age, gender distribution, and the frequency of DKA ( pH <7.3) at onset did not differ between the countries (Table 2).
Figure 1.
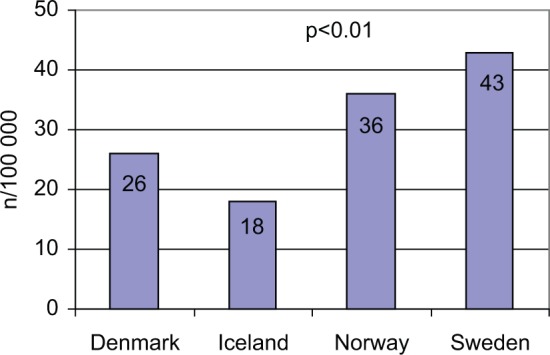
Diabetes incidence among children < 15 years of age per 100 000, in 2009. P value refers to the difference among the 4 countries.
Table 2.
Descriptive Data of Type 1 Diabetes in Children < 15 Years of Age in Denmark (DK), Iceland (I), Norway (N), and Sweden (S).
| DK | I | N | S | P | |
|---|---|---|---|---|---|
| New onset patients, n | 283 | 14 | 325 | 688 | |
| At onset | |||||
| Mean age (onset), years | 9.0 | 7.8 | 8.9 | 8.5 | ns |
| Gender (onset), female % | 45 | 36 | 45 | 46 | ns |
| Patients with DKA (onset) ph < 7.3% | 20 | 36 | 22 | 18 | ns |
| At follow-up | |||||
| Mean age (year mean), years | 11.3 | 11.8 | 10.9 | 10.7 | <.001 |
| Gender (annual registration), female % | 49 | 56 | 49 | 48 | .11 |
| HbA1c (year mean), mmol/mol (IFCC)/%(NGSP) | 64.6/8.1 | 69/8.5 | 69/8.5 | 63/7.9 | <.001 |
| Insulin dose (year mean), U/kg/24h | 0.96 | Not recorded | 0.86 | 0.87 | — |
| Numbers of insulin doses/24h (year mean) | 4.6 | Not recorded | 5 | 4.6 | — |
| CSII (proportion of patients), % | 40 | 47 | 55 | 34 | <.001 |
| Severe hypoglycemia in all children, number per 100 patient years | 8.3 | 6.4 | 5.6 | 7.5 | .04 |
| Range of center mean HbA1c, mmol/mol / % | 60-68/7.6-8.4 | NA | 58-75/7.5-9.0 | 50-69/6.7-8.5 | |
| Completeness of registration, % | |||||
| HbA1c | 93 | 100 | 97 | 100 | |
| Insulin dose | 93 | Not recorded | 98 | 92 | |
| Severe hypoglycemia | 96 | Unknown | 100 | 94 | |
| Number of inhabitants (millions) | 5.56 | 0.32 | 4.9 | 9.4 | |
The table also includes the number of inhabitants. Data from 2009. HbA1c is presented in International Federation for Clinical Chemistry and Laboratory Medicine (IFCC) and National Glycohemoglobin Standardization Program (NGSP) values. P value refers to the difference among the 4 countries.
HbA1c is the major quality indicator in each registry. At follow-up, HbA1c mean values (Table 2), as well as the proportion of children with HbA1c < 57 mmol/mol (NGSP/DCCT 7.4%) (Figure 2), showed clear variations between the countries, P < .001. Intercenter differences in mean HbA1c were found within Denmark, Norway, and Sweden, with the greatest differences occurring in Sweden (Table 2). Iceland has only 1 center.
Figure 2.
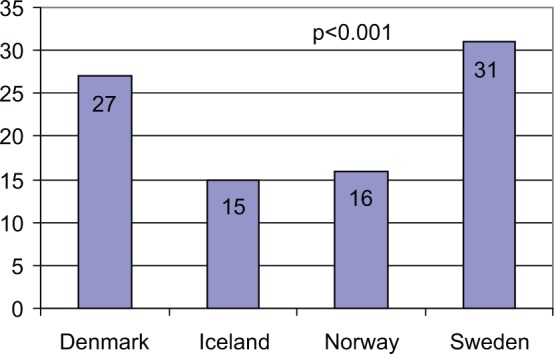
Proportion of patients with HbA1c < 57 mmol/mol (IFCC)/7.5% (NGSP) at annual registration in 2009. Numbers in the columns are the exact percentages. P value refers to the difference among the 4 countries.
The insulin dose was highest in Denmark and lower in Norway and Sweden, with no data recorded in Iceland. The number of insulin doses in patients using insulin pens was somewhat lower in Denmark and Sweden than in Norway, with data not recorded in Iceland. CSII was most frequently used in Norway and least in Sweden (Table 2).
Frequencies of SH varied significantly and were lowest in Norway.
Discussion
This is the first collaboration study for the NordicDiabKids group and its aim was to compare the registries. Future studies will focus on care outcomes. We found that several clinical variables can be used to compare the Nordic registries in Denmark, Iceland, Norway, and Sweden, but some have to be harmonized and validated. The registries were established at different times, which can explain the different methodologies to some extent. But new variables, such as the planned instrument for measuring health related quality of life, can be introduced with the same methodologies and time periods.
This study’s strength is the nationwide population-based design that includes large populations in defined geographic areas. As the national coverage of diabetes quality data is high in all 4 countries, the data are representative. In the Nordic countries, pediatric diabetes centers treat all children and adolescents with diabetes, which means that it is not difficult to achieve virtually complete data coverage. And as the pediatric diabetes populations in the Nordic countries are large, the results are valid and usable. However, increasing concerns about privacy and confidentially may have led some individual patients or their parents to refuse to participate and consent to data registration. Nevertheless, this number is considered to be very low and had no effect on the results. For example, in Danish patients can have their data registered without their consent.
The use of different HbA1c laboratory methods in the countries may have affected the level of HbA1c reported, but these are all validated by external laboratories, which minimizes this risk. Different HbA1c values—the first value registered in 2009 or the value closest to the patient’s birthday—were used to compare the mean value for each country. It is unlikely that this would bias the results, as a recent study found no seasonal variations in HbA1c.19 Furthermore, the registries’ annual reports show that there were different methods for calculating mean HbA1c value between countries, for example, regarding HbA1c values (IFCC or NGSP values) between the countries, as only Sweden had adopted IFCC values in 2009. This study presents both IFCC and NGSP values and the calculations were performed according to Hoelzel et al.14 To provide robust data from the registries and improve research, NordicDiabKids should focus on the issue of standardization.
A high proportion of the patients in all countries did not reach the treatment target of HbA1c < 57 mmol/mol (NGSP/DCCT 7.4%), indicating a need for improved diabetes care in this population. We found significant country variations between yearly HbA1c mean values and the proportion of children with HbA1c <57 mmol/mol (NGSP/DCCT 7.4%) at follow-up. Annual mean HbA1c also differed between the centers in each country.
Differences in HbA1c between centers have been found in other studies and were not explained by clinical variables such as gender, age and diabetes duration.5,20
Our collaboration network provides us with opportunities to carry out further studies, to discuss differences in treatment, policies and approaches by health care professionals and to compare guidelines on action to be taken at different HbA1c levels.
Different ways of registering data were found, such as the frequency of hypoglycemia, but they were considered to be comparable. The insulin regime differed between the countries, including the use of insulin pumps and different treatment traditions. However, this did not appear to correlate to yearly mean HbA1c in the respective country. Registered insulin doses and number of injections were reported by patients or their parents, but data can also be obtained from the information stored by CSII pumps. There is probably a discrepancy between the registered dose and the actual dose, but this is likely to be the similar between countries. Registration of insulin regimen needs to be discussed by the steering committees and harmonized to increase validity. SH is also a patient-reported variable that may be affected by recall bias and the data needs to be carefully interpreted. In our clinical experience, BP is a parameter where measurement needs to be harmonized.
Data are registered for different age groups in the different countries, with Denmark going up to 16-years-of-age and the other countries going up to 18. In this study, only children and adolescents aged between birth and 14.9 years were included. As it has been established that HbA1c tends to increase between the ages of 16 and 18, all countries should include data on adolescents up to the age of 18 years.
The highest incidence of childhood-onset type 1 diabetes was found in Sweden, and the lowest in Iceland, which is in line with earlier studies.2 Finland, which was not included in this study, is the only other Nordic country to have a higher incidence than Sweden.1 Various environmental risk exposures have been suggested as the main factors that contribute to the differences in incidence.1,4 No differences between the countries were found regarding age, gender and DKA at onset. Compared with other countries in Europe and the United States, the prevalence of DKA at onset is lower in the 4 Nordic countries.21 Unfortunately, HbA1c at onset was not available from all 4 countries. The similarities between the Nordic countries may indicate a common disease process at onset, but it may also indicate high public awareness and comprehensive, accessible health care systems.
Registering the results of fundus photography, screening for albuminuria and coeliac disease, and thyroid morbidity provide an opportunity to study the prevalence of complications in a large population. Working with a diabetes registry that includes adults is desirable and could provide data for studies on diabetes complications later in life. It is also possible to evaluate the care process, such as whether the guidelines are followed and patients have access to equal care.
Printed or Internet-based annual reports play an important role in providing easy access to national results in each country. They make it easier for each team of health care providers and stakeholders to compare quality of care and follow up on quality improvement measures. Experiences within other medical specialties have shown that combining systematic collaborative programs for improving quality of care with national quality registers can improve clinical results.22,23 In Sweden, this formalized collaboration is already in place for pediatric diabetes teams. Team members can improve the quality of pediatric diabetes care, thereby increasing the number of patients who reach treatment goals.
The lack of epidemiological data in pediatric diabetes care24 means that observational studies using data from quality registries are required. The benefit of the network between the 4 Nordic countries is that it offers the opportunity to track long-term trends, patterns of treatment, and quality of care. It also provides the opportunity to study differences between geographical areas, such as north and south, urban and rural areas, and areas with different ethnicities. Standardization, harmonization of data, and how data are collected should facilitate comparison and research. NordicDiabKids also provides important informal opportunities to discuss and exchange experiences and the collaboration enables diabetes professionals to learn from each other and to improve data collection. The aim of all registries is to improve the quality of care. In Sweden and Iceland the registries are used as clinical tools for follow up, while in Denmark and Norway data are centrally collected and mainly used for bench marking. Data in the quality registries will never reach the same standard as in research projects, as the collection and reporting of data are dependent on health professionals who are doing this as a daily task and it is not possible to audit the data provided.
Great effort have gone into the development of the 4 countries registries and the registry keeper is often a driving force, with the steering committee members getting involved in addition to their normal clinical work. It might be necessary to develop strategies for recruiting new committee members and formalizing their involvement in the context of their everyday role, if their work is to be developed and improved in the future.
Conclusions
Diabetes is an important public health problem. A high proportion of children do not reach their treatment targets, indicating a need to improve the quality of pediatric diabetes care. The quality registries provide the opportunity to investigate and compare large national cohorts and international collaboration helps to identify areas that need special attention. Collaboration and comparison are also needed to develop and harmonize quality indicators so that they can be compared between different countries, as methods of measurement and registration vary between the different country registries. To our knowledge, this is the first study to compare national quality registers in different countries and the collaboration between the countries.
Acknowledgments
We would like to thank the Icelandic Thorvaldsens Foundation, Danish Society for Diabetes in Childhood and Adolescence (DSBD), Norwegian Study Group in Childhood Diabetes, and Steering Committee of the Swedish Paediatric Diabetes Quality Registry, SWEDIABKIDS. Our thanks also go to other members of the NordicDiabKids steering committee of the group, Jannet Svensson and Karin Åkesson, who were not included as authors.
Footnotes
Abbreviations: BP, blood pressure; CSII, continuous subcutaneous insulin infusion; DCCT, Diabetes Control and Complication Trial; DKA, diabetes ketoacidosis; HbA1c, hemoglobin A1c; IFCC, International Federation of Clinical Chemistry; ISPAD, International Society for Pediatric and Adolescent Diabetes; NGSP, National Glycohemoglobin Standardization Program; SH, severe hypoglycemia.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was supported by the Swedish Board of Health and Welfare and the Swedish Association of Local Authorities and Regions. The Norwegian Childhood Diabetes Registry is financed by the South-Eastern Norway Regional Health Authority. The funding source has no role in data collection or analysis.
References
- 1. Diamond Project Group. Incidence and trends of childhood Type 1 diabetes worldwide 1990-1999. Diabet Med. 2006;23(8):857-866. [DOI] [PubMed] [Google Scholar]
- 2. Patterson CC, Dahlquist GG, Gyurus E, Green A, Soltesz G. Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet. 2009;373(9680):2027-2033. [DOI] [PubMed] [Google Scholar]
- 3. Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977-986. [DOI] [PubMed] [Google Scholar]
- 4. Nordwall M, Arnqvist HJ, Bojestig M, Ludvigsson J. Good glycemic control remains crucial in prevention of late diabetic complications-the Linkoping Diabetes Complications Study. Pediatr Diabetes. 2009;10(3):168-176. [DOI] [PubMed] [Google Scholar]
- 5. Hanberger L, Samuelsson U, Lindblad B, Ludvigsson J. A1C in children and adolescents with diabetes in relation to certain clinical parameters: the Swedish Childhood Diabetes Registry SWEDIABKIDS. Diabetes Care. 2008;31(5):927-929. [DOI] [PubMed] [Google Scholar]
- 6. Mortensen HB, Hougaard P. Comparison of metabolic control in a cross-sectional study of 2,873 children and adolescents with IDDM from 18 countries. The Hvidore Study Group on Childhood Diabetes. Diabetes Care. 1997. May;20(5):714-720. [DOI] [PubMed] [Google Scholar]
- 7. Petitti DB, Klingensmith GJ, Bell RA, et al. Glycemic control in youth with diabetes: the SEARCH for diabetes in Youth Study. J Pediatr. 2009;155(5):668-672e1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Glaser SL, Clarke CA, Gomez SL, O’Malley CD, Purdie DM, West DW. Cancer surveillance research: a vital subdiscipline of cancer epidemiology. Cancer Causes Control. 2005;16(9):1009-1019. [DOI] [PubMed] [Google Scholar]
- 9. Pihoker C, Forsander G, Wolfsdorf J, Klingensmith GJ. The delivery of ambulatory diabetes care to children and adolescents with diabetes. Pediatr Diabetes. 2009;10(suppl 12):58-70. [DOI] [PubMed] [Google Scholar]
- 10. Swedish Diabetes Pediatric Quality Registry. SWEDIABKIDS. 2008. Available at: https://www.swedi-abkids.se/. Accessed January 8, 2014.
- 11. Danish Registry for Childhood Diabetes. Available at: http://www.dsbd.dk/. Accessed January 8, 2014.
- 12. Barnediabetesregistret. ed2007. Available at: http://www.oslo-universitetssykehus.no/omoss/avdelinger/barnediabetesregisteret/Sider/enhet.aspx. Accessed January 8, 2014.
- 13. Hanas R, John G. 2010 Consensus statement on the worldwide standardization of the hemoglobin a1c measurement. Diabetes Care. 2010;33(8):1903-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hoelzel W, Weykamp C, Jeppsson JO, et al. IFCC reference system for measurement of hemoglobin A1c in human blood and the national standardization schemes in the United States, Japan, and Sweden: a method-comparison study. Clin Chem. 2004;50(1):166-174. [DOI] [PubMed] [Google Scholar]
- 15. Craig ME, Hattersley A, Donaghue KC. Definition, epidemiology and classification of diabetes in children and adolescents. Pediatr Diabetes. 2009;10(suppl 12):3-12. [DOI] [PubMed] [Google Scholar]
- 16. Skrivarhaug T. Norwegian Childhood Diabetes Registry. Oslo, Norway: Oslo University Hospital; 2009. [Google Scholar]
- 17. Wolfsdorf J, Craig ME, Daneman D, et al. Diabetic ketoacidosis in children and adolescents with diabetes. Pediatr Diabetes. 2009;10(suppl 12):118-133. [DOI] [PubMed] [Google Scholar]
- 18. Clarke W, Jones T, Rewers A, Dunger D, Klingensmith GJ. Assessment and management of hypoglycemia in children and adolescents with diabetes. Pediatr Diabetes. 2009;10(suppl 12):134-145. [DOI] [PubMed] [Google Scholar]
- 19. Hanberger L, Akesson K, Samuelsson U. Glycated haemoglobin variations in paediatric type 1 diabetes: the impact of season, gender and age. Acta Paediatr. 2014;103(4):398-403. [DOI] [PubMed] [Google Scholar]
- 20. Elding Larsson H, Vehik K, Bell R, et al. Reduced prevalence of diabetic ketoacidosis at diagnosis of type 1 diabetes in young children participating in longitudinal follow-up. Diabetes Care. 2011;34(11):2347-2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. de Beaufort CE, Swift PG, Skinner CT, et al. Continuing stability of center differences in pediatric diabetes care: do advances in diabetes treatment improve outcome? The Hvidoere Study Group on Childhood Diabetes. Diabetes Care. 2007;30(9):2245-2250. [DOI] [PubMed] [Google Scholar]
- 22. Carlhed R, Bojestig M, Peterson A, Aberg C, Garmo H, Lindahl B. Improved clinical outcome after acute myocardial infarction in hospitals participating in a Swedish quality improvement initiative. Circ Cardiovasc Qual Outcomes. 2009;2(5):458-464. [DOI] [PubMed] [Google Scholar]
- 23. Peterson A, Carlhed R, Lindahl B, et al. Improving guideline adherence through intensive quality improvement and the use of a National Quality Register in Sweden for acute myocardial infarction. Qual Manag Health Care. 2007;16(1):25-37. [DOI] [PubMed] [Google Scholar]
- 24. Dabelea D, Mayer-Davis EJ, Imperatore G. The value of national diabetes registries: SEARCH for Diabetes in Youth Study. Curr Diab Rep. 2010;10(5):362-369. [DOI] [PubMed] [Google Scholar]


