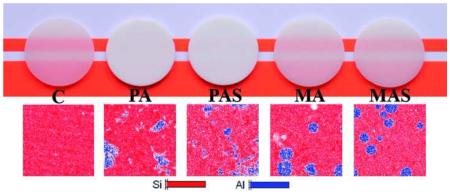
Abstract
Objectives
This study evaluated the effect of addition of alumina particles (polycrystalline or monocrystalline), with or without silica coating, on the optical and mechanical properties of a porcelain.
Methods
Groups tested were: Control (C), Polycrystalline alumina (PA), Polycrystalline alumina-silica (PAS), Monocrystalline alumina (MA), Monocrystalline alumina-silica (MAS). Polycrystalline alumina powder was synthesized using a polymeric precursor method; a commercially available monocrystalline alumina powder (sapphire) was acquired. Silica coating was obtained by immersing alumina powders in a tetraethylorthosilicate solution, followed by heat-treatment. Electrostatic stable suspension method was used to ensure homogenous dispersion of the alumina particles within the porcelain powder. The ceramic specimens were obtained by heat-pressing. Microstructure, translucency parameter, contrast ratio, opalescence index, porosity, biaxial flexural strength, roughness, and elastic constants were characterized.
Results
A better interaction between glass matrix and silica coated crystalline particles is suggested in some analyses, yet further investigation is needed to confirm it. The materials did not present significant differences in biaxial flexural strength, due to the presence of higher porosity in the groups with alumina addition. Elastic modulus was higher for MA and MAS groups. Also, these were the groups with optical qualities and roughness closer to control. The PA and PAS groups were considerably more opaque as well as rougher. Significance: Porcelains with addition of monocrystalline particles presented superior esthetic qualities compared to those with polycrystalline particles. In order to eliminate the porosity in the ceramic materials investigated herein, processing parameters need to be optimized as well as different glass frites should be tested.
Keywords: ceramics, composites, monocrystals, optical and mechanical properties, polycrystals, porcelain, silica coating, single crystals
Graphical abstract
1. Introduction
Advanced ceramics used in some highly specialized applications require both optical and mechanical properties for an excellent performance. Amongst these applications is the use of ceramics as dental biomaterials, which need to be constructed into complex and stable-during-sintering shapes, and are subjected to long-term sliding fatigue under mastication in a corrosive/moist environment [1-6]. Furthermore, dental ceramics are expected to survive for decades in the oral environment, without breaking or loosing the aesthetical natural-tooth-looking appearance [7, 8]. The ceramic materials used in dentistry that try to fulfill these expectations usually have a glass matrix with varying crystalline content [1, 9]. Final properties of such materials are affected by the amount of crystalline phase, size and type of crystals, and their dispersion within the glass matrix [10-13].
In general terms, increase in the crystalline content leads to mechanical reinforcement [11, 14, 15]. In addition to that, an effective interfacial interaction between matrix and reinforcing crystalline particles is required for improved properties [16, 17]. The presence of a gap between phases would prevent dissipation of stresses from the matrix to the tougher particles [16, 17]. Such gaps would also act as pores and therefore further compromise the material mechanical properties, as well as increase its opacity due to light scattering [10, 18-22]. When crystalline particles are nucleated and crystalized from the glass matrix, the phases are chemically alike and prone to a chemically stable interfacial interaction [14, 23]. On the other hand, the development of hybrid-ceramic materials by means of addition of second-phase crystalline particles into the glass powder before sintering [24] allows the use of non-silicate tough crystalline oxides, like zirconia or alumina, as reinforcement phase. Because these oxides have such a distinct chemical composition compared to that of the glass, the final mechanical reinforcement can only be achieved if these particles are functionalized to produce an effective interaction with the glass matrix.
To date, only two hybrid-ceramic materials with addition of second-phase crystalline particles are commercially available for dental applications, both having zirconia particles added as crystalline phase into a lithium silicate glass-ceramic system: Vita Suprinity (VITA Zahnfabrik, Bad Säckingen, Germany) and Celtra DeguDent (DeguDent, Hanau, Germany). Despite the current appeal of zirconia, alumina is a technologically important material widely used in numerous applications [25, 26], yet it is not being used in the tailoring of hybrid-ceramics for dental applications. Alumina is believed to exist in more than 15 different crystallographic phases [27, 28], which can determine distinct microstructures and properties if applied in hybrid-ceramics. Crystalline alumina has also been given a lot of attention in its transparent monocrystalline state, known as artificial sapphire [29-31]. The refractive index mismatch between phases is usually the most important aspect to be considered to estimate translucency for hybrid-materials [22]. However, no evidence was found on whether the inherent microstructure, thus inherent translucency of these alumina particles would affect final properties of hybrid-ceramic materials.
To the best of the authors’ knowledge, neither monocrystalline alumina powder nor functionalization of the surface of non-silicate particles with a silicon-rich film have been described in the scientific literature for the development of hybrid-ceramic materials. Therefore, this study was designed to apply nanostructured polycrystalline or translucent monocrystalline alumina particles in the modeling of optical and mechanical properties of a dental ceramic. A silica coating (core-shell) method was used aiming at an effective interfacial interaction between the alumina particles and the glass matrix in the tailoring of hybrid-ceramic materials as dental biomaterials.
2. Materials and methods
2.1 Materials
The dental ceramic used as matrix for the hybrid-ceramic materials in this study is commercially available under the brand name Cerabien (Noritake Dental, Aichi, Japan). The manufacturer classifies this material as porcelain, yet they do not disclose information about its composition. This porcelain was chosen because it has a coefficient of thermal expansion that matches that of alumina, and therefore less residual stresses concentrate near the interface between the two phases. Two types of alumina particles were used in the modeling of the properties of the porcelain: polycrystalline alumina sintered by means of a polymeric precursor method, and monocrystalline alumina commercially available under the brand name Sapphire Powder (GoodFellow, Huntingdon, England). The alumina particles were either silica coated or kept without any coating before being added to the porcelain powder. Coating was carried out using tetraethylorthosilicate (TEOS) as silica precursor (Sigma-Aldrich, St. Louis, MO, USA). In total, five experimental groups were tested: Porcelain only (Control, C), Porcelain + Polycrystalline alumina (PA), Porcelain + Polycrystalline alumina coated with silica (PAS), Porcelain + Monocrystalline alumina (MA), and Porcelain + Monocrystalline alumina coated with silica (MAS).
2.2 Synthesis of polycrystalline alumina by a polymeric precursor method
The nanostructured alumina powder was produced by using a variation of a previously described method [27]. Briefly, 1 M aluminum nitrate (Al(NO3)3.9H2O) P.A. (Synth, São Paulo, SP, Brazil) and 3 M anhydrous citric acid (C6H8O7) P.A. (Synth) were dissolved in water at 50°C for 1 h for the formation of aluminum citrate. Ethylene glycol (C2H6O2) P.A. (Synth) was added at a 60:40 mass ratio of the citric acid/ethylene glycol. This mixture was then stirred at 80°C for 1 h, and the temperature was then increased to 130°C to promote polymerization and remove excess solvents. The resin was then heat-treated in an atmospheric air furnace, with 10°C/min heating rate up to 300°C dwell temperature for 2 h in order to burn the organic components, resulting in a black solid mass. This material was finely hand-ground by using mortar and pestle. The powder obtained is referred as the “precursor”. In the furnace, the precursor was heat-treated at 10°C/min heating rate up to 1100°C, had dwelled for 2 h in air on an alumina boat, and then cooled to room temperature. The final product was a whitish nanostructured α-alumina powder.
2.3 Silica coating of mono and polycrystalline alumina particles
The alumina particles were used either as-received (monocrystalline) or as-synthesized (polycrystalline) and were coated with a silica layer applied by a sol–gel method. For silica coating, the particles were dispersed in an aqueous solution of 0.1 M hydrochloric acid p.a. (Synth). The suspension was kept in vigorous magnetic stirring for 15 min to favor disaggregation. TEOS was added to the suspension in the proportion of 5 mol% relative to the particles molar mass. Vigorous magnetic stirring was maintained and the temperature risen to 60°C to evaporate the aqueous content. After drying, the particles were heat-treated in an atmospheric air furnace at 5°C/min heating rate up to 900°C dwell temperature for 2 h.
2.4 Microstructural and chemical characterization of the ceramic powders
The micromorphology of the alumina particles with and without silica coating was analyzed by scanning electron microscopy – SEM (JSM 6610, JEOL, Tokyo, Japan). For elemental chemical composition, the alumina powders were analyzed using energy-dispersive spectroscopy – EDS (JSM 6610, JEOL, Tokyo, Japan). Crystalline spectra were determined by X-ray diffraction – XRD (XRD-6000; Shimadzu, Tokyo, Japan) with CuKα radiation at 40 kV and 40 mA, 4°/min scan rate, 10–80° 2θ, at room temperature.
2.5 Homogenous dispersion/mixture of the hybrid-ceramic powders
A dispersant solution [32] was prepared by solubilizing 0.5 M citric acid (Synth) in deionized water and stirring for 10 min. To this solution, 2 M triethylamine (Synth) was added and magnetic stirring was kept for 2 h, resulting in a solution with pH = 7. This solution was used as dispersant for the addition of the alumina particles to the porcelain powder. It is important to note that type of solution does not leave residues within the material after sintering. Also, it does not cause any erosion of the acid sensitive porcelain particles, and it produces an efficient electrostatic repulsion among ceramic particles [32]. In order to incorporate 10 wt% of alumina particles into the porcelain powder in a homogenous, well dispersed way, these two powders (10 wt% alumina + 90 wt% porcelain) were disaggregated together in a suspension containing isopropyl alcohol with 5% solid content (total powders). To that suspension, 10 wt% of the aforementioned dispersion solution [32] was poured. The suspension was sonicated at 9 W for 10 min in pulse mode (1 s cycles), and then placed into a round-bottom flask and taken to a rotary evaporator (RV10; IKA, Staufen, Germany) at 40°C under vacuum until complete elimination of the liquid content. The powder was kept in incubation at 150°C for 2 h to ensure complete solvent removal. After this process, the final material was a hybrid-ceramic powder ready for use. For the control group, the porcelain powder without the addition of alumina was processed similarly to the others as described herein.
2.6 Fabrication of hybrid-ceramic specimens by heat-pressing
The fabrication of both the control and hybrid-ceramic specimens followed the lost-wax technique and heat-pressing method for glass-based ceramics. For each group, five pre-sintered rod-shaped ingots (20 mm height, 12 mm diameter) were obtained by uniaxially pressing 4.5 g of each of the previously prepared (hybrid-)ceramic powders with 2 mL of distilled water at 3 ton, for 30 s. The green-bodies were heat-treated at 45°C/min heating rate from 600°C to 750°C under vacuum, 1 min dwell at 750°C with vacuum, and 1 min at 750°C without vacuum. Rod-shaped wax patterns (10 mm height, 12 mm diameter) were produced using a silicone mold. Each wax pattern was individually attached to a pressing ring using a 3 mm round wax sprue and a freshly vacuum mixed investment material was cast on a vibrating table. Following chemical setting time (45 min), the investment ring was transferred to a preheated furnace at 750°C and left for 1 h to burn out the wax.
A pre-sintered ceramic ingot was placed in the ring and transferred to the heat-pressing furnace (Kerampress; Kota, São Paulo, SP, Brazil), which was programmed according to the following sintering cycle: heating rate of 50°C/min from 700°C to 970°C, dwell 20 min + 8 min pressing at 3 bar (all under vacuum) + 1 min at 970°C without vacuum. In order to achieve higher density and translucency, pilot studies were conducted testing ideal sintering temperature and maximum percentage of alumina particles added; sintering temperature ranged from 960°C (indicated by the manufacturer of the porcelain) to 1000°C, while particle fractions varied between 5 wt% and 30 wt%. One should consider that the addition of particles changes the viscous flow behavior of the porcelain above the Tg during the heat-pressing process. After divesting and cleaning, the sintered rods were transversely sectioned using a precision circular saw coupled to a diamond-coated wafering blade under water cooling to produce disc-shaped specimens (1.1 mm thickness, 12 mm diameter). The specimens were polished down to 1 μm on both sides to a final thickness of 1 ± 0.05 mm.
2.7 Microstructural and chemical characterization of the hybrid-ceramics
The micromorphology of the sintered hybrid ceramic specimens was analyzed by SEM. The specimens were imaged both on their polished surface as well as after acid etching with 5% hydrofluoric acid for 10 s for better view of the crystals. Elemental chemical composition and mapping was carried out by EDS. The crystalline phases present in each group were determined by XRD as previously described. Surface roughness was analyzed by a contact profiler (SJ-410, Mitutoyo, Tokyo, Japan); roughness was quantified by the amplitude parameter Ra with the following test conditions: cut-off length 0.8 mm, resolution 0.0001μm (8 μm range), speed 0.5 mm/s, and total length 4 mm. Pore percentage and characterization of porosity were evaluated by using Skyscan 1272 micro-CT (computer tomography) scanner [33]. The following parameters were set for the scanning of all specimens: filter Al 0.5 & Cu 0.038; source voltage 90 kV; source current 111 μA; image pixel size 10 μm; 81 slices; lower grey threshold 60; upper grey threshold 190. A cylinder of ~4 mm3 about the center of each scanned specimen was selected for 3D reconstruction and pore evaluation. Total percentage of porosity, percentage of open pores, and percentage of closed pores were measured. In addition, 3D images contrasting dense mass of the ceramic and void spaces of pores were collected.
2.8 Optical properties
Optical properties were evaluated with a spectrophotometer (CM 3700d; Konica-Minolta, Tokyo, Japan), operating in the wavelength range of visible light (400 to 700 nm), both in reflectance and transmittance modes. Contrast ratio (CR) was calculated from the spectral reflectance of the light of the specimen (Y) on a black background (Yb) and on a white background (Yw) [34], according to the equation:
Translucency parameter (TP) was evaluated by calculating the color difference [35] of the specimens on black and white backgrounds by using the equation:
where subscript b refers to color coordinates on the black background and subscript w refers to color coordinates on the white background. Opalescence parameter (OP) was estimated as a difference in the chromaticity between the reflected and transmitted colors [36], according to the equation:
where subscripts t and r indicate the transmitted and the reflected color, respectively.
2.9 Elastic constants
The Poisson’s ratio (V) and elastic modulus (E) were determined by the ultrasonic pulse-echo method [37] using a 200 MHz ultrasonic pulser-receiver (5900 PR; Panametrics, Waltham, MA, USA), 20 MHz longitudinal and shear transducers with a delay material, and a coupling paste (Panametrics) applied between the specimen and transducer. The time of flight of ultrasonic pulse was measured with an oscilloscope (TDS 1002; Tektronix, Shanghai, China) and the thickness of the specimen was measured with a digital micrometer (Mitutoyo). Sonic velocities were calculated as two times the thickness divided by the time of flight, since in the pulse-echo method only one transducer (longitudinal or shear mode) was used to emit and capture the back-reflected wave. V and E values were calculated using the equations:
where ρ is density measured by Archimedes principle, Vl is longitudinal velocity, and Vt is shear velocity.
2.10 Biaxial flexural strength (σf)
A ball-on-ring test configuration was used to determine the failure loads of the materials tested herein. Each disc-shaped specimen was placed in contact with a flat sheet of Parafilm (Bemis NA, Neenah, WI, USA), which covered the 10 mm diameter knife-edge ring support, and centrally loaded with a 4 mm diameter ball indenter at 1 mm/min. The Parafilm compensated for geometric variation and permitted small lateral displacements to minimize friction and shear [38]. The biaxial flexure stress at failure was calculated using a monolayer analytical solution [38]:
where σf is the maximum tensile stress, P the measured fracture load, a is the radius of the knife-edge support, h is the mean specimen thickness measured from fragments at the point of fracture with a digital micrometer, and v is the Poisson's ratio measured according to section 2.9.
2.11 Sample size and statistical analysis
For σf, 20 specimens were tested in each group. For optical properties, roughness and elastic constants, 10 specimens were evaluated in each group. Significant differences among homoscedastic data (σf, E, v, and Ra) were evaluated by calculating 95% confidence intervals for the mean. Groups were considered significantly different when the 95% confidence interval bounds did not overlap. Optical properties data were heteroscedastic, thus subjected to one-way Analysis of Variances on Ranks. All pairwise multiple comparison procedures were conducted using the Student-Newman-Keuls’ method. The significance level of all analyses was set at α = 5%.
3. Results and Discussion
3.1 Microstructural and chemical characterization of the alumina powders
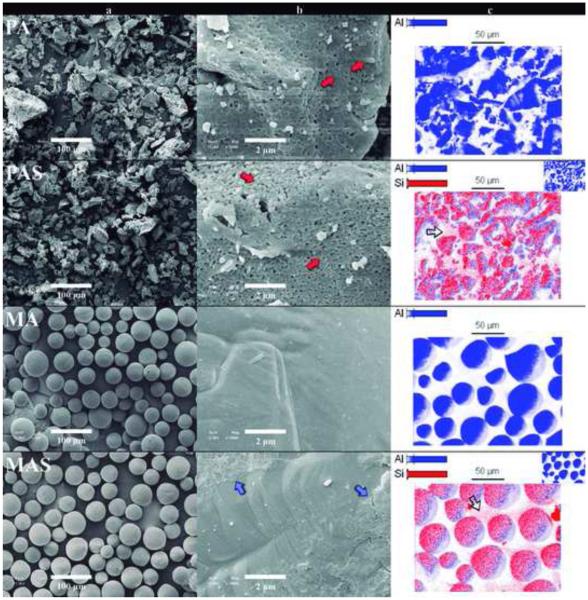
Both alumina particles had similar particle size (<60 μm) yet distinct particle shape (Figure 1): PA particles were irregular and porous, while MA particles were spherical and dense (Fig 1-a). A detailed surface observation of the PA particles revealed the boundaries of the nanosized crystals (red arrows in Fig 1-b), whereas no boundaries were seen in MA particles. The silica coating applied was only visible in the surface of the MAS group (blue arrows in Fig 1-b). The dense structure of MA particles allowed for a superficial deposition of silica during the coating process. By comparison, PA had silica deposited both at the surface and within the pores of the particles, hindering visualization of a uniform silica layer.
Figure 1.
SEM images of the alumina powders: Polycrystalline alumina (PA); Polycrystalline alumina coated with silica (PAS); Monocrystalline alumina (MA); Monocrystalline alumina coated with silica (MAS). Column (a) is the low magnification images (×200), and column (b) the high magnification images (×10k) of the alumina particles. The polycrystalline nanostructure (red arrows) of the irregular-shaped particles is clear in high magnification of PA and PAS powders. The thin silica coat layer (blue arrows) is visible comparing the highly magnified regular particles’ surfaces of MA and MAS groups. Column (c) shows the mapping of Si and Al, being Al the core of the particles for all groups, and Si identified as a coating layer covering the surface of the alumina particles for the groups PAS and MAS. Red pixels among the alumina main particles (hollow arrows) identify the presence of silica nanoparticles as a secondary result of the TEOS heat treatment.
Figure 1-c shows the mapping of Si (red pixels) and Al (blue pixels). Al was present in the core of the particles for all groups, and Si was identified as the coating layer on the surface of the alumina particles for groups PAS and MAS. Although the silica layer was not clearly visible in the SEM micrographs, the presence of Si on the surface of coated powders was confirmed by elemental mapping. The EDS analysis corroborated the SEM images showing that the size of MA and PA particles is not affected by silica coating. The hollow arrows in Figure 1-c indicate the presence of silica nanoparticles that were most likely crystallized during heat treatment of TEOS molecules that were not bonded to the alumina particles during silica coating. Formation of a secondary silica phase during coating was observed in previous analyses and will be addressed in a separate report, however, no negative effect is anticipated by the presence of these silica particles in hybrid-ceramic materials since they are chemically alike to the glass matrix (see supplementary material 1 for elemental quantification in atom % and weight % − EDS).
3.2 Microstructural and chemical characterization of the sintered specimens
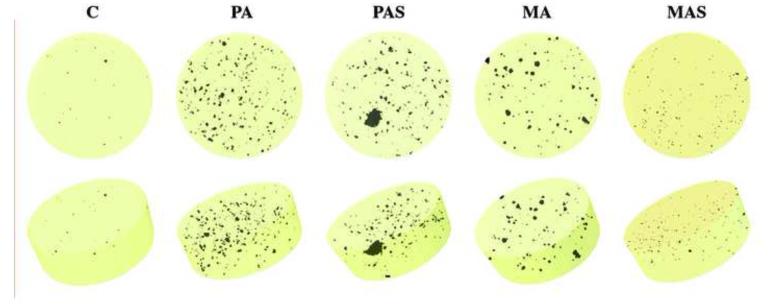
Microstructure of the sintered hybrid-ceramic specimens is shown in Figure 2. Uniform distribution of crystalline phases within the porcelain was observed in all groups (Fig 2-a). Although even the control porcelain had pores, the alumina-modified hybrid-ceramic materials showed 8× to 32× larger porosity than that seen in the control group (Table 1 & Fig. 3). Pores act as stress concentration/magnification areas and reduce the load bearing capacity of materials, and therefore cause a significant reduction in the material strength [10, 18, 19]. Porosity also scatters light and reduces the material total transmittance [20-22]. Most of the porosity identified by micro-CT scanning comprised closed pores (Table 1). This may be explained by (i) defects that remained in the microstructure after heat-pressing; or (ii) calcination of porcelain components due to sintering above the temperature recommended by its manufacturer; in fact, burnt components within the glass matrix are known to leave trapped gas bubbles, creating spherical-shaped pores [23] like some of those observed in Fig. 2. Therefore, it is clear that the porosity still needs to be reduced in order to optimize the microstructure of the modified materials. Denser specimens may be obtained by using different glass frits as matrix and/or by changing processing parameters. The use of a processing method other than heat-pressing (e.g., production of dense CAD-CAM blocks) could potentially allow the addition of higher percentages of alumina particles for the fabrication of materials with still a wider range of properties.
Figure 2.
SEM images of sintered specimens: Polycrystalline alumina (PA); Polycrystalline alumina coated with silica (PAS); Monocrystalline alumina (MA); Monocrystalline alumina coated with silica (MAS). a) Polished surfaces (×100) showing uniform distribution of crystalline phases as well as considerable porosity in groups with alumina addition. b) Elemental mapping showing Si both in the glass matrix and crystals nucleated during sintering and Al concentrated within the alumina particles. c) and d) Microstructure after acid-etching with 5% hydrofluoric acid for 10 s for better view of the crystals (magnifications ×500 and ×4k). Crystalline SiO2 (hollow arrow) nucleated and grew in all groups, while Al2O3 particles (white arrows) were not present in the control group. PA particles seemed to work as nanoclusters, with crystal dislodgement during polishing procedures (yellow arrows). Pores and defects were observed at the interface of glass matrix and alumina particles mainly for the group MA (red arrows). Bridges of glass between alumina particle and porcelain are observed (blue arrows), indicating that the silica coating was effective in improving the chemical interaction between these two phases.
Table 1.
Average of total porosity (95% confidence interval), and average opened and closed porosity for all groups
| Group | Total porosity | Closed porosity | Opened porosity |
|---|---|---|---|
| C | 0.01% (0.01) c | 0.01% | 0.00% |
| PA | 0.17% (0.04) a | 0.13% | 0.04% |
| PAS | 0.32% (0.14) a | 0.25% | 0.07% |
| MA | 0.13% (0.05) ab | 0.11% | 0.02% |
| MAS | 0.08% (0.01) b | 0.27% | 0.01% |
Distinct letters within the same column indicate significant differences among materials.
Figure 3.
3D reconstructions of micro-CT scans: Control (C); Polycrystalline alumina (PA); Polycrystalline alumina coated with silica (PAS); Monocrystalline alumina (MA); Monocrystalline alumina coated with silica (MAS). Control group clearly presents less porosity than the groups with alumina addition, amongst which, MAS possesses the lowest porosity.
Group MA showed porosity at the interface between alumina particles and porcelain matrix (red arrows in Figures 2-a and 2-d). The lack of interfacial interaction between matrix and crystalline particles is sufficient to hinder any reinforcing effect expected. A gap between the two phases prevents dissipation of stresses from the matrix to the tougher alumina particles [16, 17], and may also act as pores within the porcelain matrix [10, 18, 19]. In contrast, group MAS did not display interfacial porosity, and its higher magnification images showed glass bridging from the porcelain matrix towards the alumina particles, even after acid etching (blue arrows in Fig 2-d). These findings suggest that silica coating may improve the chemical interaction between these two phases. Nevertheless, this is just a hypothesis at this stage and further research is needed to understand whether or not silica coating has a positive effect on interfacial interaction between matrix and particles.
PA particles did not work as discrete particles, but rather as nanoclusters, as indicated by the crystal dislodgement observed during polishing (yellow arrows in Fig 2-d). The use of nanoclusters as reinforcing phase of polymeric dental biomaterials has been previously described [39, 40]. This approach considers that the progressive dislodgment of nanocrystals, instead of the loss of a whole microparticle during sliding fatigue under mastication, would benefit wear, fatigue, and esthetic properties [39, 40]. However, none of these effects has yet been tested in hybrid-ceramic materials, and the PA particles depicted herein may offer that possibility.
The elemental mapping for Si and Al across the polished surface of the specimens (Fig 2-b) showed that Si is present both in the glass matrix and in the crystals nucleated during sintering, while Al is concentrated within the added alumina particles (see supplementary material 1 for elemental quantification in atom % and weight % − EDS; and, supplementary material 2 for crystalline phase characterization − XRD). Surface imaging after 5% hydrofluoric acid-etching for 10 s was able to show the following crystalline phases (Figs 2-c and 2-d): (1) SiO2 crystals (hollow arrows) nucleated and grown in all groups during sintering and (2) Al2O3 particles added (white arrows). It is important to point out that the silica crystals are not related to the silica nanoparticles observed within the alumina powders, as they were also seen in the control group.
3.3 Optical properties
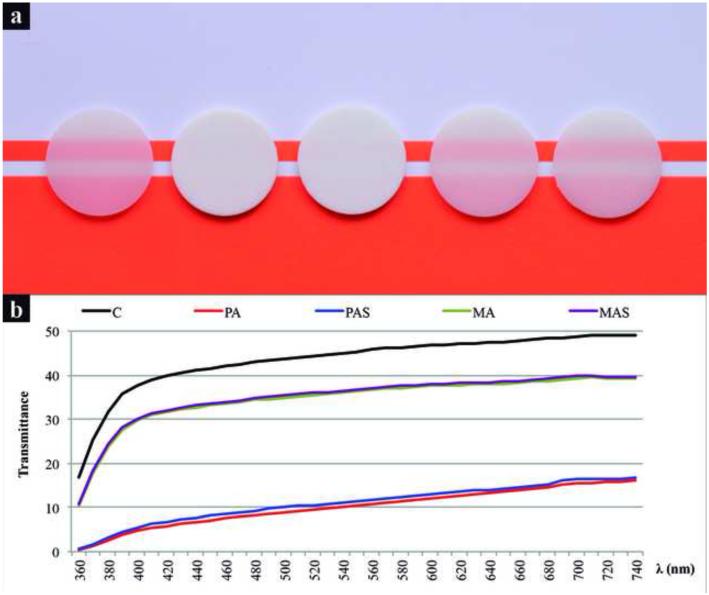
Figure 4-a shows the apparent translucency of specimens and Figure 4-b shows the spectra of total light transmittance. C, MA, and MAS were the most translucent materials, whereas PA and PAS were significantly more opaque, having less than 50% of the total light transmittance of C. Light transmittance generally increased with the increase in wavelength. Silica coating resulted in slightly higher light transmittance, which is likely explained by a better interfacial interaction between porcelain and particles, reducing light scattering at the interface of these phases [20, 21]. The translucency of groups containing alumina particles could be improved by using the previously mentioned strategies to reduce their total porosity [20-22].
Figure 4.
a) Apparent translucency of specimens from various groups: Control (C), Polycrystalline alumina (PA), Polycrystalline alumina coated with silica (PAS), Monocrystalline alumina (MA), and Monocrystalline alumina coated with silica (MAS). b) Spectra of total light transmittance, indicating that transmittance increases with the increase in light wavelength. MA and MAS have similar translucency to C, while PA and PAS are noticeably more opaque.
Results for TP and CR (Table 2) corroborate the observations of light transmittance and visual translucency: C was the most translucent group, closely followed by MA and MAS. PA and PAS were significantly more opaque than the other groups. Silica coating yielded a slight increase in translucency. CR was ~40% lower and TP was ~85% higher for monocrystalline compared to polycrystalline groups. The addition of second-phase particles into a matrix increases the material opacity mostly due to increased light scattering [41]. For hybrid-materials with the same crystalline content and particles of similar size, the refractive index mismatch between the two phases is usually the most important parameter to estimate translucency [22]. PA and MA particles have similar refractive indices (between 1.71 and 1.79), which are quite different from the refractive index of porcelain (between 1.50 and 1.53). Therefore, one could expect all hybrid-ceramics tested to have high opacity, but this was only true for PA-modified materials. Two aspects are hypothesized to explain this effect: (i) MA particles are intrinsically translucent and dense [30], thus minimizing light scattering, while PA particles are intrinsically opaque and porous, which increases light scattering within the particles [42]; and (ii) the difference in particle shape may play a role in light scattering, affecting the translucency of the experimental materials [43-45].
Table 2.
Median (25% - 75%) of optical properties: CR = contrast ratio; TP = translucency parameter; OP = opalescence parameter
| CR | TP | OP | |
|---|---|---|---|
| C | 0.32 (0.31-0.32) e | 35.1 (35.0-35.3) a | 5.7 (5.6-5.9) c |
| PA | 0.94 (0.94-0.95) a | 2.9 (2.8-3.0) d | 9.1 (8.9-9.4) a |
| PAS | 0.94 (0.93-0.94) b | 3.2 (3.2-3.3) c | 8.6 (8.2-8.9) b |
| MA | 0.55 (0.54-0.55) c | 21.6 (21.2-21.8) b | 4.1 (4.0-4.1) e |
| MAS | 0.54 (0.54-0.54) d | 21.9 (21.7-22.0) b | 4.2 (4.2-4.2) d |
Data were transformed to ranks before the analysis. Distinct letters within the same column indicate significant differences among materials (p<0.05). Note that the multiple comparisons on ranks do not include adjustment for ties.
A previous study [46] showed that spherical particles cause more light scattering than irregular particles, what goes against the results found herein, further suggesting that it was not the shape of the particles, but the intrinsic translucency of the MA particles that granted higher translucency for MA and MAS groups in comparison to PA and PAS. It is important to notice that the porcelain used in this study is highly translucent, and therefore should be used in thin increments in dental restorative applications (usually less than 0.5 mm) in order to avoid a low-value and unpleasing appearance to the restoration [47-50]. In this application context, the lower transmittance achieved with the addition of MA particles resulted in a degree of opacity similar to that generally used to build up the dentin-shade layers of dental ceramic restorations [47-50]. This opacity level may also be considered ideal for the fabrication of monolithic restorations with a natural tooth-like appearance. On the other hand, the opaque shade of PA and PAS groups might be proper for situations in which masking a discolored background is needed [47-50].
Addition of PA increased OP, while MA particles decreased OP (Table 2). All OP values measured for the materials under investigation are within the range of values previously reported for commercial dental ceramics [36, 51, 52]. The phenomenon of opalescence is related to the light scattering of the shorter wavelengths of the visible spectrum in translucent materials [36, 51, 52]. The presence of PA particles containing nanocrystals and their multiple boundaries is most likely to cause the scattering of short wavelength photons, which would explain the increase in OP for groups PA and PAS [22]. The presence of dense and translucent MA particles only slightly attenuated the opalescence. Considering the same type of particle, the very discreet variation observed in OP values for groups with and without silica coating is not expected to cause any significant effect in the final optical appearance.
3.4 Mechanical and physical properties
Table 3 shows comparisons for σf, with similar results for all groups except for PA, which showed lower σf than the control. The effect of addition of alumina particles was not noticeable on the σf of the porcelain due to the relatively high porosity observed for these groups. As previously mentioned, pores are areas of stress concentration during loading, which cause unstable crack propagation resulting in fracture at lower applied loads [10, 18, 19]. Porous structures have reduced cross-section load bearing area of material, resulting a significant reduction in strength [10, 18, 19]. Table 3 also shows comparisons for elastic constants and surface roughness across the groups. Addition of MA to the porcelain increased its elastic modulus (E), while addition of PA particles resulted in lower E. While higher crystalline content can increase E, higher porosity might decrease it [15, 19]. The higher E of MAS in comparison to all other groups indicates that the silica coating was successful in promoting better interaction of the MA particles with the glass matrix. In that case, the positive effect of increased crystalline content overcame the negative effect of porosity [15, 19]. For PA and PAS groups, the lower E compared to the control can be explained by the negative effect of porosity within the glass matrix combined with the inherent porosity of the particles [15, 19]. No variation in the Poisson’s ratio (V) with the addition of alumina particles was observed though, meaning that the effect of crystalline content and porosity are much less important in the variation of V than E [15, 19]. Finally, regarding surface roughness, MA and MAS groups were rougher than the control, although these three groups showed Ra values consistent with polished commercial dental ceramics [53]. This was not the case for PA and PAS groups, which were markedly rougher than the others. For these two groups, the cluster-like PA particles played a key role in increasing surface roughness by allowing crystal dislodgment during polishing. The same effect is likely to occur if these materials are subjected to any type of friction process during function.
Table 3.
Means (95% confidence intervals) of biaxial flexural strength (σf), elastic modulus (E), Poisson’s ratio (v), and surface roughness (Ra)
| σf (MPa) | E (GPa) | v | Ra (nm) | |
|---|---|---|---|---|
| C | 137 (10) a | 70.1 (0.4) c | 0.18 (0.01) a | 33 (10) c |
| PA | 117 (6) b | 66.7 (0.6) d | 0.16 (0.01) a | 273 (52) a |
| PAS | 125 (6) ab | 65.7 (0.8) d | 0.17 (0.01) a | 184 (37) a |
| MA | 128 (7) ab | 73.4 (0.5) b | 0.18 (0.01) a | 54 (9) b |
| MAS | 120 (7) ab | 74.7 (0.6) a | 0.17 (0.01) a | 56 (7) b |
Distinct letters within the same column indicate significant differences among materials.
4 Conclusion
Mono or polycrystalline alumina hybrid-ceramic materials having distinct characteristics and, therefore, varied potential applications were prepared by heat-pressing. Polycrystalline nanostructured alumina powder was synthesized using a polymeric precursor method, while commercial monocrystalline alumina particles were used. Silica coating of the alumina particles was carried out by heat treatment of tetraethylorthosilicate precursor and confirmed by SEM-EDS. The presence of glass bridging from porcelain to the alumina particles, along with other results, suggested that silica coating method may improve the chemical interaction between these two phases; however a thorough investigation is required before any conclusions can be drawn. Polycrystalline alumina particles worked as nanoclusters showing crystal dislodgement from the surface due to friction. The addition of monocrystalline particles yielded more translucent hybrid-ceramic, while addition of polycrystalline particles resulted in higher opacity. The effect of addition of alumina particles on the strength of the hybrid-ceramic materials was not clear due to porosity. In order to eliminate the porosity in the materials investigated herein, processing parameters need to be further optimized.
Supplementary Material
Highlights.
Mono or polycrystalline alumina particles were added to a dental porcelain.
Particle coating was carried out by heat treatment of a silica precursor.
The addition of monocrystalline particles yielded a more translucent hybrid ceramic.
Polycrystalline alumina particles worked as nanoclusters.
Glass bridging from porcelain to alumina particles suggested chemical interaction between the two phases.
Acknowledgments
The work was supported by: CNPq – Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil (Grants 141129/2011-5, 241021/2012-0, and 475462/2012-2); The United States National Institute of Dental & Craniofacial Research (Grant – 2R01 DE017925). The authors thank CEME-Sul – Centro de Microscopia Eletrônica da Zona Sul at Federal University of Rio Grande – Brazil for SEM equipment support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Denry I, Kelly JR. Emerging ceramic-based materials for dentistry. Journal of Dental Research. 2014;93(12):1235–42. doi: 10.1177/0022034514553627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrari M, Vichi A, Zarone F. Zirconia abutments and restorations: from laboratory to clinical investigations. Dental Materials. 2015;31(3):e63–76. doi: 10.1016/j.dental.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 3.Joshi GV, Duan Y, Della Bona A, Hill TJ, St John K, Griggs JA. Contributions of stress corrosion and cyclic fatigue to subcritical crack growth in a dental glass-ceramic. Dental Materials. 2014;30(8):884–90. doi: 10.1016/j.dental.2014.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rekow ED, Silva NR, Coelho PG, Zhang Y, Guess P, Thompson VP. Performance of dental ceramics: challenges for improvements. Journal of Dental Research. 2011;90(8):937–52. doi: 10.1177/0022034510391795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Sailer I, Lawn BR. Fatigue of dental ceramics. J Dent. 2013;41(12):1135–47. doi: 10.1016/j.jdent.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim JW, Kim JH, Thompson VP, Zhang Y. Sliding contact fatigue damage in layered ceramic structures. Journal of Dental Research. 2007;86(11):1046–50. doi: 10.1177/154405910708601105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beier US, Dumfahrt H. Longevity of silicate ceramic restorations. Quintessence Int. 2014;45(8):637–44. doi: 10.3290/j.qi.a32234. [DOI] [PubMed] [Google Scholar]
- 8.Haff A, Lof H, Gunne J, Sjogren G. A retrospective evaluation of zirconia-fixed partial dentures in general practices: an up to 13-year study. Dental Materials. 2015;31(2):162–70. doi: 10.1016/j.dental.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 9.Li RW, Chow TW, Matinlinna JP. Ceramic dental biomaterials and CAD/CAM technology: state of the art. Journal of prosthodontic research. 2014;58(4):208–16. doi: 10.1016/j.jpor.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Albakry M, Guazzato M, Swain MV. Biaxial flexural strength, elastic moduli, and x-ray diffraction characterization of three pressable all-ceramic materials. J Prosthet Dent. 2003;89(4):374–80. doi: 10.1067/mpr.2003.42. [DOI] [PubMed] [Google Scholar]
- 11.Borba M, de Araujo MD, Fukushima KA, Yoshimura HN, Cesar PF, Griggs JA, et al. Effect of the microstructure on the lifetime of dental ceramics. Dental Materials. 2011;27(7):710–21. doi: 10.1016/j.dental.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cesar PF, Soki FN, Yoshimura HN, Gonzaga CC, Styopkin V. Influence of leucite content on slow crack growth of dental porcelains. Dental Materials. 2008;24(8):1114–22. doi: 10.1016/j.dental.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Della Bona A, Nogueira AD, Pecho OE. Optical properties of CAD-CAM ceramic systems. J Dent. 2014;42(9):1202–9. doi: 10.1016/j.jdent.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Denry IL, Holloway JA. Elastic constants, Vickers hardness, and fracture toughness of fluorrichterite-based glass-ceramics. Dental Materials. 2004;20(3):213–9. doi: 10.1016/S0109-5641(03)00094-0. [DOI] [PubMed] [Google Scholar]
- 15.Yoshimura HN, Gonzaga CC, Cesar PF, Miranda WG. Relationship between elastic and mechanical properties of dental ceramics and their index of brittleness. Ceramics International. 2012;38(6):4715–22. [Google Scholar]
- 16.Liao WH, Tien HW, Hsiao ST, Li SM, Wang YS, Huang YL, et al. Effects of multiwalled carbon nanotubes functionalization on the morphology and mechanical and thermal properties of carbon fiber/vinyl ester composites. Acs Applied Materials & Interfaces. 2013;5(9):3975–82. doi: 10.1021/am400811p. [DOI] [PubMed] [Google Scholar]
- 17.Ni Y, Chen L, Teng K, Shi J, Qian X, Xu Z, et al. Superior Mechanical Properties of Epoxy Composites Reinforced by 3D Interconnected Graphene Skeleton. Acs Applied Materials & Interfaces. 2015;7(21):11583–91. doi: 10.1021/acsami.5b02552. [DOI] [PubMed] [Google Scholar]
- 18.Quinn GD, Hoffman K, Quinn JB. Strength and fracture origins of a feldspathic porcelain. Dental Materials. 2012;28(5):502–11. doi: 10.1016/j.dental.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshimura HN, Molisani AL, Narita NE, Cesar PF, Goldenstein H. Porosity dependence of elastic constants in aluminum nitride ceramics. Materials Research. 2007;10(2):127–33. [Google Scholar]
- 20.Lee YK. Influence of scattering/absorption characteristics on the color of resin composites. Dental Materials. 2007;23(1):124–31. doi: 10.1016/j.dental.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Pecho OE, Ghinea R, Ionescu AM, Cardona JC, Della Bona A, Perez Mdel M. Optical behavior of dental zirconia and dentin analyzed by Kubelka-Munk theory. Dental Materials. 2015;31(1):60–7. doi: 10.1016/j.dental.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Yoshimura HN, Goldenstein H. Light scattering in polycrystalline alumina with bidimensionally large surface grains. Journal of the European Ceramic Society. 2009;29(2):293–303. [Google Scholar]
- 23.Fredericci C, Yoshimura HN, Molisani AL, Pinto MM, Cesar PF. Effect of temperature and heating rate on the sintering of leucite-based dental porcelains. Ceramics International. 2011;37(3):1073–8. [Google Scholar]
- 24.Lohbauer U, Frankenberger R, Krämer N. Surface quality controls mechanical strength and fatigue lifetime of dental ceramics and resin composites. In: Wunderlich W, editor. Ceramic Materials. Vol. 9. 2010. pp. 176–97. [Google Scholar]
- 25.Cava S, Beninca R, Tebcherani SM, Souza IA, Paskocimas CA, Longo E, et al. Structural and spectroscopic characterization of Al2-xCrxO3 powders obtained by polymeric precursor method. Journal of Sol-Gel Science and Technology. 2007;43(1):131–6. [Google Scholar]
- 26.Delbrucke T, Gouvea RA, Moreira ML, Raubach CW, Varela JA, Longo E, et al. Sintering of porous alumina obtained by biotemplate fibers for low thermal conductivity applications. Journal of the European Ceramic Society. 2013;33(6):1087–92. [Google Scholar]
- 27.Cava S, Tebcherani SM, Souza IA, Pianaro SA, Paskocimas CA, Longo E, et al. Structural characterization of phase transition of Al2O3 nanopowders obtained by polymeric precursor method. Materials Chemistry and Physics. 2007;103(2-3):394–9. [Google Scholar]
- 28.Levin I, Brandon D. Metastable alumina polymorphs: Crystal structures and transition sequences. Journal of the American Ceramic Society. 1998;81(8):1995–2012. [Google Scholar]
- 29.Ozturk IK, Basar G, Er A, Guzelcimen F, Basar G, Kreger S. New energy levels of atomic niobium by laser induced fluorescence spectroscopy in the near infrared. Journal of Physics B-Atomic Molecular and Optical Physics. 2015;48(1) [Google Scholar]
- 30.Scott C, Kaliszewski M, Greskovich C, Levinson L. Conversion of polycrystalline AI(2)O(3) into single-crystal sapphire by abnormal grain growth. Journal of the American Ceramic Society. 2002;85(5):1275–80. [Google Scholar]
- 31.Yao G, Tang YQ, Fu YJ, Jiang ZQ, An XY, Chen Y, et al. Fabrication of high-quality ZnCdO epilayers and ZnO/ZnCdO heterojunction on sapphire substrates by pulsed laser deposition. Applied Surface Science. 2015;326:271–5. [Google Scholar]
- 32.De Riccardis MF, Carbone D, Rizzo A. A novel method for preparing and characterizing alcoholic EPD suspensions. J Colloid Interface Sci. 2007;307(1):109–15. doi: 10.1016/j.jcis.2006.10.037. [DOI] [PubMed] [Google Scholar]
- 33.Renghini C, Komlev V, Fiori F, Verne E, Baino F, Vitale-Brovarone C. Micro-CT studies on 3-D bioactive glass-ceramic scaffolds for bone regeneration. Acta Biomaterialia. 2009;5(4):1328–37. doi: 10.1016/j.actbio.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 34.Anusavice KJ, Zhang NZ, Moorhead JE. Influence of P205, AgNO3, and FeCl3 on color and translucency of lithia-based glass-ceramics. Dental Materials. 1994;10(4):230–5. doi: 10.1016/0109-5641(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 35.CIE Technical Committee 1.3 CIE colorimetry committee-working program on color differences Journal of the Optical Society of America. 1974;64:894–5. [Google Scholar]
- 36.Cho MS, Yu B, Lee YK. Opalescence of all-ceramic core and veneer materials. Dental Materials. 2009;25(6):695–702. doi: 10.1016/j.dental.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 37.Japanese Industrial Standards Testing methods for elastic modulus of high performance ceramics. JIS R 1602. 1986 [Google Scholar]
- 38.Addison O, Fleming GJ. Application of analytical stress solutions to bi-axially loaded dental ceramic-dental cement bilayers. Dental Materials. 2008;24(10):1336–42. doi: 10.1016/j.dental.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 39.Kaizer MR, de Oliveira-Ogliari A, Cenci MS, Opdam NJ, Moraes RR. Do nanofill or submicron composites show improved smoothness and gloss? A systematic review of in vitro studies. Dental Materials. 2014;30(4):e41–78. doi: 10.1016/j.dental.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Mitra SB, Wu D, Holmes BN. An application of nanotechnology in advanced dental materials. The Journal of the American Dental Association. 2003;134(10):1382–90. doi: 10.14219/jada.archive.2003.0054. [DOI] [PubMed] [Google Scholar]
- 41.Lee YK. Influence of filler on the difference between the transmitted and reflected colors of experimental resin composites. Dental Materials. 2008;24(9):1243–7. doi: 10.1016/j.dental.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y. Making yttria-stabilized tetragonal zirconia translucent. Dental Materials. 2014;30(10):1195–203. doi: 10.1016/j.dental.2014.08.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Foss CA, Hornyak GL, Stockert JA, Martin CR. Template-Synthesized Nanoscopic Gold Particles - Optical-Spectra and the Effects of Particle-Size and Shape. Journal of Physical Chemistry. 1994;98(11):2963–71. [Google Scholar]
- 44.Kelly KL, Coronado E, Zhao LL, Schatz GC. The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment. Journal of Physical Chemistry B. 2003;107(3):668–77. [Google Scholar]
- 45.Turssi CP, Ferracane JL, Vogel K. Filler features and their effects on wear and degree of conversion of particulate dental resin composites. Biomaterials. 2005;26(24):4932–7. doi: 10.1016/j.biomaterials.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 46.Arikawa H, Kanie T, Fujii K, Takahashi H, Ban S. Effect of filler properties in composite resins on light transmittance characteristics and color. Dent Mater J. 2007;26(1):38–44. doi: 10.4012/dmj.26.38. [DOI] [PubMed] [Google Scholar]
- 47.Barizon KT, Bergeron C, Vargas MA, Qian F, Cobb DS, Gratton DG, et al. Ceramic materials for porcelain veneers. Part I: Correlation between translucency parameters and contrast ratio. J Prosthet Dent. 2013;110(5):397–401. doi: 10.1016/j.prosdent.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 48.Barizon KT, Bergeron C, Vargas MA, Qian F, Cobb DS, Gratton DG, et al. Ceramic materials for porcelain veneers: part II. Effect of material, shade, and thickness on translucency. J Prosthet Dent. 2014;112(4):864–70. doi: 10.1016/j.prosdent.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 49.Heffernan MJ, Aquilino SA, Diaz-Arnold AM, Haselton DR, Stanford CM, Vargas MA. Relative translucency of six all-ceramic systems. Part II: core and veneer materials. J Prosthet Dent. 2002;88(1):10–5. [PubMed] [Google Scholar]
- 50.Heffernan MJ, Aquilino SA, Diaz-Arnold AM, Haselton DR, Stanford CM, Vargas MA. Relative translucency of six all-ceramic systems. Part I: core materials. J Prosthet Dent. 2002;88(1):4–9. [PubMed] [Google Scholar]
- 51.Primus CM, Chu CC, Shelby JE, Buldrini E, Heckle CE. Opalescence of dental porcelain enamels. Quintessence Int. 2002;33(6):439–49. [PubMed] [Google Scholar]
- 52.Yu B, Lee YK. Difference in opalescence of restorative materials by the illuminant. Dental Materials. 2009;25(8):1014–21. doi: 10.1016/j.dental.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 53.Flury S, Lussi A, Zimmerli B. Performance of different polishing techniques for direct CAD/CAM ceramic restorations. Oper Dent. 2010;35(4):470–81. doi: 10.2341/09-373-L. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.