Abstract
Unlike mammals and birds, teleost fish undergo external embryogenesis, and therefore their embryos are constantly challenged by stresses from their living environment. These stresses, when becoming too harsh, will cause arrest of cell proliferation, abnormal cell death or senescence. Such organisms have to evolve a sophisticated anti-stress mechanism to protect the process of embryogenesis/organogenesis. However, very few signaling molecule(s) mediating such activity have been identified. liver-enriched gene 1 (leg1) is an uncharacterized gene that encodes a novel secretory protein containing a single domain DUF781 (domain of unknown function 781) that is well conserved in vertebrates. In the zebrafish genome, there are two copies of leg1, namely leg1a and leg1b. leg1a and leg1b are closely linked on chromosome 20 and share high homology, but are differentially expressed. In this report, we generated two leg1a mutant alleles using the TALEN technique, then characterized liver development in the mutants. We show that a leg1a mutant exhibits a stress-dependent small liver phenotype that can be prevented by chemicals blocking the production of reactive oxygen species. Further studies reveal that Leg1a binds to FGFR3 and mediates a novel anti-stress pathway to protect liver development through enhancing Erk activity. More importantly, we show that the binding of Leg1a to FGFR relies on the glycosylation at the 70th asparagine (Asn70 or N70), and mutating the Asn70 to Ala70 compromised Leg1’s function in liver development. Therefore, Leg1 plays a unique role in protecting liver development under different stress conditions by serving as a secreted signaling molecule/modulator.
Author Summary
Although being challenged by stresses from their living environment during embryogenesis, teleost fish harbor a robust genetic program dictating liver development as long as any environmental change, including temperature or natural UV irradiation, is not detrimental. It is therefore of interest to explore the mechanism(s) behind this phenomenon. We showed that Liver-enriched gene 1 (Leg1) plays a unique role in protecting liver development under different stress conditions by serving as a secretory signaling molecule/modulator that binds to FGF receptor and activates the Erk signaling pathway. This finding may explain the adaption of teleost fish in coping with environmental changes.
Introduction
The process of liver development includes 1) the specification of hepatoblasts from the endoderm, 2) proliferation of hepatoblasts to form the liver primordium (liver bud), and 3) differentiation and proliferation of hepatocytes to form the embryonic liver [1–5]. Liver organogenesis is not only controlled by intrinsic transcription factors such as FoxA factors [6], GATA factors [7], Hhex[8] and Prox1 [9] but also by secreted signaling molecules [10] including FGF [11], BMP [12], Wnt[13] and RA [14] produced by neighboring mesodermal cells/tissues. Strikingly, studies of mouse, chick/quail, Xenopus and zebrafish have shown that the molecular events controlling liver development are robustly conserved across these different species although evolutionally, these species are distantly related, especially when considering the obvious anatomic differences in organ initiation and patterning [1–4] and the differences in the circumstances of their embryogenesis. Unlike mammals and birds, teleost fish complete the process of embryogenesis externally. To cope with the stresses brought about by environmental changes, teleost fish have to evolve anti-stress mechanism(s) to protect the process of embryogenesis/organogenesis. However, the molecule(s) mediating such activity is(are) currently unknown. Therefore, it is of great interest to determine whether other such factors, in addition to the aforementioned common factors, protect liver development during external embryogenesis in teleost fish.
leg1 (liver enriched gene 1) is an evolutionally conserved gene in vertebrates that encodes a novel secreted protein Leg1, which contains only a domain of unknown function 781 (DUF781) [15–17]. In zebrafish, there are two copies of the leg1 gene, namely leg1a and leg1b, which are closely linked on chromosome 20 [15]. Previous reports showed that knockdown of total Leg1 results in defective liver development, and the expression of leg1 is modulated by hypoxia conditions [18]. On the basis of the detailed analysis of leg1a mutants generated by the TALEN method, we provide strong evidence to demonstrate that Leg1 functions probably as a novel signaling molecule/modulator to protect liver development through Erk phosphorylation under stress conditions, and glycosylation at N70 in Leg1a is essential for this function.
Results
Loss-of-function of Leg1aconfers a small liver phenotype under different stress conditions
We reported previously that leg1a but not leg1b was the predominant form expressed during the embryonic stage in zebrafish and that knockdown of leg1a resulted in a small liver phenotype [15]. To unequivocally prove the role of leg1a in liver development, we generated two leg1a mutant alleles, one with a 13-bp insertion (leg1azju1) and another with a 12-bp deletion (leg1azju2) (leg1b is intact in these two leg1a alleles), via the TALEN technique[19] by targeting exon 1 of leg1a (Fig 1A). To our surprise, unlike the leg1a morphants[15], the leg1a homozygous mutant obtained from a cross between either leg1azju1/+ or leg1azju2/+ heterozygous male and female did not show an obvious small liver phenotype at 3.5 days post fertilization (dpf) when examined with a liver-specific molecular marker fatty acid binding protein 10a (fabp10a) (Fig 1B), and both leg1azju1 and leg1azju2 homozygous mutants could grow to adulthood and were fertile. We examined the total Leg1 levels by western blot analysis and found no drastic difference between unfertilized eggs from wild-type (WT) and leg1azju1/+ heterozygous females (Fig 1C). In fact, the leg1azju1 homozygous mutant obtained from leg1azju1/+ crosses retained, though showing variations, a considerable level of Leg1 at 4 dpf(Fig 1D), suggesting that maternal Leg1 compensated for the need for Leg1a during early hepatogenesis[20]. The leg1azju1 homozygous mutant was propagated and allowed to produce progenies. We determined that such leg1azju1 homozygous progenies (maternal-zygotic mutants) lacked Leg1 (Fig 1E) at 1 dpf but started to express the Leg1b homolog at 3.5 and 7 dpf. Surprisingly, whole-mount in situ hybridization (WISH) using the fabp10a probe revealed that the maternal-zygotic mutant exhibited a small liver phenotype in a season-dependent manner (Fig 1F, S1A Fig). For example, majority of the maternal-zygotic mutants exhibited a small liver phenotype in 14 cases recorded during the cold season whereas the mutant liver showed a great variation in sizes ranging from normal to small in 18 cases recorded during the warm/hot seasons (Fig 1G, S1B and S1C Fig).
Fig 1. Liver development in the maternal-zygotic leg1azju1 mutant is amenable to the environmental changes.
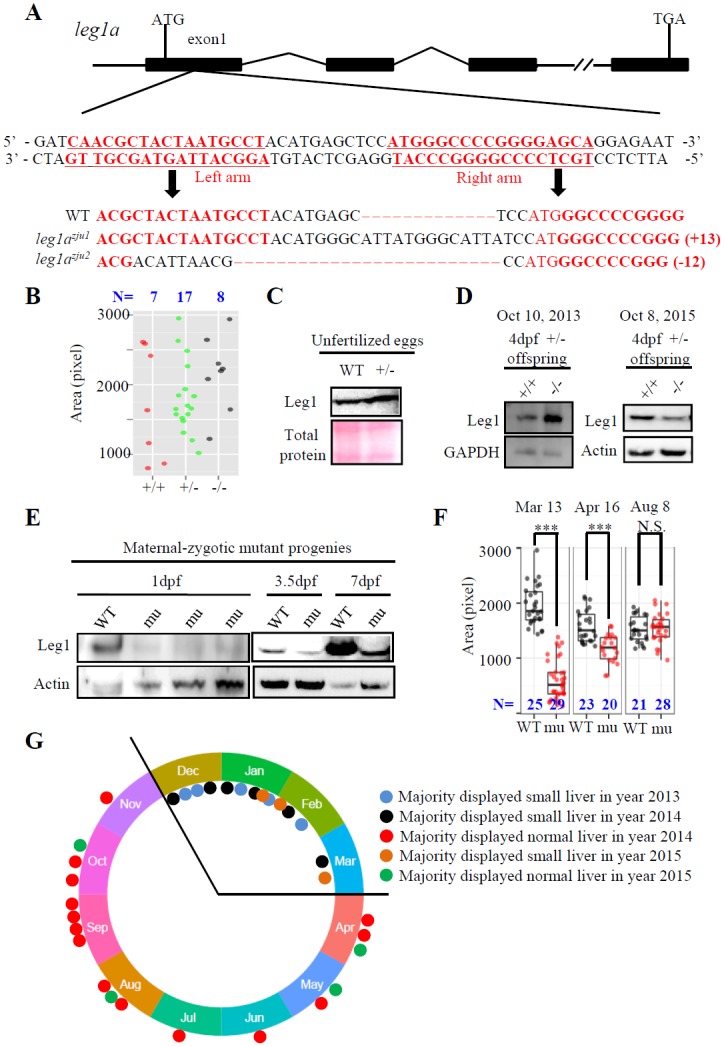
(A) Top panel: Schematic diagram showing the genomic structure of the leg1a gene. Black box: exons; solid line: introns; double slashes: omitted genomic region. Middle panel: The left and right TALEN targeting sequences in the exon1 are lettered in red. Bottom panel: Comparison of the genomic DNA sequence among WT, leg1azju1 (with 13 bp insertion) and leg1azju2 (with 12 bp deletion) two mutant alleles. TALEN target sequences are in red. (B-D) The leg1azju1 homozygotes (-/-) obtained from a cross between heterozygous (+/-) parents showed normal liver development (B). Each dot represents the liver size (measured based on the signal area of fabp10a) of a single embryo. Three independent experiments were carried and a representative one is shown here. Total Leg1 was detected in unfertilized eggs (C) and in two independent samples of 4-dpf WT (+/+) and mutant (-/-) embryos collected at different dates (D). (E-G) The maternal-zygotic leg1azju1 homozygotes (mu) obtained from a cross between homozygous parents lacked Leg1 at 1 dpf, but expressed Leg1b at 3.5 dpf and 7 dpf (E), and showed a small liver phenotype on March 13, an intermediate-sized liver on April 16, and a normal sized liver on August 8, 2015. (F). Recordings of liver phenotype in 32 cases from December 30 2013 to October 4 2015 showed majority of the maternal-zygotic leg1azju1 homozygotes (mu) exhibited a small liver in 14 cases recorded in cold seasons but a big variation in 18 cases recorded in warm/hot seasons (G). ***, p<0.001, N.S., not significant. Western blot was repeated three times for (C), five times for (D) and (E).
These results suggest that liver development in the maternal-zygotic leg1azju1 mutant is amenable to its living environment. We tested this hypothesis by growing fish in different mild stress conditions. Growing maternal-zygotic leg1azju1 mutants in relative high temperature (32°C) and high density (200 embryos per 10-cm diameter Petri dish) (Fig 2A) or briefly treating the maternal-zygotic leg1azju1 mutants at 24 hpf with 2.5 mJ/cm2 ultraviolet (UV) irradiation (UV25) (Fig 2B, S2A and S2B Fig) sharply increased the proportion of the mutant embryos displaying the small liver phenotype at 3.5 dpf. High density alone also caused a small liver phenotype to the maternal-zygotic leg1azju1 mutants (S2C Fig). In addition, we found that incubating the zygotic leg1azju1 mutant in the egg water containing a mild but not lethal dose of H2O2 (0.5mM) also led to the small liver phenotype (Fig 2C). Interestingly, the maternal-zygotic leg1azju1 mutant embryos did not exhibit a small liver phenotype at 3.5 dpf when they were grown in the egg water containing 0.5% or 1% ethanol starting at 24 dpf (S2D Fig), a concentration range not causing overall abnormality [21]. UV25 treatment also enhanced the small liver phenotype in leg1azju2, another mutant allele of the leg1a gene (S2E Fig).
Fig 2. Liver development in the maternal-zygotic leg1azju1 mutant is amenable to different stresses.
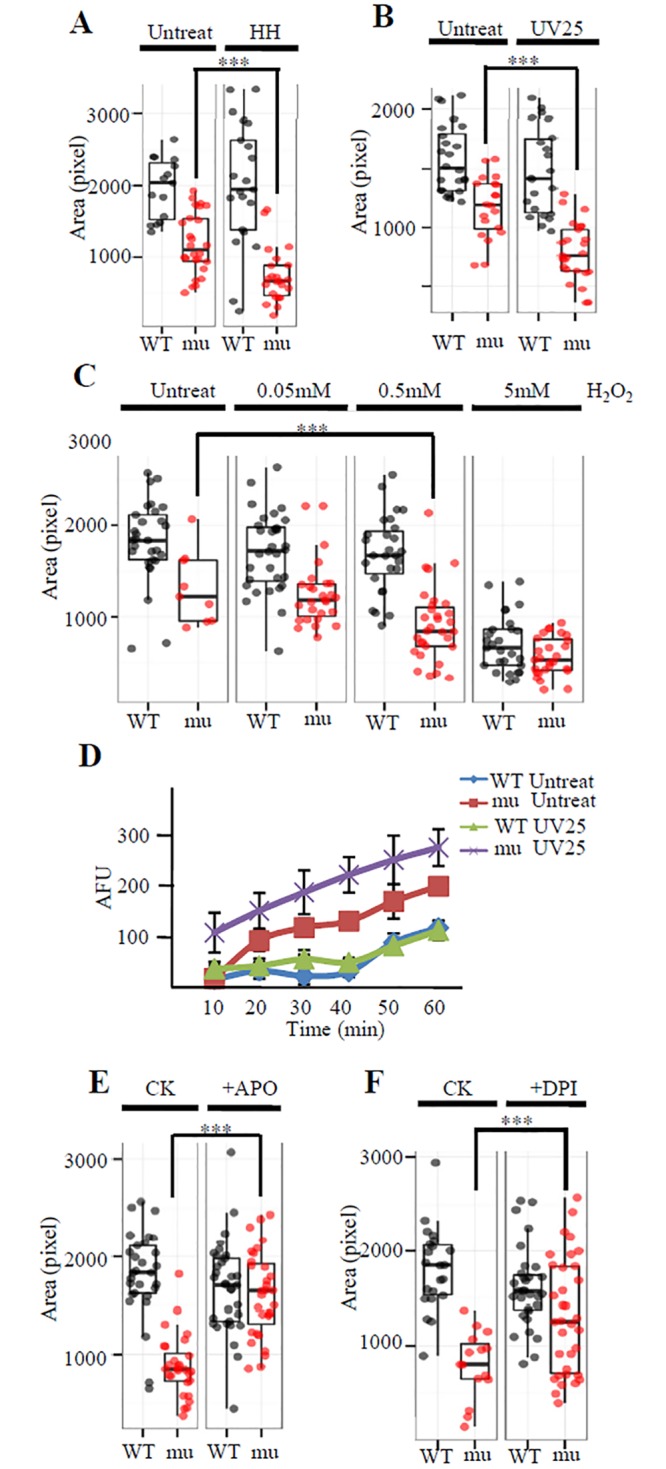
(A and B) When growing in high temperature (32°C) and high density (200 embryos per 10-cm diameter Petri dish) (HH) starting from 10 hpf till to 3.5 dpf(A) or briefly treated with 2.5 mJ UV/cm2 (UV25) at 24 hpf(B) the maternal-zygotic leg1azju1 embryos (mu) consistently exhibited a more severe small liver phenotype when compared with the untreated mutant embryos. (C) Comparison of liver development in WT and the maternal-zygotic leg1azju1(mu) embryos treated with 0.05, 0.5 and 5 mM H2O2 at 24 hpf for half an hour. (D) Upon UV25 treatment, the maternal-zygotic leg1azju1 (mu) embryos accumulated a higher level of ROS when compared to the treated WT or untreated mutant embryos within 60 min. (E and F) Incubation with APO (E) and DPI (F), two inhibitors of Duox/Nox enzyme for O2- biosynthesis, prior to UV25 treatment prevented the effect of UV on liver development in the maternal-zygotic leg1azju1(mu) embryos. Liver size was measured at 3.5dpf. Quartile boxplot was used to present the data. Each dot represents the liver size of an individual embryo. CK, the APO or DPI untreated control group. ***, p<0.001.
UV treatment, high temperature, andH2O2 treatment all would lead to oxidative stress [22]. To find out whether the maternal-zygotic leg1azju1 mutant is compromised in scavenging ROS caused the oxidative stress, we compared the ROS level at different time points between the UV25 treated WT and maternal-zygotic leg1azju1 embryos by DCFH-DA [23]. The result showed that the maternal-zygotic leg1azju1 embryos accumulated a higher ROS level at all time points examined within the first hour after UV treatment(Fig 2D). We wondered whether the development of the small liver phenotype in leg1a mutants could be prevented by blocking the production of reactive oxygen species (ROS). Diphenyleneiodonium (DPI) and apocynin (APO) are two specific inhibitors of the Duox/Nox enzyme often used to block the production of ROS[24,25]. We treated the maternal-zygotic leg1azju1 mutants with DPI or APO one hour prior to the UV25 treatment and found that both DPI and APO prevented the mutants from developing the small liver phenotype (Fig 2E and 2F).
Leg1a protects liver development under stress conditions
Liver, exocrine pancreas and intestine are all derived from the endoderm [26]. Previous genetic screening found that mutants with defects in liver development often showed defective development of the exocrine pancreas and/or intestine [27,28], likely because liver and exocrine pancreas share common progenitors [29,30]. Leg1a expression is enriched in the embryonic liver, but meanwhile, Leg1a is also a secretory protein [15]. Considering this fact, we wanted to determine whether leg1azju1 also affects development of the pancreas and other digestive organs. We used fabp10a, trypsin, insulin and fabp2a probes in WISH to mark the liver, exocrine pancreas, endocrine pancreas and intestine, respectively. Interestingly, it appeared that UV25 treatment drastically reduced the liver size, but only subtly affected the exocrine pancreas development and did not show observable effect on the intestinal tube development in the maternal-zygotic leg1azju1 mutant (Fig 3A). We then used prox1 and hhex, two earlier hepatic markers, in WISH to examine the effect of UV25 treatment on liver bud formation at 30 hpf[13]. The result showed that UV25 treatment halted liver bud formation in most of the mutant embryos but not the WT (Fig 3B).
Fig 3. Loss-of-function of Leg1 blocks the liver bud formation.
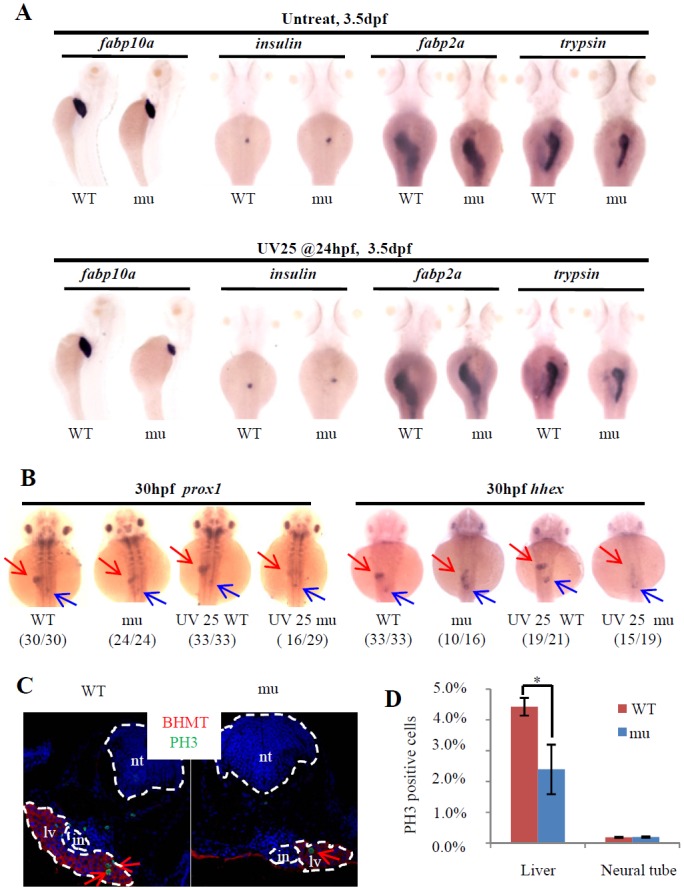
(A) Loss of maternal zygotic Leg1a affects the liver development. Embryos were treated with or without UV25 at 24hpf, WISH was performed to assess the development of the liver (fabp10a), exocrine (trypsin) and endocrine (insulin) pancreas and intestine (fabp2a) in the maternal-zygotic leg1azju1(mu) embryos at 3.5 dpf. At least three independent WISH was performed, each time with 24–31 embryos for each sample, and representative embryos were shown. (B) WISH using prox1 and hhex to examine liver bud formation at 30 hpf in embryos treated with or without UV25 treatment at 24 hpf. Representative pictures in each group are presented. The number of embryos exhibiting the phenotype over total embryos examined are shown in the bracket. Red arrow: liver bud, blue arrow: pancreatic bud. (C and D) Images (C) and statistical analysis (D) of PH3 immunostaining to compare cell proliferation of hepatocytes in WT and maternal-zygotic leg1azju1 embryos (mu) after UV25 treatment. BHMT is an enzyme highly expressed in the liver and was used to mark out the hepatocytes. DAPI was used to stain the nuclei. Red arrows indicate PH3 positive cells in the liver. PH3 positive cells in the neural tube in WT and the mutant were also recorded in parallel. *, p<0.05. in, intestinal tube, lv, liver, nt, neural tube.
A TUNEL assay did not reveal any obvious differences in the apoptotic activity between the UV25-treated WT and mutant liver cells (S3 Fig, total 12cryosections from 6 embryos examined). Immunostaining of phosphorylated histone 3 (PH3, a molecular marker for cell proliferation) showed that leg1azju1 liver cells (defined by immunostaining of the hepatic marker Betaine homocysteine S-methyltransferase (BHMT), in red) contained significantly fewer (p<0.05) PH3 positive cells (12 of 604 total cells counted or 2.4%, data obtained from 6 embryos) when compared with those in the WT (30 of 684 total cells counted or 4.43%, data obtained from 6 embryos) at 54 hpf after UV25 treatment (Fig 3C and 3D). Therefore, the maternal-zygotic leg1azju1 mutant develops a small liver phenotype under UV stress due to cell cycle arrest.
Leg1a activates the Erk pathway to promote liver development
One possible explanation for the inhibitory effect of UV25 treatment on liver development in the maternal-zygotic leg1azju1 mutant is that UV25 treatment induces the expression of Leg1. However, we observed that the UV- or H2O2-treatment of the WT embryos at 24 hpf did not cause significant changes to the levels of total leg1 transcripts at 3 and 6 hours after treatment (S4 Fig). UV25 treatment of the WT embryos at 24 hpf neither affected the level of total Leg1 protein at 3, 6, 9 and 12 hours after treatment (Fig 4A). In zebrafish, 24–34 hpf is a crucial stage for hepatogenesis when signaling molecules including FGF, BMP, Wnt2bb and RA orchestrate the initiation of the liver bud [13,31–33]. Based on all of the above, we speculated that the signaling pathway promoting cell proliferation is probably impaired due to loss of the maternal-zygotic Leg1a. This prompted us to investigate whether Leg1, being a secretory protein, is involved in known signaling pathways. We treated 24hpf WT and maternal-zygotic leg1azju1 mutant embryos with UV25 and found that UV25 treatment up-regulated the level of p-Erk in WT but showed an inhibitory effect on the mutant at 6 hours post treatment (i.e. at 30 hpf) (Fig 4B). Notably, UV25 treatment did not affect the Bmp signaling as indicated by the level of pSmad1/5/8 (Fig 4B). Importantly, upon UV25 treatment, Leg1a over-expression by leg1a mRNA injection at one-cell stage increased the level of p-Erk but did not alter the level of pSmad1/5/8 (Fig 4C). Considering that the activation of the expression of Bmp2 by heatshock of Tg(hsp70l:bmp2b) [34] embryos at 18 or 24 hpf increased only the level of pSmad1/5/8 and not that of p-Erk(Fig 4D and 4E), we speculated that Leg1 does not signal through the Bmp pathway but probably through the Erk signaling pathway to protect liver development under stress conditions.
Fig 4. Leg1 promotes the phosphorylation of Erk.
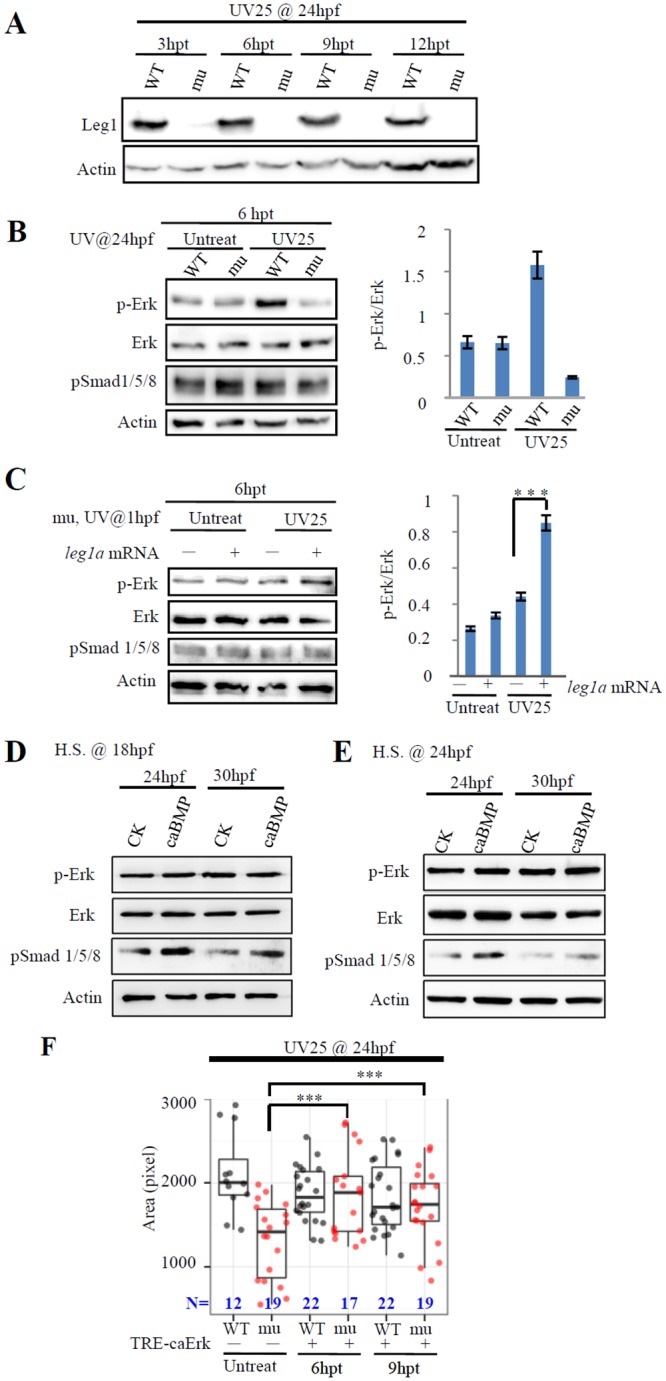
(A) UV25 treatment did not affect the Leg1 protein level. WT and maternal-zygotic leg1azju1 embryos were treated with UV25 at 24 hpf. UV25 treated embryos were harvested for total protein extraction for western blot analysis of Leg1 at 3, 6, 9 and 12 hours post treatment. (B)24-hpf WT and maternal-zygotic leg1azju1 mutant (mu) embryos were treated with or without UV25. Total protein was extracted at 6 hours post treatment (hpt) and subjected to western blot analysis of p-Erk, total Erk, and pSmad 1/5/8. (C) leg1azju1 embryos at one-cell stage were injected with or without leg1a mRNA, then treated with or without UV25 at 1 hpf. Total protein was extracted at 6 hours post treatment (hpt) and subjected to western blot analysis of p-Erk, total Erk, and pSmad 1/5/8. (D and E) Over-expression of Bmp2a does not activate the phosphorylation of Erk. Tg(hsp70l:bmp2b) embryos were heat-shocked at 18 hpf (D) or 24 hpf (E). Total protein was extracted from embryos 6 or 12 hours post heatshock and subjected to western blot analysis of pSmad1/5/8, p-Erk and total Erk. Over-expression of Bmp2 by heatshock increased only the level of pSmad1/5/8 and not that of p-Erk. CK, wild type embryos; caBMP, Tg(hsp70l:bmp2b) embryos.(F) TRE-caErk plasmid was injected into WT and mutant (mu) embryos at one-cell stage. Injected embryos were treated with Dox at 24 hpf for 6 or 9 hours (6hpt or 9hpt) and were then harvested at 3.5 dpf for WISH using the fabp10a probe.(A-E) Western blot was repeated six times for (A), four times for (B), five times for (C) and twice for (D) and (E). Actin was used as a loading control. ***, p<0.001.
To test whether Leg1 acts through the Erk-signaling pathway to protect liver development, we generated a constitutively active form of Erk mutant (caErk) by substituting L84 to P84 (L84P), S162 to D162 (S162D), D330 to N330 (D330N) simultaneously[35]. It has been shown that over-activating Erk signaling at the early stage (up to 80% epiboly) negatively regulates the endoderm formation[36]. Indeed, we found that injection of caErk mRNA into one-cell stage embryos caused small liver both in WT and mutant embryos (S5A Fig). To overcome the effect of Erk-signaling on early embryogenesis we injected caErk mRNA or fgf8 mRNA into the yolk at 22hpf and treated the embryos with UV25 at 24hpf. The effectiveness of this way of injection is demonstrated by the fact that the injected Cy3-labled oligo-dT can successfully reach to the prospective liver bud region (S5B Fig). We found that such injection rescued the mutant liver development to a great extent (S5C Fig). Next, we cloned the caErk gene downstream of the doxycycline (Dox) inducible promoter tetracycline response element(TRE)promoter[37,38](S6A Fig). The expression of caErk is effectively induced by Dox after a low dosage (10 pg) of plasmid injection although a weak leakage of the TRE promoter was observed (S6B Fig). The TRE-caErk plasmid (10 pg) was injected into one-cell stage maternal-zygotic leg1zju1mutant embryos and the injected embryos were treated with UV25 at 24 hpf followed immediately by addition of the drug Dox (final concentration 30 μg/mL). The liver development in these embryos was examined with the fabp10a probe at 3.5 dpf. The result showed that induction of the caErk expression between 24hpf and 33hpf achieved a significant rate of rescue of the liver growth in maternal-zygotic leg1zju1mutant (Fig 4F) while overall features of the injected embryos appeared relatively normal (S6C Fig).
Leg1 is modified by glycosylation at N70
We showed previously that Leg1 is a classical secretory protein [15]. Because glycosylation is a common modification for a secretory protein [39], we checked whether Leg1 is also modified by glycosylation. There are two types of glycosylation, N-glycosylation and O-glycosylation [40]. N-glycosylation can be cleaved by PNGase F [41]whereas O-glycosylation can be cleaved by the combination of endo-α-N-acetylgalactosaminidase plus neuraminidase [42]. We previously reported that adult fish serum contains a high level of Leg1 [15]. We used these enzymes to treat the serum protein and also total protein extracted from embryos at 3dpf, respectively, and found that only PNGase F treatment caused a band shift (Fig 5A and 5B). Because both leg1a and leg1b are expressed in the adult liver to produce the serum Leg1 [15], the fact that PNGase F treatment caused a clear band shift to total Leg1 protein from the serum suggests that both Leg1a and Leg1b are modified by N-glycosylation. To confirm this hypothesis, leg1a and leg1b were cloned into the expression vector PCS2+, and the obtained plasmids were used to transfect the human liver cancer cell line HepG2. PNGase F treatment caused a band shift to both the expressed Leg1a and Leg1b in HepG2 (Fig 5C).
Fig 5. Leg1 is modified by glycosylation at N70.
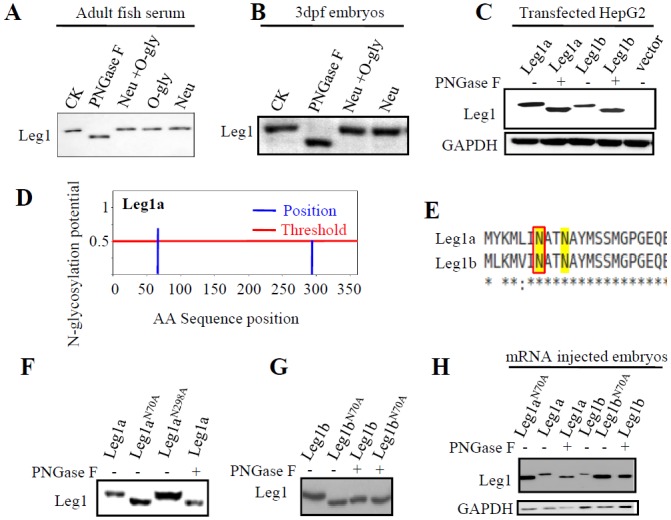
(A and B) Checking the status of glycosylation in the endogenous Leg1 by treating protein extracted from the serum (A) or 3dpf embryos (B) with PNGase F, neuraminidase (Neu), or Neu plus endo-α-N-acetylgalactosaminidase (O-gly), respectively. CK, enzyme untreated control sample.(C) Checking the status of glycosylation of Leg1a or Leg1b in plasmid transfected HepG2 cells by treating the protein samples with PNGase F.(D-H) Determination of N70 as the glycosylation site in Leg1. Prediction of the site(s) of glycosylation in Leg1a based on the Asn-Xaa-Ser/Thr motif using the software program NetNGly (http://www.cbs.dtu.dk/services/NetNGlyc/) (D).N70 is predicted to be a putative N-glycosylation site by NetNGly in both Leg1a and Leg1b (E). Mutating N70 to A70 in either Leg1a (F,H) or Leg1b (G,H) caused a gel mobility shift of Leg1 like that did the PNGase F-treated Leg1. Total protein was extracted from the plasmid transfected HepG2 cells (F and G) or mRNA injected embryos (H). Western blot was repeated twice for (A), three times for (B), (C) (F), (G) and four times for (H).
To determine which amino acid residue is glycosylated in Leg1a and Leg1b, we used a web-based platform, NetNGly (http://www.cbs.dtu.dk/services/NetNGlyc/), to predict the site of modification(s) based on the Asn-Xaa-Ser/Thr motif [43]. The prediction showed that the 70th asparagine (N70) was a putative glycosylation site for both Leg1a and Leg1b (Fig 5D and 5E). For Leg1a, N298 was also predicted to be a candidate site for glycosylation (Fig 5D). We then mutated N70 and N298 in Leg1a to alanine (A) to obtain the leg1aN70A and leg1aN298A plasmids. We transfected the leg1a WT plasmid and the two leg1aN70A and leg1aN298A mutant plasmids into the HepG2 cell line, respectively, and performed western blot analysis of Leg1 in the extracted total protein at 24 hours post transfection. The result showed that leg1aN70A produced a product with a mobility like that of Leg1a treated with PNGse F, whereas leg1aN298A produced a product with a mobility like that by the leg1a WT plasmid (Fig 5F). We also mutated the N70 to A70 in Leg1b and found that Leg1bN70A was no longer sensitive to PNGase F treatment and exhibited a mobility like that of Leg1b treated with PNGase F (Fig 5G). In addition, we injected leg1aN70A and leg1bN70A mRNA and their respective WT control mRNA into zebrafish embryos at the one-cell stage and extracted total protein at 9 hours post injection. Western blot analysis of the protein samples showed that both Leg1aN70A and Leg1bN70A exhibited a mobility like that of Leg1a or Leg1b treated with PNGase F (Fig 5H). All of these results demonstrated that N70 was the only N-linked glycosylation site for both Leg1a and Leg1b.
N70-glycosylation is necessary for the secretion of Leg1b but not Leg1a
Glycosylation in secretory proteins often facilitates the proper folding of the protein so that the protein can be licensed to be transported to the Golgi apparatus [44]. To assess the secretory ability of Leg1aN70A and Leg1bN70A, WT leg1a, WT leg1b, leg1aN70A or leg1bN70A plasmid was each co-transfected with HA-tagged rnasel1 plasmid into HepG2 cells. Rnasel1 (NCBI accession no. AI476973) encodes a known secretory protein Rnasel1 [45] and was used as a control here. Total proteins were extracted from the culture medium and the cell pellet, respectively. Western blot analysis of the protein samples showed that Leg1a, Leg1b, Leg1aN70A and Leg1bN70A were all detected in the cell pellet fraction (Fig 6A). Leg1a, Leg1b and Leg1aN70A were also detected in the culture medium fraction (Fig 6A), whereas no Leg1bN70A was detected (Fig 6A). Meanwhile, we noticed that the secretion of HA-Rnase1l in the cells expressing Leg1bN70A was also greatly reduced (Fig 6A).
Fig 6. N70-glycosylation is necessary for the secretion of Leg1b but not Leg1a.
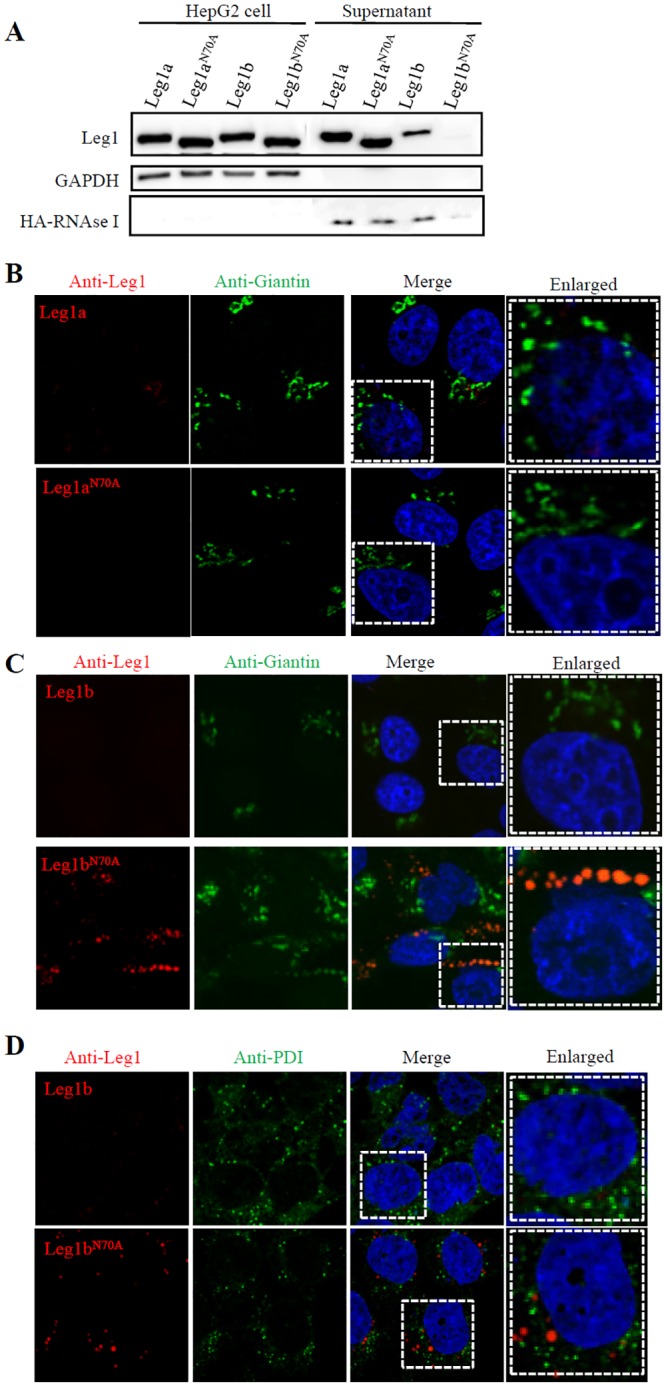
(A) Western blot analysis of secretion of Leg1a, Leg1aN70A, Leg1b and Leg1bN70A. Mutating N70 to A70 does not affect the secretion of Leg1aN70A but does affect Leg1bN70A. Total protein was extracted from cell pellets or supernatant. HA-tagged RNaseI was used as a control. Western blot was repeated three times.(B) Co-immunostaining of Leg1a or Leg1aN70A with the cis- and medial-Golgi marker Giantin in HepG2 cells transfected with WT leg1a and leg1aN70A plasmids, respectively. (C) Co-immunostaining of Leg1b or Leg1bN70Awith Giantin showed that Leg1b is secreted (upper panels) but Leg1bN70A is retained in the cis-Golgi network (lower panels). (D) Co-immunostaining of Leg1b or Leg1bN70A with the ER marker PDI in HepG2 cells transfected with WT leg1b and leg1bN70A plasmids, respectively. The enlarged view of the area highlighted with dashed lines are shown on the right.
To determine where the un-secreted Leg1bN70A was located in the protein trafficking route, we co-immunostained Leg1 with ER and Golgi markers, respectively. Consistent with western blot analysis, Leg1a and Leg1aN70A were secreted normally (Fig 6B), as was the WT Leg1b, which was hardly detectable in the leg1b plasmid-transfected cells (Fig 6C and 6D, upper panels). In contrast, Leg1bN70A nicely co-localized with the cis-Golgi indicated by cis- and medial-Golgi marker Giantin(Fig 6C, lower panels) but not with the ER marker PDI [46,47](Fig 6D, lower panels). Strikingly, cells transfected with the leg1bN70A plasmid appeared to harbor more cis-Golgi components (revealed by Giantin staining) than those in the WT leg1b-transfected cells (Fig 6C), indicating that the Leg1bN70A mutant protein is retained in the cis-Golgi apparatus, which caused a traffic jam in the cells such that the secretion of Rnase1l was also severely blocked in these cells (Fig 6A). The fact that accumulation of Leg1bN70Amutant protein in the cis-Golgi but not in the ER might explain why we did not observe an activation of the markers (including Bip, Chop, and p-eIF2a) for the ER-stress response either in the cultured cells (S7A Fig) or in the maternal-zygotic leg1azju1 mutants(S7B and S7C Fig).
N70-glycosylation is required for Leg1a to protect liver development under stress condition
Next, we tested whether N70 glycosylation in Leg1a is required to promote liver development by injecting leg1a andleg1aN70A mRNA, respectively, into the maternal-zygotic leg1azju1 mutant embryos at the one-cell stage (S8 Fig). These injected embryos were briefly treated with UV25. WISH analysis using the fabp10a probe showed that leg1aN70A mRNA injection failed to rescue the mutant liver development (Fig 7A). In fact, Leg1aN70A was greatly compromised in promoting Erk phosphorylation under UV25 (Fig 7B).
Fig 7. Leg1a interacts with FGFR and glycosylation at N70 is crucial for Leg1 to interact with FGFR3 and to protect liver development under stress conditions.
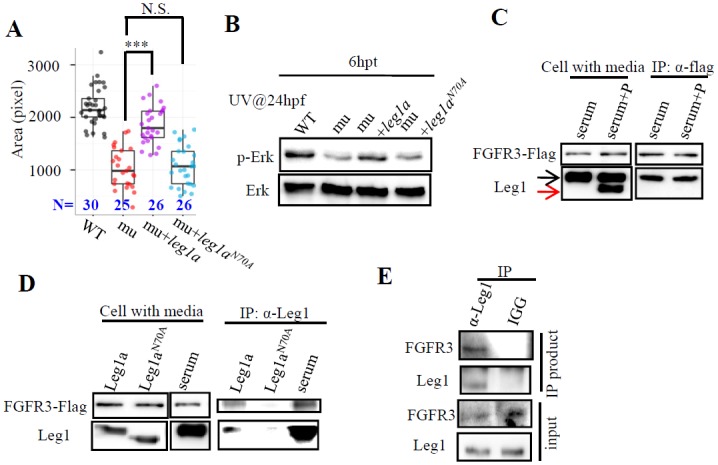
(A and B) Maternal-zygotic leg1azju1 embryos (mu) at one-cell stage were injected with leg1a or leg1bN70A mRNA. Injected embryos were treated with UV25 at 24 hpf and allowed to grow to 3.5 dpf for comparison of liver development by WISH using fabp10a probe (A) or to 30 hpf for protein extraction for western blot analysis of p-Erk and total Erk (B). A representative data set of three independent experiments was shown. ***, p<0.001, N.S., not significant. (C and D) Leg1 but not de-glycosylated Leg1 interacts with FGFR3. 293T cells over-expressing FLAG-tagged FGFR3 were incubated with total serum containing Leg1 (serum) or total serum Leg1 partially de-glycosylated by PNGase F (serum+P) (C), with supernatant containing secreted Leg1a or Leg1aN70A respectively from leg1a or leg1aN70A plasmids transfected 293T cells (D). In (C), black arrow, total Leg1, red arrow, de-glycosylated Leg1. An anti-FLAG antibody was used to perform the Co-IP, FGFR3 was detected with the anti-FLAG antibody and Leg1 with the Leg1 antibody. (E) Co-IP assay of interaction between Leg1a and FGFR3 in zebrafish. 200pg leg1 mRNA and 400 pgfgfr3 mRNA were co-injected into one-cell stage embryos. Total protein was harvested 7 hours after injection and was subjected to Co-IP using the Leg1 antibody or mouse IGG antibody. FGFR3 was detected by an anti-FRFR3 antibody. Western blot was repeated three times for (B), four times for (C), three times for (D) and (E).
Leg1a interacts with FGFR3 that depends on Leg1a N70-glycosylation
FGFis a key effector of the Erk signaling pathway. We wanted to determine whether Leg1 interacts with the FGF receptor (FGFR) to activate the phosphorylation of Erk. Extracted serum protein containing Leg1a and Leg1b (total Leg1) was incubated with human 293T cells transfected with a plasmid expressing FLAG-tagged FGFR3. Co-immunoprecipitation (Co-IP) analysis showed that Leg1 interacted with FGFR3 (Fig 7C). To determine whether N70-glycosylation is necessary for Leg1 to bind to FGFR3, we treated total serum proteins (containing both Leg1a and Leg1b) with PNGase F to get a mixture of Leg1 and de-glycosylated Leg1 under the undenaturized condition (Fig 7C, left panels). The mixture of Leg1 plus de-glycosylated Leg1 was incubated with 293T cells expressing FGFR3. Co-IP analysis showed that only Leg1 but not the de-glycosylated Leg1 interacted with FGFR3 (Fig 7C, right panels). We also over-expressed Leg1a and Leg1aN70A in 293T cells (Fig 7D, left panels) and harvested the culture medium containing Leg1a or Leg1aN70A to incubate with 293T cells overexpressing FGFR3, respectively. Co-IP showed that Leg1a but not Leg1aN70A interacted with FGFR3 (Fig 7D, right panels). In zebrafish, FGFR3 was co-immunoprecipitated by the Leg1 antibody when Leg1a and FGFR3 were co-expressed by their mRNA co-injection (Fig 7E).
The zebrafish transgenic line Tg(hsp70:dnfgfr1-gfp) expresses the dominant-negative Fgfr1(dn-Fgfr1) by the hsp70heakshock promoter[48]. The expressed dn-Fgfr1, whose tyrosine kinase domain is replaced by GFP (as a reporter of the transgenic embryos), can form heterodimer with all FGFR subtypes so that to block the FGF signaling. When this line was treated with UV25 only we found that the level of p-Erk was increased (S9 Fig). However, when the embryos were heat-shocked at 22 hpf to express dn-Fgfr1 followed by treatment with UV25 at 24 hpf, the effect of UV25 on activation of p-Erkin the GFP+ embryos was down-regulated to a similar level to that observed in the UV25 untreated GFP+ embryos. This result further suggests that the activation of Erk by UV25-treatment is through the FGF pathway,
Compensatory mechanism is activated in leg1azju1 mutant
Considering the importance of the liver for a living organism and the viable and fertile nature of theleg1a mutant, we wondered whether the small liver in the leg1a mutant would be recovered to normal during later growing stages. We treated WT and maternal-zygotic leg1azju1 mutant with UV25 at 24hpf, and check the liver size at 3.5dpf and 10dpf. While, as expected, almost all the maternal-zygotic leg1azju1 mutant displayed a small liver compared to the WT at 3.5dpf, the liver sizes in the mutants were recovered to normal at 10dpf (Fig 8A). However, the maternal-zygotic leg1azju1 mutant exhibited a lower survival rate ~32% (28/87) when compared to 66% (50/76) for the WT counted at 10 dpf. Examining the expression of leg1b, the homolog of leg1a, in the leg1azju1 mutant revealed that the levels of leg1b transcripts were up-regulated both in zygotic homozygous mutant at 4 dpf(Fig 8B) and maternal-zygotic mutant at 3-, 5-, and 7-dpf (Fig 8C). WISH using the leg1 probe also showed that the total leg1 transcripts in the maternal-zygotic leg1azju1 mutant was enriched in the liver (S10B Fig).These data suggest that the compensatory mechanism[49] is activated in the leg1azju1 mutant to support the liver development at the later stages. At the adult stage, although the liver to body ratio of the leg1azju1 mutant fish did not show significant difference to that of the WT fish (Fig 8D) the leg1azju1 mutant fish exhibited a shorter stature and higher mortality compared to the WT fish (Fig 8E and 8F), suggesting that the Leg1a anti-stress pathway also functions in the adult fish and that theLeg1bonly partially compensates for the function of Leg1a.
Fig 8. The compensatory mechanism is activated in leg1a mutant.
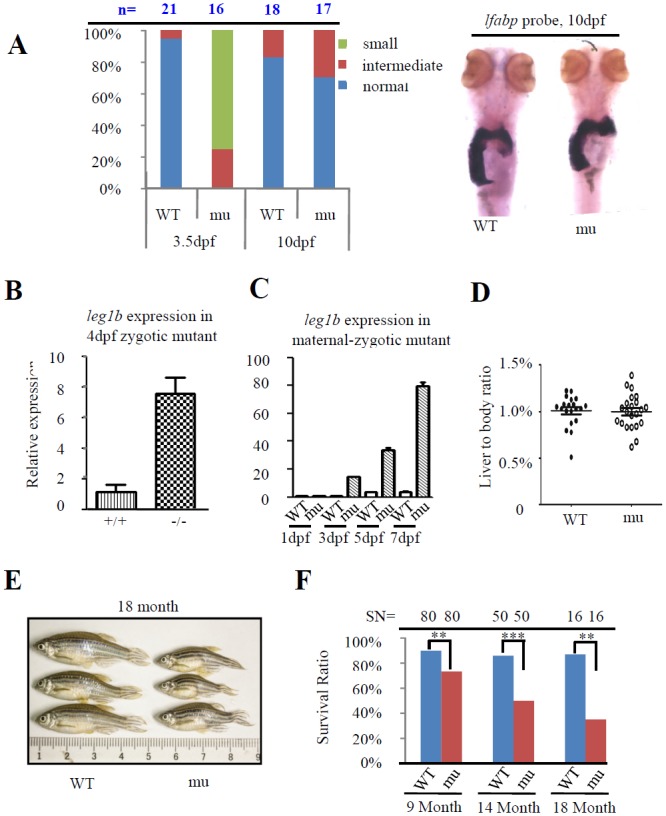
(A) WISH analysis of the liver sizes in WT and maternal-zygotic leg1azju1(mu) at 3.5 and 10 dpf using the fabp10a probe. Left panel: histogram showing the percentage of normal, intermediate and small liver in the WT and leg1a mutant embryos at 3.5 and 10 dpf. The number of embryos (n) was shown on the top. Right panel: representative image of 10-dpf embryos after WISH. (B and C) leg1b expression in the zygotic mutant embryos at 4 dpf (B) and maternal-zygotic mutant embryos at 1, 3, 5 and 7dpf(C). (D) Comparison of the liver to body ratio between WT and leg1azju1mutant in the adult fish. Each dot represents the ratio for an individual adult fish. Error bar stands for the standard error. (E) Representative images of the WT and leg1azju1mutant adult fish. (F) Survival rate of the WT and leg1azju1mutant adult fish aged at 9, 14 and 18 months. The starting number of the larvae fish for each counting (SN) are shown on the top.
Discussion
In addition to the precise spatial and temporal control of genetic programs instructing oganogenesis, successful completion of organogenesis also relies on the maintenance of an optimal environment through the elimination or neutralization of the stress-induced harmful reagents, and how this is achieved is of tremendous interest in the field of developmental biology [50]. Although undergoing external embryogenesis, teleost fish harbor a robust genetic program dictating liver development as long as any environmental change, including temperature or natural UV irradiation, is not detrimental. It is therefore of interest to explore the mechanism(s) behind this phenomenon. We showed that Leg1 plays a unique role in protecting liver development under different stress conditions by serving as a secretory signaling molecule/modulator to activate the Erk pathway. This finding may explain the adaption of teleost fish in coping with environmental changes.
The process of liver organogenesis is governed by key transcription factors (e.g., HNF, GATA, Prox and Hhex) and signaling molecules (e.g. FGF, Bmp and Wnt) [1–3,10]. Meanwhile, each of these stages has to deal with the oxidative stress constantly imposed intrinsically or externally. In zebrafish, hepatoblasts are specified at around 24 hpf and start to form the liver primordium at around 30 hpf. Both FGF and Bmp play crucial roles during this period [10,31,32]. In general, FGF acts through the FGFR-RAS-ERK signaling pathway [51], Bmp through activation of Smad1/5/8 phosphorylation [52] and Wnt2bb through the β-catenin-TCF pathway to control organ/tissue development, respectively [53]. It is envisaged that molecules mediating anti-oxidative stress during liver organogenesis might act as a tuner of the pathways controlling cell proliferation or elimination. Based on the facts that 1) Leg1a expression is enriched in the yolk syncytial layer between 24–48 hpf(S10A Fig)and this layer is directly exposed to external stress such as UV irradiation or low level of oxygen, 2) Leg1a expression is obviously enriched in the embryonic liver at 48 hpf, 3) Leg1a is a secretory protein, 4) the leg1azju1 maternal-zygotic mutant exhibited a small liver only under the cold season, UV irradiation, high temperature, or H2O2 treatment, and 5) the small liver phenotype was rescued by the antioxidant chemicals DPI and APO, we conclude that Leg1a defines a novel anti-stress pathway to protect the liver development. Besides, we noticed that the leg1azju1 maternal-zygotic adults displayed a shortened body length and reduced survival rate, suggesting that the Leg1-meidated anti-stress pathway is also necessary for wellbeing of an adult fish.
Then, the question is how there is a season in a fish facility which is maintained at relatively constant temperature throughout the year? Since the small liver exhibited by the maternal-zygotic leg1azju1 mutant is ROS-dependent we speculate that the difference in the oxygen content in the fish water in different seasons might be the cause of the stress-related phenotype although the temperature is maintained in the facility. We know that the oxygen content in the water is related to atmospheric pressure and that the atmospheric pressure is higher in the cold seasons and lower in the warm seasons. We checked the weather record between Dec 30, 2013 to Jan 30, 2015 in Hangzhou and plotted the liver size against the record of atmospheric pressure. The small liver phenotype in leg1a mutant nicely correlates with high atmospheric pressure in the cold seasons (S1D Fig). However, we cannot exclude other possibilities at this moment.
Utilizing specific morpholinos (MOs), we previously showed that leg1a is required for liver bud outgrowth[15]. However, zygotic leg1azju1 mutants do not show this phenotype, and maternal-zygotic leg1azju1 mutant phenotype is milder than the phenotype generated by the leg1a-MO. The discrepancy between the phenotype caused by MO-injection and the phenotype exhibited by a loss-of-function mutant is indeed a concern in the zebrafish community. The explanations for the discrepancy observed could be: 1) previous used leg1-MO might have yielded an off-target effect on the liver development in the morphants, this fits with the observation that leg1a or leg1b mRNA or even combination of leg1a and leg1b mRNA only partially rescued the morphant small liver phenotype[15]; 2) injecting morpholino itself works as a stress cue to induce the small liver phenotype when Leg1 is knocked down; and 3) since the leg1b gene is still intact in the leg1azju1 mutant, the mild phenotype exhibited by the maternal-zygotic leg1azju1 mutant might be due to the functional compensation by Leg1b[49]. To narrow down the possibilities, we tried to get the leg1a and leg1b double knockout mutant, however, failed in obtaining such double mutant. We also injected standard control morpholino (ST-MO, derived from human β-globin antisense morpholino) into the leg1azju1 mutant embryos and found that ST-MO did not enhance the leg1azju1 mutant phenotype (S11 Fig). We then compared the expression of leg1b in the WT and leg1azju1 and found that the expression of leg1b is elevated in the leg1azju1 mutant embryos. These data suggest that the expression of leg1b is mobilized to compensate, at least partially, for the loss of function of Leg1a in the leg1azju1 mutant.
Mechanistically, it appears that Leg1a does not signal through the Bmp pathway because Leg1a over-expression does not promote the phosphorylation of Smad1/5/8 as done by the over-expression of Bmp at 18 or 24 hpf. However, Leg1a over-expression does promote the phosphorylation of Erk upon UV25 treatment. Furthermore, UV-treatment caused up-regulation of the phosphorylation of Erk in WT but not in the maternal-zygotic leg1azju1 mutant. In addition, as revealed by immunostaining, it appeared that more p-Erk cells were in the WT endoderm than that in the maternal-zygotic leg1azju1 mutant after UV25 treatment (S12 Fig). The intriguing question is why Leg1a promotes Erk phosphorylation after UV exposure. We speculate that it maybe because UV causes certain modification or conformation change to Leg1a that facilitates the interaction between Leg1a and Fgfr3 to promote Erk phosphorylation. Nevertheless, these data suggest that Leg1a signals through the Erk pathway. Since FGF is a key effector of the Erk pathway and is essential for liver development, our data suggest that there might be a crosstalk between the FGF and Leg1-meidated anti-stress signaling pathways. Therefore, it is of great interest to determine how Leg1 promotes Erk phosphorylation in the future. For example, being a secretory protein, does Leg1a have its own receptor or shares the FGF receptor to mediate its activity? If Leg1a does share the FGF receptor with FGF, then which type of receptor do they share? Or does Leg1a simply serve as an agonist to facilitate the binding of FGF to its receptor? Leg1 is an evolutionally conserved protein across the vertebrates[15]. A recent report showed that Leg1 homologs in monotreme is highly expressed in monotreme milk and appears to be modified by N-glycosylation[17]. This implies that the tissue expression specificity and function of Leg1 might vary among different animal species. Here we showed that zebrafish Leg1a is glycosylated at N70. Although this glycosylation modification is not essential for the secretion of Leg1a, it is important for Leg1a in the promotion of liver development, for the phosphorylation of Erk and interaction with FGFR3. All available data have suggest that Leg1a is a novel signaling molecule/modulator, which has urged us to identify more downstream signaling molecules involved in this pathway, which may ultimately reveal the importance of this pathway in the evolution of vertebrates.
Materials and Methods
Ethics statement
All animal procedures were performed in full accordance to the requirement by ‘Regulation for the Use of Experimental Animals in Zhejiang Province’. This work is specifically approved by the Animal Ethics Committee in the School of Medicine, Zhejiang University (ETHICS CODE Permit NO. ZJU2011-1-11-009Y, issued by the Animal Ethics Committee in the School of Medicine, Zhejiang University).
Fish lines and maintenance
The zebrafish (Danio rerio) AB strain was used as WT in this study. To generate the leg1a mutant, we constructed a TALEN vector against the first exon of the leg1a gene (Fig 1A) according to the “Unit Assembly” protocol[19]. The TALEN mRNA was synthesized using the SP6 mMESSAGEmMACHINE Kit (Ambion) and was injected into the WT embryos at one-cell stage. These embryos were bred to the adulthood as founders to mate with a WT fish. Eight embryos from each cross were genotyped using the primer pair leg1a 4244 Fw (CTTACAAGTTACAGCAGCTCC) and legg1a 7748 Rv (CACAACGGACCAGTACATCG) followed by the second primer pair TALEN ID fw (CTCCCAGAGGATGACCATGT) and TALEN ID Rv (ACTCCAGAGCGGATTCTCCT) to identify leg1a mutants, and the rest embryos were bred to adulthood for identification of individuals carrying the mutation. The Tg(hsp70:dnfgfr1-gfp) and Tg(hsp70l:bmp2b) fish lines were obtained from Dr Feng Liu. Fish was raised and maintained in the fish facility (Ai-Sheng Zebrafish Facility Manufacturer Company, Beijing, China) in Zhejiang University according to the standard procedure.
Cell lines and plasmid transfection
HepG2 cells were grown in the DMEM medium (high glucose, GIBCO), supplemented with 10% newborn calf serum (NBCS, GIBCO). Plasmids were transfected into cells mediated with lipofectamine 2000 (InVitrogen) according to the manufacturer’s instruction. Total protein was extracted 24 hours after transfection and was subjected to western blotting analysis.
Plasmid construction and mRNA in vitro transcription
The ORF region of leg1and erk was cloned into PCS2+ vector. All leg1and erk point mutations were generated by site-directed mutagenesis. The primers for leg1 mutant used in the PCR reaction were designed by the webtoolPrimerX (http://bioinformatics.org/primerx/index.htm). The sequences of primers are listed in S1 Table. All primers used for erk point mutation was designed as previously described[35]. mRNAs were obtained via in vitro transcription using the mMessagemMachine (Ambion) according to the manufacturer’s instruction.
Immunofluorescence staining
Cells were seeded in glass slide when the optimal cell density was achieved and fixed by 3% PFA for half an hour at 4°C. The cells were washed twice with 50mM NH4Cl and three times with PBS and were then penetrated with PBST (PBS+0.1% Triton X 100) for 15min and blocked with blocking buffer (5% goat serum, 5% fetal bovine serum and 2% bovine serum albumin) for 30min, sequentially. Cells were finally incubated with corresponding primary antibody and then Alexa Fluor conjugated second antibody. The samples were visualized under a confocal microscope. Leg1 antibody [54] and BHMT antibody [55] was generated as described. PDI antibody (Sigma,P7496), Giantin antibody (Abcam, ab24586), PH3 antibody (Santa Cruz, SC-8656-R), Actin antibody (Huabio, R1207-1), GAPDH antibody (Huabio, M1211-1), p-Erk antibody (Cell Signalling Technology, 9101), total Erk antibody (Cell Signalling Technology, 4695), pSmad1/5/8 (Cell Signalling Technology, 9511) antibody were purchased from the companies as indicated.
Whole-mount in situ hybridization (WISH) and liver size measurement
WISH was performed as previously described [27]. prox1, hhex, fabp10a (liver fatty acid binding protein 10), insulin and trypsin, fabp2 (intestinal fatty acid binding protein 2) were cloned into expression vectors, respectively [27,31]. Corresponding probes were synthesized via in vitro transcription and were labeled with digoxigenin (DIG, Roche, Diagnostics). Liver size was measured as previously described [54]. Briefly, liver was marked out after WISH suing the fabp10a probe, and imaged by Nikon AZ100 from left lateral view after aligning two eyes of the embryo vertically. The fabp10a signal area in each image was calculated by Nikon image system (NIS-elements D v3.0)and used as the index of the liver size.
Glycan cleavage
PNGase F (NEB, P0704) was used to cleave N-linked glycosylation, and a combination of Endo-alpha-α-Acetylgalactosaminidase (NEB, P0733) and neuraminidase (NEB, P0720) was used to cleave O-linked glycosylation. All the enzyme treatment was performed according to the manufacturer’s instruction.
Western blot
For either fish embryo or cultured cells, total protein was extracted using an extraction buffer (63mM Tris-HCl, PH6.8, 10% glycerol, 5% β-Mercaptoethanol, 3.5% SDS) containing 1X Complete (Roche, 11873580001). Western blotting was performed as described previously [27] using corresponding antibodies as indicated in the figures. Actin antibody (Huabio, R1207-1), GAPDH antibody (Huabio, M1211-1), p-Erk antibody (Cell Signalling Technology, 9101), total Erk antibody (Cell Signalling Technology, 4695), pSmad1/5/8 antibody (Cell Signalling Technology, 9511), Bip antibody (Sigma, G9043), Chop antibody (Sigma, G6916), phosphorylated eIF2a (p-eIF2a) antibody (Cell Signaling Technology, 9721S), and total eIF2a antibody (Cell Signaling Technology, 9720S), and Flag antibody (Sigma, F1804) were purchased from the companies as indicated. Signal intensity of a desired band was calculated by ImageJ software (v.1.48).
UV and drug treatment
Embryos were treated with different dosage of UV energy supplied by Ultraviolet Crosslinker (UVP, CL-1000) at 24 hpf and then allowed to grow in the egg water. For H2O2 treatment, 24 hpf old embryos were treated with different concentration of H2O2 for half an hour. For APO and DPI treatment, embryos were incubated with 0.5 μM APO (Sigma,W508454) or 10μM DPI (Sigma, D2926) for 1 hour at 23 hpf, followed by UV25 treatment, and then allowed to grow in normal egg water. Embryos injected with TRE-caErk plasmid were treated with Dox at 24hpf, and replaced with fresh egg water at 30hpf (6hpt) and 33hpf (9hpf), respectively.
Measurement of ROS level
ROS content measurement was performed as described previously [56] with some modifications. Briefly, embryos were sunk in 100μl of 10μM DCFH-DA(Beyotime, S0033) solution for one hour prior to UV25 treatment. For each sample, embryos were divided into four groups (containing three embryos in each group) and placed in a 96-well plate. After UV25 treatment the fluorescence signal was measured at a 10 min interval for one hour on a Synergy H1 Reader (Biotek) (excitation 485 nm, emission 560 nm).
Co-immunoprecipitation (Co-IP)
For studying the interaction between Leg1 and FGFR3 in the cell culture system, Leg1 sample was prepared either by diluting 30μl of the fish serum with 1000μl of serum-free DMEM media (Gbico) or by transfecting 293T cells with the leg1 plasmid (cloned into the PCS2+ expression vector) and collecting the culture media 30 hrs after transfection. The Leg1 samples were incubated with the 293T cells expressing FGFR3 (cells transfected with the FGFR3 plasmid in the PLX304 expression vector, the vector is provided by Dr Bing Zhao) at 4°C for one hour. After incubation, the cells were washed with PBS for three times, and lysed with NP40 lysis buffer (50mM Tris-Hcl, PH 8.0, 150mM NaCl, 1% NP40, 2mM EDTA). For studying the interaction between Leg1 and FGFR3 in the embryos, embryos injected with leg1a and fgfr3 mRNA at one cell stage were harvested at 7hpf and lysed with NP40 lysis buffer. All lysates were incubated with Leg1 antibody or Flag antibody at 4°C overnight, followed by incubation with Protein A/G argrose beads (beyotime, Cat.No.P2012) for further 2 hrs. The beads were washed with cold PBS for three times and eluted by 100mM PH2.2 glycine. The elution was subjected to western blot analysis.
Quantitative Real Time-PCR (qPCR)
More than 50 embryos were pooled for total RNA extraction. Reverse transcription was performed by SuperScript II Reverse Transcriptase (Invitrogen, 18064–014) according to the manufacturer’s protocol. The transcribed cDNA was used as the template in qPCR with SYBR Green Master Mix (Vazyme). The CFX96 real time system (Bio-Rad) was used to obtain the threshold cycle (Ct) value, and the relative expression of each gene was determined after being normalized to the actin gene. Primer pairs used are listed in S2 Table.
Statistics
In considering of relative small sizes of samples with skewed phenotype distribution among individuals in this study, the conventional statistical analysis by showing mean and standard error/derivation is apparently not suitable for presenting the liver size measurement data. Instead, quartiles are more intuitive in presenting data with relative small sample size with skewed distributions [57]. Therefore, we used the quartile boxplot to present our data [58]. The box plot was drawn by ggplot2 [59]. Survival ratio statistical analyses were carried by Chi-squared test. Other statistical analyses were performed with the Student’s T-test. *, p<0.05, **, p<0.01, ***, p<0.001, N.S, no significant difference.
Supporting Information
(DOCX)
(DOCX)
(A) Representative images of embryos after WISH using the fabp10a probe corresponding to the result shown in Fig 1F. (B and C) Among the 32 cases shown in Fig 1G, the number of embryos exhibiting a small versus normal liver in 5 cases recorded in cold seasons (B) and 6 cases recorded in warm/hot seasons (C) were shown. (D) Plotting the liver sizes (majority normal or majority small) against daily atmospheric pressure in Hangzhou recorded during 30/12/2013 and 20/02/2015 (obtained from http://www.wunderground.com/).
(TIFF)
(A) Images showing an example of determining the liver size by WISH using the fabp10a probe. WT: wild type; mu: leg1azju1 mutant; mu+UV25: leg1azju1 mutant treated with UV25. (B) WT and maternal-zygotic leg1azju1(mu) embryos were treated with 1 mJ/cm2 (UV10), 2.5 mJ/cm2(UV25) and 5 mJ/cm2 (UV50) UV at 24 hpf and grew to 3.5 dpf for WISH analysis of liver development. (C) Comparison of liver sizes between the WT and maternal-zygotic leg1azju1 (mu) embryos growing in a high density condition (200 embryos per 10-cm diameter Petri dish). (D) Growing the maternal-zygotic leg1azju1 (mu) embryos in the egg water containing 0.5% or 1% ethanol did not cause a small liver phenotype. (E) Upon UV25 treatment the maternal-zygotic leg1azju2 embryos also exhibited a small liver phenotype at 3.5 dpf. *, p<0.05, **, p<0.01, ***, p<0.001, N.S., no significance.
(TIFF)
Images of TUNEL assay in the 54-hpf WT and maternal-zygotic leg1azju1 embryos (mu) after UV25 treatment at 24 hpf. No abnormal apoptotic activity was observed near the endodermal region including liver (lv) and intestine (in) in the maternal-zygotic leg1azju1 embryos (mu) compared to the WT. 12 sections from six embryos for each genotype were examined. nc, notochord, nt, neural tube.
(TIFF)
The 24-hpf WT embryos were treated with UV25 or 0.5 mM H2O2. Total leg1 or leg1b RNA level were measured by quantitative PCR (qPCR) using leg1a and leg1b common primers or leg1b specific primers, respectively. Error bar stands for the standard error. N.S., no significance.
(TIFF)
(A) Injection of constitutively active form of Erk (caErk) mRNA into one-cell stage embryos impaired liver development both in WT and leg1azju1 mutant when examined with the fabp10a probe at 3.5 dpf. (B) 200 pg Cy3 labeled oligo-dT(50) was injected into the yolk at 22 hpf, and the Cy3 signal was checked at 27 hpf. CK, oligo-dT(50) uninjected control. (C) Embryos were injected with caErk or fgf8 mRNA into the yolk at 22 hpf and were then treated with UV25 at 24 hpf. The liver development in the treated embryos at 3.5 dpf was examined with the fabp10a probe at 3.5 dpf.
(TIFF)
(A) Schematic drawing showing the structure of the TRE-caErk plasmid. RTTA expression was driven by the β-actin gene promoter. Dox binds to RTTA and the Dox-RTTA complex binds to the TRE promoter to drive the expression of caErk. (B) 10 pgTre-caErk plasmid DNA was injected into one-cell stage maternal-zygotic leg1azju1 embryos (mu). These embryos were treated with Dox at 6 hpf and total protein was harvested at 12 hpf. The protein samples were subjected to western blot analysis. The total Erk versus Tubulin ratios were shown on the right. Dox, doxycycline. Error bar stands for the standard error. ***, p<0.001. Western blot was repeated three times. (C) Images of representative 3.5-dpf embryos after WISH using the fabp10a probe. Embryos was first injected with Tre-caErk plasmid at one-cell stage, then treated with UV25 at 24 hpf and followed by Dox treatment for 6 hours (6 hpt) or 9 hours (9 hpt). After Dox treatment, embryos were transferred to the normal egg water to grow to 3.5 dpf for WISH (n = 20).
(TIFF)
(A) HepG2 cells were transfected with the leg1b, leg1bN70A, and the vector plasmid DNA. Total protein was extracted 30 hours post transfection and subjected to western analysis of Bip, Chop, phosphorylated eIF2α (p-eIF2α), and total eIF2α. These ER-stress response markers were not activated by the hypoglycosylated Leg1bN70A. Vector, the PCS2+ vector transfected cell. (B) Western blot analysis of Bip, Chop, phosphorylated eIF2α (p-eIF2α), and total eIF2α in the WT and maternal-zygotic leg1azju1 mutant embryos at 2 dpf and 3 dpf. (C) qPCR analysis of the transcript levels of ER-stress response markers including atf6, bip, perk, chop, ire1a, and grp94 in 3 dpf WT and maternal-zygotic leg1azju1 mutant embryos. Error bar stands for the standard error. Primers for analyzing these ER stress marker was as previously reported (S2 Table). Western blot was repeated three times each for A and B.
(TIFF)
Corresponding to Fig 7A and 7B. Western blot analysis of Leg1a or Leg1aN70A protein in 3 dpf old maternal-zygotic leg1azju1 mutant embryos injected with leg1a (mu+1a) orleg1aN70A(mu+1aN70A) mRNA at the one-cell stage. Protein samples from the WT and maternal-zygotic leg1azju1 mutant (mu) embryos were used as controls. Western blot was repeated three times.
(TIFF)
Tg(hsp70:dnfgfr1-gfp) embryos were heatshocked at 22 hpf to induce the expression of dominant negative FGFR1 (dn-Fgfr1). GFP signal was used to distinguish the dn-Fgfr-expressed (GFP+) and non-dn-Fgfr-expressed (GFP-) embryos. Embryos were treated with UV25 at 24 hpf, and total protein was extracted from embryos at 30 hpf (6 h post treatment) and was subjected to western analysis of the level of p-Erk. Tublin was used as a loading control. H.S., heatshock. *, p<0.05, **, p<0.01, N.S., no significance. Western blot was repeated two times.
(TIFF)
n = 25.
(TIFF)
One nanolitre of 0.5 mM standard control mopholino (ST-MO) was injected into one-cell stage embryos. The liver development was examined at 3.5 dpf using the fabp10a probe. N.S., no significance.
(TIFF)
Serial cryosections (S1 to S4) from three WT embryos (WT-1, WT-2 and WT-3) and three mutant embryos (mu-1, mu-2 and mu-3) treated with UV25 at 24 hpf were shown. DAPI was used to stain the nuclei.
(TIFF)
Acknowledgments
We thank Drs. Li Jan Lo, Sheng Ye, Bing Zhao, Xinhua Feng and Zhengping Xu for their valuable suggestions and excellent technique help, and Ms. Yixin Ye and Xiaocai Du for maintenance of the fish facility.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was funded by the "973 Program" of the Ministry of Science and Technology of China (http://www.most.gov.cn/) (2015CB942802, 2012CB944500) and the National Natural Science Foundation of China (http://www.nsfc.gov.cn/) (31330050, 30825025) to JP. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Stainier DY (2002) A glimpse into the molecular entrails of endoderm formation. Genes Dev 16: 893–907. [DOI] [PubMed] [Google Scholar]
- 2.Zaret KS (2002) Regulatory phases of early liver development: paradigms of organogenesis. NatRevGenet 3: 499–512. [DOI] [PubMed] [Google Scholar]
- 3.Duncan SA (2003) Mechanisms controlling early development of the liver. Mechanisms of Development 120: 19–33. [DOI] [PubMed] [Google Scholar]
- 4.Tao T, Peng J (2009) Liver development in zebrafish (Danio rerio). JGenetGenomics 36: 325–334. [DOI] [PubMed] [Google Scholar]
- 5.Gordillo M, Evans T, Gouon-Evans V (2015) Orchestrating liver development. Development 142: 2094–2108. 10.1242/dev.114215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee CS, Friedman JR, Fulmer JT, Kaestner KH (2005) The initiation of liver development is dependent on Foxa transcription factors. Nature 435: 944–947. [DOI] [PubMed] [Google Scholar]
- 7.Zhao RO, Watt AJ, Li JX, Luebke-Wheeler J, Morrisey EE, et al. (2005) GATA6 is essential for embryonic development of the liver but dispensable for early heart formation. Molecular and Cellular Biology 25: 2622–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bort R, Signore M, Tremblay K, Martinez Barbera JP, Zaret KS (2006) Hex homeobox gene controls the transition of the endoderm to a pseudostratified, cell emergent epithelium for liver bud development. DevBiol 290: 44–56. [DOI] [PubMed] [Google Scholar]
- 9.Sosa-Pineda B, Wigle JT, Oliver G (2000) Hepatocyte migration during liver development requires Prox1. Nature Genetics 25: 254–255. [DOI] [PubMed] [Google Scholar]
- 10.Wandzioch E, Zaret KS (2009) Dynamic signaling network for the specification of embryonic pancreas and liver progenitors. Science 324: 1707–1710. 10.1126/science.1174497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung J, Zheng M, Goldfarb M, Zaret KS (1999) Initiation of mammalian liver development from endoderm by fibroblast growth factors. Science 284: 1998–2003. [DOI] [PubMed] [Google Scholar]
- 12.Rossi JM, Dunn NR, Hogan BL, Zaret KS (2001) Distinct mesodermal signals, including BMPs from the septum transversum mesenchyme, are required in combination for hepatogenesis from the endoderm. Genes Dev 15: 1998–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ober EA, Verkade H, Field HA, Stainier DY (2006) Mesodermal Wnt2b signalling positively regulates liver specification. Nature 442: 688–691. [DOI] [PubMed] [Google Scholar]
- 14.Stafford D, Prince VE (2002) Retinoic acid signaling is required for a critical early step in zebrafish pancreatic development. Current Biology 12: 1215–1220. [DOI] [PubMed] [Google Scholar]
- 15.Chang C, Hu M, Zhu Z, Lo LJ, Chen J, et al. (2011) Liver-Enriched Gene 1a and 1B Encode Novel Secretory Proteins Essential for Normal Liver Development in Zebrafish. PloS one 6: e22910 10.1371/journal.pone.0022910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng W, Guo L, Zhang Z, Soo HM, Wen C, et al. (2006) HNF factors form a network to regulate liver-enriched genes in zebrafish. Developmental biology 294: 482–496. [DOI] [PubMed] [Google Scholar]
- 17.Enjapoori AK, Grant TR, Nicol SC, Lefevre CM, Nicholas KR, et al. (2014) Monotreme lactation protein is highly expressed in monotreme milk and provides antimicrobial protection. Genome Biol Evol 6: 2754–2773. 10.1093/gbe/evu209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin TY, Chou CF, Chung HY, Chiang CY, Li CH, et al. (2014) Hypoxia-Inducible Factor 2 Alpha Is Essential for Hepatic Outgrowth and Functions via the Regulation of leg1 Transcription in the Zebrafish Embryo. Plos One 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang P, Xiao A, Zhou M, Zhu Z, Lin S, et al. (2011) Heritable gene targeting in zebrafish using customized TALENs. Nat Biotechnol 29: 699–700. 10.1038/nbt.1939 [DOI] [PubMed] [Google Scholar]
- 20.Rossi A, Kontarakis Z, Gerri C, Nolte H, Holper S, et al. (2015) Genetic compensation induced by deleterious mutations but not gene knockdowns. Nature. [DOI] [PubMed] [Google Scholar]
- 21.Passeri MJ, Cinaroglu A, Gao C, Sadler KC (2009) Hepatic steatosis in response to acute alcohol exposure in zebrafish requires sterol regulatory element binding protein activation. Hepatology 49: 443–452. 10.1002/hep.22667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim BM, Rhee JS, Lee KW, Kim MJ, Shin KH, et al. (2015) UV-B radiation-induced oxidative stress and p38 signaling pathway involvement in the benthic copepod Tigriopus japonicus. Comp Biochem Physiol C Toxicol Pharmacol 167: 15–23. 10.1016/j.cbpc.2014.08.003 [DOI] [PubMed] [Google Scholar]
- 23.Walker SL, Ariga J, Mathias JR, Coothankandaswamy V, Xie X, et al. (2012) Automated reporter quantification in vivo: high-throughput screening method for reporter-based assays in zebrafish. PLoS One 7: e29916 10.1371/journal.pone.0029916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ellis JA, Cross AR, Jones OT (1989) Studies on the electron-transfer mechanism of the human neutrophil NADPH oxidase. Biochem J 262: 575–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stolk J, Hiltermann TJ, Dijkman JH, Verhoeven AJ (1994) Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am J Respir Cell Mol Biol 11: 95–102. [DOI] [PubMed] [Google Scholar]
- 26.Ober EA, Field HA, Stainier DYR (2003) From endoderm formation to liver and pancreas development in zebrafish. Mechanisms Of Development 120: 5–18. [DOI] [PubMed] [Google Scholar]
- 27.Chen J, Ng SM, Chang C, Zhang Z, Bourdon JC, et al. (2009) p53 isoform delta113p53 is a p53 target gene that antagonizes p53 apoptotic activity via BclxL activation in zebrafish. Genes Dev 23: 278–290. 10.1101/gad.1761609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niu X, Gao C, Jan LL, Luo Y, Meng C, et al. (2012) Sec13 safeguards the integrity of the endoplasmic reticulum and organogenesis of the digestive system in zebrafish. DevBiol 367: 197–207. [DOI] [PubMed] [Google Scholar]
- 29.Deutsch G, Jung J, Zheng M, Lora J, Zaret KS (2001) A bipotential precursor population for pancreas and liver within the embryonic endoderm. Development 128: 871–881. [DOI] [PubMed] [Google Scholar]
- 30.Wallace KN, Pack M (2003) Unique and conserved aspects of gut development in zebrafish. Dev Biol 255: 12–29. [DOI] [PubMed] [Google Scholar]
- 31.Huang H, Ruan H, Aw MY, Hussain A, Guo L, et al. (2008) Mypt1-mediated spatial positioning of Bmp2-producing cells is essential for liver organogenesis. Development 135: 3209–3218. 10.1242/dev.024406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shin D, Shin CH, Tucker J, Ober EA, Rentzsch F, et al. (2007) Bmp and Fgf signaling are essential for liver specification in zebrafish. Development 134: 2041–2050. [DOI] [PubMed] [Google Scholar]
- 33.Negishi T, Nagai Y, Asaoka Y, Ohno M, Namae M, et al. (2010) Retinoic acid signaling positively regulates liver specification by inducing wnt2bb gene expression in medaka. Hepatology 51: 1037–1045. 10.1002/hep.23387 [DOI] [PubMed] [Google Scholar]
- 34.Wilkinson RN, Pouget C, Gering M, Russell AJ, Davies SG, et al. (2009) Hedgehog and Bmp polarize hematopoietic stem cell emergence in the zebrafish dorsal aorta. Dev Cell 16: 909–916. 10.1016/j.devcel.2009.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rian H, Krens SFG, Spaink HP, Snaar-Jagalska BE (2013) Generation of Constitutive Active ERK Mutants as Tools for Cancer Research in Zebrafish. ISRN Cell Biology 2013: 1–11. [Google Scholar]
- 36.Poulain M, Furthauer M, Thisse B, Thisse C, Lepage T (2006) Zebrafish endoderm formation is regulated by combinatorial Nodal, FGF and BMP signalling. Development 133: 2189–2200. [DOI] [PubMed] [Google Scholar]
- 37.Loew R, Heinz N, Hampf M, Bujard H, Gossen M (2010) Improved Tet-responsive promoters with minimized background expression. BMC Biotechnol 10: 81 10.1186/1472-6750-10-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gossen M, Freundlieb S, Bender G, Muller G, Hillen W, et al. (1995) Transcriptional activation by tetracyclines in mammalian cells. Science 268: 1766–1769. [DOI] [PubMed] [Google Scholar]
- 39.Apweiler R, Hermjakob H, Sharon N (1999) On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochimica Et Biophysica Acta-General Subjects 1473: 4–8. [DOI] [PubMed] [Google Scholar]
- 40.Moremen KW, Tiemeyer M, Nairn AV (2012) Vertebrate protein glycosylation: diversity, synthesis and function. Nat Rev Mol Cell Biol 13: 448–462. 10.1038/nrm3383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gonzalez J, Takao T, Hori H, Besada V, Rodriguez R, et al. (1992) A Method for Determination Of N-Glycosylation Sites In Glycoproteins by Collision-Induced Dissociation Analysis In Fast-Atom-Bombardment Mass-Spectrometry—Identification Of the Positions Of Carbohydrate-Linked Asparagine In Recombinant Alpha-Amylase by Treatment with Peptide-N-Glycosidase-F In O-18-Labeled Water. Analytical Biochemistry 205: 151–158. [DOI] [PubMed] [Google Scholar]
- 42.Koutsioulis D, Landry D, Guthrie EP (2008) Novel endo-alpha-N-acetylgalactosaminidases with broader substrate specificity. Glycobiology 18: 799–805. 10.1093/glycob/cwn069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.R. Gupta EJaSB (2004) Prediction of N-glycosylation sites in human proteins.
- 44.Molinari M (2007) N-glycan structure dictates extension of protein folding or onset of disposal. Nat Chem Biol 3: 313–320. [DOI] [PubMed] [Google Scholar]
- 45.Haugg M, Schein CH (1992) The DNA sequences of the human and hamster secretory ribonucleases determined with the polymerase chain reaction (PCR). Nucleic Acids Res 20: 612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Linstedt AD, Hauri HP (1993) Giantin, a novel conserved Golgi membrane protein containing a cytoplasmic domain of at least 350 kDa. Mol Biol Cell 4: 679–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Touret N, Paroutis P, Terebiznik M, Harrison RE, Trombetta S, et al. (2005) Quantitative and dynamic assessment of the contribution of the ER to phagosome formation. Cell 123: 157–170. [DOI] [PubMed] [Google Scholar]
- 48.Lee Y, Grill S, Sanchez A, Murphy-Ryan M, Poss KD (2005) Fgf signaling instructs position-dependent growth rate during zebrafish fin regeneration. Development 132: 5173–5183. [DOI] [PubMed] [Google Scholar]
- 49.Rossi A, Kontarakis Z, Gerri C, Nolte H, Holper S, et al. (2015) Genetic compensation induced by deleterious mutations but not gene knockdowns. Nature 524: 230–233. 10.1038/nature14580 [DOI] [PubMed] [Google Scholar]
- 50.Gilbert SF (2012) Ecological developmental biology: environmental signals for normal animal development. Evol Dev 14: 20–28. 10.1111/j.1525-142X.2011.00519.x [DOI] [PubMed] [Google Scholar]
- 51.Goldfarb M (2001) Signaling by fibroblast growth factors: the inside story. Sci STKE 2001: pe37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miyazono K, Kamiya Y, Morikawa M (2010) Bone morphogenetic protein receptors and signal transduction. J Biochem 147: 35–51. 10.1093/jb/mvp148 [DOI] [PubMed] [Google Scholar]
- 53.Rao TP, Kuhl M (2010) An updated overview on Wnt signaling pathways: a prelude for more. Circ Res 106: 1798–1806. 10.1161/CIRCRESAHA.110.219840 [DOI] [PubMed] [Google Scholar]
- 54.Chang C, Hu M, Zhu Z, Lo LJ, Chen J, et al. (2011) liver-enriched gene 1a and 1b encode novel secretory proteins essential for normal liver development in zebrafish. PLoSOne 6: e22910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang SL, Aw SS, Chang C, Korzh S, Korzh V, et al. (2011) Depletion of Bhmt elevates sonic hedgehog transcript level and increases beta-cell number in zebrafish. Endocrinology 152: 4706–4717. 10.1210/en.2011-1306 [DOI] [PubMed] [Google Scholar]
- 56.Anichtchik O, Diekmann H, Fleming A, Roach A, Goldsmith P, et al. (2008) Loss of PINK1 function affects development and results in neurodegeneration in zebrafish. J Neurosci 28: 8199–8207. 10.1523/JNEUROSCI.0979-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Krzywinski M, Altman N (2013) Points of significance: error bars. Nat Methods 10: 921–922. 10.1038/nmeth.2659 [DOI] [PubMed] [Google Scholar]
- 58.Krzywinski M, Altman N (2014) Points of Significance: Visualizing samples with box plots. Nat Meth 11: 119–120. [DOI] [PubMed] [Google Scholar]
- 59.Ginestet C (2011) ggplot2: Elegant Graphics for Data Analysis. Journal Of the Royal Statistical Society Series a-Statistics In Society 174: 245–245. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(A) Representative images of embryos after WISH using the fabp10a probe corresponding to the result shown in Fig 1F. (B and C) Among the 32 cases shown in Fig 1G, the number of embryos exhibiting a small versus normal liver in 5 cases recorded in cold seasons (B) and 6 cases recorded in warm/hot seasons (C) were shown. (D) Plotting the liver sizes (majority normal or majority small) against daily atmospheric pressure in Hangzhou recorded during 30/12/2013 and 20/02/2015 (obtained from http://www.wunderground.com/).
(TIFF)
(A) Images showing an example of determining the liver size by WISH using the fabp10a probe. WT: wild type; mu: leg1azju1 mutant; mu+UV25: leg1azju1 mutant treated with UV25. (B) WT and maternal-zygotic leg1azju1(mu) embryos were treated with 1 mJ/cm2 (UV10), 2.5 mJ/cm2(UV25) and 5 mJ/cm2 (UV50) UV at 24 hpf and grew to 3.5 dpf for WISH analysis of liver development. (C) Comparison of liver sizes between the WT and maternal-zygotic leg1azju1 (mu) embryos growing in a high density condition (200 embryos per 10-cm diameter Petri dish). (D) Growing the maternal-zygotic leg1azju1 (mu) embryos in the egg water containing 0.5% or 1% ethanol did not cause a small liver phenotype. (E) Upon UV25 treatment the maternal-zygotic leg1azju2 embryos also exhibited a small liver phenotype at 3.5 dpf. *, p<0.05, **, p<0.01, ***, p<0.001, N.S., no significance.
(TIFF)
Images of TUNEL assay in the 54-hpf WT and maternal-zygotic leg1azju1 embryos (mu) after UV25 treatment at 24 hpf. No abnormal apoptotic activity was observed near the endodermal region including liver (lv) and intestine (in) in the maternal-zygotic leg1azju1 embryos (mu) compared to the WT. 12 sections from six embryos for each genotype were examined. nc, notochord, nt, neural tube.
(TIFF)
The 24-hpf WT embryos were treated with UV25 or 0.5 mM H2O2. Total leg1 or leg1b RNA level were measured by quantitative PCR (qPCR) using leg1a and leg1b common primers or leg1b specific primers, respectively. Error bar stands for the standard error. N.S., no significance.
(TIFF)
(A) Injection of constitutively active form of Erk (caErk) mRNA into one-cell stage embryos impaired liver development both in WT and leg1azju1 mutant when examined with the fabp10a probe at 3.5 dpf. (B) 200 pg Cy3 labeled oligo-dT(50) was injected into the yolk at 22 hpf, and the Cy3 signal was checked at 27 hpf. CK, oligo-dT(50) uninjected control. (C) Embryos were injected with caErk or fgf8 mRNA into the yolk at 22 hpf and were then treated with UV25 at 24 hpf. The liver development in the treated embryos at 3.5 dpf was examined with the fabp10a probe at 3.5 dpf.
(TIFF)
(A) Schematic drawing showing the structure of the TRE-caErk plasmid. RTTA expression was driven by the β-actin gene promoter. Dox binds to RTTA and the Dox-RTTA complex binds to the TRE promoter to drive the expression of caErk. (B) 10 pgTre-caErk plasmid DNA was injected into one-cell stage maternal-zygotic leg1azju1 embryos (mu). These embryos were treated with Dox at 6 hpf and total protein was harvested at 12 hpf. The protein samples were subjected to western blot analysis. The total Erk versus Tubulin ratios were shown on the right. Dox, doxycycline. Error bar stands for the standard error. ***, p<0.001. Western blot was repeated three times. (C) Images of representative 3.5-dpf embryos after WISH using the fabp10a probe. Embryos was first injected with Tre-caErk plasmid at one-cell stage, then treated with UV25 at 24 hpf and followed by Dox treatment for 6 hours (6 hpt) or 9 hours (9 hpt). After Dox treatment, embryos were transferred to the normal egg water to grow to 3.5 dpf for WISH (n = 20).
(TIFF)
(A) HepG2 cells were transfected with the leg1b, leg1bN70A, and the vector plasmid DNA. Total protein was extracted 30 hours post transfection and subjected to western analysis of Bip, Chop, phosphorylated eIF2α (p-eIF2α), and total eIF2α. These ER-stress response markers were not activated by the hypoglycosylated Leg1bN70A. Vector, the PCS2+ vector transfected cell. (B) Western blot analysis of Bip, Chop, phosphorylated eIF2α (p-eIF2α), and total eIF2α in the WT and maternal-zygotic leg1azju1 mutant embryos at 2 dpf and 3 dpf. (C) qPCR analysis of the transcript levels of ER-stress response markers including atf6, bip, perk, chop, ire1a, and grp94 in 3 dpf WT and maternal-zygotic leg1azju1 mutant embryos. Error bar stands for the standard error. Primers for analyzing these ER stress marker was as previously reported (S2 Table). Western blot was repeated three times each for A and B.
(TIFF)
Corresponding to Fig 7A and 7B. Western blot analysis of Leg1a or Leg1aN70A protein in 3 dpf old maternal-zygotic leg1azju1 mutant embryos injected with leg1a (mu+1a) orleg1aN70A(mu+1aN70A) mRNA at the one-cell stage. Protein samples from the WT and maternal-zygotic leg1azju1 mutant (mu) embryos were used as controls. Western blot was repeated three times.
(TIFF)
Tg(hsp70:dnfgfr1-gfp) embryos were heatshocked at 22 hpf to induce the expression of dominant negative FGFR1 (dn-Fgfr1). GFP signal was used to distinguish the dn-Fgfr-expressed (GFP+) and non-dn-Fgfr-expressed (GFP-) embryos. Embryos were treated with UV25 at 24 hpf, and total protein was extracted from embryos at 30 hpf (6 h post treatment) and was subjected to western analysis of the level of p-Erk. Tublin was used as a loading control. H.S., heatshock. *, p<0.05, **, p<0.01, N.S., no significance. Western blot was repeated two times.
(TIFF)
n = 25.
(TIFF)
One nanolitre of 0.5 mM standard control mopholino (ST-MO) was injected into one-cell stage embryos. The liver development was examined at 3.5 dpf using the fabp10a probe. N.S., no significance.
(TIFF)
Serial cryosections (S1 to S4) from three WT embryos (WT-1, WT-2 and WT-3) and three mutant embryos (mu-1, mu-2 and mu-3) treated with UV25 at 24 hpf were shown. DAPI was used to stain the nuclei.
(TIFF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.


