Abstract
Background:
Pharmacokinetic/pharmacodynamic (PK/PD) studies of human regular U-500 insulin (U-500R) at high doses commonly used in clinical practice (>100 units) have not been performed. The current analysis applied PK/PD modeling/simulation to fit the data and simulate single-dose and steady-state PK/PD of U-500R high-dose regimens.
Method:
Data from 3 single-dose euglycemic clamp studies in healthy obese and normal-weight patients, and normal-weight patients with type 1 diabetes were used to build the model. The model was sequential (PK inputs fed into PD component). PK was described using a 1-compartment model with first-order absorption and elimination. The model estimated separate absorption rate constants for U-500R and human regular U-100 insulin. The PD component used an effect compartment model, parameterized in terms of maximum pharmacologic effect (Emax) and concentration to achieve 50% of Emax.
Results:
The model described the data well. Steady-state PK for once-daily (QD), twice-daily (BID), or thrice-daily (TID) administration appeared to be reached 24 hours after the first dose. At steady-state, QD dosing showed the greatest fluctuations in PK/PD. BID dosing showed a gradual increase in insulin action with each dose and a fairly stable basal insulin effect. For TID dosing, activity was maintained throughout the dosing interval.
Conclusions:
PK/PD modeling/simulation of high U-500R doses supports BID or TID administration with an extended duration of activity relative to QD. TID dosing may provide slightly better full-day insulin effect. Additional PK/PD studies and randomized controlled trials of U-500R are needed to validate model predictions in patients with insulin-resistant diabetes requiring high-dose insulin.
Keywords: dosing, pharmacodynamics, pharmacokinetics, modeling, simulation, time action profile, U-500 regular human insulin
Human regular U-500 insulin (U-500R; Humulin® R U-500, Eli Lilly and Company, Indianapolis, IN) offers severely insulin-resistant and high-dose insulin-treated patients the ability to administer large doses (500 U/mL) at one-fifth the volume of that of human regular U-100 insulin (U-100R; 100 U/mL).1 In 9 case series and case reports describing 310 patients, U-500R treatment by multiple daily injections (MDI: twice daily [BID] or thrice daily [TID]) has resulted in improved glycemic control, with a mean reduction in glycated hemoglobin (HbA1c) of 1.59% (range, 1.0% to 3.3%) over a mean follow-up duration of 20 months (range of means 6 to 36 months).2 No randomized controlled trials using U-500R have been completed; 1 is ongoing.3
Despite the dramatic recent increase in the use of U-500R,4 there have been limited data reported on the pharmacokinetics (PK) and pharmacodynamics (PD) of this formulation.5-7 The first robust euglycemic clamp PK/PD study with U-500R demonstrated similar overall exposure (AUC0-t’) and effect (Gtot) of U-500R relative to U-100R after single-dose administration of 50 U and 100 U doses of each formulation to 24 healthy obese patients without diabetes.7 This study also showed significantly lower peak insulin concentration (Cmax) and effect (Rmax), and longer duration of action (late tRmax50, tRlast) for U-500R relative to U-100R. For the 100 U dose, both time-to-peak concentration (tmax) and time-to-peak effect (tRmax) were significantly longer for U-500R compared to U-100R. Onset of action (within 11-16 minutes) was similar for both formulations. Earlier studies had suggested an inverse relationship between insulin concentration and its rate of absorption in both animal and human studies.8,9
To date, there is no information on the PK/PD of U-500R at steady-state pertaining to daily dosing regimens for this formulation. Moreover, PK/PD studies for higher U-500R doses (MDI: 1.5-3.0 U/kg/day)10-12 commonly used in clinical practice, including unevenly divided dosing (BID or TID),2,11-14 have not been performed. The objective of the current analysis was to apply PK/PD modeling and simulation to predict the single-dose and steady-state PK and PD of high doses up to 750 U as single daily dose (QD) and 500 U as total daily dose to provide insights to health care professionals regarding the potential time-action profiles of high-dose U-500R.
Methods
Data from 3 different single-dose, crossover euglycemic clamp studies that evaluated the PK/PD of regular human insulin were used to build the population PK/PD model to characterize the dose-response relationships of U-500R and U-100R. Studies were conducted according to the Declaration of Helsinki and all subjects provided written informed consent. The studies included (1) healthy obese subjects without diabetes: 50 U dose, 0.4-0.6 U/kg, N = 22, and 100 U dose, 0.8-1.3 U/kg, N = 247; (2) healthy normal-weight subjects without diabetes: 0.05 to 0.4 U/kg, N = 18 (data on file, Eli Lilly and Company); and (3) normal-weight patients with type 1 diabetes: 12 U dose, 0.1 to 0.2 U/kg, N = 30 (data on file, Eli Lilly and Company). All studies had intensive PK sampling, and the glucose infusion rate (GIR) data for the clamps was modeled as a measure of PD effect. In all studies, serum insulin concentrations were measured using validated radioimmunoassays that were commercially available at the time of each study conduct. The populations were combined to provide a robust data set that would allow to describe the PK and PD dose-responses of regular human insulin.
The model used to simultaneously describe the PK/PD of U-500R and U-100R was developed based on a published model by Landersdorfer and Jusko15 using NONMEM (Version VII). In the present sequential model, the PK model was developed independently and the fixed PK parameters used as input for the PD model (Figure 1).
Figure 1.

Pharmacokinetic/pharmacodynamic model. PK component: 1-compartment model with first-order absorption and different Ka for U-500R and U-100R, proportional error structure, covariates: dose identified as a significant covariate contributing to interindividual variability of Ka and Clearance; and weight identified as significant for Vd. Sequential model: Pharmacokinetics fixed inputs into pharmacodynamic model. PD component: Effect compartment model with Emax; proportional error structure, covariates: BMI identified as a significant covariate contributing to interindividual variability of Emax. Abbreviations: Ce, concentration in effect compartment; Cs, serum insulin concentration; Ka1, absorption rate constant for U-100R; Ka2, absorption rate constant for U-500R; K20, elimination rate constant from the central (serum) compartment; K23 and K32, distribution rate constants between the serum and effect compartments; SC, subcutaneous.
Due to the data-rich nature of the data sets for 2 of the studies, PD data were averaged every 15 minutes prior to modeling, which reduced the noise in the data and allowed for shorter model run times. Both U-500R and U-100R concentration-time profiles show a single exponential decrease; 1-compartment and 2-compartment PK models were tested. Different absorption models were also tested to better describe the absorption phase, including zero-order absorption, zero- and first-order combined absorption, dual first-order absorption, and transit absorption models.15,16 There was a delay between the PK and the PD components of the model (glucose infusion rate; GIR); an effect compartment was used to account for the delay. An Emax model between effect compartment concentration and GIR best described the PK/PD relationship. The effect compartment model was parameterized in terms of the concentration in the effect compartment (Ce), the GIR at time zero (E0), Emax, and the concentration to achieve 50% of Emax (EC50), as described by the equation
Between-subject variability was assumed to have a log-normal distribution, and a proportional error model was used for the estimation of residual variability (within subject variability). Both PK and PD models were fitted using first-order conditional estimation (FOCE) methods with interaction. The goodness of fit of the model was evaluated by a visual predictive check (VPC) that was performed by simulating 200 replicates using the final model with estimated parameters.
The combined PK data from the 3 studies appeared to be linear in the 0.05-1.3 U/kg dose range, while the PD data showed an Emax (nonlinear) profile. Thus, the assumptions used for performing the simulations were that (1) PK would continue to be linear beyond 1.3 U/kg; (2) PD would be nonlinear, and follow an Emax model at doses higher than 0.3 U/kg; (3) PD differences are driven by PK; and (4) EC50 may need to be higher for patients with type 2 diabetes due to insulin resistance.
PK/PD simulation profiles were performed for U-500R only at QD doses of 165 U, 250 U, 500 U, and 750 U, and at steady-state for a total daily dose of ~500 units/day in dosing regimens of 500 U QD, 250 U BID, and 165 U TID. Unevenly divided dosing using the same total daily dose for BID 300:200 U and TID 200:150:150 U (60:40 and 40:30:30 proportions, respectively) was also evaluated.
Results
The PK profiles were best described by a 1-compartment model with first-order absorption and first-order elimination. First-order absorption was selected based on the goodness of fit of the model, with 2 different absorption rate constants (Ka) to account for the difference in absorption rates between U-500R and U-100R. The VPC plot shows no major deviation between the simulations and individual observations, and the estimated intersubject variability adequately captured the observed variability from the study. The VPC of the model for the PD (GIR) components is represented in Figure 2.
Figure 2.

Pharmacokinetic/pharmacodynamic model goodness of fit for U-500R and U-100R, 50 U and 100 U doses. The orange line and shaded area represent the model-estimated median and 90th prediction interval respectively; the solid circles represent individual data points.
Of the covariates tested (age, sex, body mass index [BMI], body weight), dose was identified as a significant covariate contributing to the interindividual variability of absorption rate constant (Ka) and apparent clearance (CL/F), and weight was significant for apparent volume of distribution (Vd/F). Only BMI was identified as a significant covariate contributing to interindividual variability of Emax. PK/PD simulations of single 165, 250, 500, and 750 U doses of U-500R are shown in Figure 3.
Figure 3.
Pharmacokinetic/pharmacodynamic modeling of single (QD) daily doses of U-500R: 165 U, 250 U, 500 U, 750 U. The hatched area represents the 90th prediction interval from the model; the thicker line represents the median.
As expected based on the assumption of linearity in PK, for single doses, median Cmax increased approximately 5-fold in the 165 U to 750 U dose range. However, the PD effect is not linear, therefore the maximum effect reflected by the maximum GIR (Rmax), was predicted to increase approximately 1.5-fold across the simulated dose range based on the Emax model.
Simulations of the PK and PD of U-500R at steady-state for approximately the same total daily dose (~500 U) administered as QD, BID, or TID daily doses are shown in Figure 4.
Figure 4.
Pharmacokinetic/pharmacodynamic modeling of U-500R doses at steady-state: 500 U QD, 250 U BID, 165 U TID. QD doses were administered at 7 am, BID doses at 7 am and 6 pm, and TID doses at 7 am, 12 noon, and 6 pm. The hatched area represents the 90th prediction interval from the model; the thicker line represents the median.
Steady-state PK appeared to be reached rapidly after 24 hours postdose for all 3 dosing regimens. As expected, the time-concentration and time-action profiles of the QD regimen at steady-state showed the greatest fluctuations for both PK and PD with peak concentration and effect at approximately 5 and 7 hours after 7 am administration, and pronounced reductions during evening hours. PD at steady-state for BID dosing was characterized by a gradual increase in insulin action with each dose and a fairly stable background insulin effect. For the TID regimen, activity was maintained throughout the dosing interval. The steady-state, potential postprandial/basal actions of U-500R are evident from the PK/PD profiles during the 24 hours of day 5, starting with the breakfast dose (Figure 5).
Figure 5.
Pharmacokinetic/pharmacodynamic modeling of U-500R doses at steady-state during 24 hours of Day 5: 500 U QD, 250 U BID, 165 U TID. Arrows represent dose administration times: for QD at 7 am, BID at 7 am and 6 pm, and TID at 7 am, 12 noon, and 6 pm.
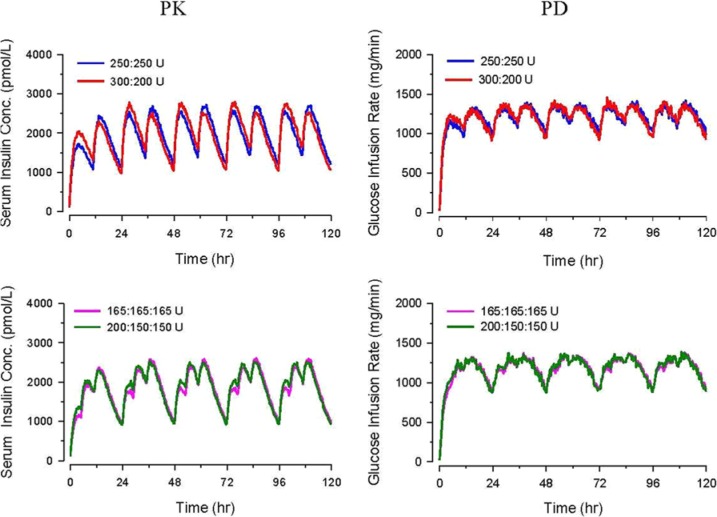
PK/PD simulation profiles of unevenly divided dosing with the same total daily dose (BID 300:200 U and TID 200:150:150 U) at steady-state were nearly identical to those of evenly proportioned doses (Figure 6).
Figure 6.
Pharmacokinetic/pharmacodynamic modeling of U-500R insulin at steady state: varied proportion dosing (BID doses at 7 am and 6 pm, and TID doses at 7 am, 12 noon, and 6 pm).
Discussion
Despite the increasing use of high-dose U-500R therapy to manage a growing population of insulin-resistant patients with diabetes mellitus in the US, the PK/PD characteristics of U-500R at clinically relevant doses and dosing regimens, especially higher doses commonly used in clinical practice (MDI up to 3.0 U/kg/day), have not been completely elucidated. Published case series have reported using U-500R by MDI with BID6,14,17,18 and TID14,18,19 or unspecified dosing.18,20-25 Most of the series reported use of U-500R without concomitant use of U-100 basal or prandial insulins.6,14,17,18,20-23 Expert reviews have supported the clinical use of U-500R by MDI in patients requiring high-dose insulin therapy, with broad dosing recommendations (BID, TID, and 4 times daily [QID]) and clinical advice.2,11-13,26-29 According to the most recent treatment algorithm proposed by Reutrakul et al,2 U-500R by MDI (BID, TID, or QID depending on total daily dose), given at least 30 minutes before meals, can be used as the sole insulin preparation without U-100 basal insulins to meet both basal and prandial insulin requirements of highly insulin-resistant patients. Recently reported PK/PD results support the use of U-500R without use of other basal insulins due to the long duration of effect of U-500R.7 However, these results were limited in that this was a single-dose study in healthy obese subjects with a maximum dose of 100 units, at the lower end of doses commonly used in clinical settings. In the current analysis, sophisticated population PK/PD modeling and simulation methods using a meta–data set of 3 insulin studies were applied to predict the exposure and effect at steady-state of clinically relevant high doses and steady-state dosing regimens of U-500R. Patients with type 1 diabetes are anticipated to have insulin resistance approximating that of healthy normal weight subjects. The study in patients with type 1 diabetes that was included in the data set used lower doses; these patients were included to allow to characterize the dose-response for PK and PD, but their contribution to the predicted effect would be smaller. The PD effects due to the higher doses was driven by the obese population with higher insulin resistance, who received doses of 50 and 100 units. The effect observed in the obese population is anticipated to be similar to what would be expected in type 2 diabetes, which is the main population of interest. The PK/PD models described both the PK data and the nonlinear PD data well (Figure 2); the lack of linearity with dose in PD data is a known phenomenon,30 partly due to insulin resistance in the higher weight population receiving higher doses.
The model predictions in this manuscript have a number of important potential clinical implications. PK/PD modeling and simulation of high single doses of U-500R (up to 750 U) displayed the expected dose-dependent kinetics and saturable (nonlinear) insulin dynamics31 with longer time-to-peak insulin concentrations and effect and duration of action, which may be relevant for dose titration based on home plasma glucose monitoring and management of hypoglycemic episodes. Simulations of QD dosing at steady-state exhibited greater fluctuations of insulin concentration and action profiles between day and evening hours. For BID and TID dosing at steady state, there is an associated increase in insulin concentration and action with each dose, although this effect was rather late and prolonged with a gradual decline over evening hours, reflecting basal insulin characteristics. This effect is highly exaggerated with QD dosing at steady-state, which might suggest a slightly greater risk of early-daytime hypoglycemia but less risk of nocturnal hypoglycemia. BID dosing at steady-state appears to have more of a postinjection elevation or gradual peak effect than TID dosing and as such, might potentially indicate some advantage for patients who prefer fewer injections (especially if they were to only consume 2 meals a day). The model suggests that overall insulin action (Gtot) would not be affected by giving a higher proportion of TID (40:30:30) or BID (60:40) doses earlier in the day, a common clinical practice.2,11-13
While it is convenient and customary to give BID or TID insulin associated with meals, the model suggests that this may not be completely necessary when using U-500R BID or TID. In addition, although there is some gradual increase in insulin action after a dose, it is by no means closely tied to carbohydrate absorption if U-500R is taken 30 to 60 minutes before meals. An insulin regimen that requires fewer injections without the need to be combined with U-100 insulins may facilitate patient adherence21,24 and reduce the risk of dosing confusion.1 The improved glycemic control observed with U-500R in various case series2,11,14,17,19-25 is possibly related to better patient adherence with the need for fewer injections rather than the widespread perception of improved absorption with U-500R,2 which has not been demonstrated.7
Limitations of the current model include the use of pooled PK/PD data from healthy obese subjects, healthy normal-weight subjects, and patients with type 1 diabetes instead of insulin-resistant patients with diabetes requiring high-dose insulin therapy. The model extrapolated beyond the available data: only single-dose data were available, and simulations were based on doses up to 7.5-fold higher than the available 100 U dose data. Moreover, the 100 U doses are at the low end of those typically used in clinical practice. Other potential limitations include the lack of being able to incorporate interoccasion variability and insulin receptor saturation into the model; and the potential bias introduced from combining U-100R and U-500R data in different populations and across a wide range of doses, for model development. Dosing regimens modeled did not include BID and TID in other proportions from those presented, and did not include QID dosing regimens. Also, the relationship of injection volume to absorption rate of insulin is poorly understood,5,32,33 the effects of severe obesity on insulin absorption33 were not specifically incorporated in the model, and the link between glucose infusion rate and blood glucose was not established.
Conclusions
In summary, modeling and simulation of the PK/PD of high doses of U-500R appear to substantiate the widespread use of this formulation as BID or TID administration, with development of steady-state levels within 24 hours and an extended duration of activity, suggesting a lack of need for concomitant use of U-100 basal insulins. Our results suggest that with either BID or TID dosing of U-500R, there is a gradual increase in insulin action with each dose and a fairly stable background insulin effect. TID dosing may provide slightly better full-day insulin effect. Our results also predict that because once-daily U-500R does not have a flat profile, it would not be advantageous for use as a basal insulin, and that bedtime dosing may increase the risk of nocturnal hypoglycemia. The challenge of achieving glycemic control, particularly in the target population of often severely obese and severely insulin-resistant patients with diabetes, warrants the conduct of randomized controlled clinical trials to provide clinicians further information on U-500R efficacy and safety and to evaluate specific titration regimens/algorithms to attain fasting and postprandial plasma glucose targets. Additional PK/PD studies of U-500R are needed to validate the model predictions in the target population of patients with insulin-resistant diabetes. Modeling work as presented in this article can be instrumental in informing the design of further studies, and can contribute to clinical research being performed in a more effective and efficient manner.
Acknowledgments
We thank Mary Alice Miller, PhD, and Jeff Bonner, PhD, of Eli Lilly and Company who provided assistance in the preparation and writing of the manuscript.
Footnotes
Abbreviations: BID, twice daily; BMI, body mass index; Conc, concentration; FOCE, first-order conditional estimate; GIR, glucose infusion rate; HbA1c, glycated hemoglobin; MDI, multiple daily injections; PD, pharmacodynamics; PK, pharmacokinetic; QD, once daily; QID, 4 times daily; TID, thrice daily; U, units; U-100R, human regular U-100 insulin; U-500R, human regular U-500 insulin; VPC, visual predictive check.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AD, XM, SR, and JAJ are full-time employees and are minor stockholders of Eli Lilly and Company. FO received grants for research support from Eli Lilly, Bristol-Myers Squibb, AstraZeneca, Merck, Boehringer Ingelheim, NovoNordisk, Sanofi, Janssen, and GlaxoSmithKline pharmaceutical companies. In addition, FO has served in scientific advisory panels for Janssen, NovoNordisk, Sanofi, and Medtronic. FO does not own any stock in any pharmaceutical company. RMB has served on a scientific advisory board, consulted or performed clinical research with Abbott Diabetes Care, Amylin, Bayer, Becton Dickinson, Boehringer Ingelheim, Intuity, Calibra, DexCom, Eli Lilly, Halozyme, Helmsley Trust, Hygieia, Johnson & Johnson, Medtronic, Merck, the National Institute of Health, Novo Nordisk, ResMed, Roche, Sanofi, and Takeda. His employer, Park Nicollet Health Services, has contracts with the listed companies for his services and no personal income goes to RMB. RMB has inherited Merck stock, and has been a volunteer officer of the American Diabetes Association.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Eli Lilly and Company.
References
- 1. Humulin R® U-500 [product label]. Indianapolis, IN: Eli Lilly and Company; 2013. [Google Scholar]
- 2. Reutrakul S, Wroblewski K, Brown RL. Clinical use of U-500 regular insulin: review and meta-analysis. J Diabetes Sci Technol. 2012;6:412-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. NIH Clinical Trials Study Record Detail. U.S. National Institutes of Health website. Available at: http://clinicaltrials.gov/ct2/show/NCT01774968. NLM Identifier: NCT01774968. Accessed July 21, 2013.
- 4. IMS Health National Prescription Audit Monthly, January 2007-August 2010. Data on file. Indianapolis, IN: Eli Lilly and Company. [Google Scholar]
- 5. Galloway JA, Spradlin CT, Nelson RL, Wentworth SM, Davidson JA, Swarner JL. Factors influencing the absorption, serum insulin concentration, and blood glucose responses after injections of regular insulin and various insulin mixtures. Diabetes Care. 1981;4:366-376. [DOI] [PubMed] [Google Scholar]
- 6. Davidson MB, Navar MD, Echeverry D, Duran P. U-500 regular insulin: clinical experience and pharmacokinetics in obese, severely insulin-resistant type 2 diabetic patients. Diabetes Care. 2010;33:281-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de la, Peña A, Riddle M, Morrow LA, et al. Pharmacokinetics and pharmacodynamics of high-dose human regular U-500 insulin versus human regular U-100 insulin in healthy obese subjects. Diabetes Care. 2011;34:2496-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Binder C. Absorption of injected insulin. A clinical-pharmacological study. Acta Pharmacol Toxicol (Copenh). 1969;27(suppl 2):1-84. [DOI] [PubMed] [Google Scholar]
- 9. Jorgensen KH, Hansen AK, Buschard K. Fivefold increase in insulin concentration delays the absorption of subcutaneous injected human insulin suspensions in pigs. Diabetes Res Clin Pract. 2000;50:161-167. [DOI] [PubMed] [Google Scholar]
- 10. Ovalle F. Clinical approach to the patient with diabetes mellitus and very high insulin requirements. Diabetes Res Clin Pract. 2010;90:231-242. [DOI] [PubMed] [Google Scholar]
- 11. Lane WS, Cochran EK, Jackson JA, et al. High-dose insulin therapy: is it time for U-500 insulin? Endocr Pract. 2009;15:71-79. [DOI] [PubMed] [Google Scholar]
- 12. Segal AR, Brunner JE, Burch FT, Jackson JA. Use of concentrated insulin human regular (U-500) for patients with diabetes. Am J Health-Sys Pharm. 2010;67:1526-1535. [DOI] [PubMed] [Google Scholar]
- 13. Cochran EK, Valentine V, Samaan K, Corey IB, Jackson JA. U-500 regular insulin therapy in high-dose insulin-requiring patients with diabetes: what diabetes educators need to know. Diabetes Educator. 2005;28:1240-1244. http://tde.sagepub.com/cgi/content/abstract/0145721713508822v1?papetoc. [Google Scholar]
- 14. Quinn SL, Lansang C, Mina D. Safety and effectiveness of U-500 insulin therapy in patients with insulin-resistant type 2 diabetes mellitus. Pharmacotherapy. 2011;31:695-702. [DOI] [PubMed] [Google Scholar]
- 15. Landersdorfer CB, Jusko WJ. Pharmacokinetic/pharmacodynamic modeling of glucose clamp effects of inhaled and subcutaneous insulin in healthy volunteers and diabetic patients. Drug Metab Pharmacokinet. 2010;25(5):418-429. [DOI] [PubMed] [Google Scholar]
- 16. Potocka E, Baughman RA, Derendorf H. Population pharmacokinetic model of human insulin following different routes of administration. J Clin Pharmacol. 2011;51:1015-1024. [DOI] [PubMed] [Google Scholar]
- 17. Ballani P, Tran MT, Navar MD, Davidson MB. Clinical experience with U-500 regular insulin in obese, markedly insulin-resistant type 2 diabetic patients. Diabetes Care. 2006;29:2504-2505. Erratum 2007;30:455. [DOI] [PubMed] [Google Scholar]
- 18. Ziesmer AE, Kelly KC, Guerra PA, George KG, Dunn FL. U-500 Regular insulin use in insulin resistant type 2 diabetic veteran patients. Endocr Pract. 2012;18:34-38. [DOI] [PubMed] [Google Scholar]
- 19. Neal JM. Analysis of effectiveness of human U-500 insulin in patients unresponsive to conventional insulin therapy. Endocr Pract. 2005;11:305-307. [DOI] [PubMed] [Google Scholar]
- 20. Wafa WS, Khan MI. Use of U-500 regular insulin in type 2 diabetes. Diabetes Care. 2006;29:2175-2176. [DOI] [PubMed] [Google Scholar]
- 21. Dailey AM, Williams S, Taneja D, Tannock LR. Clinical efficacy and patient satisfaction with U-500 insulin use. Diabetes Res Clin Pract. 2010;88:259-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Garg R, Lawrence IG, Akinsola MO, Davies MJ, McNally PG. Improved glycaemic control in severely insulin resistant, insulin-treated diabetic patients with U500 Human Actrapid over two year follow-up [abstract]. Diabetologia. 2004;47(suppl 1):A58. [Google Scholar]
- 23. Nayyar V, Jarvis J, Lawrence I, et al. Long-term follow-up of patients U-500 insulin: a case series. Pract Diab Int. 2010;27:194-197. [Google Scholar]
- 24. Boldo A, Comi RJ. Clinical experience with U500 insulin: risks and benefits. Endocr Pract. 2012;18:56-61. [DOI] [PubMed] [Google Scholar]
- 25. Lowery JB, Donihi AC, Korytkowski MT. U-500 insulin as a component of basal bolus insulin therapy in type 2 diabetes. Diabetes Technol Ther. 2012;14:505-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cochran E, Musso C, Gorden P. The use of U-500 in patients with extreme insulin resistance. Diabetes Care. 2005;28:1240-1244. [DOI] [PubMed] [Google Scholar]
- 27. Garg R, Johnston V, McNally PG, Davies MJ, Lawrence IG. U-500 insulin: why, when and how to use in clinical practice. Diabetes Metab Res Rev. 2007;23:265-268. [DOI] [PubMed] [Google Scholar]
- 28. Cochran E. U-500 insulin: when more with less yields success. Diabetes Spectr. 2009;22:116-121. [Google Scholar]
- 29. Cochran E, Gorden P. Use of U-500 insulin in the treatment of severe insulin resistance. Insulin. 2008;3:211-218. [Google Scholar]
- 30. Woodworth JR, Howey DC, Bowsher RR. Establishment of time-action profiles for regular and NPH insulin using pharmacodynamics modeling. Diabetes Care. 1994;17(1):64-69. [DOI] [PubMed] [Google Scholar]
- 31. Woodworth JR, Howey DC, Bowsher RR, Lutz S, Santa PF, Brady P. [Lys(B28), Pro(B29)] human insulin (K): dose-ranging vs. Humulin R (H) [abstract]. Diabetes. 1993;42(suppl 1):54A. [Google Scholar]
- 32. Trajanoski Z, Wach P, Kotanko P, Ott A, Skraba F. Pharmacokinetic model for the absorption of subcutaneously injected soluble insulin and monomeric insulin analogues. Biomed Tech (Berl). 1993;38:224-231. [DOI] [PubMed] [Google Scholar]
- 33. Gagnon-Auger M, du Souich P, Baillargeon JP, et al. Dose-dependent delay of the hypoglycemic effect of short-acting insulin analogs in obese subjects with type 2 diabetes: a pharmacokinetic and pharmacodynamic study. Diabetes Care. 2010;33:2502-2507. [DOI] [PMC free article] [PubMed] [Google Scholar]