Abstract
The growing incidence of serious infections mediated by methicillin-resistant Staphylococcus aureus (MRSA) strains poses a significant risk to public health. This risk is exacerbated by a prolonged void in the discovery and development of truly novel antibiotics and the absence of a vaccine. These gaps have created renewed interest in the use of biologics in the prevention and treatment of serious staphylococcal infections. This review focuses on efforts towards the discovery and development of antibody-based biologic agents and their potential as clinical agents in the management of serious S. aureus infections. Recent promising data for monoclonal antibodies (mAbs) targeting anthrax and Ebola highlight the potential of antibody-based biologics as therapeutic agents for serious infections.
Keywords: MRSA, Biologics, Antibody, Infectious Diseases, Immune Evasion
Staphylococcus aureus disease burden
Staphylococcus aureus (S. aureus) is a human pathogen that greatly impacts individuals in both hospital and community settings and is capable of causing a wide spectrum of diseases, ranging from mild, and usually self-limiting conditions like impetigo to severe, and potentially life-threatening diseases like pneumonia, endocarditis, and sepsis. In 2011, the Centers for Disease Control (CDC) identified 80,461 cases of diagnosed, severe infections mediated by MRSA in the U.S resulting in 11,285 deaths and was associated with the highest case fatality rate of all bacterial pathogen threats recognized (http://www.cdc.gov/drugresistance/threat-report-2013). While the overall prevalence of invasive MRSA infections in the hospital setting has shown a downward trend since 2005, the incidence rate of community-acquired infections has remained relatively constant and community-onset, hospital-treated infections account for the greatest number of MRSA related deaths [1]. Such infections commonly occur in individuals who are receiving outpatient dialysis treatment or who have been recently discharged from acute hospital care. Given their high risk for severe infection, these patients would be potential candidates that may benefit the most from prophylactic treatment with a biologic agent targeting S. aureus.
S. aureus pathogenesis
S. aureus is a commensal bacterium carried by 30–50% of the human population, and while colonization is associated with an increased risk for infection, natural carriers generally exhibit less severe infections than what is typically seen in non-carriers [2]. This observation demonstrates that that pre-exposure to the bacterium provides an advantage in thwarting off invasive infections. While it has not been conclusively shown what mediates this protection, it is known that persistent S. aureus carriers typically have higher levels of antistaphylococcal IgG (immunoglobulin G) than non-carriers[3, 4]. The pathogenesis of this bacterium is mediated by a vast array of surface associated proteins, carbohydrate structures, and secreted factors that are capable of suppressing complement activity, inhibiting antibody function, lysing host cells, and exerting toxic effects at sub-lytic concentrations (Figure 1) [5, 6]. Having a multifaceted set of virulence proteins facilitates the inhabitation of multiple anatomical sites within the human body and helps counter both innate and adaptive arms of the host immune system. A number of these S. aureus proteins have been the targets of monovalent vaccine and immunization strategies, yet none have yet progressed to approval for clinical use. In this review, we will highlight the pitfalls of previous immunization strategies and move onto discussing how novel anti-staphylococcal antibody-based molecules hold great promise for reversing the trend of failed clinical trials seen with previous S. aureus candidate therapies.
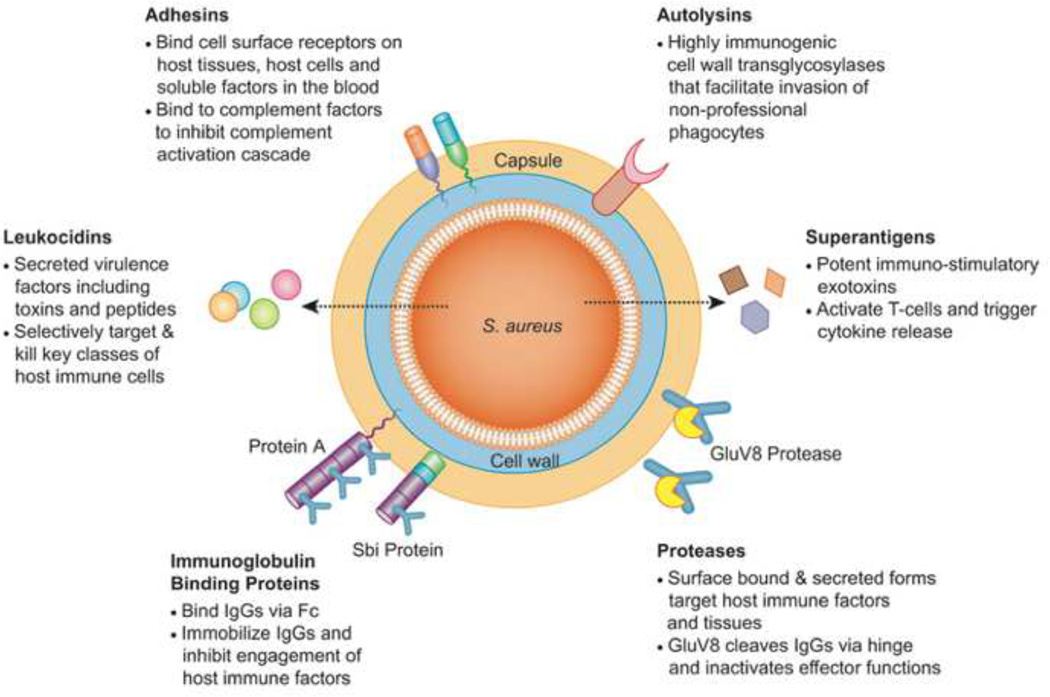
Figure 1. S. aureus Immune Evasion Factors Targeted by Experimental Biologic Agents.
S. aureus possesses an elaborate arsenal of extracellular virulence factors that serve as targets for the current class of anti-staphylococcal biologics being developed. These targets include: (1) surface bound adhesins that promote host colonization and disruption of complement pathways, (2) immunoglobulin binding proteins (Protein A, Sbi) that bind to IgGs and prevent engagement of host immune factors, (3) surface-associated and secreted proteases (GluV8) that digest IgG antibody components and diminish effector function, (4) a family of immune-stimulatory exotoxins called superantigens (SAgs), (5) potent leukocidal toxins that kill critical classes of immune cells, and (6) immunogenic cell wall autolysins that are important for bacterial uptake into non-professional phagocytes.
Antibody therapies evaluated in patients
The increasing prevalence of antibiotic resistant S. aureus strains has bolstered the need for a dependable S. aureus immunization strategy. Unfortunately, all active and passive immunization (See glossary) approaches to date have failed in clinical trial. While this review primarily focuses on antibody-based passive immunization approaches, it should be recognized and it has been reviewed elsewhere, that both passive and active immunization strategies have implemented similar S. aureus targeting tactics and criteria for preclinical proof-of-efficacy [7–11]. Commonalities that exist within these studies include the use of single, cell surface-associated S. aureus antigens as targets, in vitro opsonophagocytosis assays that demonstrate bacterial uptake and/or killing as readouts for opsonic potential of antibodies, and animal models of infection that serve as indicators of in vivo efficacy.
Passive immunization candidates that have been evaluated for efficacy are listed in Table 1. The human polyclonal immunoglobulin G (IgG), Altastaph, was the first antibody-based S. aureus therapy to go into Phase II clinical trial [12]. Altastaph is IgG obtained from the plasma of human donors immunized with a vaccine composed of S. aureus type 5 and type 8 capsular polysaccharide (CPS) [13]. Altastaph was shown to provide protection in mice infected with S. aureus and to enhance opsonophagocytosis of S. aureus [13]. However, in a study that was primarily designed to study the safety and kinetics of Altastaph, there was no indication that Altastaph protected against the development of bacteremia [12, 13]. While likely not the sole reason for its failure, in targeting S. aureus CPS 5 and 8, Altastaph has a limited potential for broad utilization, as 10–15% of the contemporary strains do not produce CP5 or CP8 due to mutations in the capsule coding genes or in capsule regulatory loci [14, 15]. Interestingly, it was recently shown in a large geographical screen of isolates from the S. aureus USA300 lineage, which is responsible for the current community-associated (CA) MRSA outbreak in the USA, that the strain has multiple mutations in the cap5 locus (cap5 promoter, cap5D nucleotide 994, and cap5E nucleotide 223) that makes it negative for CPS type 5 [16]. Veronate™ was an additional anti-Staphylococcus candidate therapy to make it to clinical trial with the use of pooled human antibodies [17]. Veronate™ is composed of human sera exhibiting high antibody titers to the staphylococcal fibrinogen-binding proteins clumping factor A (ClfA) of S. aureus and to the Ser-Asp dipeptide repeat G (SdrG) of S. epidermidis [18]. Despite demonstrating an opsonic driven ability to promote bacterial uptake, protection in mouse infections with S. aureus, and the ability to disrupt S. aureus fibrinogen binding, Veronate™ failed to reduce the incidence of late-onset sepsis in Phase III clinical trial [17, 18].
Table 1.
Antibody-based Biologics Agents Evaluated for Clinical Efficacy
| Agent | Sponsor(s) | Product Class | Target or Mechanism |
Primary Clinical Indication |
Status* |
|---|---|---|---|---|---|
| Altastaph | Nabi Biotherapeutics | Pooled human immunoglobulin | Cap5 & Cap8 | Treatment of bacteremia | Not in active development |
| Veronate | Inhibitex / Bristol Myers-Squibb | Pooled human immunoglobulin | Surface adhesins ClfA & SdrG | Prevention of infection s in neonates | Not in active development |
| Aurexis Tefibazumab) | Inhibitex / Bristol Myers-Squibb | Humanized mAb | Surface adhesin ClfA | Treatment of bacteremia | Not in active development |
| Pagibaximab (BSYX-A110) | Biosynexus Inc. / GlaxoSmithKline / MedImmune | Humanized mouse chimeric mAb | Lipoteichoic acid | Prevention of sepsis in very low birth weight infants | Not in active development |
| Aurograb | NeuTec Pharma / Novartis | Single chain variable antibody fragment | ABC transporter GrfA | Treatment of severe, deep seated infections | Not in active development |
| SAR279356 (F598) | Alopexx Pharm / Sanofi -aventis | Human mAb | PNAG | Prevention of pneumonia | Not in active development |
| Salvecin AR-301, KBSA301) | Aridis Pharm. / Kenta Biotech. | Human mAb | Alpha-toxin | Prevention of pneumonia | Phase 1/2a ongoing |
| MEDI4893 | MedImmune | Human mAb | Alpha-toxin | Prevention of pneumonia | Phase 1/2a ongoing |
| 514G3 | Xbiotech | Human mAb | Not disclosed | Treatment of bacteremia | Phase 1/2a ongoing |
Status based on publications and/or review of Sponsor website information and materials accessible via www.clinicaltrials.gov on 06-30-2015.
Attempts to treat infections with mAbs targeting S. aureus surface proteins have also been evaluated in clinical trials but have thus far failed to progress to regulatory approval. Like Veronate™, the mAb tefibazumab targets ClfA and was evaluated as an adjunctive therapy to standard antibiotic treatment in S. aureus bacteremia patients. Tefibazumab was shown to protect rabbits from S. aureus infection and while the authors elected to not publish the data, Tefibazumab also demonstrated opsonophagocytic activity [19, 20]. Tefibazumab was shown to be tolerated well in a Phase II trial focused on safety and pharmacokinetics. However, the makers of Tefibazumab indefinitely suspended trials pending the outcome of licensing negotiations [19, 21, 22]. Pagibaximab, a mAb that targets lipoteichoic acid (LTA), a key component of the bacterial Gram-positive cell wall, was shown to enhance opsonophagocytosis of Staphylococcus epidermidis (no data on S. aureus). Despite showing promise in Phase II studies for the prevention of S. aureus sepsis in low birth weight neonates, Pagibaximab ultimately failed in a more comprehensive Phase III sepsis trial [23–25]. The single-chain mAb fragment, Aurograb, targets the S. aureus ATP-binding cassette (ABC) transporter GrfA. This molecule was selected from an innovative screen that identified immunodominant antigens in the sera of septic patients [26]. While Aurograb was demonstrated to work synergistically with vancomycin to reduce S. aureus burden in the organs of infected mice, the drug was shown to be ineffective in a Phase II trial [26–28]. Of note, no peer-reviewed preclinical data has been made available for Aurograb. Finally, the exploratory efficacy of SAR279356 (F598), a mAb from the company Sanofi, targets poly-N-acetylated glucosamine (PNAG) and was investigated in the prevention of infections in mechanically ventilated patients in the intensive care unit. However, according to a press release from Sanofi, the clinical trial was prematurely terminated due to a slow enrollment rate [29].
Currently, there are three anti-staphylococcal mAbs being evaluated for clinical efficacy. In contrast to previous failed passive immunization studies, which have targeted surface-attached antigens, two of these mAbs target a secreted virulence factor, specifically alpha-toxin (AT). AT binds to host target cells in a receptor-dependent manner and forms permeable pores in their plasma membranes, which eventually lead to cell death [30]. Isogenic mutants for hla, the gene encoding AT, are attenuated in their ability to cause disease in MRSA pneumonia, SSTI, and bloodstream infection models [30–34]. The human mAb MEDI4893 from MedImmune (a division of AstraZeneca) targets a specific domain of the AT protein, which blocks AT-receptor binding [35]. This potential therapy has been shown to be protective prophylactically in the S. aureus mouse model for acute pneumonia [36]. Similarly, Aridis Pharmaceuticals states that the human mAb AR-301 prevents AT from generating pores in the host membrane, thus preventing cell death [37]. Both agents are being studied in the prevention of staphylococcal pneumonia. Lastly, Xbiotech is testing a human-derived mAb, for which the target has not been disclosed, as an adjunctive therapy to standard of care antibiotics in patients with diagnosed staphylococcal bacteremia [38].
Why so many clinical failures?
Antibody-based passive immunization agents that have thus far failed in clinical trial share a number of commonalities that might explain their lack of apparent efficacy. Firstly, they have all targeted a single cell-surface associated antigen [7–11]. Given the vast array of extracellular factors that S. aureus possesses, it is unlikely that focusing on a single antigen will cripple the bacterium. Another potential issue with targeting antibodies to surface proteins arose with the vaccine V710, which targets the cell wall-anchored iron-responsive surface determinant B (IsdB) [39]. In the Phase III clinical trial, patients who were administered this vaccine and developed a S. aureus infection were five times more likely to die than unvaccinated patients who developed a S. aureus infection [40]. This result reflects the general inadequacy of S. aureus therapeutic clinical trials to date and reiterates the complexities of S. aureus pathogenesis.
The second issue with previous antibody-based strategies is the emphasis placed on the demonstration of phagocytic uptake and/or opsonophagocytic killing [13, 18, 20, 23, 41, 42]. The use of opsonophagocytosis as an indicator for the efficacy of an antimicrobial immunotherapy became mainstream with the success of pneumococcal vaccines targeting capsular polysaccharides. These vaccines induce the production of opsonizing antibodies that lead to pneumococcal killing [43] and for which opsonophagocytotic antibody titers represent an accepted correlate of clinical protection. However, while a number of preclinical anti-S. aureus antibodies have demonstrated both opsonophagocytic activity and antibody efficacy in protecting mice from S. aureus infection, there has been no protection reported in human studies. Given the high levels of naturally occurring antibodies directed at S. aureus found in humans, it is possible that human serum may provide adequate opsonization and that increasing the amount makes no appreciable difference for S. aureus killing [3]. This opens up the concept of S. aureus’s often-underappreciated ability to survive within phagocytic cells. It has been demonstrated that S. aureus, classically regarded as an extracellular pathogen, is capable of surviving within phagocytic cells [44–48]. S. aureus is able to alter its transcriptional profile once inside human neutrophils and upregulate a number of secreted factors such as hemolysins and leukotoxins in response to neutrophil-derived anti-microbial components, which promote bacterial survival [45, 48]. It was also recently demonstrated that CA-MRSA strains utilize the bi-component pore-forming leukocidin AB (LukAB) and the alpha-phenol soluble modulin (PSM-α) to survive and escape from neutrophils and human monocytes [47, 49, 50]. Thus, when designing immunization strategies, it may be more prudent to place less of an emphasis on S. aureus uptake within phagocytic cells, but rather shift the focus to neutralizing S. aureus secreted factors that promote bacterial survival within phagocytes.
The lack of translation from S. aureus animal models to clinical studies has been the third major issue with failed immunization strategies. The mouse model for S. aureus has been especially nefarious, as vaccine and therapeutic antibodies that have shown protection in mice have not proved similarly efficacious in humans. One potential explanation for this paradox is the surplus of human-specific virulence factors produced by S. aureus. The deficiency exhibited by mouse models is also echoed in other models including rabbits, rats and non-human primates (e.g., Java monkey and cynomolgus macaques) [39, 51–54]. Thus, the reality is that faithful representative non-clinical models of staphylococcal infections in humans are lacking.
Next generation biologics target immune evasion mechanisms
The failure of first-generation antibody-based biologic agents and the inadequacy of antibiotics in severe, invasive S. aureus diseases may in part be accounted for by the array of different mechanisms used by the bacterium to circumvent components of the host immune system [5, 55, 56]. A number of such mechanisms being currently targeted by experimental antibody-based biologic agents are shown in Figure 1. These include: (i) surface-bound proteins that typically mediate adhesion to host tissues, cells and soluble factors, but can also impact the normal complement activation pathway [57], (ii) surface-anchored and secreted forms of immunoglobulin binding proteins, Protein-A and the second immunoglobulin-binding protein (Sbi), that sequester host IgG’s and impair their ability to engage elements of the host immune system via their Fc domain [58], (iii) surface-anchored and secreted forms of the GluV8 protease, that cleaves IgGs in the antibody hinge region and inactivates effector function [59], (iv) superantigens (SAgs), a family of potent immuno-stimulatory exotoxins that impair normal host immune functions via a number of distinct mechanisms [60], (v) leukocidal toxins that selectively target and kill immune cells including neutrophils and macrophages [61] and (vi) highly immunogenic cell wall autolysins that promote bacterial uptake into non-professional phagocytes, which facilitates the avoidance of the immune response and antibiotic treatment [62, 63]. Table 2 lists a number of preclinical programs described in recent meetings and publications wherein one or more of these immune evasion mechanisms are targeted via antibody-based biologic agents. This is table is meant to broadly depict the state of preclinical anti-staphylococcal therapeutics and does not comprehensively encompass all patents and publications.
Table 2.
Preclinical Antibody-based Biologics
| Target(s) or Mechanism(s) | Class | Sponsor(s) | Key Reference |
|---|---|---|---|
| Wall Techoic Acid (mAb) RNA synthesis or other targets (antibiotic) | mAb-drug conjugate | Genentech | Lehar et al., (2015) |
| Bi-component leukotoxins plus alpha hemolysin | mAb | Arsanis Biosciences | Rouha et al., (2015) |
| Surface adhesins, Bi-component leukotoxins, protein A, Sbi & GluV8 | Multi-valent biologic: mAb-centyrin fusion (‘mAbtyrin’) | Janssen Research & Development / NYU | U.S Patent Application US 2014/069347 |
| Protein A | mAb | NA | Thammavongsa et al., (2015) |
| Staphylococcal enterotoxin B (SEB) | mAb cocktail | NA | Dutta et al., (2015) |
| Immunodominant staphylococcal antigen A (IsaA) | mAb | Top Institute Pharma | van den Berg et al., (2015) |
| Glucosaminidase (Gmd) subunit of autolysin (Atl) | mAb | Telephus Medical | Varrone et al., (2014) |
| Staphylococcal enterotoxin B (SEB) | mAb | Integrated BioTherapeutics | Karauzum et al., (2012) |
| Panton-Valentine leukocidin (PVL) and γ-hemolysin C (HlgC) | Multi-valent heavy chain only antibody (HCAb) | NA | Laventie et al., (2011) |
The repeated inadequacies and lack of solid epidemiology displayed by antibody-based biologics that have targeted single S. aureus surface antigens are indicative of the likely futility of such a strategy. Hence, numerous novel pre-clinical programs are focused on the combination of antibodies targeting different antigens or in the design of multivalent biologic agents. One such approach from Dutta et al. (2015) utilizes a mAb cocktail consisting of two distinct antibodies to the SAg protein staphylococcal enterotoxin B (SEB) that yield synergistic efficacy in mice. This cocktail was shown to neutralize toxin and provide protection from intoxication with purified SEB in mice. One potential limitation in targeting SEB is the geographical driven variation that exists in the frequency of this toxin [65]. Precedents for the use of mAb cocktails in passive immunization therapy include the investigational anti-Ebola treatment ZMapp [66] and the combination of actoxumab and bezlotoxumab that neutralize the cytotoxic/cytopathic effects of Clostridium difficile toxins TcdA and TcdB, which are currently being evaluated by Merck in Phase III studies [67]. Given the complexity of S. aureus, it is unreasonable to think that the successful tactics used to combat other pathogens will directly translate to S. aureus, but the efficacy shown in targeting toxins with biologics is encouraging. In addition to passive immunization approaches that exploit multivalent biologic agents, a number of anti-staphylococcal vaccines are in development that are composed of multiple antigens. For example, 4C-Staph is an experimental multivalent vaccine developed by Novartis (now GSK) that leads to the production of antibodies that target three secreted virulence factors and two surface-attached proteins [68]. This vaccine shows protection in multiple mouse models for infection and all antigens were needed to generate an optimal immune response.
Aside from the afore-mentioned agents that singly target AT, several of groups have reported the identification and characterization of antibodies that neutralize the cytolytic activity of additional S. aureus toxins and therein may protect further classes of immune cells (Figure 2). For instance, Rouha et al. recently described a single mAb that targets AT and 3 bi-component leukocidins; gamma hemolysin (HlgABC), leukocidin ED (LukED), and Panton-Valentine Leukocidin (PVL) [69]. This multivalent mAb was shown to inhibit the cytotoxicity of these molecules in vitro and to provide protection in mouse sepsis and pneumonia models, reflecting the activity of this mAb against AT [69]. Additional current examples of targeting immune evasion/altering factors, include the staphylococcal super-antigen SEB (Dutta et al., 2015) [70], Protein-A [71, 72], the glucosaminidase subunit of autolysin (Atl) [73], and the immunodominant staphylococcal antigen A (IsaA) [74].
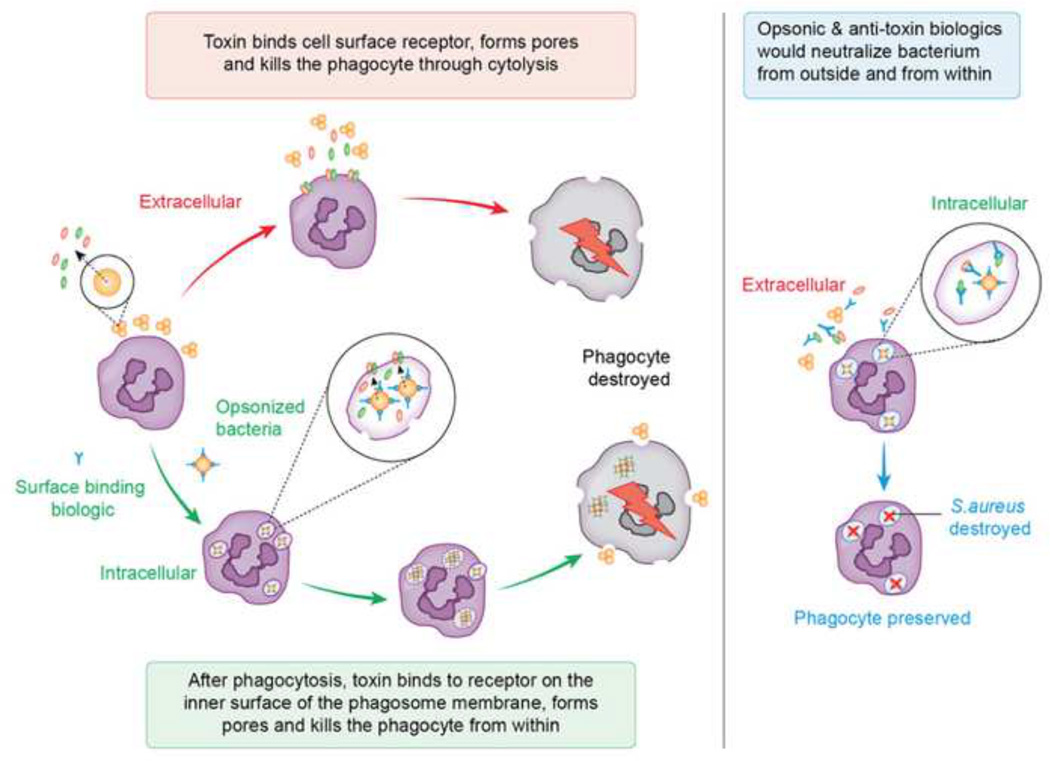
Figure 2. Extracellular and Intracellular killing mediated by S. aureus toxins.
S. aureus implements a multi-pronged strategy to kill host immune cells from both outside of the cell and from within. Extracellular cytolysis: On the surface of the host cell, S. aureus delivers pore-forming toxins that target specific host receptors and destroy the phagocyte through cell lysis. Intracellular cytolysis: S. aureus is opsonized with immunglobulins and complement, which leads to phagocytosis of a subset of this population. Intracellular bacteria then promote their survival by releasing toxins that disrupt the integrity of the phagolysosome, promote bacterial escape into the cytoplasm, and destroy the phagocyte from within. Approaches to block extracellular and intracellular cytolysis: Current biologics under development attempt to account for all facets of this strategy using molecules that opsonize the bacterium and neutralize the toxins that are responsible for causing cell death, thus disarming the bacterium from the outside and from within. With this approach, the phagocyte is preserved and S. aureus is destroyed in the phagolysosome.
Finally, there is a growing appreciation of the ability of S. aureus to persist intracellularly within different classes of host cells and therein is protected from the host immune system [5, 56]. With this understanding, a number of approaches are being explored towards the tailoring of therapeutics that attack S. aureus in both extracellular and intracellular environments. In one such effort, Genentech has described an antibody-drug conjugate that combines an anti-staphylococcal antibiotic with an opsonic antibody that facilitates phagocyte uptake and antibiotic-mediated killing within the phagosome [75, 76]. This novel antibody-antibiotic conjugate specifically targets both replicating and non-replicating intracellular bacteria, as the conjugated rifamycin-class antibiotic is not activated through proteolytic release until reaching the phagolysosome. In contrast, Janssen Research and Development has recently described a series of multi-valent biologics that combine as fusion proteins anti-staphylococcal mAbs with novel protein binding domains referred to as ‘centyrins’ [77, 78]. Specifically, these IgG-centyrin fusion proteins (referred to as ‘mAbtyrins’) target a family of Serine-Aspartate Repeat (SDR) surface-anchored adhesin proteins via the mAb portion of the molecule, and bind and neutralize the leukocidins via appended centyrin domains. In addition, the Fc portion of the mAb has been further engineered to resist hinge-directed proteolysis via GluV8 protease and eliminate Protein A and Sbi binding while retaining normal Fc-mediated interactions with the host immune system (Brezski et al., Pat. App. US 2014/069347)). These novel multi-valent biologics exhibit activity against MRSA in vitro, ex vivo and in vivo and are thought to be active intracellularly via combination of effective opsonization via the mAb portion of the molecule and neutralization of LukAB-mediated phagosome lysis (Sause et al., unpublished data).
Clinical utility of antibody-based biologics targeting S. aureus: potential indications and concerns
In the treatment of S. aureus infections, mAb-based biologics offer the promise to decrease unacceptably high levels of morbidity and mortality in severe, invasive disease states like pneumonia and bacteremia as agents adjunctive to available antibiotics. Clinical benefit of such adjunctive therapy could arise directly through synergy at the mechanistic level and/or indirectly through effective boosting of antibiotic efficacy through enhanced assistance from host innate and adaptive immune defense pathways. Should such success be realized, additional benefits would include providing physicians with alternate options to limit the unnecessary use of antibiotic agents “of last resort” and potentially preserve such agents with regard to the development and spread of antibiotic resistance. Indeed, for antibody-based biologic agents used in acute critical care treatment settings, the development of resistance by S. aureus is not anticipated through traditional mechanisms and therein one can anticipate a long shelf-life of clinical utility. Furthermore, developments in the field of molecular diagnostics that enable the rapid identification and characterization of infectious pathogens should facilitate targeted therapy in patients with predicted susceptibility to antibody-based biologic agents (see Outstanding Questions). Finally, should trends in the increasing emergence and spread of S. aureus isolates that exhibit reduced susceptibility to vancomycin, daptomycin or linezolid continue, the use of antibody-based biologic agents could conceivably play a role in the first-line management of serious infections in the absence of the development of truly novel classes of antibiotic agents that circumvent existing resistance mechanisms.
Outstanding Questions Box.
Will targeting of S. aureus host immune evasion factors by antibody-based biologic agents enhance antibiotic efficacy in serious invasive disease states through effective boosting of host immune functions?
How will new advances in molecular diagnostics facilitate the clinical development and use of new S. aureus vaccines and biologic agents?
If approved, will S. aureus vaccines and repeated use of antibody-based biologic agents select for insensitive phenotypic variants through antigenic drift?
Antibody-based biologic agents also have potential clinical utility in the prevention of disease in subjects at high-risk for S. aureus infections. In this setting, acute (single dose prophylaxis) use in patients undergoing elective or emergency invasive surgical procedures at highest-risk for hospital-acquired infections, represent a key target population with initial clinical development likely as therapy adjunctive to prophylactic antibiotics. Additional clinical utility may also be realized in patients that undergo periodic procedures that place them at risk of S. aureus infections; for instance, hemodialysis in patients with chronic kidney disease. In such settings, chronic (repeat dose) use of antibody-based biologic agents may require monitoring of patients to determine that anti-drug antibodies are not generated in patients that trigger adverse immune reactions to, or diminish the effectiveness of, such agents. Such a phenomenon would likely be more problematic for antibody-based biologics that are elaborated over and above simple human-derived or humanized mAbs. Relatedly, the chronic use of antibody-based biologics in individual patients could conceivably result in the emergence of genetic “escape” variants with reduced susceptibility to the agent through antigenic drift (see Outstanding Questions). Such a concern also applies to anti-staphylococcal vaccines along with the potential that broad community use of vaccines may facilitate the selection and emergence of new epidemic clades that evade such vaccines. In considering the future overall potential roles of anti-staphylococcal vaccines and antibody-based biologic agents, the use of the latter would presumably be favored in both prophylactic and treatment setting in acute care patients that do not have time to develop an effective immunological response to a vaccine. Similarly, antibody-based biologics would presumably also be favored in immune-suppressed patients or in elderly patients with immune senescence.
The practicalities of drug discovery and development in the modern era dictate that new antibody-based therapies for bacterial infections will necessitate relatively high initial pricing equivalent to, or increased over, current “premium” priced antibiotics currently reserved for use in second or third line therapy. However, over and above the benefits afforded to individual patients receiving mAb-based anti-staphylococcal therapy, the anticipated high costs of a truly effective agent would presumably be offset through projected reductions in the overall healthcare costs associated with shorter hospitalization stays and a lower incidence of infections or secondary complications. In the longer term, it is hoped that technological developments in the manufacturing of mAb-based biologics will facilitate reductions in the costs of production and enable their broader adoption and utilization.
Concluding remarks
The first successful therapeutic serum treatment of a child suffering from a bacterial infection, diphtheria caused by Corynebacterium diphtheriae, occurred in 1891 and for this success and later accomplishments Emil von Behring was recognized by the award of the first Nobel Prize in medicine in 1901. However, despite more than a century of research in understanding the pathophysiology of key human pathogens and developments in the synthesis and production of antibodies, approval of the first monoclonal antibody produced by recombinant DNA technology did not occur until 1998 with the approval of Synagis® (MedImmune). Indeed, Synagis® remains the only mAb approved today for routine infectious disease clinical practice and is limited to use in the prevention of serious lower respiratory tract disease caused by respiratory syncytial virus (RSV) in children at high risk of RSV disease.
With regard to staphylococcal disease, this review has highlighted potential reasons underlying the failures of past efforts to develop effective vaccines and antibody-based biologics and reasons to believe that a new generation of mAb therapies may offer hope in the prevention and/or treatment of severe, invasive disease for which antibiotic therapy is too often inadequate. These include agents that seek to neutralize virulence factors that target elements of the host immune system and therein should boost or restore host immunity and potentiate antibiotic effectiveness and multi-valent agents designed to target the bacterium’s elaborate extracellular and intracellular lifestyles (see Outstanding Questions). While additional clinical failures are likely, lessons learned from the preclinical and clinical evaluation of these new agents, combined with further advances in our understanding of the pathophysiology of S. aureus, hold real promise for the future utility of mAb-based biologic agents in the prevention and treatment of serious disease mediated by this remarkable human-centric pathogen.
Trends Box.
Community-onset, invasive infections mediated by Staphylococcus aureus infections represent a growing concern in global healthcare
The paucity of novel antibiotics for the treatment of serious invasive diseases caused by S. aureus has spurred renewed interest in the discovery and development of alternate therapies including antibody-based biologic agents
Past failures in the clinical development of anti-staphylococcal antibodies for the treatment and prevention of S. aureus infections have focused on single antigens displayed at the bacterial cell surface
Two antibody-based biologic agents currently under clinical evaluation target the secreted virulence factor alpha-hemolysin
A series of novel approaches are being explored towards the discovery and development of antibody-based biologic agents that target multiple S. aureus antigens including host immune evasion factors and intracellular reservoirs of the bacterium
Glossary
- S. aureus
Staphylococcus aureus
- MRSA
Methicillin-resistant Staphylococcus aureus
- mAbs
monoclonal antibodies
- Active immunity
immunity provided by antibodies that are generated in response to an antigen, such as a vaccine.
- Bacteremia
bacteria in the bloodstream
- Opsonophagocytosis
phagocytosis of an opsonized pathogen.
- Passive immunity
short-term immunity provided by exogenous antibodies.
- SSTI
skin and soft tissue infection
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dantes R, Mu Y, Belflower R, Aragon D, Dumyati G, Harrison LH, et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA internal medicine. 2013;173(21):1970–1978. doi: 10.1001/jamainternmed.2013.10423. PubMed PMID: 24043270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verkaik NJ, de Vogel CP, Boelens HA, Grumann D, Hoogenboezem T, Vink C, et al. Anti-staphylococcal humoral immune response in persistent nasal carriers and noncarriers of Staphylococcus aureus. J Infect Dis. 2009;199(5):625–632. doi: 10.1086/596743. Epub 2009/02/10. PubMed PMID: 19199541. [DOI] [PubMed] [Google Scholar]
- 3.Dryla A, Prustomersky S, Gelbmann D, Hanner M, Bettinger E, Kocsis B, et al. Comparison of antibody repertoires against Staphylococcus aureus in healthy individuals and in acutely infected patients. Clin Diagn Lab Immunol. 2005;12(3):387–398. doi: 10.1128/CDLI.12.3.387-398.2005. PubMed PMID: 15753252; PubMed Central PMCID: PMCPMC1065207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holtfreter S, Roschack K, Eichler P, Eske K, Holtfreter B, Kohler C, et al. Staphylococcus aureus carriers neutralize superantigens by antibodies specific for their colonizing strain: a potential explanation for their improved prognosis in severe sepsis. J Infect Dis. 2006;193(9):1275–1278. doi: 10.1086/503048. PubMed PMID: 16586365. [DOI] [PubMed] [Google Scholar]
- 5.Spaan AN, Surewaard BG, Nijland R, van Strijp JA. Neutrophils Versus Staphylococcus aureus: A Biological Tug of War. Annu Rev Microbiol. 2013 doi: 10.1146/annurev-micro-092412-155746. Epub 2013/07/10. PubMed PMID: 23834243. [DOI] [PubMed] [Google Scholar]
- 6.Thammavongsa V, Kim HK, Missiakas D, Schneewind O. Staphylococcal manipulation of host immune responses. Nature Reviews Microbiology. 2015;13(9):529–543. doi: 10.1038/nrmicro3521. PubMed PMID: WOS:000359653100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scully IL, Liberator PA, Jansen KU, Anderson AS. Covering all the Bases: Preclinical Development of an Effective Staphylococcus aureus Vaccine. Front Immunol. 2014;5:109. doi: 10.3389/fimmu.2014.00109. PubMed PMID: 24715889; PubMed Central PMCID: PMCPMC3970019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fowler VG, Jr, Proctor RA. Where does a Staphylococcus aureus vaccine stand? Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2014;20(Suppl 5):66–75. doi: 10.1111/1469-0691.12570. Epub 2014/01/31. PubMed PMID: 24476315; PubMed Central PMCID: PMC4067250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jansen KU, Girgenti DQ, Scully IL, Anderson AS. Vaccine review: "Staphyloccocus aureus vaccines: problems and prospects". Vaccine. 2013;31(25):2723–2730. doi: 10.1016/j.vaccine.2013.04.002. PubMed PMID: 23624095. [DOI] [PubMed] [Google Scholar]
- 10.Daum RS, Spellberg B. Progress toward a Staphylococcus aureus vaccine. Clin Infect Dis. 2012;54(4):560–567. doi: 10.1093/cid/cir828. PubMed PMID: 22186773; PubMed Central PMCID: PMCPMC3404717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salgado-Pabon W, Schlievert PM. Models matter: the search for an effective Staphylococcus aureus vaccine. Nat Rev Microbiol. 2014;12(8):585–591. doi: 10.1038/nrmicro3308. PubMed PMID: 24998740. [DOI] [PubMed] [Google Scholar]
- 12.Benjamin DK, Schelonka R, White R, Holley HP, Bifano E, Cummings J, et al. A blinded, randomized, multicenter study of an intravenous Staphylococcus aureus immune globulin. J Perinatol. 2006;26(5):290–295. doi: 10.1038/sj.jp.7211496. Epub 2006/04/07. PubMed PMID: 16598296. [DOI] [PubMed] [Google Scholar]
- 13.Fattom AI, Sarwar J, Ortiz A, Naso R. A Staphylococcus aureus capsular polysaccharide (CP) vaccine and CP-specific antibodies protect mice against bacterial challenge. Infect Immun. 1996;64(5):1659–1665. doi: 10.1128/iai.64.5.1659-1665.1996. Epub 1996/05/01. PubMed PMID: 8613375; PubMed Central PMCID: PMC173976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verdier I, Durand G, Bes M, Taylor KL, Lina G, Vandenesch F, et al. Identification of the capsular polysaccharides in Staphylococcus aureus clinical isolates by PCR and agglutination tests. J Clin Microbiol. 2007;45(3):725–729. doi: 10.1128/JCM.01572-06. Epub 2007/01/05. PubMed PMID: 17202275; PubMed Central PMCID: PMC1829147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cocchiaro JL, Gomez MI, Risley A, Solinga R, Sordelli DO, Lee JC. Molecular characterization of the capsule locus from non-typeable Staphylococcus aureus. Molecular microbiology. 2006;59(3):948–960. doi: 10.1111/j.1365-2958.2005.04978.x. PubMed PMID: 16420363. [DOI] [PubMed] [Google Scholar]
- 16.Boyle-Vavra S, Li X, Alam MT, Read TD, Sieth J, Cywes-Bentley C, et al. USA300 and USA500 Clonal Lineages of Staphylococcus aureus Do Not Produce a Capsular Polysaccharide Due to Conserved Mutations in the cap5 Locus. Mbio. 2015;6(2) doi: 10.1128/mBio.02585-14. doi: ARTN e02585 PubMed PMID: WOS:000355312400012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeJonge M, Burchfield D, Bloom B, Duenas M, Walker W, Polak M, et al. Clinical trial of safety and efficacy of INH-A21 for the prevention of nosocomial staphylococcal bloodstream infection in premature infants. J Pediatr. 2007;151(3):260–265. 5 e1. doi: 10.1016/j.jpeds.2007.04.060. Epub 2007/08/28. PubMed PMID: 17719934. [DOI] [PubMed] [Google Scholar]
- 18.Vernachio JH, Bayer AS, Ames B, Bryant D, Prater BD, Syribeys PJ, et al. Human immunoglobulin G recognizing fibrinogen-binding surface proteins is protective against both Staphylococcus aureus and Staphylococcus epidermidis infections in vivo. Antimicrobial agents and chemotherapy. 2006;50(2):511–518. doi: 10.1128/AAC.50.2.511-518.2006. Epub 2006/01/27. PubMed PMID: 16436704; PubMed Central PMCID: PMC1366878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall AE, Domanski PJ, Patel PR, Vernachio JH, Syribeys PJ, Gorovits EL, et al. Characterization of a protective monoclonal antibody recognizing Staphylococcus aureus MSCRAMM protein clumping factor A. Infect Immun. 2003;71(12):6864–6870. doi: 10.1128/IAI.71.12.6864-6870.2003. Epub 2003/11/26. PubMed PMID: 14638774; PubMed Central PMCID: PMC308922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patti JM. A humanized monoclonal antibody targeting Staphylococcus aureus. Vaccine. 2004;22(Suppl 1):S39–S43. doi: 10.1016/j.vaccine.2004.08.015. Epub 2004/12/04. PubMed PMID: 15576200. [DOI] [PubMed] [Google Scholar]
- 21.Weems JJ, Jr, Steinberg JP, Filler S, Baddley JW, Corey GR, Sampathkumar P, et al. Phase II, randomized, double-blind, multicenter study comparing the safety and pharmacokinetics of tefibazumab to placebo for treatment of Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2006;50(8):2751–2755. doi: 10.1128/AAC.00096-06. Epub 2006/07/28. PubMed PMID: 16870768; PubMed Central PMCID: PMC1538656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.John JF., Jr Drug evaluation: tefibazumab--a monoclonal antibody against staphylococcal infection. Current opinion in molecular therapeutics. 2006;8(5):455–460. Epub 2006/11/03. PubMed PMID: 17078388. [PubMed] [Google Scholar]
- 23.Weisman LE, Thackray HM, Garcia-Prats JA, Nesin M, Schneider JH, Fretz J, et al. Phase 1/2 double-blind, placebo-controlled, dose escalation, safety, and pharmacokinetic study of pagibaximab (BSYX-A110), an antistaphylococcal monoclonal antibody for the prevention of staphylococcal bloodstream infections, in very-low-birth-weight neonates. Antimicrobial agents and chemotherapy. 2009;53(7):2879–2886. doi: 10.1128/AAC.01565-08. PubMed PMID: 19380597; PubMed Central PMCID: PMCPMC2704668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weisman LE, Thackray HM, Steinhorn RH, Walsh WF, Lassiter HA, Dhanireddy R, et al. A randomized study of a monoclonal antibody (pagibaximab) to prevent staphylococcal sepsis. Pediatrics. 2011;128(2):271–279. doi: 10.1542/peds.2010-3081. Epub 2011/07/27. PubMed PMID: 21788224. [DOI] [PubMed] [Google Scholar]
- 25.Safety and efficacy of Pagibaximab injection in very low birth weight neonates for prevention of staphylococcal sepsis [Internet] 2011 Available from: https://clinicaltrials.gov/ct2/show/study/NCT00646399.
- 26.Burnie JP, Matthews RC, Carter T, Beaulieu E, Donohoe M, Chapman C, et al. Identification of an immunodominant ABC transporter in methicillin-resistant Staphylococcus aureus infections. Infect Immun. 2000;68(6):3200–3209. doi: 10.1128/iai.68.6.3200-3209.2000. Epub 2000/05/19. PubMed PMID: 10816464; PubMed Central PMCID: PMC97562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Otto M. Novel targeted immunotherapy approaches for staphylococcal infection. Expert opinion on biological therapy. 2010;10(7):1049–1059. doi: 10.1517/14712598.2010.495115. Epub 2010/06/10. PubMed PMID: 20528609; PubMed Central PMCID: PMC2885018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baker M. Anti-infective antibodies: finding the path forward. Nat Biotechnol. 2006;24(12):1491–1493. doi: 10.1038/nbt1206-1491. PubMed PMID: 17160047. [DOI] [PubMed] [Google Scholar]
- 29.Sanofi. A randomized, double-blind, placebo-controlled trial to assess the pharmacokinetics, pharmacodynamics, and safety of a single dose of SAR279356 in patients hospitalized in intensive care unit and on mechanical ventilation. 2012 Available from: http://en.sanofi.com/img/content/study/PKD11791_summary.pdf. [Google Scholar]
- 30.Berube BJ, Bubeck Wardenburg J. Staphylococcus aureus alpha-toxin: nearly a century of intrigue. Toxins (Basel) 2013;5(6):1140–1166. doi: 10.3390/toxins5061140. PubMed PMID: 23888516; PubMed Central PMCID: PMCPMC3717774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bubeck Wardenburg J, Schneewind O. Vaccine protection against Staphylococcus aureus pneumonia. J Exp Med. 2008;205(2):287–294. doi: 10.1084/jem.20072208. PubMed PMID: 18268041; PubMed Central PMCID: PMCPMC2271014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kennedy AD, Bubeck Wardenburg J, Gardner DJ, Long D, Whitney AR, Braughton KR, et al. Targeting of alpha-hemolysin by active or passive immunization decreases severity of USA300 skin infection in a mouse model. J Infect Dis. 2010;202(7):1050–1058. doi: 10.1086/656043. PubMed PMID: 20726702; PubMed Central PMCID: PMCPMC2945289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Powers ME, Kim HK, Wang Y, Bubeck Wardenburg J. ADAM10 mediates vascular injury induced by Staphylococcus aureus alpha-hemolysin. J Infect Dis. 2012;206(3):352–356. doi: 10.1093/infdis/jis192. PubMed PMID: 22474035; PubMed Central PMCID: PMCPMC3392186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sampedro GR, DeDent AC, Becker RE, Berube BJ, Gebhardt MJ, Cao H, et al. Targeting Staphylococcus aureus alpha-toxin as a novel approach to reduce severity of recurrent skin and soft-tissue infections. J Infect Dis. 2014;210(7):1012–1018. doi: 10.1093/infdis/jiu223. PubMed PMID: 24740631; PubMed Central PMCID: PMCPMC4207862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oganesyan V, Peng L, Damschroder MM, Cheng L, Sadowska A, Tkaczyk C, et al. Mechanisms of Neutralization of a Human Anti-alpha-toxin Antibody. Journal of Biological Chemistry. 2014;289(43):29874–29880. doi: 10.1074/Jbc.M114.601328. PubMed PMID: WOS:000344370800031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hua L, Hilliard JJ, Shi Y, Tkaczyk C, Cheng LI, Yu X, et al. Assessment of an Anti-Alpha-Toxin Monoclonal Antibody for Prevention and Treatment of Staphylococcus aureus-Induced Pneumonia. Antimicrobial Agents and Chemotherapy. 2014;58(2):1108–1117. doi: 10.1128/Aac.02190-13. PubMed PMID: WOS:000330637500059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pharmaceuticals A. AR-301: Fully Human mAb Against Staphylococcus aureus. 2015 Available from: http://www.aridispharma.com/ar301.html. [Google Scholar]
- 38.XBiotech. MRSA Antibody by Xbiotech MRSA therapeutic antibody. 2015 Available from: http://www.xbiotech.com/clinical/mrsa.html. [Google Scholar]
- 39.Kuklin NA, Clark DJ, Secore S, Cook J, Cope LD, McNeely T, et al. A novel Staphylococcus aureus vaccine: iron surface determinant B induces rapid antibody responses in rhesus macaques and specific increased survival in a murine S. aureus sepsis model. Infect Immun. 2006;74(4):2215–2223. doi: 10.1128/IAI.74.4.2215-2223.2006. Epub 2006/03/23. PubMed PMID: 16552052; PubMed Central PMCID: PMC1418914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fowler VG, Allen KB, Moreira ED, Moustafa M, Isgro F, Boucher HW, et al. Effect of an investigational vaccine for preventing Staphylococcus aureus infections after cardiothoracic surgery: a randomized trial. JAMA. 2013;309(13):1368–1378. doi: 10.1001/jama.2013.3010. Epub 2013/04/04. PubMed PMID: 23549582. [DOI] [PubMed] [Google Scholar]
- 41.Pozzi C, Wilk K, Lee JC, Gening M, Nifantiev N, Pier GB. Opsonic and protective properties of antibodies raised to conjugate vaccines targeting six Staphylococcus aureus antigens. PloS one. 2012;7(10):e46648. doi: 10.1371/journal.pone.0046648. PubMed PMID: 23077517; PubMed Central PMCID: PMCPMC3471903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fattom AI, Horwith G, Fuller S, Propst M, Naso R. Development of StaphVAX, a polysaccharide conjugate vaccine against S. aureus infection: from the lab bench to phase III clinical trials. Vaccine. 2004;22(7):880–887. doi: 10.1016/j.vaccine.2003.11.034. PubMed PMID: 15040941. [DOI] [PubMed] [Google Scholar]
- 43.Romero-Steiner S, Frasch C, Concepcion N, Goldblatt D, Kayhty H, Vakevainen M, et al. Multilaboratory evaluation of a viability assay for measurement of opsonophagocytic antibodies specific to the capsular polysaccharides of Streptococcus pneumoniae. Clin Diagn Lab Immunol. 2003;10(6):1019–1024. doi: 10.1128/CDLI.10.6.1019-1024.2003. Epub 2003/11/11. PubMed PMID: 14607861; PubMed Central PMCID: PMC262452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DuMont AL, Yoong P, Liu X, Day CJ, Chumbler NM, James DB, et al. Identification of a crucial residue required for Staphylococcus aureus LukAB cytotoxicity and receptor recognition. Infection and immunity. 2014;82(3):1268–1276. doi: 10.1128/IAI.01444-13. PubMed PMID: 24379286; PubMed Central PMCID: PMCPMC3958006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Voyich JM, Braughton KR, Sturdevant DE, Whitney AR, Said-Salim B, Porcella SF, et al. Insights into Mechanisms Used by Staphylococcus aureus to Avoid Destruction by Human Neutrophils. J Immunol. 2005;175(6):3907–3919. doi: 10.4049/jimmunol.175.6.3907. PubMed PMID: 16148137. [DOI] [PubMed] [Google Scholar]
- 46.Gresham HD, Lowrance JH, Caver TE, Wilson BS, Cheung AL, Lindberg FP. Survival of Staphylococcus aureus inside neutrophils contributes to infection. J Immunol. 2000;164(7):3713–3722. doi: 10.4049/jimmunol.164.7.3713. Epub 2000/03/22. PubMed PMID: 10725730. [DOI] [PubMed] [Google Scholar]
- 47.Surewaard BG, de Haas CJ, Vervoort F, Rigby KM, Deleo FR, Otto M, et al. Staphylococcal alpha-phenol soluble modulins contribute to neutrophil lysis after phagocytosis. Cell Microbiol. 2013 doi: 10.1111/cmi.12130. Epub 2013/03/09. PubMed PMID: 23470014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palazzolo-Ballance AM, Reniere ML, Braughton KR, Sturdevant DE, Otto M, Kreiswirth BN, et al. Neutrophil microbicides induce a pathogen survival response in community-associated methicillin-resistant Staphylococcus aureus. J Immunol. 2008;180(1):500–509. doi: 10.4049/jimmunol.180.1.500. PubMed PMID: 18097052. [DOI] [PubMed] [Google Scholar]
- 49.DuMont AL, Yoong P, Surewaard BG, Benson MA, Nijland R, van Strijp JA, et al. Staphylococcus aureus elaborates leukocidin AB to mediate escape from within human neutrophils. Infect Immun. 2013;81(5):1830–1841. doi: 10.1128/IAI.00095-13. Epub 2013/03/20. PubMed PMID: 23509138; PubMed Central PMCID: PMC3648020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Melehani JH, James DB, DuMont AL, Torres VJ, Duncan JA. Staphylococcus aureus Leukocidin A/B (LukAB) Kills Human Monocytes via Host NLRP3 and ASC when Extracellular, but Not Intracellular. PLoS Pathog. 2015;11(6):e1004970. doi: 10.1371/journal.ppat.1004970. PubMed PMID: 26069969; PubMed Central PMCID: PMCPMC4466499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee JC, Park JS, Shepherd SE, Carey V, Fattom A. Protective efficacy of antibodies to the Staphylococcus aureus type 5 capsular polysaccharide in a modified model of endocarditis in rats. Infect Immun. 1997;65(10):4146–4151. doi: 10.1128/iai.65.10.4146-4151.1997. Epub 1997/10/08. PubMed PMID: 9317020; PubMed Central PMCID: PMC175596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Malachowa N, Kobayashi SD, Braughton KR, Whitney AR, Parnell MJ, Gardner DJ, et al. Staphylococcus aureus Leukotoxin GH Promotes Inflammation. J Infect Dis. 2012;206(8):1185–1193. doi: 10.1093/infdis/jis495. Epub 2012/08/09. PubMed PMID: 22872735; PubMed Central PMCID: PMC3448972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spaan AN, Henry T, van Rooijen WJ, Perret M, Badiou C, Aerts PC, et al. The staphylococcal toxin Panton-Valentine Leukocidin targets human C5a receptors. Cell Host Microbe. 2013;13(5):584–594. doi: 10.1016/j.chom.2013.04.006. Epub 2013/05/21. PubMed PMID: 23684309. [DOI] [PubMed] [Google Scholar]
- 54.Loffler B, Hussain M, Grundmeier M, Bruck M, Holzinger D, Varga G, et al. Staphylococcus aureus panton-valentine leukocidin is a very potent cytotoxic factor for human neutrophils. PLoS Pathog. 2010;6(1):e1000715. doi: 10.1371/journal.ppat.1000715. Epub 2010/01/15. PubMed PMID: 20072612; PubMed Central PMCID: PMC2798753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Okumura CYM, Nizet V. Subterfuge and Sabotage: Evasion of Host Innate Defenses by Invasive Gram-Positive Bacterial Pathogens. Annual Review of Microbiology. 2014;68:439–458. doi: 10.1146/Annurev-Micro-092412-155711. Vol 68. PubMed PMID: WOS:000348452900024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Foster TJ. Immune evasion by staphylococci. Nat Rev Microbiol. 2005;3(12):948–958. doi: 10.1038/nrmicro1289. PubMed PMID: 16322743. [DOI] [PubMed] [Google Scholar]
- 57.Sharp JA, Echague CG, Hair PS, Ward MD, Nyalwidhe JO, Geoghegan JA, et al. Staphylococcus aureus Surface Protein SdrE Binds Complement Regulator Factor H as an Immune Evasion Tactic. Plos One. 2012;7(5) doi: 10.1371/journal.pone.0038407. oi: ARTN e38407 PubMed PMID: WOS:000305338500145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Atkins KL, Burman JD, Chamberlain ES, Cooper JE, Poutrel B, Bagby S, et al. S. aureus IgG-binding proteins SpA and Sbi: host specificity and mechanisms of immune complex formation. Mol Immunol. 2008;45:1600–1611. doi: 10.1016/j.molimm.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 59.Brezski RJ, Jordan RE. Cleavage of IgGs by proteases associated with invasive diseases An evasion tactic against host immunity? mAbs. 2010;2(3):212–220. doi: 10.4161/mabs.2.3.11780. PubMed PMID: WOS:000281388200002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu SX, McCormick JK. Staphylococcal superantigens in colonization and disease. Front Cell Infect Microbiol. 2012;2:52. doi: 10.3389/fcimb.2012.00052. Epub 2012/08/25. PubMed PMID: 22919643; PubMed Central PMCID: PMC3417409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alonzo F, 3rd, Torres VJ. The Bicomponent Pore-Forming Leucocidins of Staphylococcus aureus. Microbiology and molecular biology reviews : MMBR. 2014;78(2):199–230. doi: 10.1128/MMBR.00055-13. PubMed PMID: 24847020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van der Kooi-Pol MM, de Vogel CP, Westerhout-Pluister GN, Veenstra-Kyuchukova YK, Duipmans JC, Glasner C, et al. High Anti-Staphylococcal Antibody Titers in Patients with Epidermolysis Bullosa Relate to Long-Term Colonization with Alternating Types of Staphylococcus aureus. Journal of Investigative Dermatology. 2013;133(3):847–850. doi: 10.1038/Jid.2012.347. PubMed PMID: WOS:000315008500040. [DOI] [PubMed] [Google Scholar]
- 63.Hirschhausen N, Schlesier T, Schmidt MA, Gotz F, Peters G, Heilmann C. A novel staphylococcal internalization mechanism involves the major autolysin Atl and heat shock cognate protein Hsc70 as host cell receptor. Cell Microbiol. 2010;12(12):1746–1764. doi: 10.1111/j.1462-5822.2010.01506.x. Epub 2010/07/21. doi: CMI1506 [pii] 10.1111/j.1462-5822.2010.01506.x. PubMed PMID: 20642807. [DOI] [PubMed] [Google Scholar]
- 64.Dutta K, Varshney AK, Franklin MC, Goger M, Wang X, Fries BC. Mechanisms mediating enhanced neutralization efficacy of staphylococcal enterotoxin B by combinations of monoclonal antibodies. J Biol Chem. 2015;290(11):6715–6730. doi: 10.1074/jbc.M114.630715. PubMed PMID: 25572397; PubMed Central PMCID: PMCPMC4358096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nienaber JJ, Sharma Kuinkel BK, Clarke-Pearson M, Lamlertthon S, Park L, Rude TH, et al. Methicillin-susceptible Staphylococcus aureus endocarditis isolates are associated with clonal complex 30 genotype and a distinct repertoire of enterotoxins and adhesins. J Infect Dis. 2011;204(5):704–713. doi: 10.1093/infdis/jir389. PubMed PMID: 21844296; PubMed Central PMCID: PMCPMC3156104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qiu XG, Wong G, Audet J, Bello A, Fernando L, Alimonti JB, et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature. 2014;514(7520):47. doi: 10.1038/Nature13777. -+PubMed PMID: WOS:000342420800032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hernandez LD, Racine F, Xiao L, DiNunzio E, Hairston N, Sheth PR, et al. Broad coverage of genetically diverse strains of Clostridium difficile by actoxumab and bezlotoxumab predicted by in vitro neutralization and epitope modeling. Antimicrob Agents Chemother. 2015;59(2):1052–1060. doi: 10.1128/AAC.04433-14. Epub 2014/12/03. PubMed PMID: 25451052; PubMed Central PMCID: PMC4335902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bagnoli F, Fontana MR, Soldaini E, Mishra RPN, Fiaschi L, Cartocci E, et al. Vaccine composition formulated with a novel TLR7-dependent adjuvant induces high and broad protection against Staphylococcus aureus. P Natl Acad Sci USA. 2015;112(12):3680–3685. doi: 10.1073/Pnas.1424924112. PubMed PMID: WOS:000351477000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rouha H, Badarau A, Visram ZC, Battles MB, Prinz B, Magyarics Z, et al. Five birds, one stone: neutralization of alpha-hemolysin and 4 bi-component leukocidins of Staphylococcus aureus with a single human monoclonal antibody. MAbs. 2015;7(1):243–254. doi: 10.4161/19420862.2014.985132. PubMed PMID: 25523282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Karauzum H, Chen G, Abaandou L, Mahmoudieh M, Boroun AR, Shulenin S, et al. Synthetic Human Monoclonal Antibodies toward Staphylococcal Enterotoxin B (SEB) Protective against Toxic Shock Syndrome. Journal of Biological Chemistry. 2012;287(30):25203–25215. doi: 10.1074/Jbc.M112.364075. PubMed PMID: WOS:000306651700030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim HK, Emolo C, DeDent AC, Falugi F, Missiakas DM, Schneewind O. Protein A-specific monoclonal antibodies and prevention of Staphylococcus aureus disease in mice. Infect Immun. 2012;80(10):3460–3470. doi: 10.1128/IAI.00230-12. Epub 2012/07/25. PubMed PMID: 22825452; PubMed Central PMCID: PMC3457578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thammavongsa V, Rauch S, Kim HK, Missiakas DM, Schneewind O. Protein A-neutralizing monoclonal antibody protects neonatal mice against Staphylococcus aureus. Vaccine. 2015;33(4):523–526. doi: 10.1016/J.Vaccine.2014.11.051. PubMed PMID: WOS:000349196100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Varrone JJ, Bentley KLD, Bello-Irizarry SN, Nishitani K, Mack S, Hunter JG, et al. Passive Immunization With Anti-Glucosaminidase Monoclonal Antibodies Protects Mice From Implant-Associated Osteomyelitis by Mediating Opsonophagocytosis of Staphylococcus aureus Megaclusters. Journal of Orthopaedic Research. 2014;32(10):1389–1396. doi: 10.1002/Jor.22672. PubMed PMID: WOS:000340588000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van den Berg S, Bonarius HPJ, van Kessel KPM, Elsinga GS, Kooi N, Westra H, et al. A human monoclonal antibody targeting the conserved staphylococcal antigen IsaA protects mice against Staphylococcus aureus bacteremia. International Journal of Medical Microbiology. 2015;305(1):55–64. doi: 10.1016/J.Ijmm.2014.11.002. PubMed PMID: WOS:000348957700007. [DOI] [PubMed] [Google Scholar]
- 75.Brown MD Eric J, Flygare John, Hazenbos Wouter, Lee Byoung Chul, Lehar Sophie M, Mariathasan Sanjeev, MORISAKI John Hiroshi, Pillow Thomas H, Staben Leanna, Vandlen Richard, Koefoed Klaus, Strandh Magnus, Andersen Peter S. inventorAnti-wall teichoic antibodies and conjugates. United States. 2014 [Google Scholar]
- 76.Lehar SM, Pillow T, Xu M, Staben L, Kajihara KK, Vandlen R, et al. Novel antibody-antibiotic conjugate eliminates intracellular S. aureus. Nature. 2015 doi: 10.1038/nature16057. PubMed PMID: 26536114. [DOI] [PubMed] [Google Scholar]
- 77.Jacobs SA, Diem MD, Luo JQ, Teplyakov A, Obmolova G, Malia T, et al. Design of novel FN3 domains with high stability by a consensus sequence approach. Protein Engineering Design & Selection. 2012;25(3):107–117. doi: 10.1093/Protein/Gzr064. PubMed PMID: WOS:000300717300003. [DOI] [PubMed] [Google Scholar]
- 78.Diem MD, Hyun L, Yi F, Hippensteel R, Kuhar E, Lowenstein C, et al. Selection of high-affinity Centyrin FN3 domains from a simple library diversified at a combination of strand and loop positions. Protein Engineering Design & Selection. 2014;27(10):419–429. doi: 10.1093/Protein/Gzu016. PubMed PMID: WOS:000343893400017. [DOI] [PubMed] [Google Scholar]