Abstract
Background:
Hypoglycemia is a common and serious side effect of insulin therapy in patients with diabetes. Early detection and prediction of hypoglycemia may improve treatment and avoidance of serious complications. Continuous glucose monitoring (CGM) has previously been used for detection of hypoglycemia, but with a modest accuracy. Therefore, our aim was to investigate whether a novel algorithm that adds information of the complex dynamic/pattern of heart rate variability (HRV) could improve the accuracy of hypoglycemia as detected by a CGM device.
Methods:
Data from 10 patients with type 1 diabetes studied during insulin-induced hypoglycemia were obtained. Blood glucose samples were used as reference. HRV patterns and CGM data were combined in a mathematical prediction algorithm. Detection of hypoglycemic periods, performed by the algorithm, was treated as a pattern recognition problem and features/patterns derived from HRV and CGM prior to each blood glucose sample were used to decide if that particular point in time was below the hypoglycemic threshold of 3.9 mmol/L.
Results:
A total of 903 samples were analyzed by the novel algorithm, which yielded a sensitivity of 79% and a specificity of 99%. The algorithm was able to detect 16/16 hypoglycemic events with no false positives and had a lead time of 22 minutes as compared to the CGM device.
Conclusions:
Detection accuracy and lead time were significantly improved by the novel algorithm compared to that of CGM alone.
Keywords: continuous glucose monitoring, heart rate variability, hypoglycemia, diabetes
Patients with type 1 diabetes (T1D) adhering to strict glycemic control are at increased risk of developing severe hypoglycemia. Recent reports have indicated that the incidence of hypoglycemia requiring emergency assistance is as high as 7.1% per year1 and that as many as 6-10% of all deaths in patients with T1D result from hypoglycemia.2-4
Patients with T1D are known to have increased risk of sudden, unexpected death during sleep. This phenomenon, known as “dead-in-bed syndrome,” occurs due to mechanisms that are poorly understood. However, hypoglycemia, ECG abnormalities, and autonomic dysfunction have been suggested to be involved. A new theory suggests a link between sleep apnoea and hypoglycemia as repeated episodes of hypoglycemia may induce adaptation of orexin-neurons, causing defective awakening and hypotonia of upper airway muscles during sleep.5
Severe hypoglycemia is also common in patients with type 2 diabetes (T2D) across all stages of glycemic control. In a large trial of patients with T2D, more than 10% suffered from severe events of hypoglycemia and required assistance within the past year.6 Furthermore, a link exists between severe hypoglycemia and a higher risk of cardiovascular disease and therefore avoiding hypoglycemia is central in preventing cardiovascular disease in patients with T2D.7
Heart rate variability (HRV) is an established tool for studying cardiac autonomic activity over time and HRV has been used to detect autonomic dysfunction in clinical settings. Autonomic dysfunction has been linked to hypoglycemic unawareness,8 increased mortality after myocardial infarction9 as well as cardiac failure.10 Furthermore, altered autonomic regulation during hypoglycemia may contribute to cardiac events.11 A recent study by Koivikko et al12 demonstrated a reduction in HRV during spontaneous nocturnal hypoglycemia in patients with T1D.
Nocturnal hypoglycemia is a frequent event in patients with diabetes13 and feared by patients and their relatives.14 Prior attempts to notify patients before hypoglycemia develops have typically been made using continuous glucose monitoring (CGM) devices15,16 or other physiological measures, for example, ECG, heart rate, QT intervals, or skin impedance. However, the sensitivity and specificity of these approaches are far from impressive.17-21
Ly et al and Bergenstal et al have shown the potential for CGM/insulin pump to reduce incidences of moderate and severe hypoglycemia.22,23
We wanted to test the hypothesis that HRV patterns in combination with CGM data could be used as an improved method to real-time early detection of hypoglycemia.
If early real-time detection is possible, patients can be warned in advance about an upcoming hypoglycemic event and take steps to prevent it. This approach has formerly been successfully used in patients with chronic obstructive pulmonary disease to predict upcoming exacerbations.24 The present study investigated whether HRV patterns combined with CGM data could improve the accuracy of hypoglycemia as detected by a CGM device.
Methods
Study Design and Participants
Data for this study were obtained from a trial performed at Steno Diabetes Center (Gentofte, Denmark). Ten adult males with long-lasting T1D were recruited: age 44 ± 15 years (mean ± SD), BMI 24 ± 1.4 kg/m2, duration of diabetes 18 ± 14 years, baseline heart rate of 64 [53;67] and mean HbA1c 7.4 ± 0.9% (57 mmol/mol). None of the patients had a history of cardiovascular disease or autonomic neuropathy or were taking drugs affecting the cardiovascular system. All patients had a normal electrocardiogram.
Each patient was studied on 2 similar experimental days during insulin induced hypoglycemia. On study days, the subjects were placed in a hospital bed with the back rest elevated to a comfortable position. Equipment for measuring ECG (lead II) and a CGM device (Guardian RT; Minimed Inc, Northridge, CA, USA) producing a reading every 5 min, were attached. The CGM was calibrated by a nurse according to the device instructions. Blood samples for measurements of insulin and blood glucose were taken at 9 am and 9:30 am, constituting the start and end of the baseline period. Throughout the experiment bedside measurements of blood glucose were made from earlobe capillary blood, approximately every 10 minutes. The blood samples were analyzed with a HemoCue Glucose 201+ glucose analyzer (HemoCue, Sweden), which has been shown to have very good correlation with the YSI system.25 Hypoglycemia was induced by a single subcutaneous bolus of insulin aspart (NovoRapid; NovoNordisk A/S, Bagsværd, Denmark); bolus size was determined by an experienced physician. When blood glucose readings reached a nadir of 2.5 mmol/L (45 mg/dL), the subjects were given juice to increase blood glucose above the hypoglycemic threshold of 3.9 mmol/L (70 mg/dL).
The study protocol was approved by the local Danish ethics committee and the study was conducted according to the principles of the Helsinki Declaration II. All patients gave their written informed consent.
Preprocessing
In total, 18 sessions with insulin induced hypoglycemia were obtained during the trial. For this study we excluded 2 sessions due to missing CGM data, blood glucose readings, or unusable noisy ECG signals.
The ECG signal was used for detection of peaks and calculation of RR intervals (time between R to R of the ECG QRS-complex). RR intervals were divided in epochs of 5 minutes during the trial. RR interval outliers from each epoch were replaced with the mean from that particular epoch. Outliers were defined as RR intervals deviating 50% from previous RR intervals or when being outside mean ± 3 standard deviations. Epochs were analyzed using HRV analysis software (HRVAS) and measures ranging from time domain, Poincare, Nonlinear, time-frequency and frequency domain—all derived from the HRV epochs. Two different models were used to estimate power spectral density: Welch and auto regression.
The blood glucose readings were spline resampled with a rate of 5 minutes equivalent to each reading of the CGM device.
Modeling derivation and development
Early detection or prediction according to a predetermined threshold of hypoglycemia (3.9 mmol/L) was treated as a pattern recognition problem, which equals the assignment of a label to a given input value. This means that blood glucose reading from each patient served as reference and was used to categorize each 5-minute sample into 2 classes, that is, normal glucose level (Cn) or hypoglycemia (Chy).
The modeling approach of the algorithm, as shown in Figure 1, was based on extracting and selecting (reducing) discriminative features from CGM and HRV data prior to the point in time, which categorized as either Cn or Chy. Examples of discriminative features used in the algorithm is (1) the median heart rate averaged from epochs 10-20 prior to a sample or (2) the skewness of SDNN (standard deviation of normal-to-normal intervals) from epochs 10-40 prior to a sample. The calculation used to combine different HRV epoch measures is shown in Table 1.
Figure 1.
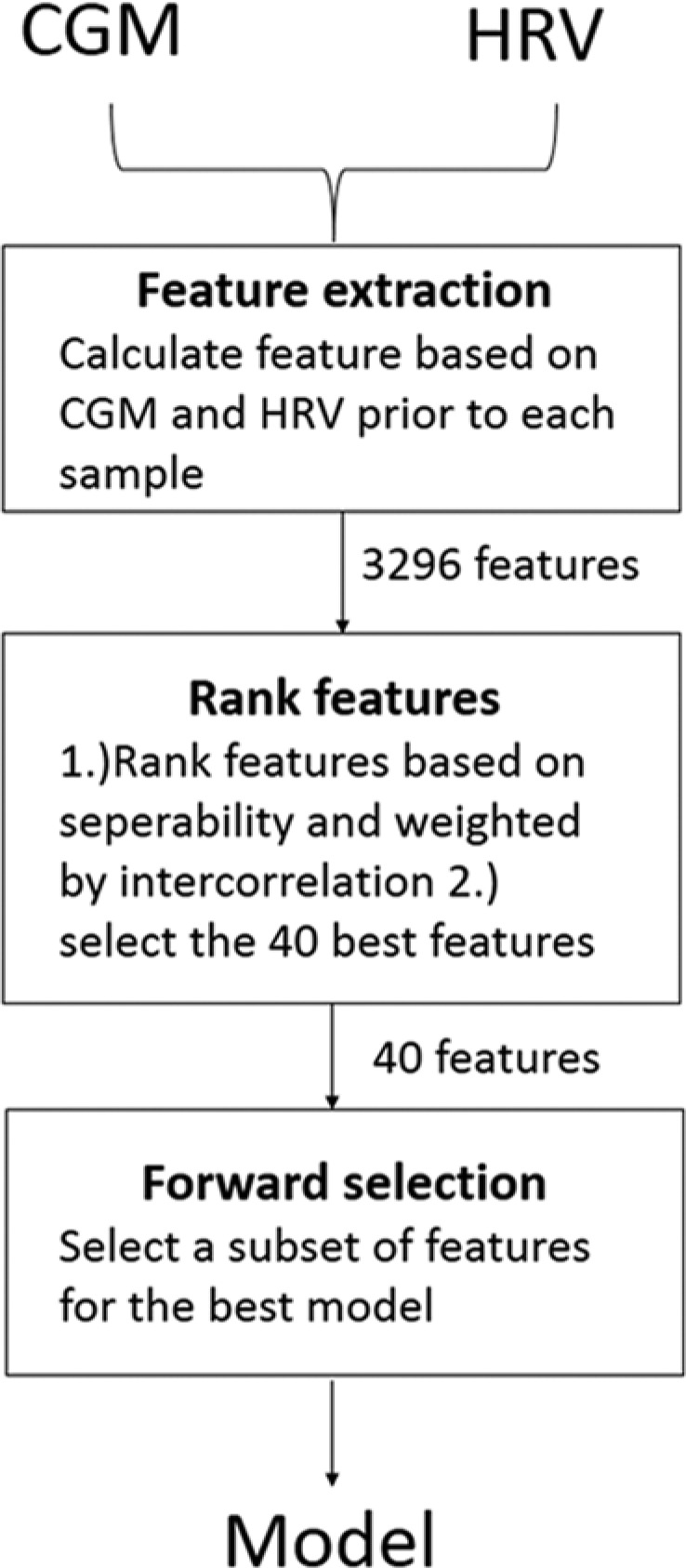
The concept of developing the model: (1) Features were extracted from CGM and HRV prior to each sample. (2) Features were ranked based on ROC curve seperability and weighted by feature intercorrelation—features outside the best 40 were discarded. (3) Forward selection was used to select a subset of features for the best classification. Steps 2 and 3 were done using cross-validation. CGM, continuous glucose monitoring; HRV, heart rate variability; ROC, receiver operating characteristics curve.
Table 1.
Equations Used to Combine Heart Rate Variability (HRV) Measures Into Features.
| Description | Equation |
|---|---|
| Differentiation | Myepcx1 – Myepcx2 |
| Average(ing) | µ (Myepcx1 – Myepcxn) |
| Slope | α (Myepcx1 – Myepcxn) |
| Standard deviation | σ (Myepcx1 – Myepcxn) |
| Skewness | γ1 (Myepcx1 – Myepcxn) |
| Ratio | Myepcx1 / Myepcx2 |
My represents an HRV measure y; epcx1 represents a 5-minute RR interval epoch; µ is the arithmetic mean; α is the slope regression coefficients; σ is the standard deviation; ϒ1 is the third standardized moment.
The goal of feature extraction and reduction is to find preferably small numbers of features that are particularly distinguishing or informative for the classification, that is, normal glucose level (Cn) or hypoglycemia (Chy). Measures derived from RR interval epochs and CGM reading 0-30 minutes prior to an event were used for calculating multiple features in different time intervals.
The extraction of CGM was much more sparse and restricted to the current reading, the difference between the current reading and the reading made 30 minutes previously, the slope of all readings in the interval and the slope relative to the current reading.
To classify the patterns, 3296 different features were evaluated for their discrimination abilities. A ranking algorithm based on a receiver operating characteristics curve (ROC) area under the curve (AUC) and intercorrelation weighting of features was used to eliminate uninformative features. Afterward, forward selection was used to find a subset of features for the final classification algorithm including leave-one-out cross-validation, that is, samples from the same subject were not used for both validation and training of the algorithm. This method ensures an accurate estimate of how the algorithm will generalize to an independent data set. A binary linear logistic regression classifier was used to classify the samples into either Cn or Chy.
All data processing was done using custom analysis software developed in MATLAB® R2011b (MathWorks, Natick, MA, USA).
Algorithm performance
Sample-based sensitivity, specificity, accuracy and ROC AUC were used to evaluate the classification algorithm. The algorithm was allowed to make early prediction of a hypoglycemic event, up to 10 minutes prior to the blood reference reaching the predefined hypoglycemic level of 3.9 mmol/L (70 mg/dL). The algorithm produces a belonging probability to a given class for each sample. The ROC AUC is calculated by changing the probability threshold stepwise for a given class between 0-1.
Furthermore, we also evaluated the algorithm event-based, that is, where 2 or more consecutive samples classified as hypoglycemic were considered as a hypoglycemic event. The event-based performance was evaluated in absolute numbers of true-positive and false-positive events. In addition, we also calculated lead time for the detection of a true-positive hypoglycemic event where the lead time was defined as the time between the detection and the nadir blood glucose of that specific hypoglycemic event.
For comparison, we also calculated the sample-based and event-based evaluation for the CGM alone, purely based on the current readings.
Statistics
We used classical statistics to test results for the proposed algorithm and that of CGM alone.
Differences in lead time, sensitivity and specificity between CGM and the algorithm were tested using a 2-sample t test or the McNemar test. Statistical comparisons were considered significant when P values were < .05. Coefficient of variation (CV) was calculated for lead-time differences in those patients who had multiple successful days. Non-normal-distributed measures are presented as median [5%;95% percentile]—normal distribution was tested using QQ-plots.
Results
The time from insulin injection to nadir glucose was 110 [91;147] minutes. Blood glucose at nadir was 2.4 [2.2;2.9] mmol/l, baseline glucose level was 9.0 [7.6;13.3] mmol/l, and time in hypoglycemia (blood glucose ≤ 3.9 mmol/l) was 31 [26;39] minutes. Heart rate increased significantly (P < .01) 64 [53;67] to 69 [62;74] from baseline to nadir glucose level.
Sample-based evaluation
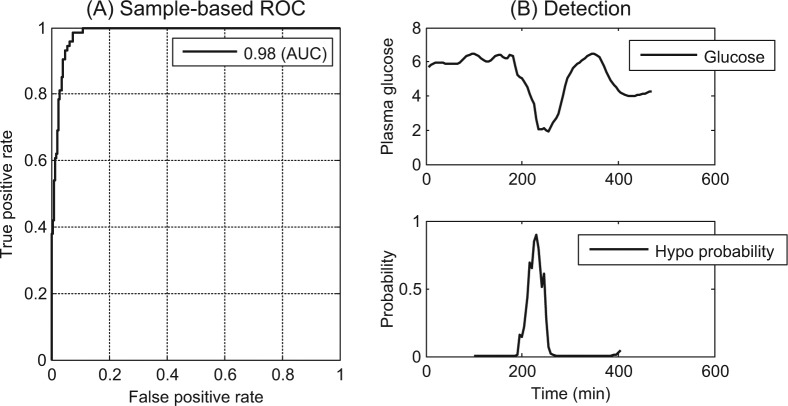
A total of 903 samples equivalent to 4515 minutes among 10 patients with 16 hypoglycemic events were analyzed and classified by the novel algorithm. Figure 2 shows the sample-based evaluation, which yielded a ROC AUC of 0.98. Furthermore, Figure 3 shows a blood glucose profile for a subject during a hypoglycemic event and the algorithms probability output for each sample. The results are presented in Table 2. With a specificity of 99% the algorithm had a sensitivity of 79% versus 33% for CGM alone which was a significant improvement (P < .05).
Figure 2.
(A) Sample-based receiver operating characteristics curve with area under the curve of 0.98. (B) Top image shows the blood glucose drop during 1 session; and (B) bottom image shows the algorithm probabilistic output for samples belonging to an hypoglycemic event.
Figure 3.
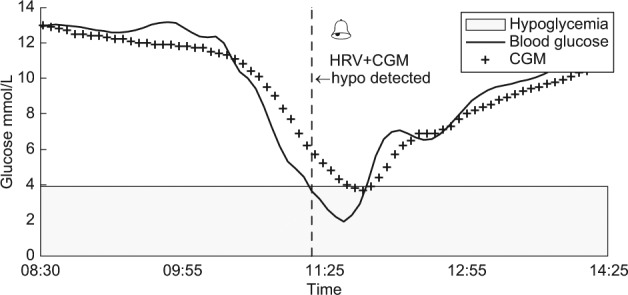
Glucose levels represented for 1 patient with a hypoglycemic episode by blood glucose levels (solid curve) and corresponding CGM (++ curve). The CGM alone detects the episode late after the onset, while the CGM+HRV algorithm detects the episode close to the onset (algorithm detection represented by the vertical dotted line marked “hypo detected”). CGM, continuous glucose monitoring; HRV, heart rate variability.
Table 2.
The Classification Algorithm Compared With Continuous Glucose Monitoring alone.
| Sample-based |
Event-based |
||||
|---|---|---|---|---|---|
| Sensitivity | Specificity | True positive | False positive | Lead time (minutes) | |
| HRV/CGM algorithm | 79 | 99 | 16/16 | 0 | 22 ± 12 |
| CGM | 33 | 98 | 12/16 | 0 | 0 ± 11 |
CGM, continuous glucose monitoring; HRV, heart rate variability.
Event-Based Evaluation
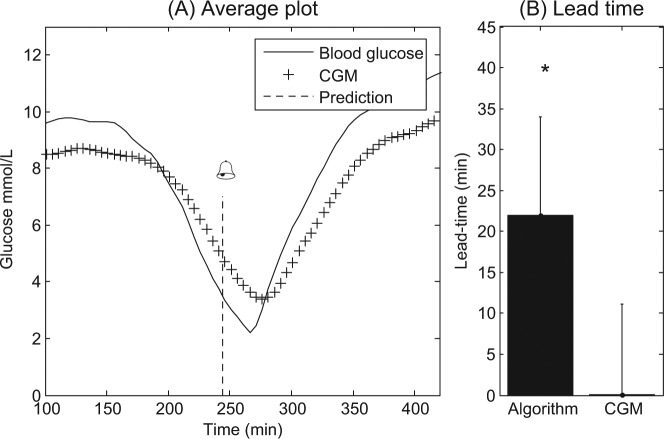
The algorithm classified all hypoglycemic events correctly, and did not detect any false-positive events, whereas the CGM alone detected 12/16 events (P < .05). Importantly, the algorithm had a lead time of 22 ± 12 minutes as compared with the CGM device, which had a lead time of 0 ± 11 minutes (P < .05). Figure 4A shows the patients average CGM and blood glucose curve. Figure 4B illustrates the difference in lead time between the algorithm and that of the CGM alone. Only 6 patients had 2 days included and the difference in lead time between days ranged from 0 to 25 minutes with a CV of 0.65.
Figure 4.
(A) Average for the CGM signal, average blood glucose level, and average predicted time point for hypoglycemia among all patients. Important to notice is the lag between the CGM and the actual glucose level. The dashed line and bell represent the average lead time. The data are centered on the nadir blood glucose level for each patient. (B) Lead time difference between the algorithm and CGM. *Significant difference. CGM, continuous glucose monitoring.
Discussion
There is evidence that the onset of moderate hypoglycemia is preceded by release of counter-regulatory hormones such as growth hormone, glucagon, epinephrine, and cortisol,26 causing signals such as rise in pulse and lowering of the HRV. Accordingly, the purpose of this study was to investigate whether the HRV patterns in combination with CGM could be used to predict the onset of insulin induced hypoglycemia. Early detection and intervention may decrease or eliminate the events themselves and hence any damage caused by hypoglycemic episodes. Based on the results set forth in this article, our algorithm can improve early detection or even predict the occurrence of hypoglycemic events.
Our proposed algorithm yielded significantly higher sample-based sensitivity with equally high specificity compared to that of CGM alone. Furthermore, the algorithm was able to detect 16/16 hypoglycemic events without any false-positives and with a lead time of 22 min. With CGM alone the true-positive rate was much lower (12/16) without a lead time. Recently, Jensen et al27 published an article on the same data as our study but with the aim of detecting hypoglycemia from CGM readings and insulin data. Our algorithm with inclusion of HRV features appears to have a better specificity (99% vs 93%), higher ROC AUC (0.98 vs 0.96) and a larger lead time, 22 versus 14 minutes while the sensitivity was slightly lower (79% vs 81%). In addition, the model by Jensen et al had 1 false-positive event. Our model has considerable better lead time and specificity compared to that previously proposed. Thus, based on the present results it seems beneficial to add HRV data to reduce false positives, which is a recurrent problem when using CGM for hypoglycemic detection.16,27-29 However, high sensitivity of the system is likewise important, otherwise the system could give the patient a false sense of safety.
This trial was conducted in 10 patients with T1D who were bedbound during insulin-induced hypoglycemia. It is known that diurnal variation influences both glucose excursions and HRV.30 In addition, HRV is affected by gender, age, nightly activities, and blood pressure.31-33 If the proposed prediction algorithm was to be used in a real-life setting, these variations should be taken into consideration. However, the strength of our algorithm is that the features used were more related to relative changes in HRV than absolute values—which make it more robust to diurnal variations.
Despite the consistent use of cross validation, the combination of features selected in this algorithm is not definitively the best selection for generalization. The patient sample number is small and not necessarily representative. Selecting optimal subset of features should ideally be performed in a larger set of patients than included in this proof of concept study. One possible approach could be to replace the current static algorithm which is applied on all patients with a dynamic adaptive algorithm individually tailored to each patient or to a group of similar patients.
The performance of this study’s classifier was good. However, in the real world real-time surveillance would be the ultimate goal, as this would identify the majority of events with normoglycemia and only a few dangerous events with hypoglycemia. In addition, the amount of data and events needed to be evaluated would be larger and more divergent. Furthermore, Koivikko et al12 state that symptoms at the time of hypoglycemia induced by clamp may contribute to an autonomic response. In future studies the ideal setup would consist of data obtained during spontaneous nocturnal hypoglycemia.
A nonlaboratory setup is challenging when it comes to the use of HRV as a standalone predictor for hypoglycemia. HRV is an indirect measure of the autonomic modulation and thereby a result of both internal and external changes in breathing, blood pressure, hormonal activity, as well as mental and physical status.34 For example, a sudden change in body position from lying to standing will result in a sudden change in HRV measurements, which in a standalone application could result in a false-positive hypoglycemia alarm. Because of difficulties in extracting the information of interest that is embedded in the HRV measurements, it seems inappropriate to reply only on HRV in the prediction of hypoglycemia. To circumvent this challenge, the novel algorithm only uses the dynamic/pattern of HRV to clarify whether a change in the measurements conducted with the CGM device is of clinical interest. In the example with the change in body position from laying to standing, this change would not affect the CGM measurement and in this case the HRV values are ignored. In patients with both T1D and T2D, autonomic imbalance is prevalent and may progress to autonomic neuropathy which is associated with hypoglycemic unawareness. However, it is expected that the algorithm cannot be used in patients with severe cardiovascular autonomic neuropathy, which encompasses damage to the autonomic nerve fibers that innervate the heart, resulting in abnormalities in heart rate control and significantly reduced HRV.
CGM has been used in several applications as hypoglycemia alarms and it has been shown to reduce the incidence.35 This comes with a trade-off partly because of the 10-minute lag time from changes in blood glucose and partly because of poor accuracy of the CGM systems in measuring glucose values within the hypoglycemic range.36,37 The result is an increased number of false alarms or even false security. The combination of CGM and our algorithm seems attractive—as the CGM could provide information regarding blood glucose levels and sudden drops and the novel algorithm could be used to classify if a drop should be treated as a hypoglycemic incidence.
Conclusions
Detection accuracy and lead time were significantly improved by the novel algorithm compared to that of CGM alone. Real time moving surveillance and prediction is the goal; therefore, the novel algorithm needs to be evaluated in a larger and more divergent population.
Footnotes
Abbreviations: AUC, area under the curve; CGM, continuous glucose monitoring; CV, coefficient of variation; HRV, heart rate variability; ROC, receiver operating characteristics curve; T1D, type 1 diabetes; T2D, type 2 diabetes.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The algorithm used in this article was developed by Medicus Engineering. SLC is a consultant for Medicus Engineering. JF is a stock owner and consultant for Medicus Engineering.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by funding from Novo Nordisk A/S.
References
- 1. Leese G, Wang J, Broomhall J. Frequency of severe hypoglycemia requiring emergency treatment in type 1 and type 2 diabetes: a population-based study of health service resource use. Diabetes Care. 2003;(August):1176-1180. Available at: http://care.diabetesjournals.org/content/26/4/1176.short. Accessed November 18, 2013. [DOI] [PubMed]
- 2. Musen G, Jacobson A, Ryan C. Impact of diabetes and its treatment on cognitive function among adolescents who participated in the Diabetes Control and Complications Trial. Diabetes Care. 2008;(10):10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Skrivarhaug T, Bangstad H-J, Stene LC, Sandvik L, Hanssen KF, Joner G. Long-term mortality in a nationwide cohort of childhood-onset type 1 diabetic patients in Norway. Diabetologia. 2006;49(2):298-305. [DOI] [PubMed] [Google Scholar]
- 4. Feltbower R, Bodansky H. Complications and drug misuse are important causes of death for children and young adults with type 1 diabetes results from the Yorkshire Register of Diabetes. Diabetes. 2008;31(5):922-926. [DOI] [PubMed] [Google Scholar]
- 5. Parekh B. The mechanism of dead-in-bed syndrome and other sudden unexplained nocturnal deaths. Curr Diabetes Rev. 2009;4(5):210-215. [DOI] [PubMed] [Google Scholar]
- 6. Lipska KJ, Warton EM, Huang ES, et al. HbA1c and Risk of Severe Hypoglycemia in type 2 diabetes: the Diabetes and Aging Study. Diabetes Care. 2013;36:3535-3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goto A, Arah O, Goto M. Severe hypoglycaemia and cardiovascular disease: systematic review and meta-analysis with bias analysis. BMJ. 2012;4533(July):1-11. [DOI] [PubMed] [Google Scholar]
- 8. Cryer P. Mechanisms of hypoglycemia-associated autonomic failure and its component syndromes in diabetes. Diabetes. 2005;54:3592-3601. [DOI] [PubMed] [Google Scholar]
- 9. Kleiger RE, Miller JP, Bigger JT, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 1987;59(4):256-262. [DOI] [PubMed] [Google Scholar]
- 10. Malik M. Guidelines heart rate variability. Eur Heart J. 1996;17:354-381. [PubMed] [Google Scholar]
- 11. Koivikko ML, Salmela PI, Airaksinen KEJ, et al. Effects of sustained insulin-induced hypoglycemia on cardiovascular autonomic regulation in type 1 diabetes. Diabetes. 2005;54(3):744-750. [DOI] [PubMed] [Google Scholar]
- 12. Koivikko ML, Tulppo MP, Kiviniemi AM, et al. Autonomic cardiac regulation during spontaneous nocturnal hypoglycemia in patients with type 1 diabetes. Diabetes Care. 2012;35(7):1585-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Buckingham BA, Cameron F, Calhoun P, et al. Outpatient safety assessment of an in-home predictive low-glucose suspend system with type 1 diabetes subjects at elevated risk of nocturnal hypoglycemia. Diabetes Technol Ther. 2013;15(8):622-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jørgensen HV, Pedersen-Bjergaard U, Rasmussen AK, Borch-Johnsen K. The impact of severe hypoglycemia and impaired awareness of hypoglycemia on relatives of patients with type 1 diabetes. Diabetes Care. 2003;26(4):1106-1109. [DOI] [PubMed] [Google Scholar]
- 15. Tsalikian E, Kollman C. GlucoWatch® G2TM biographer (GW2B) alarm reliability during hypoglycemia in children. J Diabetes Sci Technol. 2004;6(5):559-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Palerm CC, Bequette BW. Hypoglycemia detection and prediction using continuous glucose monitoring-a study on hypoglycemic clamp data. J Diabetes Sci Technol. 2007;1(5):624-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Skladnev V, Ghevondian N. Clinical evaluation of a noninvasive alarm system for nocturnal hypoglycemia. Diabetes Sci Technol. 2010;4(1):67-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. San PP, Ling SH, Nguyen HT. Block based neural network for hypoglycemia detection. Conf Proc Annu Int Conf IEEE Eng Med Biol Soc. 2011;2011(2):5666-5669. [DOI] [PubMed] [Google Scholar]
- 19. Nguyen HT, Ghevondian N, Jones TW. Real-time detection of nocturnal hypoglycemic episodes using a novel non-invasive hypoglycemia monitor. Conf Proc Annu Int Conf IEEE Eng Med Biol Soc. 2009;2009:3822-3825. [DOI] [PubMed] [Google Scholar]
- 20. Skladnev VN, Tarnavskii S, McGregor T, Ghevondian N, Gourlay S, Jones TW. Hypoglycemia alarm enhancement using data fusion. J Diabetes Sci Technol. 2010;4(1):34-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Georga EI, Protopappas VC, Ardigò D, Polyzos D, Fotiadis DI. A glucose model based on support vector regression for the prediction of hypoglycemic events under free-living conditions. Diabetes Technol Ther. 2013;15(8):634-643. [DOI] [PubMed] [Google Scholar]
- 22. Ly TT, Nicholas JA, Retterath A, Lim EM, Davis EA, Jones TW. Effect of sensor-augmented insulin pump therapy and automated insulin suspension vs standard insulin pump therapy on hypoglycemia in patients with type 1 diabetes: a randomized clinical trial. JAMA. 2013;310(12):1240-1247. [DOI] [PubMed] [Google Scholar]
- 23. Bergenstal RM, Klonoff DC, Garg SK, et al. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med. 2013;369(3):224-232. [DOI] [PubMed] [Google Scholar]
- 24. Jensen MH, Cichosz SL, Dinesen B, Hejlesen OK. Moving prediction of exacerbation in chronic obstructive pulmonary disease for patients in telecare. J Telemed Telecare. 2012;18:99-103. [DOI] [PubMed] [Google Scholar]
- 25. Stork ADM, Kemperman H, Erkelens DW, Veneman TF. Comparison of the accuracy of the HemoCue glucose analyzer with the Yellow Springs Instrument glucose oxidase analyzer, particularly in hypoglycemia. Eur J Endocrinol. 2005;153(2):275-281. [DOI] [PubMed] [Google Scholar]
- 26. Zammitt N, Frier B. Hypoglycemia in type 2 diabetes pathophysiology, frequency, and effects of different treatment modalities. Diabetes Care. 2005;28(12):2946-2961. [DOI] [PubMed] [Google Scholar]
- 27. Jensen MH, Christensen TF, Tarnow L, Seto E, Dencker Johansen M, Hejlesen OK. Real-time hypoglycemia detection from continuous glucose monitoring data of subjects with type 1 diabetes. Diabetes Technol Ther. 2013;15(7):1-6. [DOI] [PubMed] [Google Scholar]
- 28. Eren-Oruklu M, Cinar A, Quinn L, Smith D. Estimation of future glucose concentrations with subject-specific recursive linear models. Diabetes Technol Ther. 2009;11(4):243-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pappada SM, Cameron BD, Rosman PM, et al. Neural network-based real-time prediction of glucose in patients with insulin-dependent diabetes. Diabetes Technol Ther. 2011;13(2):135-141. [DOI] [PubMed] [Google Scholar]
- 30. Cichosz SL, Fleischer J, Hoeyem P, et al. Assessment of postprandial glucose excursions throughout the day in newly diagnosed type 2 diabetes. Diabetes Technol Ther. 2013;15(1):78-83. [DOI] [PubMed] [Google Scholar]
- 31. Zhang J. Effect of age and sex on heart rate variability in healthy subjects. J Manipulative Physiol Ther. 2007;30(5):374-379. [DOI] [PubMed] [Google Scholar]
- 32. Antelmi I, de Paula RS, Shinzato AR, Peres CA, Mansur AJ, Grupi CJ. Influence of age, gender, body mass index, and functional capacity on heart rate variability in a cohort of subjects without heart disease. Am J Cardiol. 2004;93(3):381-385. [DOI] [PubMed] [Google Scholar]
- 33. Atiea JA, Luzio S, Owens DR. The dawn phenomenon and diabetes control in treated NIDDM and IDDM patients. Diabetes Res Clin Pract. 1992;16(3):183-190. [DOI] [PubMed] [Google Scholar]
- 34. Fleischer J. Diabetic autonomic imbalance and glycemic variability. J Diabetes Sci Technol. 2012;6(5):1207-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Garg S, Zisser H, Schwartz S, Bailey T. Improvement in glycemic excursions with a transcutaneous, real-time continuous glucose sensor: a randomized controlled trial. Diabetes Care. 2006;29(1):44-50. [DOI] [PubMed] [Google Scholar]
- 36. Wolpert HA. Use of continuous glucose monitoring in the detection and prevention of hypoglycemia. J Diabetes Sci Technol. 2007;1(1):146-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Melki V, Ayon F, Fernandez M, Hanaire-Broutin H. Value and limitations of the continuous glucose monitoring system in the management of type 1 diabetes. Diabetes Metab. 2006;32(2):123-129. [DOI] [PubMed] [Google Scholar]