Abstract
Background
Few studies have investigated the relationship between structural brain abnormalities and dimensions of depressive symptomatology.
Methods
In the current study, we examined the relationship between cortical structural abnormalities and specific behavioral dimensions relevant to depression in a sample of unmedicated patients with major depressive disorder (MDD, n=57) and demographically similar healthy control volunteers (HC, n=29). All subjects underwent diagnostic assessment with the SCID, MRI at 3T, and behavioral dimensional assessments using the visual analog scales (VAS). Cortical regions were extracted for each subject, and group comparisons of cortical volume (CV), surface area (SA), and cortical thickness (CT) were performed controlling for multiple comparisons using a bootstrapping technique. Regions demonstrating group differences were analyzed for correlation with specific behavioral dimensions.
Results
As compared with HC, MDD subjects exhibited reduced CV within the left supramarginal gyrus, right ventrolateral prefrontal cortex (VLPFC), entorhinal cortex, parahippocampal gyrus, fusiform gyrus and pericalcarine, reduced SA in the right VLPFC, cuneus, and left temporal pole, and reduced CT in the right rostral anterior cingulate cortex (rACC) (all p’s < 0.05, corrected). The largest effect occurred within the right VLPFC CV and SA (MDD<HC; effect sizes: 0.60). CV in the right VLPFC inversely correlated with sadness, fatigue and worry, and CT in the right rACC inversely correlated with irritability and fatigue.
Limitations
Future studies will be required to further map the anatomical changes in depression to behavioral dimensions.
Conclusions
Our results indicate that specific cortical abnormalities are associated with specific behavioral components linked to depression.
Introduction
Biomarkers based on neuroimaging techniques noninvasively visualize brain abnormalities implicated in MDD and are well-suited to guide the development of novel treatments, assess treatment response, and tailor treatment approaches to biological subtypes of depression (Lener and Iosifescu 2015; Niciu et al. 2014). Structural MRI methods using cortical parcellation and morphometic analysis have allowed for the examination of subtle morphometric brain changes (eg., cortical thickness and surface area) (Tu et al. 2012; Qiu et al. 2014; Han et al. 2014) through the application of FreeSurfer (Fischl 2012), voxel-based morphometry (VBM), and voxel-based analysis (VBA) have allowed for the detection of subtle structural abnormalities in MDD within prefrontal, temporal, and limbic areas compared to healthy volunteers (Lorenzetti et al. 2009; Kempton et al. 2011; Bora et al. 2012; Du et al. 2012; Lai 2013; Sacher et al. 2012).
Given the importance of early identification of individuals at risk for depression, studies have sought to identify whether specific brain structural abnormalities can be seen prior to onset of illness. Cross-sectional studies of unaffected first-degree relatives of patients with MDD, have shown volumetric reductions in the hippocampus (Foland-Ross et al. 2015) and increases in the amygdala (Romanczuk-Seiferth et al. 2014) compared to non-depressed cohorts without familial risk. In a longitudinal study of 86 adolescents, emergence of depression was associated with attenuated growth of the hippocampus during early to mid-adolescence, suggesting that brain volumetric changes in individuals at high risk for depression occur progressively prior to the onset of depression (Whittle et al. 2014). In another longitudinal study of a large sample of unmedicated depressed adult patients (N=103), number of depressive episodes was associated with volumetric reduction in the dentate gyrus and medial prefrontal cortex (Treadway et al. 2015). And, in a study of a large sample of healthy volunteers (N=102), male but not female subjects with subclinical symptoms of depression (measured by the Beck Depression Inventory), showed volumetric reductions in limbic areas (Spalletta et al. 2014). Taken together, this suggests that structural abnormalities in prefrontal cortical and limbic areas, associated with symptoms of depression, may serve as an at-risk biomarker of MDD (Treadway et al. 2015).
Moreover, although many structural neuroimaging studies of MDD have examined associations between structural brain abnormalities and clinical variables (eg., age at onset, duration of illness, number of episodes, length of remission, effect of medication, and severity of the current depressive episode) (Lorenzetti et al. 2009; Bora et al. 2012; Du et al. 2012; Lai 2013; Grieve et al. 2013), few have examined specific symptom or behavioral dimensions of depressive illness (Chuang et al. 2014; Machino et al. 2014; Pizzagalli et al. 2004; Joffe et al. 2009). In prior studies, prominent anhedonia in patients with MDD has been associated with a significant reduction in overall gray matter density by age compared to MDD patients without anhedonia and a healthy control group (Pizzagalli et al. 2004). Negative symptoms (eg., blunted affect, alogia, and social withdrawal) have been associated with a significant reduction in gray matter volume in the cerebellum (Chuang et al. 2014). Rumination, the non-productive compulsive attention on internal mental distress (Treynor W 2003), has been associated with increased gray matter volume in the right superior temporal gyrus in patients who have failed two or more trials of antidepressants (ie., treatment-resistant depression) (Machino et al. 2014). And, in MDD patients with high levels of neuroticism, trait depression, and chronic stress, the effect of hippocampal volume reduction was mediated by BDNF 66Met carrier status (Joffe et al. 2009). If replicated, this may provide genetic links between stress-related brain morphometric changes and risk of onset or recurrence of depression.
Based on the National Institute of Mental Health (NIMH) Research Domain Criteria (R-DoC) initiative (Insel et al. 2010), the categorization of symptoms into domains or dimensions (eg., emotion regulation, cognitive processing systems, memory processing, and perception) was proposed to investigate underlying neural circuits and systems emerging from a wide array of research techniques (eg., neuroimaging, genetics, electrophysiology, etc.). From this framework, links could be examined between structural and functional brain abnormalities, pathophysiologic mechanisms, risk of illness, and likelihood of treatment response. For example, fMRI studies measuring neural activity in response to the induction of rumination have shown hyperactivity in the subgenual anterior cingulate cortex (sgACC) and dorso-medial prefrontal cortex (dmPFC) (Kross et al. 2009; Lemogne et al. 2009; Yoshimura et al. 2010), and hypoactivity in the dorsomedial thalamus and ventral striatum (Grimm et al. 2009). In a meta-analysis of fMRI studies examining neural activation correlates of antidepressant treatment response, increased neural activity in the rostral anterior cingulate cortex (rACC) robustly (Cohen’s d: 0.918) predicted an antidepressant response across 23 studies using different treatment interventions (Pizzagalli 2011). In a recently published fMRI study from our group, patients with treatment-resistant depression, in contrast to a healthy comparison group, showed reduced neural responses to positive faces in the right caudate, which appeared to increase in association with an antidepressant response to ketamine within a similar region of the right caudate (Murrough et al. 2015). Yet, in the clinical setting, the diagnosis of MDD is based on the coexistence of discrete symptoms taken from the Diagnostic and Statistical Manual of Mental Disorders (ie. DSM) (Association. 2013). Therefore, in order to implement neuroimaging biomarkers of depression into an effective diagnostic algorithm in the clinical setting, a rapid and valid method of detecting patients at high risk for depression is necessary.
In order to examine structural imaging biomarkers of depression, we performed an MRI study on 57 MDD patients and 29 healthy control subjects (HC) at 3T and investigated whether differences in cortical regions found in patients with MDD were linked to symptom domains of MDD, measured by the visual analogue scale (VAS), across the depressive spectrum. We chose the VAS, as it is a well-validated scale of an array of depressive symptoms apt in discriminating illness severity (Folstein and Luria 1973; Killgore 1999) that can be rapidly self-administered.
Materials and Methods
Overall Approach
We were interested in examining whether regionally specific structural differences found in MDD are 1) specific to individual symptoms of depression, and 2) are also found in non-depressed individuals who exhibit sub-clinical depressive symptoms. As such, our analytic approach consisted of a data-driven whole-brain cortical morphometric analysis comparing cortical volume (CV), surface area (SA), and cortical thickness (CT) of patients with MDD and HC. Extracted regions showing significant differences in CV were used to perform clinical correlations with VAS scores with two different approaches. In the first case, we performed correlations only within the MDD group to investigate whether CV correlated with depressive symptoms related to an MDD diagnosis. In the second case, we performed CV correlations with VAS scores throughout our entire cohort (MDD and HC) to investigate whether CV correlated with behavioral dimensions relevant to depression along a depressive spectrum (ie., depressive symptoms unrelated to diagnosis).
Participants
Institutional Review Board (IRB) approval for this study was obtained. Fifty-seven MDD patients and 29 healthy volunteers were recruited and screened through the Mood and Anxiety Disorders Program (MAP) at Mount Sinai. All MDD patients who met current criteria for a major depressive episode, as assessed with the Structured Clinical Interview for DSM-IV—Patient Edition (FIrst MB 1995), were free of concurrent antidepressant medication for at least 1 week before imaging and had current depressive symptoms of at least moderate severity as determined by a score of 20 or greater using the Montgomery-Asberg Depression Rating Scale (MADRS) (Montgomery and Asberg 1979). Exclusion criteria included a lifetime history of schizophrenia or other primary psychotic disorder, current Axis I diagnosis other than MDD, including current psychotic or manic symptoms, evidence of Axis II diagnoses, substance use disorder within one month of screening, a positive urine toxicology at screening, or any unstable medical illness. Physical examination, vital signs, weight, electrocardiogram, standard blood tests, and urinalysis confirmed absence of unstable medical illnesses. Women of childbearing potential were required to have a negative pregnancy test before enrollment and immediately before treatment. After complete description of the study to potential participants, written informed consent was obtained.
Data Acquisition, Processing, and Statistical Analysis
All patients and controls underwent a high-resolution T1-weighted MRI scan at 3T (Philips Achieva 3.0T X-series). Scan parameters were: 3D MPRAGE, TE/TR:3.49/7.60ms, flip angle: 8°, FOV 21 cm and voxel volume of 0.94×0.94×1mm. We performed automated segmentation of brain subvolumes and parcellation of the brain cortex using the FreeSurfer software package (Fischl 2012). CV, SA, and CT were calculated from cortical parcellation. MDD patients and HCs were compared using a two-tailed Student’s t-Test.
Multiple comparisons correction was implemented as a bootstrap technique (Efron 1979). For each measure, the bootstrap was implemented using resampling with replacement, over regions and subjects. Before resampling, the measures over subjects for each region were Z-normalized to control for systematic region variation in structure measures (ie., size, thickness, etc). Resampling with replacement was implemented by drawing indices with a randint function call over the range of integers between 0-[subjects × regions], [subjects × regions] times. After resampling, Z-values were rescaled based on the initial means and variances. A two-sample T-test was computed comparing the group difference between MDD and HC, for each iteration of resampling. The distribution of T values accumulated by the bootstrap procedure comprised a null distribution, against which the test T-statistic was ranked against. This procedure also approximately controlled for statistical bias due to sample size differences. Corrected p-values were found to agree closely with nominal p-values.
Clinical Measures of Symptoms
We computed subject-level means of cortical volume over regions with significant (p<0.05, corrected) group differences for further covariation analyses. Depression severity was measured using MADRS; Inter-rater reliability was assessed using a two-way mixed, absolute-agreement, single-measures intra-class coefficient (ICC) (McGraw 1996), which was in the very high range, ICC = 0.904 (Cicchetti 1994), suggesting that depression symptoms were rated similarly across raters. Individual depressive symptoms were assessed using the patient-reported Visual Analog Scale for mood (VAS) (Folstein and Luria 1973). For the VAS, we used anxiety, worry, irritability, sadness, and fatigue scores as they reflect core components of depression that may map to separate neural systems (Nelson and Charney 1981). We performed correlations using appropriate statistics SPSS, version 22. Correlations performed within our total sample (across HC and MDD groups) were calculated using Spearman’s rho for robustness against non-normal distributions whereas Pearson’s r was used for correlations within the MDD group.
Results
Demographics and Clinical Characteristics
The MDD and HC groups had no significant differences in age, gender, race, handedness, or level of education. As expected, the HC group demonstrated significantly lower MADRS and VAS scores than the MDD group (Table 1).
Table 1.
Demographic and clinical data characteristics
| Characteristic | MDD (N=57) | HC (N=29) | Statistic | P-value |
|---|---|---|---|---|
| Mean Age, S.D. | 40.27 ± 12.28 | 38.00 ± 11.70 | −0.82 | 0.493 |
| Male, Count (%) | 28 (51%) | 13 (45%) | 0.281 | 0.596 |
| Caucasian, Count (%) | 29 (52%) | 13 (45%) | 0.381 | 0.537 |
| Handedness (%R) | 1 | 0.91 | 2.449 | 0.118 |
| Education (yrs) | 15.79 ± 2.57 | 16.96 ± 2.96 | 1.77 | 0.361 |
| Age at Onset (yrs) | 19.59 ± 12.13 | ------------------- | ----------- | ---------- |
| Illness Duration (yrs) | 7.26 ± 13.39 | ------------------- | ----------- | ---------- |
| MADRSa | 28.07 ± 6.09 | 1.44 ± 3.08 | −21.35 | <0.0001 |
| Sadb | 6.83 ± 2.55 | 0.62 ± 1.15 | −12.40 | 0.001 |
| Anxiousb | 5.74 ± 2.81 | 1.34 ± 2.18 | −7.26 | 0.014 |
| Irritatedb | 4.68 ± 3.03 | 0.46 ± 1.04 | −7.12 | <0.0001 |
| Fatiguedb | 6.69 ± 2.54 | 1.25 ± 1.65 | −10.18 | 0.018 |
| Worriedb | 6.19 ± 2.81 | 0.79 ±1.40 | −9.51 | <0.0001 |
Statistic refers to either t-statistic and p-values from two sample t-test or chi-square and p-values from chi-square analysis (1 df for all MDD and HC);
Montgomery-Asberg Rating Scale for Depression Total (range 0–60);
Based on separate visual analogue scales (range 1–10)
Whole-Brain Analysis
Table 2 summarizes significant differences in cortical morphometry (CV, CT, and SA). Compared to HC, MDD patients had reduced CV in the pars triangularis (within a section commonly known as the ventrolateral prefrontal cortex, VLPFC), the entorhinal cortex, the parahippocampal gyrus, the fusiform gyrus, the pericalcarine cortex in the right hemisphere and the supramarginal gyrus in the left hemisphere. Reduced SA was also found in the right VLPFC, cuneus, and left temporal pole in MDD as compared to HC. The largest effect was within the VLPFC (MDD<HC) with respect to CV and SA (Cohen’s d: 0.60). Reduced CT was also found in the right rACC (See Figures 1–3).
Table 2.
Differences in cortical morphometry
| Region | Metric | MDD (Mean, S.D.) | HC (Mean, S.D) | Effect Size | p-valuecorr |
|---|---|---|---|---|---|
| Right ventrolateral prefrontal cortex | CV SA |
3982 ± 671 1431 ± 232 |
4405 ± 734 1576 ± 250 |
0.60 0.60 |
0.012 0.012 |
| Right rostral anterior cingulate cortex | CT | 2.95 ± 0.22 | 3.06 ± 0.19 | 0.49 | 0.033 |
| Left supramarginal gyrus | CV | 9952 ± 1410 | 10748 ± 1803 | 0.49 | 0.043 |
| Left temporal pole | SA | 460 ± 57 | 488 ± 54 | 0.49 | 0.037 |
| Right entorhinal cortex | CV | 1492 ± 374 | 1668 ± 356 | 0.50 | 0.039 |
| Right parahippocampal gyrus | CV | 2065 ± 298 | 2216 ± 337 | 0.47 | 0.046 |
| Right fusiform gyrus | CV | 9291 ± 1215 | 10083 ± 1587 | 0.56 | 0.022 |
| Right pericalcarine cortex | CV | 2216 ± 430 | 2383 ± 257 | 0.47 | 0.031 |
| Right cuneus | SA | 1450 ± 171 | 1531 ± 162 | 0.48 | 0.037 |
CV= Cortical Volume (mm3), SA=Surface Area (mm2), CT=Cortical Thickness (mm), Cohen’s d effect size= (meanHCmeanMDD)/SDpooled, Corrected P-value for multiple comparisons
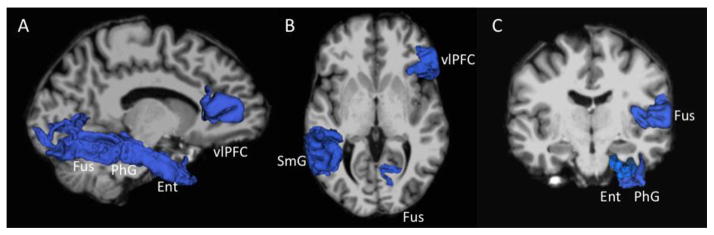
Figure 1.
Regions showing significant between-group differences (MDD < HC) in cortical volume, rendered in blue. (A) Right fusiform gyrus (Fus), parahippocampal gyrus (PhG), entorhinal cortex (Ent), and ventrolateral prefrontal cortex (vlPFC) shown against left medial surface of the brain. (B) Right vlPFC, left supramarginal gyrus (SmG), and right Fus shown in axial view. (C) Right Fus, PhG, and Ent shown in coronal view.
Figure 3.
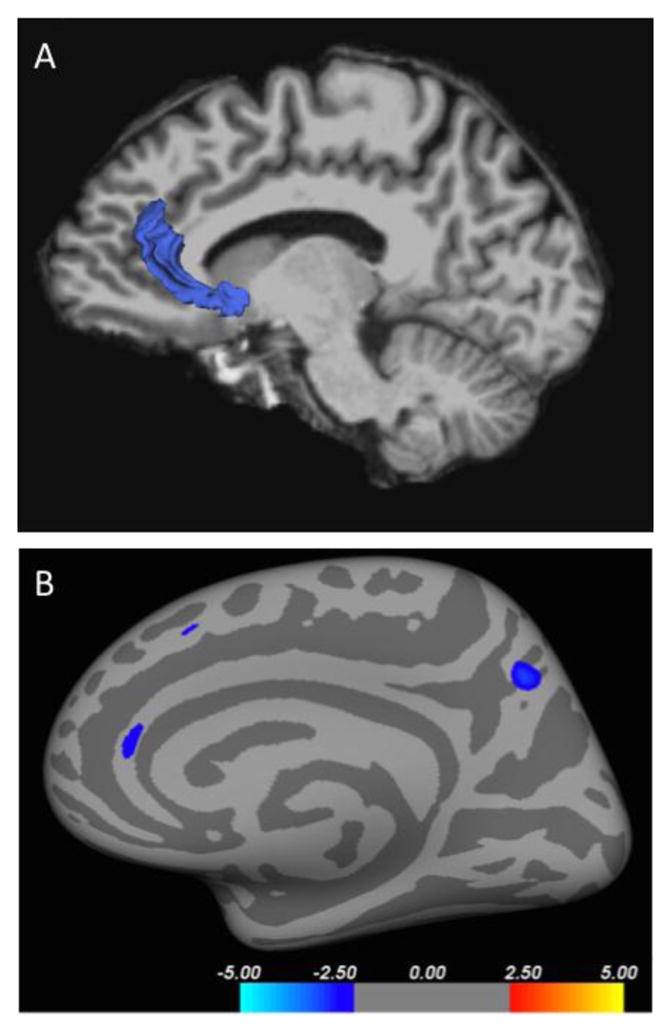
Cortical thickness differences in the right anterior cingulate cortex (A) colored blue displayed against the right medial surface of the brain, and (B) from a right mid-sagittal view in an inflated brain rendering. Red and blue denote decreased (MDD < HC) and increased (MDD > HC) cortical thickness respectively, and the color bars indicate the t value from two-sample t-test analysis of each pair of groups. Regions within the superior frontal gyrus and precuneus did not reach significance after correction with the bootstrap method.
Correlation Analysis
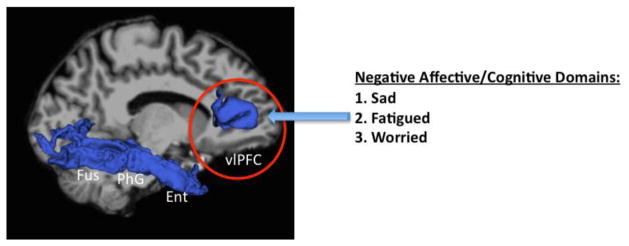
The extracted cortical regions that reached significance after correction in our whole-brain analysis were analyzed for covariation with behavioral dimensions measured using the VAS (Table 3). The assumption of non-normality across the entire sample of subjects (MDD and HC) was confirmed using Kolmogorov-Smirnov tests for normality. Across all subjects (MDD and HC), CV reductions in the right VLPFC were associated with sadness (r=−0.371), fatigue (r=−0.442), and worry (r=−0.430) (Figure 4). Given the emerging evidence of structural and functional abnormalities in the ACC in MDD (Pizzagalli 2011; Salvadore et al. 2009; Salvadore et al. 2010), we performed a post-hoc correlation of the right rACC and found that right rACC CT negatively correlated with VAS measures of irritated (p = 0.036) and fatigued (p = 0.015) across all subjects. Within the MDD group, CV reduction in the right VLPFC was associated with sadness (r=−0.362), fatigued (r=−0.393), and worry (r=−0.391) (see also Figure 4).
Table 3.
Association between cortical volume in the right ventrolateral prefrontal cortex and depressive symptoms along the depression spectrum
| Group | Depression symptomsa | Correlation Coefficientb | P-value | |
|---|---|---|---|---|
| Uncorrected | Correctedc | |||
|
| ||||
| Depressive Spectrum | Sad | −0.371 | 7.55E-4 | 0.0095 |
| Irritated | −0.387 | 4.71E-4 | 0.0075 | |
| Fatigued | −0.442 | 5.00E-5 | 0.0027 | |
| Worried | −0.430 | 8.70E-5 | 0.0031 | |
|
| ||||
| MDD | Sad | −0.362 | 0.010 | 0.038 |
| Fatigued | −0.393 | 0.005 | 0.0208 | |
| Worried | −0.391 | 0.005 | 0.0417 | |
Depression symptoms based on Visual Analogue Scale subscores;
In Depressive Spectrum: Rhospearman= Bimodal distribution was assumed when MDD and HC groups were combined; In MDD patients: Pearson ‘r’ was used;
p-valueCorr= FDR correction for multiple comparisons;
Depression symptoms based on Visual Analogue Scale subscores; Corrected p-value= FDR correction for multiple comparisons
Figure 4.
Cortical volume (CV) reduction in the ventrolateral prefrontal cortex (vlPFC) correlated with negative affective and cognitive symptom domains of depression measured by the Visual Analogue Scale (VAS). Sad, fatigued, and worried symptoms measured by VAS were found within a depressive spectrum as well as within patients with MDD.
Conclusion
In the current study, specific morphometric reductions found in patients with MDD correlate with specific behavioral dimensions of depression across a clinical spectrum. Our main findings were that cortical volume is reduced within the VLPFC and that this reduction correlates with discrete behavioral dimensions in the depressive spectrum as measured by VAS. While previous studies have investigated the relationship between structural neuroimaging abnormalities and symptom domains of depression (Pizzagalli et al. 2004; Savitz and Drevets 2009; Chuang et al. 2014; Machino et al. 2014), this is the first study to examine the relationship between cortical structural abnormalities in MDD and specific behavioral dimensions of depression in a large sample of adult MDD and HC subjects. If replicated, behavioral symptoms in addition to structural imaging abnormalities may provide an expedient method of identifying patients at high risk for depression in the clinical setting where time and financial resources are limited.
Our results from the data-driven morphometric analysis are consistent with widespread brain morphometric abnormalities associated with MDD, particularly within specific frontal and medial temporal regions of the brain (Grieve et al. 2013; Zeng et al. 2015). Animal models of depression have shown that the cellular effects of stress at the level of the hippocampus and prefrontal cortex leads to a reduction in the structure and function of both neurons and supporting glial cells via reductions in long-term potentiation (LTP) (Kullmann and Lamsa 2011), retraction of dendritic arborizations (Haber, Zhou, and Murai 2006), and reduced volume of astroglia (Lushnikova et al. 2009). Therefore, CV reduction in the MDD group, particularly in frontal, medial temporal, and limbic areas, may reflect stress-related neurotoxic effects (Treadway et al. 2015) that precede manifestation of illness. This is supported by a recent study that demonstrated an association between ACC and orbitofrontal cortical volumetric reduction and subclinical depressive symptoms in a cohort of non-depressed subjects (Webb et al. 2014). Our study extends upon this finding as CV reduction in the VLPFC associated with depressive symptoms along a spectrum from healthy subjects to patients diagnosed with depression. Although the relationship between structural and functional brain abnormalities has been less clear (Guo, Liu, Yu, et al. 2014; Guo, Liu, Zhang, et al. 2014; Drevets, Price, and Furey 2008; Price and Drevets 2010; Singh et al. 2013), CV reduction in the VLPFC may be associated with dysconnectivity in functional brain networks as reported by a recent meta-analysis of MDD studies using resting state functional connectivity(Kaiser et al. 2015). Future work using is necessary to examine pathophysiological mechanisms that may link how CV abnormalities are associated with emergence of specific symptoms.
CV reductions in the right VLPFC robustly correlated with behavioral dimensions of sadness, worry, and fatigue that correspond to components of negative affect (Insel et al. 2010). These dimension-specific findings are in line with the proposed RDoC initiative by the National Institute of Mental Health(Insel et al. 2010). From these constructs, models have been proposed to examine the complex functional relationships within prefrontal, limbic, parietal, and striatal regions in association with positive and negative valence circuits, cognitive control systems, and the mind’s “default” state (Langenecker, Jacobs, and Passarotti 2014; Woody and Gibb 2015). In our study, sadness was associated with CV reduction in the prefrontal cortex (ie., VLPFC). Depressed mood (or sadness) is the highest reported negative affective symptom of depression (Nair et al. 1999) and has been associated with overactivity in limbic regions and hypo-activity in the prefrontal cortex (Mayberg et al. 1999). Although speculative, specific symptoms of depression, particularly those that fall under the negative affective RDoC domain (eg., persistent sadness), may be associated with more severe pathology that can be detectable by current structural imaging methods.
The symptom of worry was linked to CV reductions in the right VLPFC across the depressive spectrum and within the MDD group. In a study using VBM to examine cortical gray matter volume differences in patients with MDD, comorbid MDD and anxiety disorder, in comparison with an HC group, reduction in the gray matter volume of the right inferior lateral frontal cortex was found unique to patients with MDD (van Tol et al. 2010). This may suggest that excessive worry in association with volumetric reductions in the VLPFC may indicate risk for depressive illness and recurrence. Furthermore, reductions in the right VLPFC CV may be related to disruption in prefrontal cortical cognitive control as has been shown in functional studies of worry (Owens, Derakshan, and Richards 2015) and anxiety (de Kwaasteniet et al. 2015). Moreover, rumination, a cognitive symptom related to worry, crosses diagnostic boundaries with other psychiatric conditions, has been related to abnormalities in negative valence systems (Woody and Gibb 2015), and has been shown to mediate the association between depressive and anxiety disorders (McLaughlin and Nolen-Hoeksema 2011).
We found that CV reduction in the right VLPFC and cortical thickness reduction in the right rACC correlated with symptoms of being irritated and fatigued. The interpretation of our regional volumetric correlations with irritability is a challenge due to a lack of studies examining neural correlates of irritability in the extant literature. In a large sample prospective study of patients with MDD who were observed for over 30 years, irritability and anger manifested within a depressive episode reached a prevalence of 54% and was associated with a severe, chronic, and complex form of the illness as measured by several clinical parameters (Judd et al. 2013). Irritability arises from inadequate regulation of negative affect and comprises a broader emotion regulation abnormality, which is a core feature of MDD (Etkin, Egner, and Kalisch 2011). Consistent with several structural and functional studies in MDD showing fronto-limbic abnormalities (Etkin, Egner, and Kalisch 2011; Pizzagalli 2011; Heller et al. 2013), our study showed that reductions of cortical thickness in the rACC (a limbic area), was associated with symptoms related to emotion dysregulation. This adds to previous work showing that reductions in ACC gray matter is an important neural substrate for affective instability and a potential endophenotype for depression (Boes et al. 2008; van Eijndhoven et al. 2013; Webb et al. 2014).
The interpretation of our regional volumetric correlations with fatigue also presents with challenges due to a lack of studies as well as existing semantic differences. Although fatigue is a hallmark symptom of a major depressive episode, the symptom construct may have physical, cognitive, and emotional meanings to both the patient and the clinician rater (Arnold 2008; Demyttenaere, De Fruyt, and Stahl 2005). Moreover, to add another layer of complexity, the symptom of fatigue may be used to modify the description of other depressive symptoms that can frequently co-occur in patients with MDD. For example, fatigue, as it is related to subjective energy and ensuing low productivity, is the second most prominent residual symptom of MDD, and is frequently associated with impaired concentration (ie. mental or cognitive fatigue), rumination and irritability (Carney et al. 2014; Demyttenaere, De Fruyt, and Stahl 2005; Boksem, Meijman, and Lorist 2006; Fava 2003).. In a secondary analysis of the STAR*D MDD cohort, lower baseline fatigue and remission of fatigue during antidepressant treatment in patients with MDD were associated with higher rates of remission of depressive symptoms and better function and quality of life (Ferguson et al. 2014). In a study of a non-depressed cohort, erroneous responses after undergoing a cognitive demanding task corresponded with EEG abnormalities from the ACC (Boksem, Meijman, and Lorist 2006). Although it is uncertain if this experimentally induced condition is synonymous with or analogous to fatigue, in any or all of its semantic forms, experienced by patients with MDD, the reduction of cortical thickness in the rACC could be associated with functional abnormalities in the limbic system affecting sustained attention and cognitive control. In a recent meta-analysis of structural neuroimaging studies across multiple psychiatric diagnoses (schizophrenia, bipolar disorder, depression, addiction, obsessive-compulsive disorder, and anxiety), the dorsal ACC was identified as one of three shared regions of gray matter loss. Interestingly, in their analysis of their HC comparison group, reductions in gray matter associated with poor executive functioning, suggesting that despite having likely diverse etiologies, the emergence of psychopathology may be related to disruptions in cognition across diagnoses (Goodkind et al. 2015).
This study has some limitations that merit discussion. With regard to our imaging approach, future studies utilizing MRI at higher field strength (i.e. 7T) would provide enhanced Signal-to-Noise Ratio (SNR) and attendant increased spatial resolution to measure smaller brain structures that may serve as a more sensitive marker for disease (Balchandani and Naidich 2014). Secondly, current structural MRI techniques may still lack the ability to identify subtle abnormalities of MDD (Lorenzetti et al. 2009) that affect functional neurocircuitry (Guo, Liu, Yu, et al. 2014; Guo, Liu, Zhang, et al. 2014; Sacher et al. 2012; Diener et al. 2012; Hamilton et al. 2012; Kerestes et al. 2014; Treadway and Pizzagalli 2014; Kaiser et al. 2015), which may be more proximal to underlying pathophysiological mechanisms of depressive illness. However, high signal variability associated with illness state makes it challenging to detect discernible trait related functional patterns in task-related fMRI studies. Therefore, structural imaging is more accessible, interpretable and clinically translatable, and may represent a more stable method to use in identifying individuals at risk for depression. To this end, it is uncertain whether regional cortical structural differences are associated with a pre-existing structural vulnerability prior to symptom onset (a structural trait vulnerability), concurrent with symptom onset but prior to illness onset (a structural and symptom trait vulnerability), or is associated with an early part of the pathogenesis of depression (state vulnerability). Therefore, longitudinal studies of non-depressed patients with subclinical depression may be necessary to investigate this further. Thirdly, we chose the VAS to measure behavioral dimensions relevant to depression because of its high reliability and validity specific to depression, the ease and rapidity of administration, and the sensitivity it has in capturing the subject’s subjective experience (eg., mood state) (Folstein and Luria 1973). The VAS is typically used in measuring pain or somatic symptoms in pain related disorders as well as in association with depression (Hung et al. 2014). In a recent clinical trial of patients with MDD and bipolar disorder, baseline VAS restlessness, sad, and irritated was associated with an antidepressant response to scopolamine, a potentially effective and novel agent for depression (Furey et al. 2012). In future work, we will explore other scales that enable correlation to symptom dimensions (eg., anhedonia, irritability, and fatigue).
In conclusion, cortical morphometric reductions found in depressed patients are associated with specific depressive symptoms across the illness spectrum. If replicated, structural MRI abnormalities linked with specific symptoms of depression may help to identify patients at high risk for depression and enable earlier treatment interventions. Future prospective structural MRI studies of individuals at high risk may be necessary to determine whether VLPFC volumetric reductions can be validated as a biomarker for illness risk.
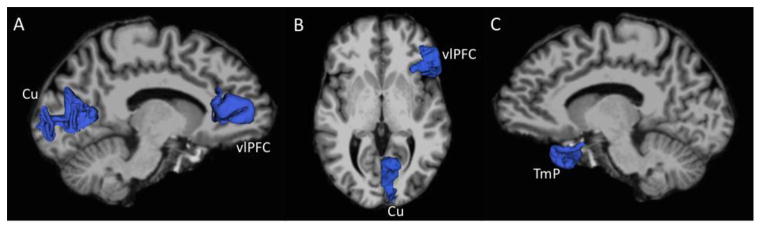
Figure 2.
Regions showing significant between-group differences (MDD < HC) in surface area rendered in blue. (A) Right cuneus (Cu) and ventrolateral prefrontal cortex (vlPFC) shown against left medal surface of the brain. (B) Right vlPFC and Cu shown in axial view. (C) Left temporal pole (TmP) shown against right medial surface of the brain.
Highlights.
Few studies have investigated the relationship between structural brain abnormalities and dimensions of depressive symptomatology
Widespread brain morphometric abnormalities were associated with major depressive disorder (MDD), particularly within specific frontal and medial temporal regions of the brain
Across the entire cohort of subjects (MDD and healthy volunteers), cortical volume reductions in the right ventrolateral prefrontal cortex robustly correlated with negative affect (sadness and worry) as well as a cognition (ie. mental fatigue)
Therefore, we found that cortical morphometric reductions found in depressed patients are associated with specific depressive symptoms across the illness spectrum
If replicated, structural MRI abnormalities linked with specific symptoms of depression may help to identify patients at high risk for depression and enable earlier treatment interventions
Acknowledgments
Role of Funding Source:
This work was supported by the National Institute of Mental Health of the National Institutes of Health under Award Number K23MH094707 (Career Development Award to JWM). Support was also provided by the Iris & Junming Le Foundation (Award to Dr. Murrough), by the Brain and Behavior Research Foundation (NARSAD grant to Dr. Murrough) and by grant UL1TR000067 from the NIH National Center for Advancing Translational Sciences to Mount Sinai. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We thank Drs. Dan Iosifescu MD, Katherine Collins PhD, and Brian Iacoviello PhD and the clinical staff at the Mood and Anxiety Disorders Program for their assistance in diagnostic and psychopathological assessments. We thank Dr. Cheuk Tang and the Translational and Molecular Imaging Institute and Department of Radiology, Icahn School of Medicine at Mount Sinai. We thank Dr. Dennis Charney MD for helpful advice. Funding agencies did not have any further role in the analysis and reporting of the results.
Footnotes
Conflicts of Interest:
There are no conflicts of interest to declare for all authors related to this current study. In the past 3 years, Dr. Murrough has served on advisory boards for Janssen Research and Development and Genentech, has provided consultation services for ProPhase, LLC and Impel Neuropharma and has received research support from Janssen and Avanir Pharmaceuticals; he is named on a patent pending for neuropeptide Y as a treatment for mood and anxiety disorders, on a patent pending for the combination of ketamine and lithium to maintain the antidepressant response to ketamine, and on a patent pending for the combination of ketamine and lithium for the treatment of suicidal ideation.
Contributors:
Drs. Marc Lener and James Murrough conceived the idea, and designed and executed the study. Mr. Wong and Dr. Lener conducted the brain cortical analysis and Dr. Kundu conducted the multiple comparisons correction in consultation with Drs. Balchandani, Tang, and Murrough who reviewed the manuscript, suggested further analyses and interpreted the results along with other authors. Ms. DeWilde provided database support. All authors took part in going through the manuscript carefully.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnold LM. Understanding fatigue in major depressive disorder and other medical disorders. Psychosomatics. 2008;49:185–90. doi: 10.1176/appi.psy.49.3.185. [DOI] [PubMed] [Google Scholar]
- Association., American Psychiatric. Diagnostic and statistical manual of mental disorders. Washington, DC: 2013. [Google Scholar]
- Balchandani P, Naidich TP. Ultra-High-Field MR Neuroimaging. AJNR Am J Neuroradiol. 2014 doi: 10.3174/ajnr.A4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boes AD, McCormick LM, Coryell WH, Nopoulos P. Rostral anterior cingulate cortex volume correlates with depressed mood in normal healthy children. Biol Psychiatry. 2008;63:391–7. doi: 10.1016/j.biopsych.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boksem MA, Meijman TF, Lorist MM. Mental fatigue, motivation and action monitoring. Biol Psychol. 2006;72:123–32. doi: 10.1016/j.biopsycho.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Bora E, Harrison BJ, Davey CG, Yucel M, Pantelis C. Meta-analysis of volumetric abnormalities in cortico-striatal-pallidal-thalamic circuits in major depressive disorder. Psychol Med. 2012;42:671–81. doi: 10.1017/S0033291711001668. [DOI] [PubMed] [Google Scholar]
- Carney CE, Moss TG, Lachowski AM, Atwood ME. Understanding mental and physical fatigue complaints in those with depression and insomnia. Behav Sleep Med. 2014;12:272–89. doi: 10.1080/15402002.2013.801345. [DOI] [PubMed] [Google Scholar]
- Chuang JY, Murray GK, Metastasio A, Segarra N, Tait R, Spencer J, Ziauddeen H, Dudas RB, Fletcher PC, Suckling J. Brain structural signatures of negative symptoms in depression and schizophrenia. Front Psychiatry. 2014;5:116. doi: 10.3389/fpsyt.2014.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti DV. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychological Assessment. 1994;6:284–90. [Google Scholar]
- de Kwaasteniet BP, Rive MM, Ruhe HG, Schene AH, Veltman DJ, Fellinger L, van Wingen GA, Denys D. Decreased Resting-State Connectivity between Neurocognitive Networks in Treatment Resistant Depression. Front Psychiatry. 2015;6:28. doi: 10.3389/fpsyt.2015.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demyttenaere K, De Fruyt J, Stahl SM. The many faces of fatigue in major depressive disorder. Int J Neuropsychopharmacol. 2005;8:93–105. doi: 10.1017/S1461145704004729. [DOI] [PubMed] [Google Scholar]
- Diener C, Kuehner C, Brusniak W, Ubl B, Wessa M, Flor H. A meta-analysis of neurofunctional imaging studies of emotion and cognition in major depression. Neuroimage. 2012;61:677–85. doi: 10.1016/j.neuroimage.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du MY, Wu QZ, Yue Q, Li J, Liao Y, Kuang WH, Huang XQ, Chan RC, Mechelli A, Gong QY. Voxelwise meta-analysis of gray matter reduction in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2012;36:11–6. doi: 10.1016/j.pnpbp.2011.09.014. [DOI] [PubMed] [Google Scholar]
- Efron B. Bootstrap methods: another look at the jackknife. The Annals of Statistics. 1979:1–26. [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M. Symptoms of fatigue and cognitive/executive dysfunction in major depressive disorder before and after antidepressant treatment. J Clin Psychiatry. 2003;64(Suppl 14):30–4. [PubMed] [Google Scholar]
- Ferguson M, Dennehy EB, Marangell LB, Martinez J, Wisniewski SR. Impact of fatigue on outcome of selective serotonin reuptake inhibitor treatment: secondary analysis of STAR*D. Curr Med Res Opin. 2014;30:2109–18. doi: 10.1185/03007995.2014.936553. [DOI] [PubMed] [Google Scholar]
- FIrst MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis Disorders (SCID) Biometrics Research, New York State Psychiatric Institute; New York, NY, USA: 1995. [Google Scholar]
- Fischl B. FreeSurfer. Neuroimage. 2012;62:774–81. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foland-Ross LC, Gilbert BL, Joormann J, Gotlib IH. Neural Markers of Familial Risk for Depression: An Investigation of Cortical Thickness Abnormalities in Healthy Adolescent Daughters of Mothers With Recurrent Depression. J Abnorm Psychol. 2015 doi: 10.1037/abn0000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Luria R. Reliability, validity, and clinical application of the Visual Analogue Mood Scale. Psychol Med. 1973;3:479–86. doi: 10.1017/s0033291700054283. [DOI] [PubMed] [Google Scholar]
- Furey ML, Nugent AC, Speer AM, Luckenbaugh DA, Hoffman EM, Frankel E, Drevets WC, Zarate CA., Jr Baseline mood-state measures as predictors of antidepressant response to scopolamine. Psychiatry Res. 2012;196:62–7. doi: 10.1016/j.psychres.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB, Ortega BN, Zaiko YV, Roach EL, Korgaonkar MS, Grieve SM, Galatzer-Levy I, Fox PT, Etkin A. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72:305–15. doi: 10.1001/jamapsychiatry.2014.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve SM, Korgaonkar MS, Koslow SH, Gordon E, Williams LM. Widespread reductions in gray matter volume in depression. Neuroimage Clin. 2013;3:332–9. doi: 10.1016/j.nicl.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm S, Ernst J, Boesiger P, Schuepbach D, Hell D, Boeker H, Northoff G. Increased self-focus in major depressive disorder is related to neural abnormalities in subcortical-cortical midline structures. Hum Brain Mapp. 2009;30:2617–27. doi: 10.1002/hbm.20693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Liu F, Yu M, Zhang J, Zhang Z, Liu J, Xiao C, Zhao J. Functional and anatomical brain deficits in drug-naive major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2014;54:1–6. doi: 10.1016/j.pnpbp.2014.05.008. [DOI] [PubMed] [Google Scholar]
- Guo W, Liu F, Zhang Z, Liu J, Yu M, Zhang J, Xiao C, Zhao J. Unidirectionally affected causal connectivity of cortico-limbic-cerebellar circuit by structural deficits in drug-naive major depressive disorder. J Affect Disord. 2014;172C:410–16. doi: 10.1016/j.jad.2014.10.019. [DOI] [PubMed] [Google Scholar]
- Haber M, Zhou L, Murai KK. Cooperative astrocyte and dendritic spine dynamics at hippocampal excitatory synapses. J Neurosci. 2006;26:8881–91. doi: 10.1523/JNEUROSCI.1302-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. Am J Psychiatry. 2012;169:693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PubMed] [Google Scholar]
- Han KM, Choi S, Jung J, Na KS, Yoon HK, Lee MS, Ham BJ. Cortical thickness, cortical and subcortical volume, and white matter integrity in patients with their first episode of major depression. J Affect Disord. 2014;155:42–8. doi: 10.1016/j.jad.2013.10.021. [DOI] [PubMed] [Google Scholar]
- Heller AS, Johnstone T, Peterson MJ, Kolden GG, Kalin NH, Davidson RJ. Increased prefrontal cortex activity during negative emotion regulation as a predictor of depression symptom severity trajectory over 6 months. JAMA Psychiatry. 2013;70:1181–9. doi: 10.1001/jamapsychiatry.2013.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung CI, Liu CY, Chen CY, Yang CH, Wang SJ. The impacts of migraine and anxiety disorders on painful physical symptoms among patients with major depressive disorder. J Headache Pain. 2014;15:73. doi: 10.1186/1129-2377-15-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–51. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Joffe RT, Gatt JM, Kemp AH, Grieve S, Dobson-Stone C, Kuan SA, Schofield PR, Gordon E, Williams LM. Brain derived neurotrophic factor Val66Met polymorphism, the five factor model of personality and hippocampal volume: Implications for depressive illness. Hum Brain Mapp. 2009;30:1246–56. doi: 10.1002/hbm.20592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd LL, Schettler PJ, Coryell W, Akiskal HS, Fiedorowicz JG. Overt irritability/anger in unipolar major depressive episodes: past and current characteristics and implications for long-term course. JAMA Psychiatry. 2013;70:1171–80. doi: 10.1001/jamapsychiatry.2013.1957. [DOI] [PubMed] [Google Scholar]
- Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-Scale Network Dysfunction in Major Depressive Disorder: A Meta-analysis of Resting-State Functional Connectivity. JAMA Psychiatry. 2015 doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempton MJ, Salvador Z, Munafo MR, Geddes JR, Simmons A, Frangou S, Williams SC. Structural neuroimaging studies in major depressive disorder. Meta-analysis and comparison with bipolar disorder. Arch Gen Psychiatry. 2011;68:675–90. doi: 10.1001/archgenpsychiatry.2011.60. [DOI] [PubMed] [Google Scholar]
- Kerestes R, Davey CG, Stephanou K, Whittle S, Harrison BJ. Functional brain imaging studies of youth depression: a systematic review. Neuroimage Clin. 2014;4:209–31. doi: 10.1016/j.nicl.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore WD. The visual analogue mood scale: can a single-item scale accurately classify depressive mood state? Psychol Rep. 1999;85:1238–43. doi: 10.2466/pr0.1999.85.3f.1238. [DOI] [PubMed] [Google Scholar]
- Kross E, Davidson M, Weber J, Ochsner K. Coping with emotions past: the neural bases of regulating affect associated with negative autobiographical memories. Biol Psychiatry. 2009;65:361–6. doi: 10.1016/j.biopsych.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann DM, Lamsa KP. LTP and LTD in cortical GABAergic interneurons: emerging rules and roles. Neuropharmacology. 2011;60:712–9. doi: 10.1016/j.neuropharm.2010.12.020. [DOI] [PubMed] [Google Scholar]
- Lai CH. Gray matter volume in major depressive disorder: a meta-analysis of voxel-based morphometry studies. Psychiatry Res. 2013;211:37–46. doi: 10.1016/j.pscychresns.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Langenecker SA, Jacobs RH, Passarotti AM. Current Neural and Behavioral Dimensional Constructs across Mood Disorders. Curr Behav Neurosci Rep. 2014;1:144–53. doi: 10.1007/s40473-014-0018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemogne C, le Bastard G, Mayberg H, Volle E, Bergouignan L, Lehericy S, Allilaire JF, Fossati P. In search of the depressive self: extended medial prefrontal network during self-referential processing in major depression. Soc Cogn Affect Neurosci. 2009;4:305–12. doi: 10.1093/scan/nsp008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lener MS, Iosifescu DV. In pursuit of neuroimaging biomarkers to guide treatment selection in major depressive disorder: a review of the literature. Ann N Y Acad Sci. 2015;1344:50–65. doi: 10.1111/nyas.12759. [DOI] [PubMed] [Google Scholar]
- Lorenzetti V, Allen NB, Fornito A, Yucel M. Structural brain abnormalities in major depressive disorder: a selective review of recent MRI studies. J Affect Disord. 2009;117:1–17. doi: 10.1016/j.jad.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Lushnikova I, Skibo G, Muller D, Nikonenko I. Synaptic potentiation induces increased glial coverage of excitatory synapses in CA1 hippocampus. Hippocampus. 2009;19:753–62. doi: 10.1002/hipo.20551. [DOI] [PubMed] [Google Scholar]
- Machino A, Kunisato Y, Matsumoto T, Yoshimura S, Ueda K, Yamawaki Y, Okada G, Okamoto Y, Yamawaki S. Possible involvement of rumination in gray matter abnormalities in persistent symptoms of major depression: an exploratory magnetic resonance imaging voxel-based morphometry study. J Affect Disord. 2014;168:229–35. doi: 10.1016/j.jad.2014.06.030. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–82. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- McGraw KO, Wong SP. Forming inferences about some intraclass correlation coefficients. Psychological Methods. 1996;1:30–46. [Google Scholar]
- McLaughlin KA, Nolen-Hoeksema S. Rumination as a transdiagnostic factor in depression and anxiety. Behav Res Ther. 2011;49:186–93. doi: 10.1016/j.brat.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Collins KA, Fields J, DeWilde KE, Phillips ML, Mathew SJ, Wong E, Tang CY, Charney DS, Iosifescu DV. Regulation of neural responses to emotion perception by ketamine in individuals with treatment-resistant major depressive disorder. Transl Psychiatry. 2015;5:e509. doi: 10.1038/tp.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair J, Nair SS, Kashani JH, Reid JC, Mistry SI, Vargas VG. Analysis of the symptoms of depression--a neural network approach. Psychiatry Res. 1999;87:193–201. doi: 10.1016/s0165-1781(99)00054-2. [DOI] [PubMed] [Google Scholar]
- Nelson JC, Charney DS. The symptoms of major depressive illness. Am J Psychiatry. 1981;138:1–13. doi: 10.1176/ajp.138.1.1. [DOI] [PubMed] [Google Scholar]
- Niciu MJ, Mathews DC, Nugent AC, Ionescu DF, Furey ML, Richards EM, Machado-Vieira R, Zarate CA., Jr Developing biomarkers in mood disorders research through the use of rapid-acting antidepressants. Depress Anxiety. 2014;31:297–307. doi: 10.1002/da.22224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens M, Derakshan N, Richards A. Trait Susceptibility to Worry Modulates the Effects of Cognitive Load on Cognitive Control: An ERP Study. Emotion. 2015 doi: 10.1037/emo0000052. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011;36:183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Oakes TR, Fox AS, Chung MK, Larson CL, Abercrombie HC, Schaefer SM, Benca RM, Davidson RJ. Functional but not structural subgenual prefrontal cortex abnormalities in melancholia. Mol Psychiatry. 2004;9:325, 93–405. doi: 10.1038/sj.mp.4001501. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu L, Lui S, Kuang W, Huang X, Li J, Li J, Zhang J, Chen H, Sweeney JA, Gong Q. Regional increases of cortical thickness in untreated, first-episode major depressive disorder. Transl Psychiatry. 2014;4:e378. doi: 10.1038/tp.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanczuk-Seiferth N, Pohland L, Mohnke S, Garbusow M, Erk S, Haddad L, Grimm O, Tost H, Meyer-Lindenberg A, Walter H, Wustenberg T, Heinz A. Larger amygdala volume in first-degree relatives of patients with major depression. Neuroimage Clin. 2014;5:62–8. doi: 10.1016/j.nicl.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher J, Neumann J, Funfstuck T, Soliman A, Villringer A, Schroeter ML. Mapping the depressed brain: a meta-analysis of structural and functional alterations in major depressive disorder. J Affect Disord. 2012;140:142–8. doi: 10.1016/j.jad.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Salvadore G, Cornwell BR, Colon-Rosario V, Coppola R, Grillon C, Zarate CA, Jr, Manji HK. Increased anterior cingulate cortical activity in response to fearful faces: a neurophysiological biomarker that predicts rapid antidepressant response to ketamine. Biol Psychiatry. 2009;65:289–95. doi: 10.1016/j.biopsych.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvadore G, Cornwell BR, Sambataro F, Latov D, Colon-Rosario V, Carver F, Holroyd T, DiazGranados N, Machado-Vieira R, Grillon C, Drevets WC, Zarate CA., Jr Anterior cingulate desynchronization and functional connectivity with the amygdala during a working memory task predict rapid antidepressant response to ketamine. Neuropsychopharmacology. 2010;35:1415–22. doi: 10.1038/npp.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz JB, Drevets WC. Imaging phenotypes of major depressive disorder: genetic correlates. Neuroscience. 2009;164:300–30. doi: 10.1016/j.neuroscience.2009.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MK, Kesler SR, Hadi Hosseini SM, Kelley RG, Amatya D, Hamilton JP, Chen MC, Gotlib IH. Anomalous gray matter structural networks in major depressive disorder. Biol Psychiatry. 2013;74:777–85. doi: 10.1016/j.biopsych.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalletta G, Piras F, Caltagirone C, Fagioli S. Hippocampal multimodal structural changes and subclinical depression in healthy individuals. J Affect Disord. 2014;152–154:105–12. doi: 10.1016/j.jad.2013.05.068. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Pizzagalli DA. Imaging the pathophysiology of major depressive disorder - from localist models to circuit-based analysis. Biol Mood Anxiety Disord. 2014;4:5. doi: 10.1186/2045-5380-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Waskom ML, Dillon DG, Holmes AJ, Park MT, Chakravarty MM, Dutra SJ, Polli FE, Iosifescu DV, Fava M, Gabrieli JD, Pizzagalli DA. Illness progression, recent stress, and morphometry of hippocampal subfields and medial prefrontal cortex in major depression. Biol Psychiatry. 2015;77:285–94. doi: 10.1016/j.biopsych.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treynor W, Gonzalez R, Nolen-Hoeksema S. Rumination reconsidered: a psychometric analysis. Cognitive Therapy and Research. 2003;27:247–59. [Google Scholar]
- Tu PC, Chen LF, Hsieh JC, Bai YM, Li CT, Su TP. Regional cortical thinning in patients with major depressive disorder: a surface-based morphometry study. Psychiatry Res. 2012;202:206–13. doi: 10.1016/j.pscychresns.2011.07.011. [DOI] [PubMed] [Google Scholar]
- van Eijndhoven P, van Wingen G, Katzenbauer M, Groen W, Tepest R, Fernandez G, Buitelaar J, Tendolkar I. Paralimbic cortical thickness in first-episode depression: evidence for trait-related differences in mood regulation. Am J Psychiatry. 2013;170:1477–86. doi: 10.1176/appi.ajp.2013.12121504. [DOI] [PubMed] [Google Scholar]
- van Tol MJ, van der Wee NJ, van den Heuvel OA, Nielen MM, Demenescu LR, Aleman A, Renken R, van Buchem MA, Zitman FG, Veltman DJ. Regional brain volume in depression and anxiety disorders. Arch Gen Psychiatry. 2010;67:1002–11. doi: 10.1001/archgenpsychiatry.2010.121. [DOI] [PubMed] [Google Scholar]
- Webb CA, Weber M, Mundy EA, Killgore WD. Reduced gray matter volume in the anterior cingulate, orbitofrontal cortex and thalamus as a function of mild depressive symptoms: a voxel-based morphometric analysis. Psychol Med. 2014;44:2833–43. doi: 10.1017/S0033291714000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S, Lichter R, Dennison M, Vijayakumar N, Schwartz O, Byrne ML, Simmons JG, Yucel M, Pantelis C, McGorry P, Allen NB. Structural brain development and depression onset during adolescence: a prospective longitudinal study. Am J Psychiatry. 2014;171:564–71. doi: 10.1176/appi.ajp.2013.13070920. [DOI] [PubMed] [Google Scholar]
- Woody ML, Gibb BE. Integrating NIMH Research Domain Criteria (RDoC) into Depression Research. Curr Opin Psychol. 2015;4:6–12. doi: 10.1016/j.copsyc.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura S, Okamoto Y, Onoda K, Matsunaga M, Ueda K, Suzuki S, Shigetoyamawaki Rostral anterior cingulate cortex activity mediates the relationship between the depressive symptoms and the medial prefrontal cortex activity. J Affect Disord. 2010;122:76–85. doi: 10.1016/j.jad.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Zeng LL, Shen H, Liu L, Fang P, Liu Y, Hu D. State-dependent and trait-related gray matter changes in nonrefractory depression. Neuroreport. 2015;26:57–65. doi: 10.1097/WNR.0000000000000301. [DOI] [PubMed] [Google Scholar]