Abstract
Background:
Today most research on pen needle design revolves around pain perception statements through clinical trials, but these are both costly, timely, and require high sample sizes. The purpose of this study was to test if tissue damage, caused by different types of needles, can be assessed by evaluating skin blood perfusion response around needle insertion sites.
Method:
Three common sized pen needles of 28G, 30G, and 32G as well as hooked 32G needles, were inserted into the neck skin of pigs and then removed. Laser Speckle Contrast Analysis was used to measure skin blood perfusion for 20 minutes after the insertions. Seven pigs were included in the study and a total of 118 randomized needle insertions were conducted. Histology was made of tissue samples inserted with 18G, 28G, and 32G needles, and stained to quantify red and white blood cell response.
Results:
Based on area under curve, calculated for each individual blood perfusion recording and grouped according to needle type, skin blood perfusion response relates to needle diameter. The response was significantly higher after insertions with 28G and hooked 32G needles than with 30G (P < .05) and 32G (P < .01) needles. Histology results were not significant, but there was a trend of an increased response with increasing needle diameter.
Conclusions:
Skin blood perfusion response to pen needle insertions rank according to needle diameter, and the tissue response caused by hooked 32G needles corresponds to that of 28G needles. The relation between needle diameter and trauma when analyzing histology was also suggested.
Keywords: laser speckle contrast analysis, diabetes, injections, pen needles, needle size, tissue damage
Approximately 382 million people worldwide have diabetes,1 half of the prevalent cases are not known or diagnosed, half of those diagnosed are not treated, and half of those treated are not controlled.2,3 One of the reasons for poor treatment compliance is injection anxiety causing 20% of insulin users to sometimes skip their injections, and 10% to restrict their number of injections.4 As many as 94% of insulin users exhibit symptoms of anxiety, distress, or phobia around blood and injury from injections,5 22% of insulin users have to mentally prepare themselves for injections,6 and 33% of insulin users dread their injections.7 This underlines why it is of high importance to develop needle designs that cause as little fear, injury, and pain as possible.
Pain perception is one of the preferred methods to evaluate new needle design.8-18 Studies have shown how needle diameter correlates with both the magnitude of the perceived pain, typically measured on visual analog scales (VASs), and with pain occurrence, that is, how often the needle causes pain sensation.11-13 However, pain is a subjective measure with a large number of biasing variables causing data with high variance. Therefore, a high sample size is needed to detect differences in pain, which makes it both costly and time-consuming to carry out the clinical trials.
One alternative way to obtain information about the needle impact on tissue is to use animal models, where histology can be used to assess tissue trauma from, for example, a needle insertion. The needle insertion can cause tissue bleeding and initiate inflammation in the tissue. Pig models are especially useful when examining skin disease and wound healing due to anatomical and biological similarities to humans.19 The magnitude of tissue trauma can be evaluated based on manual stereology of histological slides, through semiquantitative methods, or by automatic analysis of digital images20-22 where stains are used to detect specific cells of interest. For example, analyzing the presence of white blood cells (WBCs) in the tissue is one commonly used inflammation marker.23 Even though histology is a well-known method for assessing tissue reactions, it is a cumbersome and time-consuming analysis that is very examiner dependent and requires careful planning to avoid bias due to the many manual handling steps.
When mechanical trauma from an insulin pen needle insertion is applied to the skin, several biological responses occur, including release of histamine and nitric oxide.24 Because histamine and nitric oxide both act as vasodilators,24,25 it is likely that an increase of blood perfusion at the site of needle insertions could be a valid indirect measure of needle trauma.
Laser Doppler flowmetry has been demonstrated as a method to assess microvascular response after needle insertions in the skin.26 Laser speckle contrast analysis (LASCA) is a further development of the laser Doppler technology,27-29 and is experimentally and clinically used to evaluate the skin blood perfusion in animal models and humans with, for example, microvascular diseases, such as scleroderma,30 for identification of revascularization in skin grafts,31 and for measurement of skin burns,32 all with high reproducibility.33
The aim of this study is to evaluate if skin penetration with incremental needle diameter relates to skin blood perfusion measured with LASCA in pigs. Because it is well established that skin penetration with incremental needle diameter results in incremental penetration pain in humans, a positive relation between needle diameter and skin blood perfusion in pigs would indicate that this method could potentially be a novel method to obtain an easy and fast estimate of human injection pain, generated by using animal models. The findings will be supported by evaluating automated quantification of needle-induced tissue bleeding and inflammation by histology.
Methods
LASCA Study
The neck areas of 7 pigs (Landrace, Yorkshire, and Duroc, LYD) of similar weight (approximately 80-90 kg) were shaved the day before the needle insertion experiments. This was done to remove the thick and stiff bristles which otherwise could influence the optical signal. On the day of the studies, the pigs were sedated with a Zoletil mixture (see Table 1) given intramuscularly with a dose of 0.1 mL/kg. When sedated, the pigs were placed on the side with the neck facing upward. Propofol (Propolipid 10 mg/mL, Fresenius Kabi AB, Bad Homburg, Germany) was administered intravenously with 2-3 mL every 20 minutes or as needed. The experiments were carried out with a view to meet the LASCA experimental recommendations outlined by Mahé et al34 to minimize procedure responsible variation in the recordings. The experimental circumstances are outlined in Table 1.
Table 1.
Overview of the Experimental Setup Using the Laser Speckle Scanner.
| Before the data acquisition | |
|---|---|
| Room conditions | Dedicated, thermally controlled room with constant lighting and low ventilation |
| Pig placement | Always placed on the side on a roller table with neck facing upward |
| Anesthesia | Zoletil mixture given initially in dose of 0.1 mL/kg and supplement of 2-3 mL Propofol every 20 minutes, or when needed. Zoletil mixture consisting of 1 bottle of Zoletil 50 Vet. dry matter, 125 mg Tiletamine + 125 mg Zolazepam (Virbac, Carros, France) dissolved with 6.5 mL Sedator 1 mg/mL (Novartis Healthcare A/S, Basel, Switzerland), 1.25 mL Ketaminol Vet. 100 mg/mL (Intervet, Boxmeer, Netherlands), and 2.5 mL Torbugesic Vet 10 mg/mL (ScanVet, Fredensborg, Denmark). |
| LASCA scanner settings | |
| Distance from laser head to skin | Between 18.0 and 20.0 cm |
| Data sampling frequency | 10 images per second |
| Averaging | 10 images |
| Effective frame rate | 1 image per second |
| Point density | High |
| Post processing of data | |
| Region of interest sizes | 1.0 cm in diameter, corresponding to 78.54 mm2 |
| Time of interest sizes | 5 seconds |
| Baseline recording | 3 minutes |
| Signal recording | 20 minutes |
The LASCA scanner (Pericam PSI, Perimed, Stockholm, Sweden) was placed above the skin, allowing a measurement area of 12 × 12 cm. A baseline of 3 minutes was recorded to control for variations in baseline values between pigs and between insertion sites. Four different types of needles with diameters representing conventionally used diabetes needles, as well as positive and negative controls, were used for randomized insertions. The needles were 12 mm × 28G (0.36 mm), 6 mm × 30G (0.30 mm), 6 mm × 32G (0.23 mm) (NovoFine® needles, Novo Nordisk A/S, Bagsværd, Denmark), and 6 mm × 32G needles with “hooks” to resemble reuse and damage. The positive and negative controls were 40 mm × 18G (1.27 mm) needles (Microlance™, Becton, Dickinson and Company, Franklin Lakes, NJ, USA) and needle hubs (NovoFine) not containing cannulas, respectively.
Up to 9 needle insertions, or needle hub “skin touches”, were performed for each recording with a specially designed multineedle insertion device that secured simultaneous insertions in equal skin depths of 6 mm. No compound was injected. Immediately after needle removal, the recording was resumed and continued to record for 20 minutes. Considering that the procedure was repeated for both neck sides of the pig, the 20 minute signal recording was chosen since it secured an ethically sound sedation time of approximately 1 hour for each pig.
Based on experience, a large difference between the control needles and smaller differences between the test needles could be expected, thus 9 needle insertions with each of the control needles and 25 insertions with each of the test needles were planned.
Histology Study
To support the observations made from the blood perfusion recordings, a parallel study with the purpose of analysis by histology was carried out on biopsies of needle-inserted neck tissue of 4 LYD pigs. Pilot experiments revealed that the red blood cells (RBCs) and WBC in damaged tissue provided strong signals after 1 hour and 24 hours, respectively. Twenty-four biopsies extracted after 1 hour were processed: 4 biopsies from insertions with 18G needles, 8 biopsies from insertions with 28G needles, 8 biopsies from insertions with 32G needles, and 4 biopsies without needle insertion (negative controls). For feasibility reasons it was chosen to leave out the 30G needle from this part of the experiment. Same amount and grouping of biopsies extracted after 24 hours were processed.
Ten tissue slides of approximately 2 × 1 cm were produced from each biopsy to represent each needle insertion. The slides were sliced perpendicular to the skin, were 5 µm thick, and were sliced with 25 µm separation. Immunohistochemical (IHC) staining was performed with antihemoglobin (AHG) primary antibodies (ab82871, Abcam, Cambridge, UK) staining RBC (tissue bleeding) on the 1-hour biopsies, and CD45 primary antibodies (ab10558, Abcam, Cambridge, UK) staining WBC expressing the CD45 antigen (immune response) on the 24-hour biopsies.
LASCA Data Analysis
LASCA results are expressed as arbitrary perfusion units.34 The baseline recording of each measurement was used to make a perfusion unit baseline reference with which each recorded signal perfusion unit in the 20-minute signal was compared. Thus, the final data set was expressed as a percentage of the baseline.
Increasing the time of interest (ie, the number of averaged time steps) decreases the variability of the measurements35 and cancels out minor animal movements and breathing artifacts. The blood perfusion data were therefore averaged in 5-second intervals since this was the approximate breath cycle time of the sedated pigs.
Regions of interest (ROIs) were fitted around all insertion sites, measuring the signals for each needle insertion. The calculated value of a ROI is defined as the average of all the pixel perfusion values in the skin area of interest,34 thus it is important to choose a ROI size that contains the majority of the signal without being too large so that small signals “disappear.” On this basis, circular ROIs of 10.0 mm in diameter were chosen.
The blood perfusion graphs were grouped by needle type and averaged. Moreover, the area under the curve (AUC) was calculated from 0 to 20 minutes after the needle insertions. The grouped AUCs for each needle type revealed to be log-normal distributed within the groups, and statistical analysis (Fisher’s least significant difference test) was thus performed on log-transformed data.
Histology Data Analysis
Slides were scanned into 20× magnification, high-resolution images with NanoZoomer (version 2.0 HT, Hamamatsu, Hamamatsu City, Japan) and then analyzed using MATLAB® (MathWorks, Natick, MA, USA). Based on thresholding, morphological masks, and blob detection, segmentation of the scanned slide images was performed to locate tissue bleeding and immune response, see example in Figure 1. To reduce the image size while keeping the information about the cells of interest, a subfield image was constructed by moving a 250 × 250 pixels (approximately 0.5 × 0.5 mm) mask across the image and counting the number of pixels segmented to be the cells of interest inside each subfield. This count was then assigned to be the value of the subfield. By doing this, an intensity image was constructed, see Figure 2. Each intensity image was divided into 3 ROIs. The 2 lateral ROIs were used to calculate baseline background values and these values plus 2 standard deviations defined the threshold for signal counts in the center ROI. Image-to-image registration was used on images originating from the same tissue. A quantitative measure of the cells of interest was obtained for each needle insertion and compared with analysis of variance.
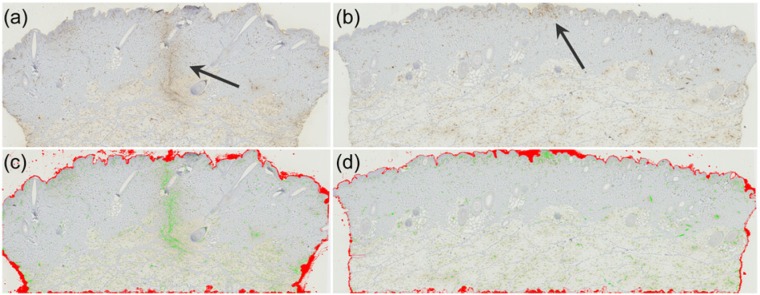
Figure 1.
Histology of tissue trauma 1 hour after needle insertion. (a) A scanned antihemoglobin stained histology slide with tissue containing the trauma of an 18G needle in the center of the tissue (arrow). (b) Same as a but with a trauma from a 32G needle. It is seen that the signal is much smaller than the signal from the 18G needle, and in this slide, only restricted to the epidermal layer. (c) Overlay image showing the complete segmentation of the slide as seen in a. The red overlay color denotes the morphological masks applied to exclude the epidermal layer as well as the local bleedings from the tissue removal procedure on the sides and bottom of the tissue. The green overlay color indicates presence of red blood cells. (d) Overlay image showing the complete segmentation of the 32G needle insertion seen in b.
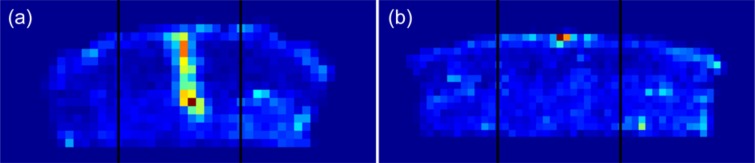
Figure 2.
Summation images of tissue trauma caused by (a) the 18G needle and (b) the 32G needle exemplified in Figure 1. Each summed image contains 10 segmented and subfield divided histology slides. Each image is divided into 3 areas, 2 lateral and 1 center area. The mean value of the subfields in the 2 lateral areas represents the baseline value of the tissue. The signal is found in the center area, where the signal threshold is defined as the baseline value plus 2 standard deviations. The number of subfields qualifying as signal subfields is defined as the signal count of the center area.
Results
Skin Blood Perfusion
The final data output consists of 118 individual responses representing the percentage change in skin blood perfusion from baseline over time from each needle insertion.
Figure 3 shows the averaged blood perfusion profiles for each needle group. All groups reach maximum within the first minute after needle insertion. The 18G needle (positive control) insertions result in the highest response with a peak blood perfusion increase of 39.9% ± 13.7 (mean ± SD) in the ROI around the insertion site. The 28G, 30G, and 32G needle groups create peak increases of 23.6% (± 14.0), 18.7% (± 14.4), and 15.6% (± 14.1), respectively. The hooked 32G needle group produces a peak increase of 27.3% (± 20.7). The mere touch of the needle hub on the skin creates an increase of 4.9% (± 5.4), but this signal vanishes after about 2 minutes. In contrast, the signals from the other groups are still increased compared to baseline 20 minutes after needle insertion.
Figure 3.
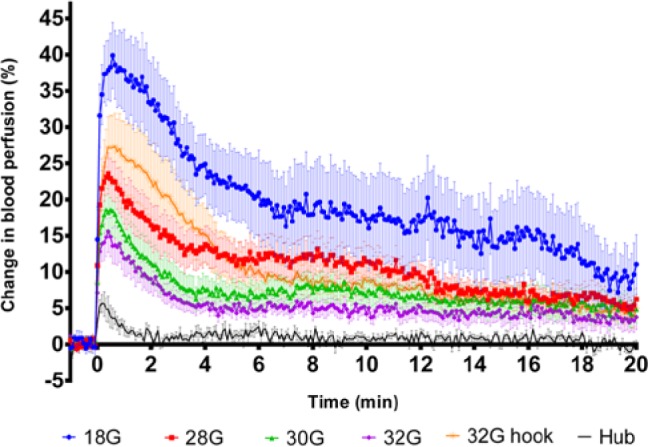
Blood perfusion following skin penetration. Averaged (mean ± SEM) skin blood perfusion profiles measured 0-20 minutes after needle insertion. Only 1 minute of the 3-minute baseline is included in the plot.
The AUC data of the blood perfusion responses reveal statistically significant difference of the positive and negative control groups compared to all other data groups (Figure 4). The 28G needle group and the 32G hooked needle group AUCs are both significantly larger than the 30G and 32G needle groups.
Figure 4.
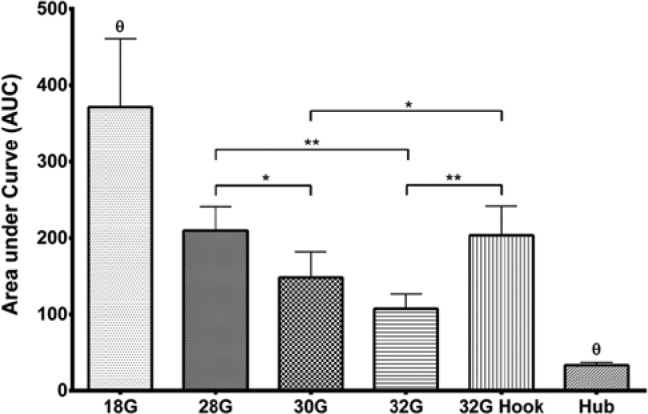
AUC (mean with SEM) calculated from 0-20 minutes for all skin blood perfusion profiles, grouped by needle type. θ data groups significantly different from all other data groups. Statistics were performed on log-transformed data, but represented in this figure as original data.
*Data groups significantly different with P < .05. **Data groups significantly different with P < .01.
Cellular Response
The quantitative measures of IHC stained RBCs and WBCs are seen in Figure 5. It is seen that the positive and negative controls provide the highest and lowest cell quantifications, respectively. Insertions with the 28G needles provide nonsignificant but slightly higher cell quantifications compared to the 32G needles.
Figure 5.
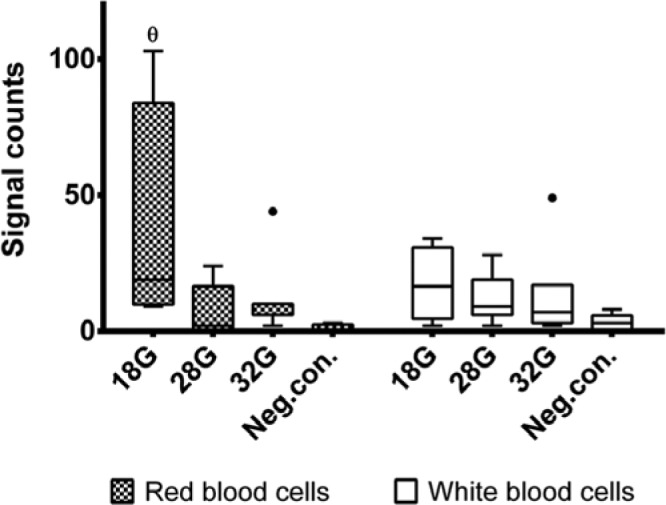
Quantified measures of immunohistochemically stained red blood cells and white blood cells, representing the needle induced tissue bleeding and inflammation, respectively. θ data group significantly different from all other data groups. The dots represent outliers defined as values with a distance from the median exceeding 1.5 times the interquartile range.
Discussion
Laser speckle technology is a novel method for quantification of the skin blood perfusion after insulin pen needle insertions. Using this method on animals could be an indirect way to perform needle testing in a faster and cheaper way compared to clinical trials, while also having the advantage of data being objectively quantifiable.
It was observed that the skin blood perfusion reacts rapidly to mechanical stress and reached maximum within the first minute. The peak within the first minute differs from the results measured by Rayman et al,26 where the peaks occurred after 10-15 minutes. One explanation to this could be biological differences between human and porcine skin. Another explanation could be that the laser Doppler flowmetry used in the study by Rayman et al has a point illuminated probe which measures blood flow in deeper tissues compared to the whole-field laser speckle imaging.36 If the majority of the immediate cellular response to tissue damage occurs in the superficial part of the skin, and the LASCA scanner covers a more superficial, but greater surface area compared to the point-illuminated laser Doppler probe, then this could be the reason for a faster response. Nevertheless, the 2 measurement methods are very different, and the results are difficult to compare directly, but they both support the hypothesis that it is possible to assess skin blood perfusion after needle insertions using a surface measurement method.
The response of the hooked needles is initially a little higher than the 28G needles, but the 2 groups have comparable AUC calculations. Interestingly, the hooks were approximately 0.15 mm, making the cross section diameter of the needle 0.38 mm, which is very close to the diameter of the 28G needle. This supports that the skin blood perfusion is related to the amount of damaged tissue. Even though there is not a significant difference between the AUCs of the 30G and 32G needles, a clear trend of lower blood perfusion is observed with the 32G needles, but a higher sample size would be needed to reliably detect a difference of that magnitude.
Twenty minutes after needle insertion the blood flow was still increased from baseline, which is concordant with observations by Rayman et al where preliminary studies indicated that the blood flow would return to normal over a period of 2 to 3 days.26 In theory, LASCA could be run for as long as signal is present, but this is limited by the ethical recommendations of total sedation time of the pig. However, it is assessed that the results are valid in terms of demonstrating significant differences in peak blood flow and AUC.
In the histology study, the 18G needle insertion calculation of RBC was statistically significant different from the other data groups, whereas statistical difference was not achieved between the other data groups. This experiment was performed to support the LASCA findings, and despite the low power due to the small sample size and expected high variance, there is still a nonsignificant trend of increased cellular response with increased needle diameter supporting the validity of the LASCA findings.
Propofol and Sedator (Medetomidin) are both known to induce cardiovascular effects,37-39 and even though the 2 drugs in combination reduce the effects,40 it must be assumed that the anesthetized pigs are subject to some residual cardiovascular effects, and we cannot rule out an influence of this on our results. However, it is assessed that a potential residual cardiovascular effect would equally influence all measurements, and that the potential drift in actual values would not have had an impact on the results in terms of demonstrating significant differences in peak blood flow and AUC.
The multineedle insertion device was used to insert all needles simultaneously in a skin depth of 6 mm. The 30G and 32G needles were 6 mm long to begin with, so at insertion, these pen needle hubs must have contributed with a part of the total signal, which is most likely similar to the magnitude of the isolated “Hub” signal (please see Figure 3). The needle hubs of the 12 mm × 28G needles were elongated to shorten the needle cannula length, so the signal from the 28G needles also contains a signal from the hub which is assumed to be of the same magnitude as the others. The 18G needles were the only cannulas not mounted in a regular pen needle hub, and even though the insertion device secured insertion lengths of 6 mm, this needle had no needle hub touching the skin. Thus, the initial peak of the 18G graph could potentially be slightly higher if this cannula was placed in a pen needle hub. However, the signal from the actual hub group is very limited compared to the signal from the 18G needle, and it is assessed that this would not have influenced the results.
The needle insertion device was handheld, and therefore not with controlled needle insertion speed for all needle insertions. However, all insertions were performed by the same experimenter to standardize it as much as possible. A previous study indicates that needle insertion speed does not influence the frequency of, or degree of, painful ratings by human subjects,12 and therefore we do not expect that small variations in needle insertion speed would influence the LASCA measurements. However, for a more controlled repeated experiment, it could be of interest to use a mechanically controlled insertion device.
Future Implications
It is well established that pain perception in humans relates to needle diameter.11-13 Thus, the fact that the magnitudes of the blood perfusion graphs obtained in animals also rank according to needle diameter, could potentially indicate that this method can be used as an indirect pain perception measurement. This hypothesis, however, needs to be validated in future studies.
This study was conducted to measure the blood perfusion increase as a result of needle insertion only, thus, it does not assess the potential increase that, for example, an insulin injection would induce. In addition, as the LASCA scanner measures blood perfusion in the superficial part of the skin, and because insulin is injected into the subcutaneous tissue, it is unknown if and how much a subcutaneous injection would affect the LASCA signal. However, this is a potential topic for future research.
Conclusions
A novel method for a quantitative biological assessment of pen needle induced tissue trauma has been introduced with the present study using LASCA technology supported by histology with cellular quantifications of RBCs and WBCs.
The results demonstrated a positive relation between needle diameter and skin blood perfusion measured with LASCA, which was supported by histology in pigs. The response caused by simulated misused 32G needles corresponds to that of 28G needles, which are both significantly larger than 30G and 32G needles. These results show that LASCA can be used as a method to objectively quantify skin trauma differences between different types of needles in a cheaper, faster way than clinical trials. In addition, the results could potentially indicate that this method can be used as an indirect pain perception measurement.
Acknowledgments
The authors acknowledge Hanne Gamst-Andersen, Hans Egon Rasmussen, Per Bo Danielsen, and Torben Jørgensen from the Animal Unit at Novo Nordisk A/S for their help with preparation and execution of the animal experiments, laboratory technicians Maibritt Pedersen, Bettina Brandrup, and Jeanette Bannebjerg Johansen at Novo Nordisk A/S for their help with histology preparation, veterinary scientist Rikke Kaae Kirk at Novo Nordisk A/S for valuable information about and help with staining procedures and histology interpretation, and Morten Lind Jensen at Novo Nordisk A/S for critical reading and academic and medical viewpoints during the writing procedure.
Footnotes
Abbreviations: AHG, antihemoglobin; AUC, area under the curve; DM, diabetes mellitus; G, gauge; IHC, immunohistochemical; LASCA, laser speckle contrast analysis; LYD, Landrace, Yorkshire, and Duroc; RBC, red blood cells; ROI, region of interest; SD, standard deviation; SEM, standard error of mean; TOI, time of interest; VAS, visual analog scale; WBC, white blood cells.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: KAP is enrolled as an industrial PhD student at Novo Nordisk A/S, CBJ is a consultant working for Novo Nordisk A/S, and NBM and JK are full-time employees of Novo Nordisk A/S.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is a part of a Danish industrial PhD program that is funded by the Danish Ministry of Science, Innovation, and Higher Education and Novo Nordisk A/S.
References
- 1. Danaei G, Finucane MM, Lu Y, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378:31-40. [DOI] [PubMed] [Google Scholar]
- 2. Hart JT. Rule of halves: implications of increasing diagnosis and reducing dropout for future workload and prescribing costs in primary care. Br J Gen Pract. 1992;42:116. [PMC free article] [PubMed] [Google Scholar]
- 3. Smith WC, Lee AJ, Crombie IK, Tunstall-Pedoe H. Control of blood pressure in Scotland: the rule of halves. BMJ. 1990;300:981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aronson R. The role of comfort and discomfort in insulin therapy. Diabetes Technol Ther. 2012;14:741-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berlin I, Bisserbe JC, Eiber R, et al. Phobic symptoms, particularly the fear of blood and injury, are associated with poor glycemic control in type I diabetic adults. Diabetes Care. 1997;20:176-178. [DOI] [PubMed] [Google Scholar]
- 6. Peyrot M, Rubin RR, Lauritzen T, et al. Resistance to insulin therapy among patients and providers results of the cross-national Diabetes Attitudes, Wishes, and Needs (DAWN) study. Diabetes Care. 2005;28:2673-2679. [DOI] [PubMed] [Google Scholar]
- 7. Peyrot M, Rubin RR, Kruger DF, Travis LB. Correlates of insulin injection omission. Diabetes Care. 2010;33:240-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hirsch L, Gibney M, Berube J, Manocchio J. Impact of a modified needle tip geometry on penetration force as well as acceptability, preference, and perceived pain in subjects with diabetes. J Diabetes Sci Technol. 2012;6:328-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hirsch LJ, Gibney MA, Albanese J, et al. Comparative glycemic control, safety and patient ratings for a new 4 mm × 32G insulin pen needle in adults with diabetes. Curr Med Res Opin. 2010;26:1531-1541. [DOI] [PubMed] [Google Scholar]
- 10. Jaber A, Bozzato GB, Vedrine L, Prais WA, Berube J, Laurent PE. A novel needle for subcutaneous injection of interferon beta-1a: effect on pain in volunteers and satisfaction in patients with multiple sclerosis. BMC Neurol. 2008;8:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Iwanaga M, Kamoi K. Patient perceptions of injection pain and anxiety: a comparison of NovoFine 32-gauge tip 6mm and Micro Fine Plus 31-gauge 5mm needles. Diabetes Technol Ther. 2009;11:81-86. [DOI] [PubMed] [Google Scholar]
- 12. Arendt-Nielsen L, Egekvist H, Bjerring P. Pain following controlled cutaneous insertion of needles with different diameters. Somatosens Mot Res. 2006;23:37-43. [DOI] [PubMed] [Google Scholar]
- 13. Egekvist H, Bjerring P, Arendt-Nielsen L. Pain and mechanical injury of human skin following needle insertions. Eur J Pain. 1999;3:41-49. [DOI] [PubMed] [Google Scholar]
- 14. Schwartz S, Hassman D, Shelmet J, et al. A multicenter, open-label, randomized, two-period crossover trial comparing glycemic control, satisfaction, and preference achieved with a 31 gauge × 6 mm needle versus a 29 gauge × 12.7 mm needle in obese patients with diabetes mellitus. Clin Ther. 2004;26:1663-1678. [DOI] [PubMed] [Google Scholar]
- 15. Kreugel G, Keers JC, Kerstens MN, Wolffenbuttel BH. Randomized trial on the influence of the length of two insulin pen needles on glycemic control and patient preference in obese patients with diabetes. Diabetes Technol Ther. 2011;13:737-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chantelau E, Lee DM, Hemmann DM, Zipfel U, Echterhoff S. What makes insulin injections painful? BMJ. 1991;303:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Miwa T, Itoh R, Kobayashi T, et al. Comparison of the effects of a new 32-gauge × 4-mm pen needle and a 32-gauge × 6-mm pen needle on glycemic control, safety, and patient ratings in Japanese adults with diabetes. Diabetes Technol Ther. 2012;14:1084-1090. [DOI] [PubMed] [Google Scholar]
- 18. Nagai Y, Ohshige T, Arai K, et al. Comparison between shorter straight and thinner microtapered insulin injection needles. Diabetes Technol Ther. 2013;15:550-555. [DOI] [PubMed] [Google Scholar]
- 19. Sullivan TP, Eaglstein WH, Davis SC, Mertz P. The pig as a model for human wound healing. Wound Repair Regen. 2001;9:66-76. [DOI] [PubMed] [Google Scholar]
- 20. Kraan MC, Haringman JJ, Ahern MJ, Breedveld FC, Smith MD, Tak PP. Quantification of the cell infiltrate in synovial tissue by digital image analysis. Rheumatology. 2000;39:43-49. [DOI] [PubMed] [Google Scholar]
- 21. Kårsnäs A, Dahl AL, Larsen R. Learning histopathological patterns. J Pathol Inform. 2011;2:S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mahmood NH, Mansor MA. Red blood cells estimation using Hough transform technique. Signal Image Processing. 2012;3:53-64. [Google Scholar]
- 23. Vana G, Meingassner JG. Morphologic and immunohistochemical features of experimentally induced allergic contact dermatitis in Göttingen minipigs. Vet Pathol. 2000;37:565-580. [DOI] [PubMed] [Google Scholar]
- 24. Rang HP. Rang and Dale’s Pharmacology. 7th ed. Edinburgh, UK: Churchill Livingstone; 2012. [Google Scholar]
- 25. Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524-526. [DOI] [PubMed] [Google Scholar]
- 26. Rayman G, Williams SA, Spencer PD, Smaje LH, Wise PH, Tooke JE. Impaired microvascular hyperaemic response to minor skin trauma in type I diabetes. BMJ. 1986;292:1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Boas DA, Dunn AK. Laser speckle contrast imaging in biomedical optics. J Biomed Optics. 2010;15:011109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Briers JD. Laser Doppler, speckle and related techniques for blood perfusion mapping and imaging. Physiol Meas. 2001;22:R35. [DOI] [PubMed] [Google Scholar]
- 29. Draijer M, Hondebrink E, van Leeuwen T, Steenbergen W. Review of laser speckle contrast techniques for visualizing tissue perfusion. Lasers Med Sci. 2009;24:639-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Murray AK, Moore TL, Manning JB, Taylor C, Griffiths CE, Herrick AL. Noninvasive imaging techniques in the assessment of scleroderma spectrum disorders. Arthritis Care Res. 2009;61:1103-1111. [DOI] [PubMed] [Google Scholar]
- 31. McGuire PG, Howdieshell TR. The importance of engraftment in flap revascularization: confirmation by laser speckle perfusion imaging. J Surg Res. 2010;164:e201-e212. [DOI] [PubMed] [Google Scholar]
- 32. Lindahl F, Tesselaar E, Sj+Âberg F. Assessing paediatric scald injuries using laser speckle contrast imaging. Burns. 2013;39:662-666. [DOI] [PubMed] [Google Scholar]
- 33. Roustit M, Millet C, Blaise S, Dufournet B, Cracowski JL. Excellent reproducibility of laser speckle contrast imaging to assess skin microvascular reactivity. Microvasc Res. 2010;80:505-511. [DOI] [PubMed] [Google Scholar]
- 34. Mahé G, Humeau-Heurtier A, Durand S, Leftheriotis G, Abraham P. Assessment of skin microvascular function and dysfunction with laser speckle contrast imaging. Circ Cardiovasc Imaging. 2012;5:155-163. [DOI] [PubMed] [Google Scholar]
- 35. Rousseau P, Mahé G, Haj-Yassin F, et al. Increasing the region of interest and time of interest, both reduce the variability of blood flow measurements using laser speckle contrast imaging. Microvasc Res. 2011;82:88-91. [DOI] [PubMed] [Google Scholar]
- 36. Thompson OB, Hirst ER, Andrews MK. Is there a difference between laser speckle and laser Doppler in depth sensitivity? Proc SPIE. 2011:78980E. [Google Scholar]
- 37. Krassioukov AV, Gelb AW, Weaver LC. Action of propofol on central sympathetic mechanisms controlling blood pressure. Can J Anaesth. 1993;40:761-769. [DOI] [PubMed] [Google Scholar]
- 38. Cullen LK. Medetomidine sedation in dogs and cats: a review of its pharmacology, antagonism and dose. Br Vet J. 1996;152:519-535. [DOI] [PubMed] [Google Scholar]
- 39. Golden AL, Bright JM, Daniel GB, Fefee D, Schmidt D, Harvey RC. Cardiovascular effects of the alpha2-adrenergic receptor agonist medetomidine in clinically normal cats anesthetized with isoflurane. Am J Vet Res. 1998;59:509. [PubMed] [Google Scholar]
- 40. Thurmon JC, Ko JC, Benson GJ, Tranquilli WJ, Olson WA. Hemodynamic and analgesic effects of propofol infusion in medetomidine-premedicated dogs. Am J Vet Res. 1994;55:363-367. [PubMed] [Google Scholar]