Abstract
Objectives
Cardiothoracic surgical leadership recently challenged the surgical community to achieve an operative mortality rate of 1.0% for the performance of isolated coronary artery bypass grafting (CABG). The possibility of achieving this goal remains unknown due to the increasing number of high-risk patients being referred for CABG. The purpose of our study was to identify a patient population in which this operative mortality goal is achievable relative to the estimated operative risk.
Methods
Patient records from a multi-institution (17 centers) Society of Thoracic Surgeons (STS) database for primary, isolated CABG operations (2001–2012) were analyzed. Multiple logistic regression modeling with spline functions for calculated STS predicted risk of mortality (PROM) was used to rigorously assess the relationship between estimated patient risk and operative mortality, adjusted for operative year and surgeon volume.
Results
A total of 34,416 patients (average patient age, 63.9 ± 10.7 years; 27% [n = 9190] women) incurred an operative mortality rate of 1.87%. Median STS predicted risk of mortality was 1.06% (interquartile range, 0.60% −2.13% ) and median surgeon CABG volume was 544 (interquartile range, 303–930) operations over the study period. After risk adjustment for the confounding influence of surgeon volume and operative year, the association between STS PROM and operative mortality was highly significant (P < .0001). More importantly, the adjusted spline function revealed that an STS PROM threshold value of 1.27% correlated with a 1.0% probability of death, accounting for 57.3% (n = 19,720) of the total study population. Further, the STS PROM demonstrated a limited predictive capacity for operative mortality for STS PROM > 25% as observed to expected mortality began to diverge.
Conclusions
Achieving the goal of 1.0% operative mortality for primary, isolated CABG is feasible in appropriately selected patients in the modern surgical era. However, this goal may be achieved in only 60% of CABG patients without other improvements in processes of care. Calculated STS PROM can be used to strongly identify patients with estimated mortality risk < 1.27% to achieve this goal, but it appears limited in its predictive capacity for those patients with estimated risk > 25.0%. These data provide a foundation for further study to determine if 1.0% mortality for CABG is achievable nationwide.
Surgical myocardial revascularization with coronary artery bypass grafting (CABG) remains 1 of the most common operations performed in the United States.1 Over the past few decades, the use of CABG for first-line treatment of coronary artery disease has declined as percutaneous coronary intervention (PCI) technology has advanced.2 Reduced mortality rates following performance of PCI over CABG as demonstrated in the SYNTAX trial and other series has become central to the argument by many proponents for PCI for coronary disease amenable to both percutaneous and surgical revascularization despite improved long-term outcomes favoring CABG.3 Current estimates of mortality following PCI have been reported at 1%, whereas those for the performance of isolated CABG are approximately 2%.1,3 As a result, the surgical community was recently challenged by leadership in the field of cardiothoracic surgery to achieve a 1% mortality rate or less for the performance of isolated CABG operations within the next 3 to 5 years.4 Although ambitious, achieving this goal would not only significantly influence the debate regarding choice for PCI versus CABG but would also provide for higher-quality care for hundreds of thousands of US patients annually.
The Society for Thoracic Surgeons (STS) maintains a nationwide database of adult cardiac surgeries performed in the United States. Representing the largest clinical database of its kind, the STS National Cardiac Database provides clinicians and researchers the ability to assess risk-adjusted outcomes for several different cardiac operations, including isolated CABG. Furthermore, the STS has developed various risk models for cardiac operations that allow for the prediction of an expected outcome for a patient based on a given set of risk factors.5,6 Perhaps the most commonly used STS risk model is that which estimates the predicted risk of mortality (PROM) for individual patients. Adjusting for the prevalence of 30 different demographic, clinical, and operative present-on-admission factors, the STS PROM can be calculated for an individual patient to determine that patient’s expected mortality risk.7 The use of the STS PROM has been validated and widely accepted by the US cardiothoracic surgery community as a reliable preoperative metric to evaluate patient risk.5,8,9 Thus, STS PROM scores should be able to identify patients with expected mortality rates ≤1%.
The Virginia Cardiac Surgery Quality Initiative (VCSQI) is a voluntary group of 17 different cardiac surgery centers, both academic and private, within the Commonwealth of Virginia. This group holds quarterly meetings to exchange and compare de-identified patient information in an effort to improve cardiac surgical care, quality, and costs. The primary objective of the organization is to identify quality improvement opportunities in cases where high cost, resource intensive, or frequently occurring preventable outcomes might occur. Collectively, the VCSQI centers perform approximately 99% of the cardiac surgeries performed in the Commonwealth, and each center individually contributes patient data to the STS National Cardiac Database.
The purpose of our study was to determine if the challenge to achieve a ≤1% operative mortality rate for primary, isolated CABG operations is feasible in the modern surgical area, to identify in which patient populations this mortality goal is achievable relative to estimated operative risk, and to identify patient- and operation-related risk factors that contribute most to mortality among patients where goal mortality was deemed not achievable to discern if certain patients should not be receiving surgical myocardial revascularization.
METHODS
This investigation was exempt from formal institutional review board review at each participating center because it represents a secondary analysis of the VCSQI data registry with the absence of Health Insurance Portability and Accountability Act patient identifiers and because the data is collected for quality analysis and purposes other than research.
Patients and Data Acquisition
De-identified patient-level data were obtained from the VCSQI database for the study period January 1, 2001, through June 30, 2012. All records included patients undergoing primary, isolated CABG operations (STS procedure type “CAB Only” and incidence type “First Cardiovascular Surgery”). All CABG procedures represent standard surgical approaches to surgical myocardial revascularization with and without the use of cardiopulmonary bypass support. Patient preoperative risk was assessed by prevalence of patient comorbid disease, extent of coronary artery disease, operative status, and individually calculated STS PROM score.
Measured Outcomes
The primary outcomes of interest was the risk-adjusted association between the probability of death (operative mortality) and STS PROM score to identify in which patients a predicted probability of death < 1% could be achieved. Secondary outcomes included estimated risk-adjusted associations between CABG mortality and established patient risk factors used to calculate the STS PROM score as well as several process of care measures endorsed by the STS and National Quality Forum. Operative mortality was defined as all patient deaths occurring during hospitalization as well those within 30 days of the date of surgery despite discharge status. Standard STS clinical definitions for all analyzed variables were used.10
Statistical Analysis
Descriptive statistics
All statistical analyses were designed to test the null hypothesis that operative mortality following primary, isolated CABG would not be significantly associated with calculated STS PROM score. Study outcomes and data comparisons were established a priori before data collection. Categorical variables are expressed as standard group percentages, whereas continuous variables are expressed as either mean ± standard deviation or median (25th, 75th percentile) depending on overall variable distribution. Descriptive, univariate statistics included either Pearson χ2 or Fisher exact test for categorical variables and either independent sample single factor analysis of variance for comparisons of normally distributed data or the Wilcoxon rank sum test for nonnormally distributed data comparisons. Calculated test statistics were used to derive all 2-tailed P values with standard statistical significance set to α < 0.05.
Estimated mortality risk and surgeon volume measurement
STS PROM was analyzed as a continuous function, using restricted cubic spline (RCS) smoothing transformations to account for both linear and nonlinear associations with operative mortality. RCS functions are beneficial because they use all data points to estimate the shape of the relationship between an exposure (STS PROM) and an outcome (operative mortality). The use of RCS transformations, therefore, provides a more robust method to determine if nonlinear relationships exist between a continuous variable and a dependent outcome. Use of RCS forces the tails of a function to be linear, which simplifies the representation. In these analyses, an RCS function was developed for both STS PROM and individual surgeon volume using a total of three knots placed at the 5th, 50th, and 95th percentiles of the distribution of both STS PROM and surgeon volume to define the tails of each function.
Hierarchical regression models
Hierarchical multiple regression models were used to estimate confounder-adjusted associations between calculated STS PROM score and the probability of operative mortality for patients undergoing primary, isolated CABG operations. In addition, similar models were used to estimate the relative predictive capacity of established patient- and operation-level risk factors on the probability of death among 2 different study cohorts based on an identified threshold value for STS PROM at which the probability of death is < 1% to identify which factors appear to influence mortality the greatest in those where goal mortality is deemed achievable.
Hierarchical multiple regression models were used to account for potentially overdispersed variance estimates associated with correlated events within hospitals. Use of such multilevel models has been shown to avoid overestimation of the statistical significance of association between modeled covariates, such as STS PROM or surgeon volume, compared with the use of conventional statistical modeling technique. Clustering at the hospital level was considered in the hierarchical structure of each logistic regression model to account for the potential confounding influence of differences in individual hospitals (eg, differences in hospital volume). Model covariates were selected a priori and were considered potential confounders for the influence of STS PROM on patient death. Performed models for the association between estimated patient risk and the dependent outcome of operative mortality were adjusted for the potential confounding influence of individual surgeon experience on patient outcomes by inclusion of surgeon operative volume during the study period as well as the influence of baseline changes in practice over the study period by inclusion of operative year. Confounder-adjusted measures of association are reported with a 95% confidence interval for all regression models.
Model parameter significance
The statistical significance of the association between STS PROM and operative mortality was measured using 2 different tests. The overall statistical significance of the relationship between STS PROM and mortality was measured using the nested model log-likelihood test, which compares the total log-likelihood obtained by the model to that obtained by an otherwise identical model, excluding the STS PROM measure. The difference in model log-likelihood yields a test statistic with a χ2 distribution with degrees of freedom equal to the number of parameters associated with the volume measure. The statistical significance of each STS PROM measure term in the models and terms for all other model factors for each of the fixed effects included in the models was assessed using the factors model effect size or likelihood ratio (Wald statistic). The likelihood ratio obtained for each modeled factor was used to determine the relative predictive strength of each factor on mortality risk. Relative differences in the overall contribution of each factor to the prediction of mortality in each study cohort was assessed by plotting the likelihood ratios.
Model performance
Model performance was assessed using the C statistic and the Nagelkerke Pseudo R2 statistic. The C statistic is equivalent to the area under the receiver operating characteristic curve for models with a dichotomous response variable, and it provides an estimate of the model’s ability to discriminate between observed instances of death and survival. A value of 0.5 indicates that the model provides predictive discrimination equal to chance, whereas a value of 1.0 indicates perfect discrimination between dependent outcomes. The Nagelkerke Pseudo R2 statistic is a log-likelihood ratio χ2 based measure that is analogous to the R2 statistic in ordinary multiple regression. The Nagelkerke Pseudo R2 statistic theoretically changes from 0 for models that provide no predictive information to 1 for models that predict perfectly. Model calibration was assessed using the Hosmer-Lemeshow χ2 test statistic. All statistical analyses were conducted using R statistical software, version 2.12.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Patient Characteristics and Operative Features for Primary, Isolated CABG
Table 1 displays descriptive statistics and frequencies for select patient risk factors used to derive the STS PROM score for isolated CABG operations. For the entire patient cohort, operative mortality following primary, isolated CABG operations was 1.9% and overall median STS PROM was 1.1%. Overall median individual surgeon volume over the entire study period was 544 operations, with the highest volume surgeons (95th percentile) performing a median of 1434 isolated CABG operations. Similar distributions in overall cardiac surgical volumes were apparent for individual surgeons, suggesting that isolated CABG volumes correlated with overall surgeon volume during this study period. Average patient age was 63.9 years, and the large majority of patients (73% ) were men. Diabetes was the most prevalent comorbid disease, occurring in 39% of patients. With respect to preoperative cardiac status, a majority of patients presented with New York Heart Association functional class II or III, underwent myocardial revascularization for 2-vessel coronary artery disease, and underwent operations in either an elective or urgent setting.
TABLE 1.
Frequency of select patient risk factors used to estimate Society of Thoracic Surgeons predicted risk of mortality (STS PROM)
| Variable | Result | % |
|---|---|---|
| Operative mortality | 666 | 1.9 |
| Median STS PROM, % | 1.1 (0.6, 2.1) | |
| Surgeon volume | 544 (303, 930) | |
| Age, y | 63.9 ± 12.2 | |
| Gender | ||
| Male | 25,226 | 73.3 |
| Female | 9190 | 26.7 |
| Weight, kg | 86 (75, 99) | |
| Height, cm | 173 (165, 180) | |
| Diabetes | 13,540 | 39.3 |
| Dialysis | 831 | 2.4 |
| Chronic lung disease | ||
| Mild | 3416 | 9.9 |
| Moderate | 1788 | 5.2 |
| Severe | 863 | 2.5 |
| Immunosuppression | 685 | 2.0 |
| Peripheral vascular disease | 4934 | 14.3 |
| Cerebrovascular disease | 4616 | 13.4 |
| Cerebrovascular accident | 1920 | 5.6 |
| Myocardial infarction | 7576 | 22.0 |
| Heart failure | 4071 | 11.8 |
| New York Heart Association functional class | ||
| I | 1622 | 4.7 |
| II | 5961 | 17.3 |
| III | 9665 | 28.1 |
| IV | 3873 | 11.3 |
| Atrial fibrillation | 1385 | 4.0 |
| Number of diseased vessels | ||
| One | 1407 | 4.1 |
| Two | 26,782 | 77.8 |
| Three | 6155 | 17.9 |
| Ejection fraction, % | 55 (45, 60) | |
| Status | ||
| Elective | 13,921 | 40.4 |
| Urgent | 19,288 | 56.0 |
| Emergent | 1207 | 3.5 |
Values are given as n, median (25th, 75th percentile), mean ± standard deviation. STS PROM, Society of Thoracic Surgeons predicted risk of mortality.
Adjusted Relationship Between Probability of Death and Estimated Mortality Risk
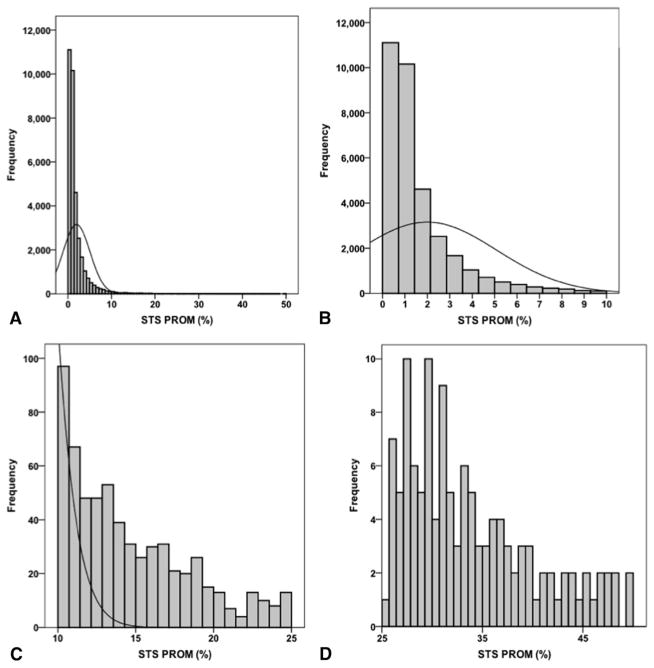
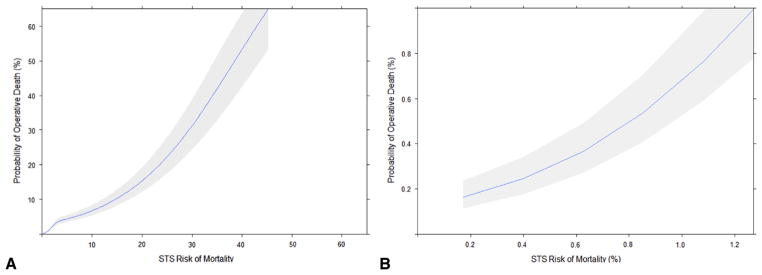
To identify a potential patient population in whom a 1% CABG mortality rate may be achievable, the relationship between observed mortality and estimated mortality risk using the STS PROM score was explored. Figure 1 displays the overall distribution of STS PROM scores for the entire patient population (Figure 1, A), those with scores ranging from 0% to 10% (Figure 1, B), those with scores ranging from 10% to 25% (Figure 1, C), and those with scores > 25% (Figure 1, D). As expected, STS PROM scores were nonnormally distributed with a prominent right ward skew in the distribution of scores. Considering these findings, operative mortality was modeled as a function of STS PROM with RCS transformations as both linear and nonlinear terms, adjusted for the confounding influence of individual surgeon volume (with RCS transformations) and operative year (Table 2). As a result, the association between STS PROM and operative mortality was highly significant (P < .0001) (Figure 2, A). More importantly, the adjusted spline function revealed that an STS PROM threshold value of 1.27% correlated with a 1.0% probability of death (Figure 2, B), which accounted for 57% (n = 19,720) of the total study population. Further examination of this relationship revealed that the STS PROM demonstrated a limited predictive capacity for operative mortality for STS PROM > 25% because the relationship between expected mortality and the probability of death began to diverge with widening confidence intervals.
FIGURE 1.
Histograms displaying the distribution of Society of Thoracic Surgeons predicted risk of mortality (STS PROM). A, Overall STS PROM distribution. B, STS PROM 0% to 10%. C, STS PROM 10% to 25%. D, STS PROM 25% to 50%.
TABLE 2.
Multivariable logistic regression results for operative mortality modeled as a function of Society of Thoracic Surgeons predicted risk of mortality (STS PROM) (with restricted cubic splines), surgeon operative volume (with restricted cubic splines), and operative year.* Ranked by covariate likelihood ratio (Wald χ2 test statistic)
| Covariate | Likelihood ratio (Wald χ2) | Odds ratio (95% confidence interval) | P value |
|---|---|---|---|
| STS PROM (linear) | 846.7 | 1.89 (1.68–2.09) | <.0001 |
| STS PROM (nonlinear) | 251.5 | 6.59 (5.35–8.12) | <.0001 |
| Operative year (reference = 2005) | 46.3 | <.0001 | |
| 2001 | 0.38 (0.15–0.96) | ||
| 2002 | 0.92 (0.55–1.53) | ||
| 2003 | 1.15 (0.72–1.83) | ||
| 2004 | 0.72 (0.49–1.07) | ||
| 2006 | 0.92 (0.67–1.28) | ||
| 2007 | 0.90 (0.65–1.24) | ||
| 2008 | 1.43 (1.04–1.97) | ||
| 2009 | 1.79 (1.32–2.43) | ||
| 2010 | 1.46 (1.06–2.01) | ||
| 2011 | 1.27 (0.90–1.78) | ||
| 2012 | 1.34 (0.75–2.39) | ||
| Surgeon operative volume (linear) | 16.3 | 0.31 (0.47–0.15) | .0003 |
| Surgeon operative volume (nonlinear) | 0.8 | 0.73 (0.62–0.86) | .36 |
STS PROM, Society of Thoracic Surgeons predicted risk of mortality.
Model performance characteristics: C statistic = 0.81, Nagelkerke Pseduo R2 = 0.16.
FIGURE 2.
Plot of adjusted association between operative mortality and (A) overall restricted cubic spline function for Society of Thoracic Surgeons (STS) predicted risk of mortality and (B) restricted cubic spline function for STS predicted risk of mortality (0%–1.27% ).
The statistical performance of this regression model achieved adequate discrimination with a C statistic = 0.81. The model explained 16% of the variance in operative mortality as reflected by a Nagelkerke Pseudo R2 value of 0.16. Model calibration was adequate across deciles of observed risk as reflected by Hosmer-Lemeshow P < .05.
Comparison of Patient Risk Profiles With Respect to STS PROM Threshold
After empirically testing the adjusted relationship between operative mortality and STS PROM, a threshold value of STS PROM ≤ 1.27% correlated with a desired goal of ≤1% operative morality. Thus, to further characterize patient risk profiles for those projected to achieve goal mortality compared with those who were not, the frequency of patient and operative risk factors were stratified into STS PROM categories: STS PROM ≤ 1.27% and STS PROM > 1.27% (Table 3). Median STS PROM scores for both cohorts were 0.7% and 2.4%, respectively. Not surprisingly, patients with STS PROM > 1.27% were older with a higher burden of comorbid disease, reduced cardiac function, an increased burden of coronary artery disease, and more frequent nonelective operations. Mortality was significantly higher in those with STS PROM > 1.27% (3.7% vs 0.6% ; P < .001).
TABLE 3.
Comparison of select patient risk factors and processes of care by Society of Thoracic Surgeons predicted risk of mortality (STS PROM) threshold
| Variable | STS PROM ≤ 1.27% | STS PROM > 1.27% | P value |
|---|---|---|---|
| Median STS PROM, % | 0.7 (0.5, 0.9) | 2.4 (1.7, 4.0) | <.001 |
| Age, y | 59.0 ± 8.7 | 70.5 ± 9.4 | <.001 |
| Gender | <.001 | ||
| Male | 82.7 | 60.6 | |
| Female | 17.3 | 39.4 | |
| Weight, kg | 90 (79, 102) | 81 (70, 93) | <.001 |
| Height, cm | 175 (168, 180) | 170 (163, 178) | <.001 |
| Diabetes | 34.9 | 45.4 | <.001 |
| Dialysis | 0.1 | 5.5 | <.001 |
| Chronic lung disease | <.001 | ||
| Mild | 7.6 | 13.1 | |
| Moderate | 2.9 | 8.2 | |
| Severe | 0.8 | 4.8 | |
| Immunosuppression | 0.8 | 3.6 | <.001 |
| Peripheral vascular disease | 7.0 | 24.2 | <.001 |
| Cerebrovascular disease | 6.7 | 22.5 | <.001 |
| Cerebrovascular accident | 2.3 | 10.0 | <.001 |
| Myocardial infarction | 15.4 | 30.8 | <.001 |
| Heart failure | 4.6 | 21.6 | <.001 |
| New York Heart Association functional class | <.001 | ||
| I | 8.8 | 6.6 | |
| II | 34.5 | 21.9 | |
| III | 46.3 | 45.2 | |
| IV | 10.4 | 26.3 | |
| Atrial fibrillation | 1.8 | 7.1 | <.001 |
| Number of diseased vessels | <.001 | ||
| One | 5.3 | 2.5 | |
| Two | 20.3 | 14.6 | |
| Three | 74.1 | 82.8 | |
| Ejection fraction, % | 55 (50, 60) | 50 (40, 60) | <.001 |
| Status | <.001 | ||
| Elective | 46.5 | 32.3 | |
| Urgent | 53.1 | 60.0 | |
| Emergent | 0.4 | 7.7 | |
| Median surgeon volume | 530 (293, 848) | 582 (317, 930) | <.001 |
| Operative mortality | 0.6 | 3.7 | <.001 |
| Beta-blocker (preoperative) | 84.0 | 81.0 | <.001 |
| Beta-blocker (discharge) | 91.7 | 83.2 | <.001 |
| Antiplatelet (discharge) | 96.5 | 91.6 | <.001 |
| Lipid-lowering medication (discharge) | 92.1 | 82.1 | <.001 |
Values are given as median (25th, 75th percentile), mean ± standard deviation, or %. STS PROM, Society of Thoracic Surgeons predicted risk of mortality.
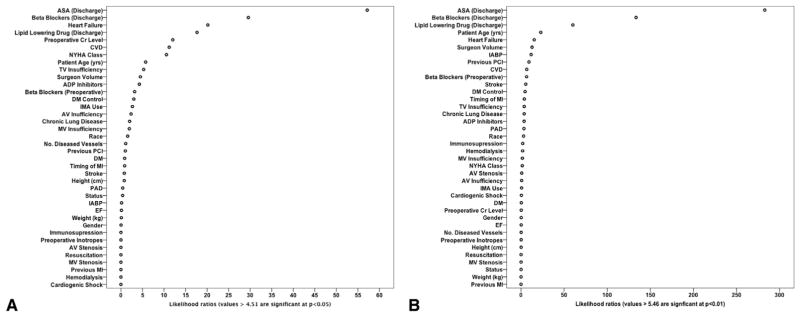
Next, the adjusted relationship between operative morality and patient risk factors and established processes of care was modeled for each STS PROM patient cohort to identify potential patient- and operation-related factors to explain differences in mortality risk. Table 4 displays the relative strength of association between modeled factors and mortality as determined by comparing the likelihood ratios (Wald χ2). Figure 3 displays dot plots depicting the relative predictive capacity of each modeled factor for the outcome of operative mortality among STS PROM study cohorts. Among both cohorts process of care measures such as perioperative medication use and cardiac protective medication at discharge were among factors with the strongest association with mortality risk. Similarly, potentially modifiable factors such as heart failure, preoperative renal function, and cerebrovascular disease had significant associations with mortality. Not surprisingly, in the cohort of patients with STS PROM > 1.27%, intra-aortic balloon pump use and previous PCI demonstrated significant relationships with mortality, whereas these factors were not associated with mortality in patients with STS PROM < 1.27% Model performance within each study cohort was strong with respect to discrimination (C statistic = 0.91 and 0.94), explanation of variance (Nagelkerke Pseudo R2 = 0.16), and calibration (Hosmer-Lemeshow P < .05).
TABLE 4.
Comparison of modeled factors for operative mortality ranked by factor likelihood ratio
| Model 1: STS PROM ≤ 1.27%
|
Model 2: STS PROM > 1.27%
|
||||
|---|---|---|---|---|---|
| Modeled factor | Likelihood ratio | P value | Modeled factor | Likelihood ratio | P value |
| ASA (discharge) | 57.162 | .000 | ASA (discharge) | 282.740 | .000 |
| Beta-blockers (discharge) | 29.577 | .000 | Beta-blockers (discharge) | 133.420 | .000 |
| Heart failure | 20.166 | .000 | Lipid-lowering drug (discharge) | 60.160 | .000 |
| Lipid-lowering drug (discharge) | 17.655 | .000 | Patient age, y | 22.877 | .000 |
| Preoperative creatinine level | 12.034 | .001 | Heart failure | 15.296 | .000 |
| Cardiovascular disease | 11.206 | .001 | Surgeon volume | 12.792 | .000 |
| New York Heart Association functional class | 10.556 | .014 | Intra-aortic balloon pump | 11.652 | .001 |
| Patient age, y | 5.729 | .017 | Previous percutaneous coronary intervention | 9.197 | .002 |
| TV insufficiency | 5.272 | .261 | Cardiovascular disease | 6.626 | .010 |
| Surgeon volume | 4.512 | .034 | Beta-blockers (preoperative) | 6.442 | .011 |
| ADP inhibitors | 4.270 | .039 | Stroke | 5.456 | .019 |
| Beta-blockers (preoperative) | 3.177 | .075 | Diabetes mellitus control | 4.647 | .200 |
| Diabetes mellitus control | 2.986 | .394 | Timing of myocardial infarction | 3.826 | .575 |
| IMA use | 2.657 | .448 | TV insufficiency | 3.722 | .445 |
| AV insufficiency | 2.343 | .673 | Chronic lung disease | 3.679 | .298 |
| Chronic lung disease | 2.019 | .568 | ADP inhibitors | 3.578 | .059 |
| MV insufficiency | 1.941 | .747 | Peripheral artery disease | 3.355 | .067 |
| Race | 1.555 | .907 | Race | 2.931 | .711 |
| Number of diseased vessels | 1.104 | .576 | Immunosuppression | 1.946 | .163 |
| Previous percutaneous coronary intervention | 1.013 | .314 | Hemodialysis | 1.817 | .178 |
| Diabetes mellitus | .857 | .355 | MV insufficiency | 1.539 | .820 |
| Timing of myocardial infarction | .848 | .974 | New York Heart Association functional class | 1.424 | .700 |
| Stroke | .779 | .378 | AV stenosis | .827 | .363 |
| Height, cm | .767 | .381 | AV insufficiency | .747 | .945 |
| Peripheral artery disease | .411 | .521 | IMA use | .668 | .881 |
| Status | .406 | .816 | Cardiogenic shock | .406 | .524 |
| Intra-aortic balloon pump | .157 | .692 | Diabetes mellitus | .356 | .551 |
| Ejection fraction | .118 | .731 | Preoperative creatinine level | .243 | .622 |
| Weight, kg | .096 | .756 | Gender | .195 | .658 |
| Gender | .025 | .874 | Ejection fraction | .178 | .673 |
| Immunosuppression | .000 | .997 | Number of diseased vessels | .161 | .923 |
| Preoperative inotropes | .000 | .997 | Preoperative inotropes | .147 | .701 |
| AV stenosis | .000 | .998 | Height, cm | .126 | .723 |
| Resuscitation | .000 | .998 | Resuscitation | .054 | .816 |
| MV stenosis | .000 | .999 | MV stenosis | .039 | .843 |
| Previous myocardial infarction | .000 | .999 | Status | .037 | .982 |
| Hemodialysis | .000 | .999 | Weight, kg | .003 | .958 |
| Cardiogenic shock | .000 | 1.000 | Previous myocardial infarction | .000 | .999 |
STS PROM, Society of Thoracic Surgeons predicted risk of mortality; ASA, American Society of Anesthesiologists; TV, tricuspid valve; ADP, adenosine diphosphate; IMA, internal mammary artery; AV, aortic valve; MV, mitral valve.
FIGURE 3.
Dot plots of likelihood ratios (Wald statistics) for each modeled factor as it relates to the probability of death for patients with (A) Society of Thoracic Surgeons predicted risk of mortality ≤1.27% and (B) Society of Thoracic Surgeons predicted risk of mortality > 1.27%. ASA, American Society of Anesthesiologists; CVD, cardiovascular disease; NYHA, New York Heart Association; TV, tricuspid valve; ADP, adenosine diphosphate; DM, diabetes mellitus; IMA, internal mammary artery; AV, aortic valve; MV, mitral valve; PCI, percutaneous coronary intervention; MI, myocardial infarction; PAD, peripheral arterial disease; IABP, intra-aortic balloon pump; EF, ejection fraction.
Comparison of Patient Risk Profiles and Factors Between Survivors and Decedents in Patients Above the STS PROM Threshold
Patient risk profiles were compared between survivors and decedents in the cohort of patients with STS PROM scores above the 1.27% threshold to aid in clinical decision making for those where 1% observed mortality is not projected to be achievable (Table 5). In this cohort of patients, decedents expectedly presented with higher preoperative risk and significantly higher median STS PROM scores, were on average older, were likely to be women, and had much higher rates of hemodialysis, peripheral vascular disease, heart failure, advanced New York Heart Association functional class, and emergent operations. Median individual surgeon volume was significantly lower for decedents compared with survivors. Significant differences in the provision of cardiovascular medications at discharge were dramatic between overall survivors and decedents after discharge. Interestingly, no significant differences were observed with respect to preoperative beta-blocker use.
TABLE 5.
Comparison of select patient risk factors and processes of care between survivors and decedents for patients with Society of Thoracic Surgeons predicted risk of mortality (STS PROM) > 1.27%
| Variable | Survivors (n = 14,138) | Decedents (n = 549) | P value |
|---|---|---|---|
| STS PROM, % | 2.4 (1.7, 3.9) | 4.6 (2.7, 9.2) | <.001 |
| Age, y | 70.4 ± 9.5 | 72.3 ± 9.4 | <.001 |
| Gender | .08 | ||
| Male | 60.8 | 57.0 | |
| Female | 39.2 | 43.0 | |
| Weight, kg | 81 (70, 92) | 80 (67, 92) | .05 |
| Height, cm | 170 (163, 178) | 168 (160, 178) | .02 |
| Diabetes | 45.4 | 45.5 | .93 |
| Dialysis | 5.3 | 11.3 | <.001 |
| Chronic lung disease | <.001 | ||
| Mild | 13.1 | 12.0 | |
| Moderate | 8.2 | 9.5 | |
| Severe | 4.6 | 9.8 | |
| Immunosuppression | 3.5 | 5.8 | .005 |
| Peripheral vascular disease | 23.9 | 32.1 | <.001 |
| Cerebrovascular disease | 22.4 | 26.2 | .04 |
| Cerebrovascular accident | 9.9 | 10.2 | .83 |
| Myocardial infarction | 30.9 | 29.7 | .57 |
| Heart failure | 21.0 | 37.3 | <.001 |
| New York Heart Association functional class | <.001 | ||
| I | 6.7 | 3.3 | |
| II | 22.1 | 16.5 | |
| III | 45.4 | 38.6 | |
| IV | 25.7 | 41.6 | |
| Atrial fibrillation | 6.9 | 11.1 | <.001 |
| Number of diseased vessels | .68 | ||
| One | 2.5 | 2.4 | |
| Two | 14.5 | 16.6 | |
| Three | 82.9 | 80.9 | |
| Ejection fraction, % | 50 (40, 60) | 45 (30, 55) | <.001 |
| Status | <.001 | ||
| Elective | 32.7 | 22.2 | |
| Urgent | 60.0 | 60.8 | |
| Emergent | 7.3 | 16.9 | |
| Surgeon volume | 582 (319, 930) | 464 (266, 739) | <.001 |
| Beta-blocker (preoperative) | 81.8 | 77.7 | .06 |
| Beta-blocker (discharge) | 85.8 | 19.7 | <.001 |
| Antiplatelet (discharge) | 94.5 | 21.1 | <.001 |
| Lipid lowering medication (discharge) | 84.7 | 19.5 | <.001 |
Values are given as median (25th, 75th percentile), mean ± standard deviation, or %. STS PROM, Society of Thoracic Surgeons predicted risk of mortality.
DISCUSSION
Our study reports on current trends in primary, isolated CABG mortality in a large, multi-institutional statewide cohort of patients with a focus of determining whether or not the challenge to achieve an observed mortality rate of ≤1% is feasible in the modern surgical era and to identify in which patients this goal may be achieved. To investigate this question, the widely accepted and validated STS PROM score was used to demonstrate a threshold value at which expected mortality risk and adjusted probability of death correlate. Patient risk profiles, operative features, and perioperative processes of care were then examined as a function of this threshold to further identify patient populations where a 1% goal mortality rate may not be achievable. Overall, these novel findings further clarify the feasibility of the charge to the cardiothoracic surgery community to reduce CABG and to demonstrate that these goals may be achievable in select patient populations. Further, these results highlight several important implications related to CABG quality assessment and reporting as well as surgical performance standards and process of care.
The principle purpose of our study was to determine if the challenge to achieve a ≤1% operative mortality rate for primary, isolated CABG is feasible in the modern surgical era. A reduction in CABG mortality over the past several years has been reported by the STS in several nationwide series.1,2,11 In a recent report of national trends in STS CABG outcomes among 1.49 million patients from 2000 to 2009, predicted mortality rates of 2.3% were consistent over the study period, whereas observed mortality rates declined from 2.4% to 1.9%, representing a 24% reduction in relative risk for the performance of CABG over a 10-year period.1 Considering these trends and that the STS PROM score has been developed and widely accepted as a method of determining surgical risk, our report empirically tested the correlation of STS PROM with adjusted probability of death, taking into account the confounding influence of individual surgeon volume and hospital as well generalized changes in practice pattern over operative years. Our results demonstrate a highly significant association between STS PROM and adjusted probability of death. More importantly, using RCS transformations of the STS PROM function, we demonstrated an important threshold value of STS PROM (PROM = 1.27% ) where the adjusted probability of death following CABG could be expected to be ≤1%. Considering this threshold, the proportion of patients represented in this statewide, multi-institutional patient cohort with STS PROM ≤ 1.27% accounted for 57% of the total study population and demonstrated an observed operative mortality rate of 0.6%. These findings may be extrapolated to suggest that the challenge to achieve ≤1% operative mortality for primary, isolated CABG may be feasible in less than two-thirds of patients requiring surgical myocardial revascularization.
The second purpose of our study was to identify in which patient populations a ≤1% operative mortality goal is achievable relative to the estimated operative risk. To answer this question, the established threshold value for STS PROM was used to stratify the study cohort to compare patient risk profiles, operative features, and the role of established perioperative processes of care. Next, all 30 components of the STS PROM score as well as STS and National Quality Forum-approved measures of performance were modeled to determine their relative importance in the prediction of CABG-related mortality. The reported findings demonstrate that those patients in whom goal mortality can be achieved are on average significantly younger, more commonly men, present with a reduced burden of comorbid disease and with superior cardiac function, and undergo significantly fewer emergent operations. Perhaps more significant than these findings were the demonstrated differences in the relative strength of the association among modeled patient risk factors and adjusted mortality risk within each study cohort. Unique in our analyses was the demonstration that within the patient cohort in which goal mortality was deemed not achievable (ie, STS PROM > 1.27% ) the relative strength of association between certain risk factors and mortality was much higher than in the cohort in which goal mortality was deemed achievable (ie, STS PROM ≤ 1.27% ). Thus, although in both cohorts of patients (STS PROM ≤ 1.27% and > 1.27% ) process of care measures, including the provision of aspirin and beta-blockers at the time of discharge, were the strongest predictors of mortality, the modeled effect sizes or likelihood ratios within the STS PROM > 1.27% cohort (Wald statistic =282 and 133, respectively) were significantly higher than those in the STS PROM ≤ 1.27% cohort (Wald statistic =57 and 29, respectively). These results imply that there exists significant potential for the improvement of CABG mortality through improved efforts to modify practice patterns and optimize perioperative cardiac surgical care.
The third purpose of our study was to identify the patient-and operation-related risk factors that contribute most to mortality among patients where goal mortality was deemed not achievable. This was done to discern if certain patients should not be receiving surgical myocardial revascularization. The results of these analyses reveal striking differences in patient risk profiles for survivors and decedents in this cohort of patients. Among this seemingly high-risk patient population, the most striking differences between survivors and decedents occurred as a function of estimated mortality risk; individual surgeon operative volume; and in the prevalence of preoperative hemodialysis requirements, preoperative heart failure, and performance of emergent versus nonemergent operations. Specifically, this result suggests that patients and surgeons should be particularly aware of the increased risk of performing isolated CABG operations in patients with STS PROM > 4.6%, patients with preoperative hemodialysis requirements, and patients with poor preoperative cardiac function. More importantly, these findings support the argument that surgeons performing CABG operations for these select patients should not be penalized for disproportionately high mortality rates compared with patients with lower risk profiles.
Critical to the reported results in our study are the methods used to analyze the relationship between the STS PROM score and adjusted probability of death as well as the results of these analyses. In our analyses the choice to model mortality as function of STS PROM was a result of several factors. STS PROM has become a validated and widely accepted tool used by surgeons to identify and predict mortality risk for individual patients. As a result, we wanted to not only quantify the number of patients in Virginia achieving a 1% mortality, but also to verify and demonstrate the reliability of using STS PROM in the future to help predict the potential to achieve this goal. The use of RCS transformations of the STS PROM variable is the most accurate and robust approach to modeling this continuous function because it characterizes the linear and nonlinear components that may exist between the relationship between STS PROM and operative mortality.12,13 A common approach used in the cardiac surgery literature is the seemingly arbitrary categorization of STS PROM into discrete categories (ie, quartiles or quintiles).9,14,15 This approach presents an inherent methodologic problem as the conversion of continuous data, such as STS PROM, into categorical data results in a crucial loss of information.12 The segmentation of continuous data is problematic because it discards information in the original variable and requires arbitrarily selected cut points for defining categories and deriving conclusions,16 ultimately resulting in a loss of explanatory power as the modeled effect of that variable is flat across the range of values included in that category. Thus, if one categorizes STS PROM for CABG into a category that includes scores ranging from 0% to 1.5%, the resultant measure of association will be interpreted as the measure for the entire range of values included in that category. Thus, a distinction in mortality for STS PROM of 0.5% compared with STS PROM of 1.3% could not be drawn. The results of our series demonstrate that a significant difference in the adjusted probability of death between such a range of STS PROM scores is not only statistically, but also clinically significant in the context of achieving a goal of ≤1% mortality for isolated CABG. Furthermore, the observation in our study that STS PROM scores > 25% do not appear to correlate well with the probability of death after adjustment for operative year and surgeon volume highlights an apparent limitation of the STS PROM score for very–high-risk patients undergoing isolated CABG operations that surgeons should be aware of during preoperative patient counseling and risk assessment.
Our results have significant surgeon- and health care system-related implications. In the current health care environment, many different organizations have become increasingly focused on risk-adjusted outcome reporting and the use of various metrics by which to rank hospitals and providers from a quality performance perspective. Thus, health insurance groups and organizations such as The Leapfrog Group, the National Quality Forum, and the Agency for Healthcare Research and Quality are attracted to easily measurable and identifiable metrics that can be used to base referral patterns and regionalization of medical care. With this in mind, the ambitious goal to achieve a ≤1% observed mortality rate for isolated CABG operations may require significant qualification based on the results of our analyses to help guide such groups in the very near future. The fear that generalized adoption of a ≤1% mortality rate for the performance of all isolated CABG operations could become a metric by which current and future generations of cardiothoracic surgeons could be held accountable leaves very little room for surgical error and creates an inhospitable environment for new surgeons and surgeons or hospitals that routinely perform operations on high risk CABG patients where the likelihood of achieving an average mortality rate of ≤1% is unlikely. Nevertheless, surgeons will undoubtedly be presented with higher-risk patients in whom a 1% operative mortality will be very difficult to achieve but who will nonetheless have a better overall survival with the operation than with alternative forms of treatment, which questions the need to compete with PCI with respect to short-term, periprocedural mortality. There are also ultra–high-risk patients who may be better candidates for PCI. With this in mind, the development of a surgeon and/or hospital quality index may be beneficial to take into account variations in patient risk profiles for the performance of isolated CABG operations.
Calculated observed to expected ratios are an alternative metric of performance for surgeons and hospitals. We believe that modeling mortality risk while taking into account factors such as surgeon experience (using volume as a surrogate), differences between hospitals, and inherent changes in practice pattern over time provide further insight into the use of commonly available and routinely used clinical tools such as the STS PROM calculator when determining patient mortality risk and assessing surgeon- and hospital-specific quality for the performance of CABG.
The role that various processes of care play in the context of our results deserves special attention. The STS and National Quality Forum have identified several different performance measures and processes of care for the performance of isolated CABG operations.17,18 The multidimensional performance measure is composed of 4 different domains consisting of 11 different operative and perioperative cardiac surgery metrics, including use of internal mammary artery grafts and perioperative medication use such as beta-blockers, antiplatelet medications, and lipid-lowering agents. Thus, in the context of our report, the role of these factors as determinants in the achievement of ≤1% operative mortality is of critical importance. The results of our analyses demonstrate that among modeled factors for adjusted probability of death, the provision of perioperative medications plays a disproportionate role and that an increase in use of these processes of care measures may serve as 1 mechanism to further reduce mortality following performance of isolated CABG.
Our results also demonstrate the benefits of statewide and/ or regional cardiac surgery quality initiatives to advance goals of the cardiothoracic and medical community. Also highlighted by leadership in the field of cardiothoracic surgery as an essential goal for cardiac surgery,4 regional and statewide cardiac surgical quality initiative groups play an important role in helping to define various processes of care and best practice techniques to affect outcomes and quality care delivered to patients undergoing cardiac surgery. As a result, an important aspect of our study concerned the statewide collaborative effort of the VCSQI to use both clinical and uniform patient-specific financial data to help identify evidence-based best practice models with the goal of improving cardiac surgery outcomes while reducing costs. Through the process of examining the relative effects of various outcomes on cardiac surgical costs and resource use, we have been able to advance several different initiatives aimed to improve overall surgical quality within the Commonwealth of Virginia, including protocols designed to address the negative effect of atrial fibrillation and blood product use.19 The results of our study, therefore, set the stage for the future development of protocol-driven approaches to reduce complication rates and other identified factors contributing to operative mortality for the performance of primary, isolated CABG operations within VCSQI participating centers to achieve lower mortality rates in an attempt to increase the number of patients where the goal of ≤1% operative mortality can be achieved.
Our study has limitations. First, the secondary analysis of the VCSQI data registry and STS data limited the performed analyses to de-identified data that are nonrandomized. The study design is subject to limitations of inherent selection bias, and the reported results are limited to describe observed associations between factors and outcomes without assessing direct cause and effect relationships. Second, the blinded nature of the data further limited the ability to scrutinize certain surgeon- and hospital-specific details, including differences in individual surgeon experience (ie, years of practice) or surgical volume as a function of university versus community hospital environment. As a result, the opportunity to identify and describe best practice models within participating hospitals is not possible at this time. Third, although the influence of correlated hospital events was modeled as a random effect within the performed hierarchical regression models, the variance attributed to the unique characteristics of participating VCSQI hospitals cannot fully be accounted for in our statistical analysis. In addition, all analyses were limited to short-term, operative outcomes, and intermediate or long-term follow-up data were not available. Finally, the potential for unrecognized miscoding of data must also be considered in any secondary analysis of a data registry.
CONCLUSIONS
Based on reported results, the goal to achieve ≤1% operative mortality for primary, isolated CABG appears feasible in appropriately selected patients in the modern surgical era. However, this goal may be achieved in < 60% of CABG patients without other improvements in processes of care. The calculated STS PROM score can be used to strongly identify patients with a threshold value of estimated mortality risk ≤1.27% to achieve this goal. However, the STS PROM appears limited in its predictive capacity for very–high-risk patients with estimated risk > 25.0%. These data provide a strong foundation for further study to determine if ≤1% mortality for CABG is achievable nationwide.
Abbreviations and Acronyms
- CABG
coronary artery bypass grafting
- PCI
percutaneous coronary intervention
- PROM
predictive risk of mortality
- RCS
restricted cubic spline
- STS
Society of Thoracic Surgeons
- VCSQI
Virginia Cardiac Surgery Quality Initiative
Footnotes
Disclosures: Authors have nothing to disclose with regard to commercial support.
Read at the 93rd Annual Meeting of The American Association for Thoracic Surgery, Minneapolis, Minnesota, May 4–8, 2013.
References
- 1.El Bardissi AW, Aranki SF, Sheng S, O’Brien SM, Greenberg CC, Gammie JS. Trends in isolated coronary artery bypass grafting: an analysis of the Society of Thoracic Surgeons adult cardiac surgery database. J Thorac Cardiovasc Surg. 2012;143:273–81. doi: 10.1016/j.jtcvs.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 2.Epstein AJ, Polsky D, Yang F, Yang L, Groeneveld PW. Coronary revascularization trends in the United States, 2001–2008. JAMA. 2011;305:1769–76. doi: 10.1001/jama.2011.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–72. doi: 10.1056/NEJMoa0804626. [DOI] [PubMed] [Google Scholar]
- 4.Mack MJ. If this were my last speech, what would I say? Ann Thorac Surg. 2012;94:1044–52. doi: 10.1016/j.athoracsur.2012.05.134. [DOI] [PubMed] [Google Scholar]
- 5.Jin R, Furnary AP, Fine SC, Blackstone EH, Grunkemeier GL. Using Society of Thoracic Surgeons risk models for risk-adjusting cardiac surgery results. Ann Thorac Surg. 2010;89:677–82. doi: 10.1016/j.athoracsur.2009.10.078. [DOI] [PubMed] [Google Scholar]
- 6.Shahian DM, O’Brien SM, Filardo G, Ferraris VA, Haan CK, Rich JB, et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 1—coronary artery bypass grafting surgery. Ann Thorac Surg. 2009;88(1 Suppl):S2–22. doi: 10.1016/j.athoracsur.2009.05.053. [DOI] [PubMed] [Google Scholar]
- 7.Society of Thoracic Surgeons. [Accessed January 5, 2013];Online risk calculator. Available at: http://riskcalc.sts.org/STSWebRiskCalc/About%20the%20STS%20Risk%20Calculator%20v2.73.pdf.
- 8.Farrokhyar F, Wang X, Kent R, Lamy A. Early mortality from off-pump and on-pump coronary bypass surgery in Canada: a comparison of the STS and the EuroSCORE risk prediction algorithms. Can J Cardiol. 2007;23:879–83. doi: 10.1016/s0828-282x(07)70843-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Puskas JD, Kilgo PD, Thourani VH, Lattouf OM, Chen E, Vega JD, et al. The society of thoracic surgeons 30-day predicted risk of mortality score also predicts long-term survival. Ann Thorac Surg. 2012;93:26–33. doi: 10.1016/j.athoracsur.2011.07.086. discussion 33–5. [DOI] [PubMed] [Google Scholar]
- 10.Ngaage DL. Off-pump coronary artery bypass grafting: the myth, the logic and the science. Eur J Cardiothorac Surg. 2003;24:557–70. doi: 10.1016/s1010-7940(03)00381-6. [DOI] [PubMed] [Google Scholar]
- 11.Lapar DJ, Mery CM, Kozower BD, Kern JA, Kron IL, Stukenborg GJ, et al. The effect of surgeon volume on mortality for off-pump coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2012;143:854–63. doi: 10.1016/j.jtcvs.2011.12.048. [DOI] [PubMed] [Google Scholar]
- 12.Greenland S. Dose-response and trend analysis in epidemiology: alternatives to categorical analysis. Epidemiology. 1995;6:356–65. doi: 10.1097/00001648-199507000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Kozower BD, Stukenborg GJ. The relationship between hospital lung cancer resection volume and patient mortality risk. Ann Surg. 2011;254:1032–7. doi: 10.1097/SLA.0b013e31821d4bdd. [DOI] [PubMed] [Google Scholar]
- 14.Puskas JD, Thourani VH, Kilgo P, Cooper W, Vassiliades T, Vega JD, et al. Off-pump coronary artery bypass disproportionately benefits high-risk patients. Ann Thorac Surg. 2009;88:1142–7. doi: 10.1016/j.athoracsur.2009.04.135. [DOI] [PubMed] [Google Scholar]
- 15.Qadir I, Salick MM, Perveen S, Sharif H. Mortality from isolated coronary bypass surgery: a comparison of the Society of Thoracic Surgeons and the EuroSCORE risk prediction algorithms. Interact Cardiovasc Thorac Surg. 2012;14:258–62. doi: 10.1093/icvts/ivr072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stukenborg GJ, Kilbridge KL, Wagner DP, Harrell FE, Jr, Oliver MN, Lyman JA, et al. Present-at-admission diagnoses improve mortality risk adjustment and allow more accurate assessment of the relationship between volume of lung cancer operations and mortality risk. Surgery. 2005;138:498–507. doi: 10.1016/j.surg.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 17.National Quality Forum. The STS CABG Composite Score. [Accessed January 5, 2013];NQF-endorsed standards. 2009 Available at: http://www.sts.org/sites/default/files/documents/pdf/ndb2010/Report_OV_NQF_44-73.pdf.
- 18.Shahian DM, O’Brien SM, Normand SL, Peterson ED, Edwards FH. Association of hospital coronary artery bypass volume with processes of care, mortality, morbidity, and the Society of Thoracic Surgeons composite quality score. J Thorac Cardiovasc Surg. 2010;139:273–82. doi: 10.1016/j.jtcvs.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 19.LaPar DJ, Crosby IK, Ailawadi G, Ad N, Choi E, Spiess BD, et al. Blood product conservation is associated with improved outcomes and reduced costs following cardiac surgery. J Thorac Cardiovasc Surg. 2013;145:796–803. doi: 10.1016/j.jtcvs.2012.12.041. [DOI] [PubMed] [Google Scholar]