Abstract
Compression of the active phase (α) during reentrainment to phase-shifted light-dark (LD) cycles is a common feature of circadian systems, but its functional consequences have not been investigated. This study tested whether α compression in Siberian hamsters (Phodopus sungorus) impaired their spatial working memory as assessed by spontaneous alternation (SA) behavior in a T-maze. Animals were exposed to a 1- or 3-h phase delay of the LD cycle (16 h light: 8 h dark). SA behavior was tested at four multi-day intervals after the phase shift and α was quantified for those days. All of the animals failed at the SA task while α was decompressing, but recovered spatial memory ability once α returned to baseline levels. A second experiment exposed hamsters to a 2-h light pulse either early or late at night to compress α without phase-shifting the LD cycle. SA behavior was impaired until α decompressed to baseline levels. In a third experiment, α was compressed by changing photoperiod (LD 16:8, 18:6, 20:4) to see if absolute differences in α were related to spatial memory ability. Animals performed the SA task successfully in all three photoperiods. These data show that the dynamic process of α compression and decompression impairs spatial working memory and suggests that α modulation is a potential biomarker for assessing the impact of transmeridian flight or shift work on memory.
Keywords: circadian, phase shift, alpha, spontaneous alternation, jet lag, shift work, sex differences, biomarker
INTRODUCTION
Disruptions in the entrainment of circadian rhythms produce substantial deficits in memory processing (see Smarr et al., 2014 for review). In humans, abrupt phase shifts of the daily illumination cycle such as those experienced after a single transmeridian flight or when chronically engaged in shift work or frequent travel also impair memory (Cho et al., 2000; Cho 2001; Rouchi et al., 2005). Given that air travel and shift work are integral parts of many peoples’ lives, efforts should be made to develop ways to ameliorate or minimize their impact on brain function. To accomplish this goal, it is necessary to relate specific parameters of circadian timing to specific memory impairments.
Memory deficits caused by jet lag and shift work can be regarded as entrainment problems in which the circadian system is put in a transitional state from which it eventually recovers by resynchronizing to the shifted zeitgeber. Similar deficits have been reported in animal studies where simulated jet lag impaired memory. When mice were exposed to a single phase-delay or phase-advance of the LD cycle, contextual fear conditioning was impaired on the day after the shift, regardless of whether the training occurred before or after the phase shift (Loh et al., 2010). Several studies have trained rats on a passive avoidance task and then exposed them to LD cycle phase advances or delays of different durations (Davies et al., 1974; Childs and Redfern, 1981; Tapp and Holloway, 1981; Fekete et al., 1985; Fekete et al., 1986). Other studies trained and tested rats on the passive avoidance task after a phase shift of the LD cycle (Fekete et al., 1985). In all of these studies, both acquisition and recall of the task was impaired in the days following the phase shifts.
Several studies have used a chronic phase-shifting protocol in which the LD cycle is phase-advanced by 3 h each day for 6 days (Devan et al., 2001; Craig and McDonald, 2008; Zelinski et al., 2013). The animals in these studies were unable to maintain entrainment during the LD cycle shifts, as expected, but eventually reentrained. This phase-advancing LD cycle protocol did not impair performance on the Morris water maze task, and visual inspection of the published actograms suggests that animals had reentrained by the time they were tested (Devan et al., 2001; Craig and McDonald, 2008). By contrast, animals that were exposed to this phase-shifting protocol and that also exhibited deficits in platform search behavior did reentrain, but did so with delayed phase angles of entrainment and a shortened duration of their active phases (Zelinski et al., 2014). Overall, memory studies that exposed animals to either acute or chronic phase shifts of the LD cycle rarely monitored circadian rhythms or quantified parameters of entrainment. In cases where those parameters were quantified, however, entrainment patterns were not the same after the phase shift and activity onset was not a reliable indicator of stable entrainment of the circadian system as a whole.
Some studies have suggested that memory deficits can persist long after animals reentrain to the LD cycle. In those studies, the LD cycle was phase-advanced by 3 or 6 h every few days for several weeks (Craig and McDonald, 2008; Gibson et al., 2010). In one study (Gibson et al., 2010), golden hamsters were found to have memory impairments that persisted for 30 days after the LD cycle was stabilized, but circadian rhythms could not be monitored at the time of memory testing. A similar study performed with rats found that phase-shifting impaired memory (Craig and McDonald, 2008), but also found that the animals exhibited a significantly delayed phase angle of entrainment nearly one month after stabilizing the LD cycle. Thus, the animals cannot be said to have resumed entrainment with the same circadian state that they exhibited prior to the phase shift protocol. This is important because it shows that the aftereffects of the phase-shifting protocol can last long after activity onset reentrains, and implies that the long-term memory deficits may be due to ongoing changes in circadian state.
The difficulty in relating memory deficits to reentrainment may lie in the way entrainment is defined. The studies discussed above defined entrainment exclusively in terms of locomotor activity onset. During reentrainment to phase shifts of LD cycles, the duration of sustained nightly locomotor activity (i.e., α) typically decreases (compresses) rapidly and then gradually returns to its pre-shifted level (Pittendrigh and Daan, 1976b; Yamaguchi et al., 2013). For delays of the LD cycle, the progressive decompression of α continues long after activity onset has stably reentrained. Likewise, activity onset takes much longer to reentrain to a phase advance of the LD cycle than does activity offset.
If entrainment is defined in terms of the stability of both activity onset and offset, then a different picture of entrainment and memory emerges. For example, an actogram of an animal exhibiting some performance impairment in the Morris water task indicates that α may still have been in the process of decompression (Zelinski et al., 2013), but α was not quantified. Tapp and Holloway (1981) trained rats in a passive avoidance task and then exposed them to a single shift of the LD cycle (6 or 12 h). Rats in which α did not compress during reentrainment performed well on the memory test, whereas those with a compressed α exhibited substantial memory deficits. The dichotomy in entrainment patterns in that study suggests that memory impairment was caused by α compression and not by the reentrainment process per se.
Given that so few memory studies have actually monitored circadian rhythms during reentrainment, it is difficult to know exactly which parameters of circadian timing might serve as markers of memory impairment, but α compression might be a good candidate. The goal of this study was to induce α compression in multiple ways and determine whether dynamic and static changes in α would be associated with impairments in a hippocampal-dependent memory test. We chose spontaneous alternation (SA) behavior as a measure of spatial working memory because task performance is stable over time, even when animals are tested multiple times per day over several weeks (Douglas, 1989). Thus, we were able to test individual animals repeatedly without the concern that the animals would benefit from practice effects or go through an extinction process of a learned event, and the SA task obviated the need to control for training-testing intervals in animals tested repeatedly. SA behavior reflects the natural tendency of animals to alternate arm choices in a maze over a short time period (Dember and Fowler, 1958; Dember, 1989). Successful alternation attempts require the animal to acquire and use spatial information during the test. As such, SA is regarded as an excellent behavioral assay of hippocampal function (Gerlai, 1998; Deacon and Rawlins, 2006).
METHODS
Animals and Housing Conditions
Male and female Siberian hamsters (Phodopus sungorus) were bred in the laboratory in a 16:8-h light-dark (LD) cycle (lights on at 0200 h, PST) at an ambient temperature of 22°C. Animals were provided with cotton batting for nesting material; food (Purina chow # 5015) and tap water were available ad libitum. All experimental procedures were approved by Stanford University’s Administrative Panel on Laboratory Animal Care (Animal Use Protocol Number: 14988) and were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Equal numbers of male and female hamsters were used in all experiments.
Housing and lighting conditions were as described previously (Ruby et al., 2013). During each experiment, animals were housed singly and locomotor activity was measured by passive infrared motion detectors mounted directly above the tip of the water bottle sipper tube (Ruby et al., 2013). Activity levels primarily reflected drinking behavior and locomotor activity that occurred directly under the sipper tube. Activity bouts were summed in 10-min intervals and stored on computer. The times of day when spatial memory was tested are given by zeitgeber time (ZT) where ZT0 = time of lights-on and ZT16 = time of lights-off in the animal rooms.
Spontaneous Alternation in the T-Maze
SA is based on the natural tendency of rodents to consecutively alternate between left and right arm choices during exploration of a T-maze (Gerlai, 1998; Deacon and Rawlins, 2006). Dimensions of the T-maze and lighting conditions during testing were as described (Ruby et al., 2013). Hamsters were placed in the start chamber located at the far end of the stem arm of a transparent acrylic T–maze. A sliding door separated the start chamber from the rest of the apparatus that is comprised of an alleyway that leads to a choice point at the intersection of the stem arm with the left and right arms of the maze. A divider panel is centered at the intersection of the “T” and extends a short distance into the stem arm. The divider panel increases the number of arm entries in a given period of time (Deacon and Rawlins, 2006). Animals were tested 3 h before dark onset in all experiments.
Hamsters were gently handled daily for 5 min over 7–10 days prior to testing using a progressive handling procedure. During the first 3 days, the experimenter’s hand was placed in the cage and the hamster was allowed to sniff and crawl over the hand. On days 4 and 5, each hamster was held in the experimenter’s hand without removing the animal from the cage. Over the remaining days, the animals were removed from the cage, held in both hands, and allowed to crawl along the experimenter’s arm. The animals were considered ready for testing when all hamsters could be picked up and handled without them retreating when approached and exhibited no obvious stress responses when handled (e.g., vocalizations, escape behavior, rapid respiration). The protocol for SA testing began with a hamster confined to the start chamber for 60 sec, and then permitted access to the rest of the maze for 7 min. An alternation attempt was scored when all four feet of a hamster entered one of the lateral arms, re-entered the stem arm, and then entered the lateral arm opposite the one previously chosen. Re-entry into the same arm was a nonalternation. Alternation performance was thus operationally defined by the percentage of time the hamster alternated upon arriving at the divider panel (i.e., the number of alternations observed/the number of alternation attempts X 100). The T–maze was cleaned with 70% ethanol, dried, and ventilated for a few minutes between trials.
Entrainment and α
We defined α as the duration of nocturnal activity between activity onset and offset. Activity onset was defined as the first bout of activity that remained above the daily mean for ≥ 30 min, and activity offset was the last bout of activity above the daily mean before activity fell below the mean for ≥ 30 min. An activity profile was created for individual days and activity onsets and offsets were determined manually for each day (ClockLab, Actimetrics, Evanston, IL); α was quantified for days on which hamsters were tested for SA behavior. To determine the length of time animals needed to reentrain to the LD cycle, animals had to exhibit clear reentrainment based on visual inspection of the actograms. The exact days on which activity onsets and offsets reentrained were determined by fitting a line over the last 10 days of entrained activity onsets and offsets on the actograms. That line was then extended upward across all previous days in the actograms (ClockLab, Actimetrics, Evanston, IL). The first day of reentrainment for activity onsets and offsets was defined as the first onset and offset to occur after the extended line. A similar procedure was also used for animals housed in constant darkness (DD) in experiment 1, but in those animals, we were limited to fitting a line over 5 days because DD tends to alter circadian period after that time.
Data Analysis
Performance on the SA test was determined by a one-sample t-test to determine whether scores were statistically different from random chance performance (i.e., alternation = 50%). A score of positional bias was created to check for left-right biases in the T-maze arms. Positional bias was calculated as: time on the right/(time on the left + time on the right) × 100, so that a score that is significantly < 50% indicates a left bias, and > 50% indicates a right bias. Changes in α, the number of arm entries, and in positional bias were evaluated by one-way analysis of variance (ANOVA) with repeated measures. Dunnett’s correction was set at P=0.05 and used for all subsequent pairwise comparisons with the baseline condition serving as the control group. Data are presented as mean ± SEM. Siberian hamsters do not exhibit any sex differences in SA performance, arm entries, or positional bias (Ruby et al., 2013), so data from males and females were combined.
The notation suggested by Aschoff et al. (1975) for stating phase shift parameters is employed here. A phase advance or delay is indicated by a plus (+) or minus (–) sign, respectively, followed by phase shift duration in hours, and “L” or “D” to indicate whether the shift was accomplished via a change in the light or dark phase, respectively. For example, a 3-h phase delay made by extending the light phase is indicated by −3L.
Experiment 1: Phase Delay of the LD Cycle by 1 or 3 Hours
This experiment simulated jet lag to determine whether small phase delays of the LD cycle were sufficient to impair spatial working memory. Hamsters were exposed to a phase delay of the LD cycle (16:8) by extending the light phase for 1 (n=10) or 3 (n=10) h for a single light cycle, and remained in the phase-delayed LD cycle (16:8) thereafter. SA behavior was assessed five days prior to the phase delay to establish a baseline (BL) alternation rate, and at 2, 7, 14, and 21 days after the shift. To test for light masking effects of activity onset, a separate group of hamsters (n=8) was exposed to the −3L phase shift and then housed in constant darkness (DD) at the end of the extended light phase.
Experiment 2: Early and Late Nighttime Light Pulses
This experiment tested whether α compression could impair memory independent of the phase-shifting effects of the LD cycle. Animals were housed in a 16:8 LD cycle and exposed to a single 2-h light pulse during the night from ZT 17–19 (n=10) or ZT 21–23 (n=10) to produce a phase delay of activity onset or a phase advance of activity offset, respectively, of the activity rhythms. SA behavior was assessed five days prior to the light pulses (BL), and at 2, 7, 14, and 21 days after the pulses.
Experiment 3: Using Photoperiod to Compress α
Experiments 1 and 2 evaluated whether transient decreases in α impaired memory, but did not rule out the possibility that simply shortening α might impair memory. This experiment tested whether the absolute duration of α was associated with deficits in spatial working memory. The time of dark offset was advanced by 2 h for each change in day length to shorten α. SA was tested while animals (n=10) were housed in a 16:8 LD cycle, then after stable reentrainment to LD 18:6, and then after stable reentrainment to LD 20:4.
RESULTS
Sex Differences
We previously showed that Siberian hamsters do not exhibit sex differences in SA scores, number of arm entries, or left-right arm preferences in the T-maze (Ruby et al., 2013). The present study retrospectively tested for sex differences in α compression in experiment 1 (−3L phase shift) and experiment 2 (late nighttime pulse) because these two photic manipulations produced the largest effects on α compression. Two-way ANOVA was used to test for sex differences with repeated measures for time after light treatment. No significant main effects for sex differences in α were found in experiment 1 (F(1,8)=2.09, P=0.186) or 2 (F(1,8)=0.22, P=0.651).
Experiment 1: Phase Delay of the LD Cycle by 1 or 3 Hours
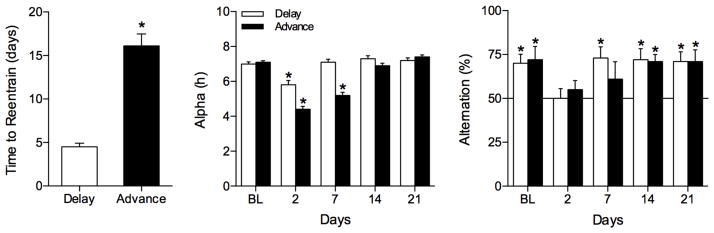
All animals stably reentrained to the LD cycle after the phase-delays (Fig. 1). Reentrainment was characterized by rapid resetting of activity onset and a gradual daily delay of activity offset (Fig. 1). Activity offsets required significantly longer to reentrain than did onsets for both 1- and 3-h phase delays (paired t-tests, P<0.0001 for both delays; Fig. 2, left panel). There were no significant differences in the number of days for activity onset to reentrain in the −1L and −3L groups (P>0.05, Fig. 2, left panel), whereas activity offset in the −3L group required approximately twice as long to reentrain compared to the −1L group (t-test, P<0.001).
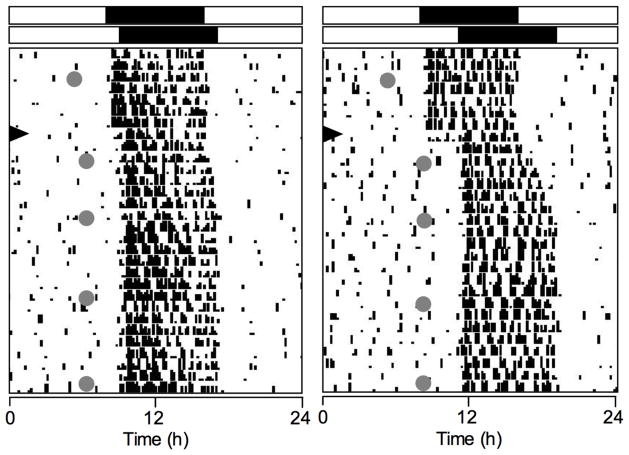
Fig. 1.
Representative single-plotted actograms of animals exposed to −1L (left) or −3L (right) phase delays of the LD cycle. The black arrowhead indicates the day of the phase delays. Gray circles indicate days and times of SA testing. Black rectangles represent the duration and timing of the dark phase of the illumination cycle before (top) and after (bottom) the phase shifts. Note the markedly different reentrainment rates for activity onset and offset.
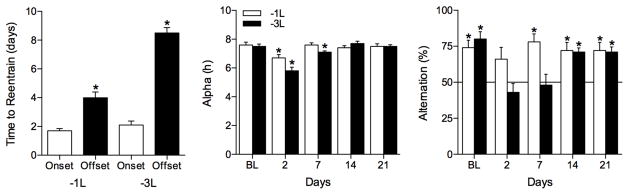
Fig. 2.
SA is impaired by phase delays and recovers once activity offset reentrains. The mean (± SE) number of days for activity onsets and offsets to reentrain (left panel) show that SA behavior recovers to normal levels only after activity offset has reentrained. Activity offsets required significantly more days to reentrain than did onsets (* 0.0001<P<0.001). Mean (± SE) α duration (middle panel) and alternation rates (right panel) are given for each day of testing during baseline (BL) and on days 2, 7, 14, and 21 after the −1L (white bars; n=10) and −3L (black bars; n=10) LD cycle phase shifts. Alternation rates significantly above chance levels indicated by asterisk (0.001<P<0.002). Horizontal line indicates chance level (i.e., 50% alternation). * indicate SA rates that were significantly above chance levels (0.0002<P<0.004), and α values that differed significantly from BL values (Dunnett’s correction, P=0.05).
Both phase shifts significantly shortened α, but it returned to baseline (BL) levels by days 7 and 14 after a 1-h or 3-h delay, respectively (−1L: F(4,32)=4.9, P=0.006; −3L: F(4,45)=24.0, P<0.0001; Fig. 2, middle panel). In both groups, performance on the SA task did not fully recover until after activity offsets had reentrained, thereby restoring α to BL levels. The mean number of arm entries did not change significantly with repeated testing from BL to day 21 (P>0.05; overall means: −1L, 8.5 ± 0.5; −3L, 7.29 ± 0.5). Positional bias did not differ significantly from 50% (P>0.05; overall means: −1L, 49.5 ± 2.1; −3L, 48.2 ± 2.6).
Both −1L and −3L phase delays impaired performance in the SA task. Two days after the 1-h phase delay, hamsters did not perform better than chance (P>0.05), but recovered by day 7 (Fig. 2, right panel). A 3-h phase delay of the LD cycle produced temporary deficits in SA behavior on days 2 and 7 that persisted for at least 7 days after the phase shift, but recovered by day 14 (Fig. 2 right panel).
Hamsters exposed to the 3-h phase delay and then housed in DD (−3L DD) exhibited significant α compression on day 2 (Fig. 3, left two panels). There were no differences in α between −3L and −3L DD groups on days BL or 2 (two-way ANOVA with repeated measures for time; P>0.05; Fig. 3, middle panel). The number of days for activity onset to stabilize (i.e., reentrain in LD or free run stably in DD) did not differ between these groups (t-test; P>0.05; Fig. 3, right panel).
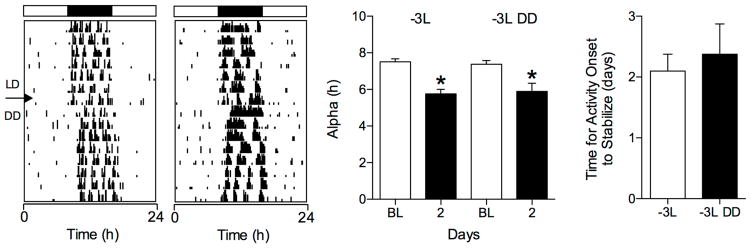
Fig. 3.
The LD cycle does not mask alpha compression or the rate of reentrainment to a −3L phase shift. Representative actograms of two hamsters exposed to a −3L phase shift and then housed in DD (left two panels). The black arrowhead indicates the day of the phase delay. On that day, the light phase was extended by 3 h. DD began at the end of the 3-h light extension. Mean (± SE) α duration during BL and on day 2 after the phase delay (middle panel). * indicates α significantly lower than BL values (P<0.0001). The number of days for α to reentrain (−3L) or to achieve a stable free run (−3L DD) (right panel) did not differ significantly (P>0.05).
Experiment 2: Early and Late Nighttime Light Pulses
Hamsters reentrained to the LD cycle after the light pulses (Fig. 4). Light pulses given early or late at night produced a transient compression of α that lasted for several days (Fig. 4). Animals required approximately 3.6x the number of days to reentrain to the phase advance than to the phase delay (t-test, P<0.0001; Fig. 5, left panel).
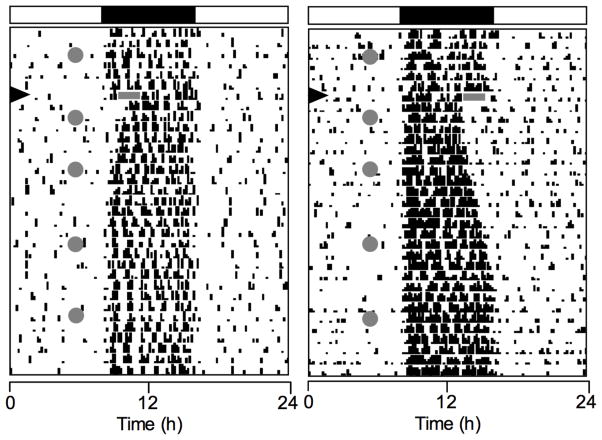
Fig. 4.
Representative actograms of animals exposed to a delay (left) or advance (right) light pulse. The timing and duration of the light pulses are indicated by the gray rectangle. The days on which the light pulse and SA testing occurred are as in figure 1. Note the difference in the number of days required for activity offsets to reentrain.
Fig. 5.
SA is impaired by α compression independently of LD cycle phase shifts. Reentrainment required significantly more time after an advance light pulse than after a delay pulse (left panel, * P<0.0001). Days to reentrain, α (middle panel), and alternation rates (right panel) are presented as in figure 1 (n=10 for both groups). Asterisks indicate SA rates significantly above chance (0.002<P<0.01) and changes in α significantly different from BL (Dunnett’s correction, P=0.05). Random alternation rates (i.e., 50%) indicated by horizontal line. Recovery of spatial working memory occurred only after both activity onsets and offsets reentrained.
There was a significant compression of α by both light pulses (delay: F(3,36)=12.0, P<0.0001; advance: F(4,40)=82.0, P<0.0001; Fig. 5, middle panel), but α returned to BL levels by days 7 and 14 for delay and advance groups, respectively. In both groups, SA performance did not recover until α decompressed to levels observed prior to the light pulses (Fig. 5). The number of arm entries did not change significantly from BL to day 21 (P>0.05; overall means: delay, 6.2 ± 0.3; advance, 5.7 ± 0.2). Positional bias did not differ significantly from 50% (P>0.05; overall means: delay, 52.3 ± 2.8; advance, 46.9 ± 2.3).
Both light pulses impaired performance on the SA task. Hamsters did not perform better than chance on day 2 after the phase delay, but recovered by day 7 (Fig. 5, right panel). The phase advance light pulse produced transient memory impairment on the SA task on days 2 and 7 that recovered by day 14 (Fig. 5, right panel). Thus, in experiments 1 and 2, the time course of α decompression mirrored the recovery of SA performance.
Experiment 3: Using Photoperiod to Compress α
All animals maintained stable entrainment to the LD cycle in all three photoperiods (Fig. 6). Adjustment to each change in photoperiod occurred within 1–2 days. Hamsters did not exhibit any deficits in SA performance in any of the photoperiods (Fig. 7). The number of arm entries was, however, significantly affected by photoperiod (F(2,27)=6.20, P=0.007). Post-hoc analysis found that the number of arm entries were significantly lower in the 20:4 LD cycle compared to 16:8 (mean number of entries: LD 16:8, 7.6 ± 0.7; LD 18:6, 5.9 ± 0.4; LD 20:4, 5.0 ± 0.4). Positional bias did not differ significantly form 50% (P>0.05; LD 16:8, 51.4 ± 4.3; LD 18:6. 49.5 ± 3.4; LD 20:4, 52.8 ± 4.0).
Fig. 6.
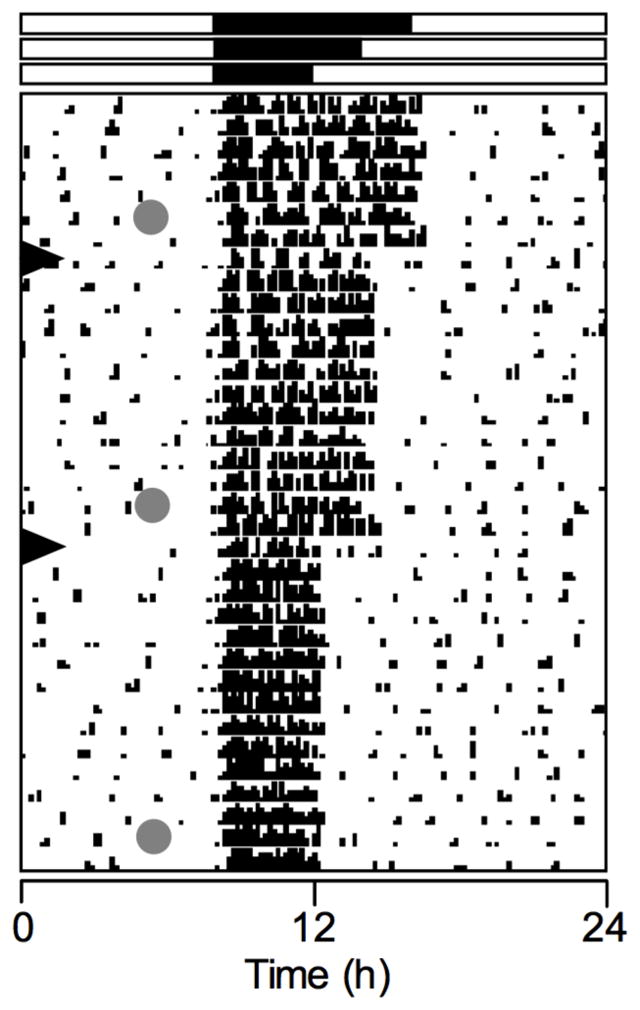
Representative actogram of a hamster exposed to sequentially shorter dark phases. The dark phase of the LD cycle was shortened from 16:8 to 18:6, and then to 20:4 on the days indicated by the arrowheads. The duration and timing of the dark phases are given by the black rectangles above the actogram. Gray circles indicate days and times of SA tests.
Fig. 7.
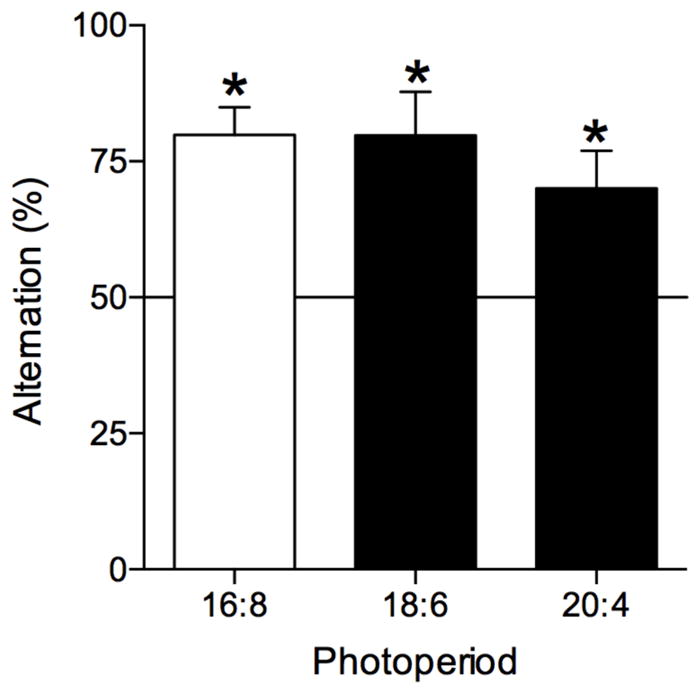
Alternation rates of animals tested in different photoperiods. Random alternation rates (i.e., 50%) indicated by horizontal line. Asterisks indicate SA rates that were significantly above chance levels (0.0004<P<0.02; n=10).
DISCUSSION
Compression of α during reentrainment is a common feature of circadian timing (Aschoff et al., 1975; Pittendrigh and Daan, 1976b; Sisk and Stephan, 1981; Stephan et al., 1982; Yamaguchi et al., 2013), but its functional consequences outside of circadian rhythms have not been investigated. Our main finding was that spatial working memory was impaired during the reentrainment process to phase-delayed LD cycles and did not recover until both activity onset and offset stably reentrained. In addition, memory can be compromised without phase-shifting the LD cycle by delivery of nighttime light pulses that compress α. By contrast to dynamic changes in α, static changes in α caused by increasing day length did not impair memory. These data emphasize the importance of defining entrainment in terms of activity onset and offset when relating circadian rhythms to cognitive function. Given that nonstandard LD cycles and chronic phase-shifting have long lasting aftereffects on rhythm period (τ) and α (Pittendrigh and Daan, 1976a; Aton et al., 2004; Craig and McDonald, 2008), these findings might also help explain past studies in which memory deficits persisted after reentrainment of activity onset to chronically phase-shifted LD cycles.
Most laboratory studies of jet lag and memory have used LD cycle phase shifts between 6 and 12 h. We used smaller phase shifts to determine the lower range of sensitivity of spatial working memory to α compression. A 1-h delay of the LD cycle is a very modest perturbation of the circadian system, and yet this delay was sufficient to transiently interfere with performance on the spontaneous alternation task. This finding demonstrates the sensitivity of working memory to α compression and distinguishes between dynamic changes in α versus static (absolute) changes in their impact on memory. This point is illustrated by our findings in which a dynamic change in α of as little as 1 h can impair memory whereas a static change in α of 8 h—brought about by changing day length—does not.
It is unlikely that our measures of α were compromised by light masking during the LD cycle phase shifts. Hamsters that were phase-delayed and then housed in DD exhibited the same degree of α compression as did animals maintained in the LD cycle. Activity onsets of animals housed in DD also achieved a stable free run in the same number of days required for activity onset to reentrain to the LD cycle. Furthermore, the nighttime light pulse experiments performed here avoided masking issues by not exposing α to the light phase of the LD cycle and still produced results similar to the phase delay experiments. Thus, our data suggest that α compression in Siberian hamsters is not a result of light masking of locomotor activity.
When evaluating performance on memory tests it is important to distinguish between cognitive and noncognitive factors such as lack of motivation to engage the test or distraction from the test environment that might explain poor performance (Dember and Richman, 1989). For these reasons, the number of arm entries in the T-maze is used as a measure of motivation and positional bias is used as an index of attention to the maze cues. A significantly lower number of arm entries would indicate that the animals are not interested in exploring the maze. Positional bias scores that are significantly different from 50% would suggest that the animal is perseverating on one side of the maze and its arm choices are not being guided by spatial exploration. Our data show that even when animals failed the spontaneous alternation test, they still exhibited a high number of arm entries and no positional biases. These measures suggest that dynamic α compression produced true cognitive deficits in spatial working memory.
The present study primarily used phase-delaying signals to examine the relationship between α and SA behavior because earlier studies with Siberian hamsters showed that a 3-h phase advance could disrupt rhythms in a significant number of animals (Ruby et al., 1998). In experiment two, hamsters were exposed to a light signal during the time of night that phase advances rhythms and compresses alpha (Ruby et al., 2004), and exhibited results similar to those in experiment one. Therefore, it is unlikely that a series of LD cycle phase-advance experiments would produce a different outcome. We also did not test a control group of animals in an unmanipulated LD cycle to see if repeated SA testing hastened their recovery because studies in rats have shown that SA behavior is stable over several weeks, even in animals tested multiple times per day (Douglas, 1989), and arrhythmic Siberian hamsters do not improve performance when tested repeatedly (Ruby et al., 2013). While longer day lengths were used to compress α in experiment three, the complementary experiments of shortening day length to decompress α were not performed. Shortening day length induces a robust suite of winter-like endocrine and metabolic changes in Siberian hamsters that negates comparison to long day animals on strictly circadian terms (Goldman, 1999). Hamsters could be allowed to spontaneously recrudesce from short day lengths and would thus match the physiological condition of animals in the present study, but they would also be much older. Future investigations will examine the effects of day length and age on α compression and memory. In summary, we report for the first time that the dynamic process of α compression impairs spatial working memory. This finding implicates α modulation as a possible biomarker for assessing the impact of transmeridian flight or shift work on cognition.
Acknowledgments
The authors thank Fabian Fernandez (University of Arizona) for helpful comments on this manuscript. This research was supported by a grant from the National Institute of Mental Health (#MH095837).
References
- Aschoff J, Hoffmann K, Pohl H, Wever R. Reentrainment of circadian rhythms after phase-shifts of the zeitgeber. Chronobiologia. 1975;2:23–78. [PubMed] [Google Scholar]
- Aton SJ, Block GD, Tei H, Yamazaki S, Herzog ED. Plasticity of circadian behavior and the suprachiasmatic nucleus following exposure to non-24-hour light cycles. J Biol Rhythms. 2004;19:198–207. doi: 10.1177/0748730404264156. [DOI] [PubMed] [Google Scholar]
- Childs G, Redfern PH. A circadian rhythms in passive avoidance behaviour: The effect of phase shift and benzodiazepines. Neuropharmacol. 1981;20:1365–1366. [PubMed] [Google Scholar]
- Cho K, Ennaceur A, Cole JC, Suh CK. Chronic jet lag produces cognitive deficits. J Neurosci. 2000;20:RC66, 1–5. doi: 10.1523/JNEUROSCI.20-06-j0005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K. Chronic “jet lag” produces temporal lobe atrophy and spatial cognitive deficits. Nat Neurosci. 2001;4:567–568. doi: 10.1038/88384. [DOI] [PubMed] [Google Scholar]
- Craig LA, McDonald RJ. Chronic disruption of circadian rhythms impairs hippocampal memory in the rat. Brain Res Bull. 2008;76:141–151. doi: 10.1016/j.brainresbull.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Davies JA, Navarathan V, Redfern PH. The effect of phase-shift on the passive avoidance response in rats and the modifying action of chlordiazepoxide. Br J Pharmacol. 1974;51:447–451. doi: 10.1111/j.1476-5381.1974.tb10681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon RMJ, Rawlins JNP. T-maze alternation in the rodent. Nat Protoc. 2006;1:7–12. doi: 10.1038/nprot.2006.2. [DOI] [PubMed] [Google Scholar]
- Dember WN, Fowler H. Spontaneous alternation behavior. Psychol Bull. 1958;55:412–428. doi: 10.1037/h0045446. [DOI] [PubMed] [Google Scholar]
- Dember WN, Richman CL. Spontaneous alternation behavior. New York, NY: Springer-Verlag; 1989. [Google Scholar]
- Devan BD, Goad EH, Petri HL, Antoniadis EA, Hong NS, Ko CH, Leblanc L, Lebovic SS, Lo Q, Ralph MR, McDonald MJ. Circadian phase-shifted rats show normal acquisition but impaired long-term retention of place information in the water task. Neurobiol Learn Mem. 2001;75:51–62. doi: 10.1006/nlme.1999.3957. [DOI] [PubMed] [Google Scholar]
- Douglas RJ. Using SAB as a tool: advice from a veteran. In: Dember WN, Richman CL, editors. Spontaneous Alternation Behavior. Springer-Verlag; New York: 1989. pp. 145–159. [Google Scholar]
- Fekete M, van Ree JM, de Wied D. The ACTH-(4–9) analog ORG 2766 and desglycinamide9-(Arg8)-vasopressin reverse the retrograde amnesia induced by disrupting circadian rhythms in rats. Peptides. 1986;7:563–568. doi: 10.1016/0196-9781(86)90027-6. [DOI] [PubMed] [Google Scholar]
- Fekete M, van Ree JM, Niesink RJM, de Wied D. Disrupting circadian rhythms in rats induces retrograde amnesia. Physiol Behav. 1985;34:883–887. doi: 10.1016/0031-9384(85)90008-3. [DOI] [PubMed] [Google Scholar]
- Gerlai R. A new continuous alternation task in T-maze detects hippocampal dysfunction in mice: A strain comparison study. Beh Brain Res. 1998;95:91–101. doi: 10.1016/s0166-4328(97)00214-3. [DOI] [PubMed] [Google Scholar]
- Gibson EM, Wang C, Tjho S, Khattar N, Kriegsfeld LJ. Experimental “jet lag” inhibits adult neurogenesis and produces long-term cognitive deficits in female hamsters. PLoS ONE. 2010;5(12):e15267. doi: 10.1371/journal.pone.0015267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman BD. The Siberian hamster as a model for study of the mammalian photoperiodic mechanism. Adv Exp Med Biol. 1999;460:155–164. doi: 10.1007/0-306-46814-x_17. [DOI] [PubMed] [Google Scholar]
- Loh DH, Navarro J, Hagopian A, Wang LM, Deboer T, Colwell CS. Rapid changes in the light/dark cycle disrupt memory of conditioned fear in mice. PLoS ONE. 2010;5(9):e12546. doi: 10.1371/journal.pone.0012546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents: I. The stability and lability of spontaneous frequency. J Comp Physiol A. 1976a;106:223–252. [Google Scholar]
- Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents: IV. Entrainment: pacemaker as clock. J Comp Physiol A. 1976b;106:291–331. [Google Scholar]
- Rouch I, Wild P, Ansiau D, Marquié J-C. Shiftwork experience, age and cognitive performance. Ergonomics. 2005;48:1282–1293. doi: 10.1080/00140130500241670. [DOI] [PubMed] [Google Scholar]
- Ruby NF, Fernandez F, Garrett A, Klima J, Zhang P, Sapolsky R, Heller HC. Spatial memory and long-term object recognition are impaired by circadian arrhythmia and restored by the GABAA antagonist pentylenetetrazole. PLoS ONE. 2013;8(8):e72433. doi: 10.1371/journal.pone.0072433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby NF, Joshi N, Heller HC. Phase shift magnitude and direction determine whether Siberian hamsters reentrain to the photocycle. J Biol Rhythms. 1998;13:506–517. doi: 10.1177/074873049801300606. [DOI] [PubMed] [Google Scholar]
- Ruby NF, Barakat MT, Heller HC. Phenotypic differences in reentrainment behavior and sensitivity to nighttime light pulses in Siberian hamsters. J Biol Rhythms. 2004;19:1–12. doi: 10.1177/0748730404268055. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Stephan FK. Phase shifts of circadian rhythms of activity and drinking in the hamster. Behav Neural Biol. 1981;33:334–344. [Google Scholar]
- Smarr BL, Jennings KJ, Driscoll JR, Kriegsfeld LJ. A time to remember: The role of circadian clocks in learning and memory. 2014;128:283–303. doi: 10.1037/a0035963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan FK, Donaldson JA, Gellert J. Retinohypothalamic tract symmetry and phase shifts of circadian rhythms in rats and hamsters. Physiol Behav. 1982;29:1153–1158. doi: 10.1016/0031-9384(82)90313-4. [DOI] [PubMed] [Google Scholar]
- Tapp WN, Holloway FA. Phase shifting circadian rhythms produces retrograde amnesia. Science. 1981;211:1056–1058. doi: 10.1126/science.7193351. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Suzuki T, Mizoro Y, Kori H, Okada K, Chen Y, Fustin J-M, Yamazaki F, Mizuguchi N, Zhang J, Dong X, Tsujimoto G, Okuno Y, Doi M, Okamura H. Mice genetically deficient in vasopressin V1a and V1b receptors are resistant to jet lag. Science. 2013;342:85–90. doi: 10.1126/science.1238599. [DOI] [PubMed] [Google Scholar]
- Zelinski EL, Hong NS, McDonald RJ. Persistent impairments in hippocampal function following a brief series of photoperiod shifts in rats. Anim Cogn. 2014;17:127–141. doi: 10.1007/s10071-013-0645-8. [DOI] [PubMed] [Google Scholar]