Abstract
We discuss here the potential mechanisms of action associated with hypertrophic (HCM) or dilated (DCM) cardiomyopathy causing mutations in the myosin regulatory (RLC) and essential (ELC) light chains. Specifically, we focus on four HCM mutations: RLC-A13T, RLC-K104E, ELC-A57G and ELC-M173V, and one DCM RLC-D94A mutation shown by population studies to cause different cardiomyopathy phenotypes in humans. Our studies indicate that RLC and ELC mutations lead to heart disease through different mechanisms with RLC mutations triggering alterations of the secondary structure of the RLC which further affect the structure and function of the lever arm domain and impose changes in the cross bridge cycling rates and myosin force generation ability. The ELC mutations exert their detrimental effects through changes in the interaction of the N-terminus of ELC with actin altering the cross talk between the thick and thin filaments and ultimately resulting in an altered force-pCa relationship. We also discuss the effect of mutations on myosin light chain phosphorylation. Exogenous myosin light chain phosphorylation and/or pseudo-phosphorylation were explored as potential rescue tools to treat hypertrophy-related cardiac phenotypes.
Keywords: Myosin light chains, Mutation, Cardiomyopathy, Structure, Function, Phosphorylation
Introduction
Hypertrophic cardiomyopathy (HCM) is an autosomal dominant disease characterized by ventricular hypertrophy, myofibrillar disarray (Maron et al. 1982) and sudden cardiac death (SCD). HCM is considered the leading cause of SCD among athletes and young adults under the age of 30 (Marian and Roberts 1998; Maron et al. 1995). Genetic studies have linked HCM to mutations in genes encoding for all major sarcomeric proteins, including both myosin regulatory light chain (RLC) (~4 %) and essential light chain (ELC) (~2 %) (Alcalai et al. 2008). Unlike HCM, dilated cardiomyopathy (DCM) is characterized by ventricular dilatation and diminished contractile function (Schonberger and Seidman 2001). The clinical symptom of DCM is heart failure (HF), which is often associated with arrhythmia and SCD (Hershberger et al. 2013). Distinct from HCM, which in most cases is a genetic disease, DCM can be caused by a variety of factors, including ischemia, alcohol toxicity and viral infections (Gomes et al. 2005; Szczesna-Cordary et al. 2012). It is estimated that only 25–48 % of all DCM cases are due to familial and genetic factors (Kamisago et al. 2000; Millat et al. 2011). To date, more than 30 genes encoding sarcomeric, cytoskeletal and nuclear proteins are associated with DCM (Dellefave and McNally 2011). Among them are mutations in at least eight genes encoding for sarcomeric proteins: β-MHC (myosin heavy chain), α-MHC, MyBP-C (myosin binding protein C), titin, cardiac actin, α-Tm (tropomyosin) and troponins (Perriard et al. 2003; Piran et al. 2012).
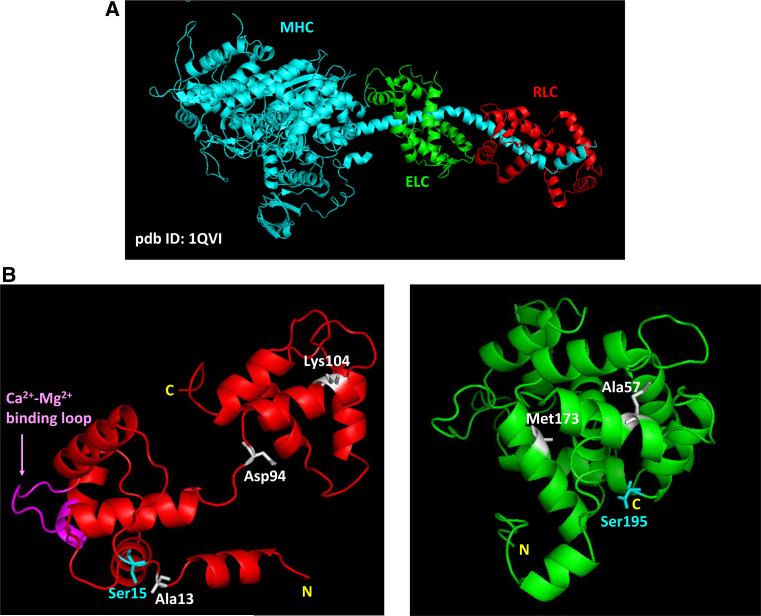
The role of muscle myosin is to form the thick filaments that slide past the thin filaments producing force and muscle contraction. Binding of myosin to actin leads to formation of the cross-bridges that hydrolyze ATP and undergo the cyclic transitions from the weakly to strongly attached states. The released energy propels myosin sliding along the actin filaments resulting in myofilament movement and muscle contraction. The myosin molecule is composed of three major structural domains (Rayment et al. 1993): (1) the motor domain, containing actin and ATP binding sites; (2) the lever arm domain, containing myosin ELC and RLC (both domains form the myosin head also called S1) (Fig. 1a); and (3) the tail region, which is responsible for thick filament formation. Since the myosin lever arm plays a pivot role in regulating the thick and thin filament interactions (Geeves 2002), it is conceivable that any mutations in its inherent subunits, either RLC or ELC (Fig. 1b) may alter the structure and function of the lever arm and force generation (Hernandez et al. 2007; Szczesna 2003).
Fig. 1.
a Structure of myosin head (S1) and the lever arm domain (pdb ID: 1QVI); b ITASSER predicted computational structures of human ventricular RLC (left) and ELC (right). Depicted: HCM/DCM mutations in RLC/ELC discussed in this report; phosphorylatable serines, Ser15-RLC and Ser195-ELC; Ca2+-Mg2+ binding site on RLC
Both myosin light chains (MLCs), the RLC and ELC attach to their respective MHC IQ motifs and structurally support the lever arm domain (Geeves 2002; Rayment et al. 1993). As an EF-hand Ca2+-binding protein, the RLC contains a Ca2+–Mg2+ binding site which can be occupied by either Ca2+ or Mg2+ (Szczesna 2003) (Fig. 1b, left panel). It has been postulated that during cardiac muscle contraction this site could act as a delayed Ca2+ buffer aiding the sarcoplasmic reticulum (SR) to sequester Ca2+ during diastole (Szczesna-Cordary et al. 2007; Wang et al. 2006). The ELC is also an EF-hand like protein (Fig. 1b, right panel); however, in the striated muscle ELC has lost its ability to bind calcium. Instead, reports have shown that the N-terminus of ELC can work as a tether between the myosin cross-bridge and actin regulating cross-bridge attachment and force development (Aydt et al. 2007; Hernandez et al. 2007; Kazmierczak et al. 2009; Lossie et al. 2014; Muthu et al. 2011; Petzhold et al. 2014; Timson 2003; Xie et al. 1994).
Another functionally important domain of MLCs is the serine phosphorylation site (Figs. 1b, 2). In the RLC, phosphorylation of Ser15 with myosin light chain kinase (MLCK) has been widely recognized to play an important role in cardiac muscle contraction under normal and disease conditions (Kamm and Stull 2011). Reduced RLC phosphorylation was reported in patients with HF (van der Velden et al. 2003a, b) and observed in animal models of cardiac disease (Abraham et al. 2009; Kerrick et al. 2009; Scruggs et al. 2009; Sheikh et al. 2012; Yuan et al. 2015). Attenuation of RLC phosphorylation in cardiac MLCK knock-out mice led to ventricular myocyte hypertrophy, fibrosis and to mild dilated cardiomyopathy (Chang et al. 2015; Ding et al. 2010). Any change in RLC phosphorylation is then expected to cause abnormal heart performance, presumably through morphological and/or functional alterations (change in force, myofilament calcium sensitivity, ATPase activity, etc.) (Huang et al. 2008; Morano 1999; Sweeney et al. 1993). Constitutive RLC-mutant pseudo-phosphorylation was recently shown to prevent the development of HCM in mice (Yuan et al. 2015). For the ELC, proteomic analysis from Van Eyk's lab has revealed that the residue Ser195 can be phosphorylated in pharmacologically preconditioned cardiomyocytes, although no specific kinase responsible for ELC phosphorylation has been identified (Arrell et al. 2001) (Fig. 2). In zebrafish cardiomyocytes, it was shown that a pseudo phosphorylation mimic of the ELC, containing serine195 mutated to aspartic acid (S195D), could rescue cardiomyocyte contractility that was lost in the zebrafish mutant lazy susan (laz) (Meder et al. 2009). This study suggested a beneficial effect of ELC phosphorylation for heart performance.
Fig. 2.
Amino acid sequences of human ventricular RLC (P10916) and ELC (P08590). Depicted: Ala → Thr (A13T-RLC/HCM), Asp → Ala (D94A-RLC/DCM and Lys → Glu (K104E-RLC/HCM) mutations on myosin regulatory light chain, and Ala → Gly (A57G-ELC/HCM) and Met → Val (M173V-ELC/HCM) mutations on myosin essential light chain. Also shown phosphorylatable P-Ser15/RLC and P-Ser195/ELC and Ca2+-Mg2+ loop on RLC
Cardiomyopathy-causing mutations in myosin light chains (MLCs)
In this report we aimed to provide insight into the molecular determinants of MLC mutations identified by population studies to cause heart disease. Specifically, we focused on the N- and C-terminal mutations in the myosin RLC (encoded by the MYL2 gene) and myosin ELC (encoded by MYL3) that are associated with HCM. We also aimed to reveal the mechanism of a newly identified DCM mutation which was found, for the first time, on the MLC (Huang et al. 2015). Due to the limited clinical information regarding the MLC mutation carriers and their families, this article mostly attends to studies performed on animal models of HCM including transgenic (Tg) A13T (Alanine → Threonine)-RLC mice, Tg-K104E (Lysine → Glutamic acid)-RLC mice and Tg-A57G (Alanine → Glycine)-ELC mice, revealing an association between the site of the mutation and the functional and morphological alterations in cardiac function. Approximately 10 % of endogenous mouse cardiac RLC was replaced by the A13T mutant of human ventricular RLC in Tg-A13T mouse myocardium (Kazmierczak et al. 2012), while ~100 % by K104E-RLC mutant in Tg-K104E mice (Huang et al. 2014). In control RLC mice, ~40 % and ~100 % of human ventricular RLC WT (wild-type) L4 and L2, respectively were replaced for endogenous mouse cardiac RLC (Wang et al. 2006). In ELC mice, ~80 % of human cardiac A57G-ELC was incorporated into mouse myocardium and the results compared to WT-ELC mice expressing ~80 % of human ventricular ELC (Muthu et al. 2011). In the absence of animal models of heart disease for D94A (Aspartate → Alanine) RLC and M173V (Methionine → Valine) ELC mutations, the studies were performed in RLC-depleted and mutant reconstituted or ELC mutant-exchanged porcine cardiac muscle preparations. Data for D94A-RLC reconstituted preparations (~60 % reconstitution) were compared with WT-RLC reconstituted muscles (~70 %); while for M173V-ELC exchanged muscles (~60 %) with ~50 % WT-ELC exchanged porcine cardiac muscle preparations (Huang 2015; Huang et al. 2015).
Mutations in MYL2
Recent genetic studies have revealed that mutations in the myosin RLC are more common than previously reported (for review see (Muthu et al. 2012; Szczesna 2003)) and in just the past few years, new mutations in MYL2 have been identified (Alvarez-Acosta et al. 2014; Olivotto et al. 2008; Santos et al. 2012), with some detected multiple times and in different ethnic populations (Andersen et al. 2009; Garcia-Pavia et al. 2011). In this review we focus on the effects of two HCM-causing mutations in the RLC, the N-terminal A13T mutation and the C-terminal K104E mutation (Figs. 1b, 2). The A13T-RLC mutation was first identified in an American family as a particular subtype of cardiac hypertrophy characterized by mid left ventricular (LV) obstruction (Poetter et al. 1996). The A13T-RLC mutation was later found to occur in HCM patients of European decent (Andersen et al. 2001; Hougs et al. 2005). The C-terminal K104E-RLC mutation was found in a Danish family presenting with a classical HCM phenotype with LV hypertrophy and diastolic dysfunction (Andersen et al. 2001). The proband diagnosed with HCM at the age of 17 developed a severe hypertrophic phenotype with pronounced inter-ventricular septal (IVS) hypertrophy and inverted transmitral flow at 35 years of age, indicating diastolic dysfunction (Andersen et al. 2001, 2009). The diagnosis of the proband was complicated by the fact that he and his two family members were also positive for a splice acceptor site mutation in MYL2 making the target allele for HCM ambiguous. Two family members who exclusively carried the K104E mutation did not have clinical phenotypes consistent with HCM further complicating the causative role of this K104E-RLC mutation in HCM (Huang et al. 2014).
In this report we also explore a novel mutation in MYL2, D94A that was identified by exome sequencing in a pedigree with familial dilated cardiomyopathy (Figs. 1b, 2) (Huang et al. 2015). Clinical characteristics of family members with the DCM-causing D94A mutation in MYL2 included decreased ejection fraction (EF) and impaired contractility. The proband and his sister were diagnosed with DCM at ages 24 and 29 after preventive cardiovascular screening revealed mild global LV systolic dysfunction. Medical therapy was initiated upon evidence of decreased EF. Their mother was diagnosed with peripartum cardiomyopathy (PPCM) at age 21. Septal wall thickness measurements were within normal range in all affected family members (Huang et al. 2015). The D94A mutation was the first mutation to be identified in any MLC to cause DCM.
Mutations in MYL3
The HCM-associated mutations in the myosin ELC (product of MYL3 gene) are quite rare, but they are also linked to malignant outcomes (Kaski et al. 2009; Lee et al. 2001; Olson et al. 2002; Poetter et al. 1996; Richard et al. 2003; Schaub et al. 1998). In this report we focused on two disease causing mutations in myosin ELC, the N-terminal A57G mutation, and the C-terminal M173V (Figs. 1b, 2). The A57G mutation was found in two unrelated Korean families with HCM and one Japanese patient presenting with a classic asymmetric septal hypertrophy (Lee et al. 2001). The M173V mutation was discovered in an adult proband who was diagnosed with HCM as a child, but the clinical information on the specific disease phenotype has not been available (Morita et al. 2008).
Structural effects of MLC mutations
In this report we aimed to compare the mechanisms of HCM associated mutations in the N-terminal vs. C-terminal mutations in both MLCs, and also relate the mechanisms specific for the RLC vs. ELC protein. We hypothesized that mutations in MLC lead to domain specific structural changes that trigger alterations in the MHC-MLC interaction and result in compromised incorporation of the MLC into the myosin lever arm domain. These changes may then affect myosin function and its ability to interact with actin to produce force and muscle contraction. For myosin RLC, all three mutations were shown to alter the domain structure by far UV CD (circular dichroism) measurements (Table 1). A significantly increased α-helical content was observed for A13T-RLC (Szczesna et al. 2001), while the K104E-RLC and D94A-RLC significantly decreased the α-helical content of RLC compared with WT-RLC (Huang 2015; Huang et al. 2014). It's important to note however that all CD spectra were acquired for recombinant isolated RLC mutant and WT proteins and that their structure may further change when attached to their immediate binding partner, the MHC.
Table 1.
Summary of structural and functional effects and disease-causing mechanisms for RLC (MYL2) and ELC (MYL3) mutations
| RLC |
ELC |
||||
|---|---|---|---|---|---|
| A13T HCM | K104E HCM | D94A DCM | A57G HCM | M173V HCM | |
| Human phenotype | Mid LV obstruction in American family (Poetter et al. 1996); cardiac hypertrophy in European patients (Andersen et al. 2001; Hougs et al. 2005) | Pronounced LV and IVS hypertrophy, inverted transmitral flow pattern, diastolic filling abnormalities (Andersen et al. 2001; Andersen et al. 2009) | Decreased EF, impaired contractility and systolic dysfunction (Huang 2015) | Classic asymmetric septal hypertrophy in unrelated Asian families with HCM (Lee et al. 2001) | Identified in adult proband diagnosed with HCM as a child (Morita et al. 2008). No specific clinical information available |
| Sarcomere | |||||
| MLCs | α-helical content ↑; endogenous RLC phosphorylation level (NC) | α-helical content ↓; endogenous RLC phosphorylation level↓ | α-helical content↓; time dependent RLC phosphorylation (NC) | Effect through the N-ELC and actin interaction; S195D ELC able to restore abnormal calcium sensitivity of A57G mutant | Effect through the N-ELC and actin interaction; S195D ELC able to partially restore decreased ATPase activity of M173V |
| Myosin | Cross bridge cycling rate (ATPase) ↓ | Cross bridge cycling rate (ATPase) ↑; load dependent mechanochemistry↓ | Cross bridge cycling rate (ATPase) ↑ | Cross bridge cycling rate (ATPase) (NC); myosin head (lever arm) stiffness↑ | Cross bridge cycling rate (ATPase) ↓ |
| Fibers | Force ↑ pCa50 (NC) | Force ↓ pCa50 (NC) Passive tension ↑ Relaxation rate↓ |
Force ↓ (NS) pCa50 (NC) |
Force ↓, pCa50 ↑ Passive tension ↑ Relaxation rate (NC) After intense exercise, pCa50, ↓ suggesting potential transition to HF |
Force (NC) pCa50↑ |
| Heart | |||||
| Histopathology | Severe IVS hypertrophy and fibrosis seen in 6 month-old mice | Fibrosis exacerbates with age; no myofilament disarray. High mitochondrial content seen in 6 month-old LV | N/A | Severe fibrosis seen in 6 month-old mice, no myofilament disarray | N/A |
| In vivo measurements | Normal cardiac morphology and function in 6 month-old mice; hypertrophy and EF ↓ in 16 month-old mice | Diastolic dysfunction: Tau↑ and E/A ratio ↓ as early as at 6 months of age. In >13 month-old animals: LV and IVS hypertrophy; LVDD↓, LVDS ↓, LVEDV ↓, LVESV ↓ | N/A | 7 month-old mice: LVEDV ↑, LVESP↑ no change in LV and septum wall thickness. SV, SW and CO↑; Ees (slope of ESPVR) ↑ | N/A |
| HCM gene expression by qPCR | N/A | NCX and L-type calcium channels, SERCA2a and RyR (NC) | N/A | SERCA2a ↑ in sedentary group; ANP, BNP and Col VIIIa ↑ after intense exercise | N/A |
| Cardiomyopathy | |||||
| Morphology | Severe IVS hypertrophy with deceased EF in 16 month-old mice | Severe HCM phenotype in >13 month-old animals | Increase in LVDD seen in patients carrying D94A mutation | Eccentric hypertrophy in 7 month-old mice | N/A |
| Function | Diastolic dysfunction reported in patients (see above) | Diastolic dysfunction and potential inefficiency energy use seen in mice; muscle fatigue↑ | Decrease in EF was seen in DCM patients (see above) | Systolic dysfunction with high risk of sudden cardiac death after intense exercise in mice | N/A |
| Possible disease causing mechanisms | Altered cross bridge kinetics due to structural changes in RLC Disease phenotype with ~ 10 % protein expression, suggesting a poison peptide mechanism |
Diastolic disturbance and inefficiency in energy usage, both of which could be caused by alterations in structure of RLC; changes in endogenous RLC phosphorylation as potential mechanism of action | Effects mainly through alterations in RLC and lever arm structure; inefficiency in energy usage may contribute to the disease mechanism | Mechanism through disrupting the N-ELC and actin interaction; disturbance of calcium homeostasis Exercise exacerbated HCM : upregulated expression of HCM-linked genes was observed |
Decreased ATPase activity and increased calcium sensitivity of force may underlie disease mechanism; possible alterations in the C-ELC-MHC and N-ELC-actin interactions |
NC no change, IVS interventricular septum, LV left ventricle, LVDD LV inner diameter at the end of diastole, LVDS LV inner diameter at the end of systole, LVEDV LV end diastolic volume, LVESV LV end systolic volume, LVESP LV end systolic pressure, EF ejection fraction, ESPVR end systolic pressure–volume relationship, SV stroke volume, SW stroke work, CO cardiac output, HF heart failure, RyR ryanodine receptor, ANP/BNP atrial/brain natriuretic peptide
Studies with RLC-depleted and RLC-mutant-reconstituted porcine cardiac muscle preparations showed that both A13T and D94A mutations decreased the RLC incorporation into the lever arm domain, while the K104E mutation did not alter the binding of RLC to MHC (Huang 2015; Huang et al. 2014; Kazmierczak et al. 2012). However, as shown by small angle X-ray diffraction patterns using transgenic mouse papillary muscle fibers, the K104E-RLC mutation significantly increased the interfilament lattice spacing (IFS) at both short and long sarcomere lengths under relaxation conditions suggesting that the K104E myosin-formed thick filaments move away from each other (Huang 2015). However, the mutation did not render any changes in the cross-bridge mass distribution reflected by no changes in the I1,1/I1,0 equatorial reflections’ intensity ratio (Table 1) (Huang 2015). It is important to note that X-ray diffraction experiments on Tg-K104E mouse fibers were compared to Tg-WT with two animal models expressing the same amount of transgene, i.e. the human ventricular K104E or WT RLC, respectively (Huang 2015). Unfortunately, no such experiments were performed for the A13T-RLC mutation incorporated into the mouse myocardium at 10 % (Kazmierczak et al. 2012) or for the D94A-RLC mutation due to the lack of transgenic D94A mice available for studies. In sum, both A13T- and K104E-RLCs triggered some structural changes in the myosin lever arm domain that ultimately affected myosin ability to generate force and muscle contraction.
For the ELC and its N-terminal molecular contacts with actin, it was anticipated that both the N-terminal A57G and C-terminal M173V mutations would affect the interaction of myosin with actin through the direct effects on the N-terminus ELC-actin interaction (Table 1). Low angle X-ray diffraction measurements using Tg-A57G mouse papillary muscle fibers showed that under rigor condition (no ATP present) and when actin and myosin were strongly bound, the A57G mutation significantly decreased IFS, suggesting a tighter packing of the thick filaments in the A57G mutated sarcomeres (Table 1) (Muthu et al. 2011). No X-ray experiments were performed with the C-terminal M173V-ELC mutation due to the lack of Tg-M173V mice available for research. However, it is anticipated that this C-terminal M173V-ELC mutation may play a critical role in the interaction of the ELC with the C-terminal lever arm region of the myosin cross-bridge and its interaction with actin during force generation. The binding of ELC to the MHC IQ motif occurs in a non-covalent manner, mainly through the van der Waals contacts (Rayment et al. 1993; Xie et al. 1994). Therefore, the C-terminus of ELC is important for the formation of stable ELC-MHC structures and sarcomere assembly (Petzhold et al. 2011). Any structural abnormalities in this C-terminal ELC region due to the M173V mutation could lead to myofilament disarray and trigger formation of fibrotic lesions in the myocardium. It is hypothesized that the C-terminal M73V mutation positioned in this critical ELC region that interacts with the lever arm domain may interfere with the structural stability of the myosin cross-bridge and affect transmission of the conformational changes between the motor domain and the lever arm domain, and in the conduction of external loads from the myosin backbone to myosin's active site. In conclusion, both A57G- and M173V- ELCs could be implicated in the structural reorganization of the myosin lever arm having structural and functional consequences and affect generation of force and muscle contraction.
Functional effects of MLC mutations
In vitro functional studies on MLC mutations included the actin activated myosin ATPase activity assays, steady state force measurements, force-pCa dependence (pCa50), muscle relaxation rates and passive tension measurements. We hypothesized that the mutations in RLC and ELC can impose significant changes in one or more of these functional parameters of force generation and muscle contraction (Table 1). The experiments were performed on either Tg mouse cardiac muscle preparations or RLC-depleted and mutant-reconstituted or ELC mutant-exchanged porcine cardiac muscles (Huang 2015; Huang et al. 2015).
All three RLC mutations altered the actin-activated myosin ATPase activity with myosin purified from mouse hearts (A13T, K104E) or from porcine hearts reconstituted with D94A-RLC. The N-terminal A13T-RLC mutation significantly decreased maximal ATPase activity (Vmax) (Kazmierczak et al. 2012), while D94A-RLC and the C-terminal K104E-RLC mutations increased Vmax (Huang et al. 2014, 2015). Vmax represents the rate constant of the transition from the weakly (A·M·ATP ↔ A·M·ADP·Pi) to strongly (A·M·ADP ↔ A·M) bound myosin cross-bridges with phosphate release being rate limiting (Rayment 1996). Therefore, the A13T-RLC mutation decreased, while D94A-RLC and K104E-RLC increased the rate of the weak to strong actin binding transition compared with WT myosin (Table 1). None of the mutations affected the Michaelis–Menten constant, Km. Unlike RLC mutations, the N-terminal ELC A57G mutation did not affect the actin activated mouse purified myosin ATPase activity (Kazmierczak et al. 2013). The C-terminal M173V mutation in the ELC was observed to decrease the actin-activated mutant-exchanged porcine myosin ATPase activity compared with WT-ELC myosin (Table 1) (Huang 2015). Since the actin activated hydrolysis of Mg·ATP by myosin fuels muscle contraction, these results suggested that the mutations which reduce Vmax (A13T-RLC, M173V-ELC) may affect the ability of the mutated cross-bridges to hydrolyze ATP decreasing chemical energy that could be used to produce mechanical work.
In functional studies, the A13T-RLC mutation significantly increased (Kazmierczak et al. 2012), while K104E-RLC decreased (Huang et al. 2014), and D94A-RLC did not affect (Huang et al. 2015) the maximal contractile force measured in skinned papillary muscle fibers from Tg-A13T and Tg-K104E mouse hearts or in D94A-porcine reconstituted papillary muscle strips (Table 1). Surprisingly, no changes in the Ca2+ sensitivity of force generation (pCa50) were observed for any studied RLC mutants. This was in contrast to other RLC mutations, namely R58Q-RLC and D166 V-RLC, which were shown to render a significant increase in the Ca2+ sensitivity of force (Kerrick et al. 2009; Wang et al. 2006; Yuan et al. 2015). It is important to note that the majority of HCM-causing sarcomeric mutations do increase the Ca2+ sensitivity of contraction (Marston 2011). In addition, K104E-RLC significantly increased passive tension and reduced muscle relaxation rates (Huang et al. 2014), suggesting the possibility of diastolic dysfunction in K104E mice (Table 1). In addition, in the in vitro motility assays, the velocity of actin filaments propelled by K104E-RLC myosin decreased faster with increasing loads than propelled by the WT-RLC myosin. This result suggested that when placed in vivo, the K104E-RLC mutation could make the heart more susceptible to muscle fatigue (Table 1) (Huang et al. 2014).
Similar to K104E-RLC, the A57G mutation in myosin ELC also decreased maximal force generation and increased passive tension compared with WT-ELC mice, but no differences were observed in muscle relaxation rates between A57G-ELC and WT-ELC mouse cardiac muscle preparations (Table 1) (Kazmierczak et al. 2013; Muthu et al. 2011). In contrast to the RLC mutations studied in this report and in accord with other HCM mutations (Xu et al. 2010), A57G-ELC resulted in a significant increase in the myofilament Ca2+ sensitivity compared with WT-ELC, as measured in mouse skinned papillary muscle strips (Kazmierczak et al. 2013) (Table 1). On the other hand, the C-terminal M173V-ELC mutation exerted no effect on maximal force generation, but similar to A57G-ELC, it also sensitized the M173V-exchanged porcine muscle fibers to Ca2+ (Table 1) (Huang 2015). Furthermore, the A57G-ELC mutation was reported to cause SCD in humans (Lee et al. 2001). Since SCD often happens after intense exercise, the effects of exercise of Tg-A57G versus Tg-WT mice were investigated (Kazmierczak et al. 2014). Compared to the sedentary group, in which only SERCA2a was upregulated in A57G versus WT mice, quantitative PCR results showed that the HCM-related genes, e.g. atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP) and Col VIIIa (collagen) were all upregulated in exercised A57G-ELC mice (Table 1). On the other hand, studies on sedentary and exercised mouse fibers showed a significant decrease in calcium sensitivity of force in exercised vs. sedentary A57G-ELC mice (Kazmierczak et al. 2014). The steady state force of exercised A57G mice was significantly lower than in WT, which in combination with decreased Ca2+ sensitivity could indicate a potential for heart failure (Table 1) (Huang 2015). As shown in Kazmierczak et al. (2014), no significant changes in endogenous MyBP-C, Tm, TnT, TnI, or RLC phosphorylation were found in sedentary or exercised A57G-ELC animals compared to WT-ELC mice. The lack of the effect of exercise on protein phosphorylation was somewhat surprising but the similar observations have been reported by other laboratories and no effect of exercise on phosphorylation of myosin RLC in the ventricles or atria of mice was found by Fewell et al. (1997). Similarly, no changes in phosphorylation of titin or TnI in mice upon exercise training were observed in Hidalgo et al. study (2014).
Effect of MLC mutations on heart morphology and cardiac function in vivo
Histopathological staining with hematoxylin and eosin (H&E) and Masson's trichrome, and the in vivo heart measurements by echocardiography and invasive hemodynamics were conducted on transgenic mouse models of HCM (Huang et al. 2014; Kazmierczak et al. 2012; Kazmierczak et al. 2013). For RLC mutant animals, a significantly larger IVS mass was observed in 6 month-old Tg-A13T mice compared with Tg-WT or NTg (non-transgenic) littermates (Kazmierczak et al. 2012). In addition, A13T-RLC animals demonstrated severe fibrotic lesions in their LV walls compared with controls suggesting exaggerated activation of extracellular collagen.
Histology studies on 6 month-old Tg-K104E hearts showed minor fibrosis which exacerbated in >13 month-old animals suggesting that the K104E-RLC HCM phenotype was age dependent (Huang et al. 2014). Echocardiography measurements conducted on senescent 16 month-old A13T-RLC animals showed significantly reduced EF compared with WT-RLC mice (Huang 2015). The echocardiography assessment of K104E-RLC mice showed no HCM in 6 month-old animals, but a pronounced HCM phenotype in senescent > 13 month-old K104E hearts (Huang et al. 2014). Doppler measurements on 6 month-old K104E-RLC mice showed a decrease in E/A ratio, and invasive pressure–volume (PV-loops) measurements revealed prolonged deceleration time (tau), both indicative of potential diastolic disturbance in K104E-RLC mutant animals (Huang et al. 2014) (Table 1). Comparing these two mouse phenotypes associated with A13T-RLC and K104E-RLC mutations and given that ~10 % versus ~100 % Tg protein is expressed in A13T versus K104E mouse hearts, one has to anticipate that the A13T-RLC mutation would result in a much more severe HCM phenotype than K104E-RLC when both were incorporated into mouse hearts at ~100 % (Table 1).
Similar to A13T-RLC, the A57G-ELC mutation resulted in severe fibrosis with no myofilament disarray in the hearts of 6 month-old A57G-ELC mice (Kazmierczak et al. 2013). However, unlike in RLC mice, the echocardiography measurements showed a rare HCM phenotype in A57G-ELC mice manifested by no changes in IVS or LV wall thickness but with substantially increased LV chamber size compared with WT hearts (Kazmierczak et al. 2013). Hemodynamics measurements showed a significant increase in systolic indices such as stroke work (SW), stroke volume (SV) and cardiac output (CO). The load-independent end-systolic elastance, Ees (indicator of cardiac contractility) was also increased in A57G-ELC compared with WT-ELC mice (Table 1). The time constant of ventricular relaxation tau was two-fold faster in A57G than in WT mice, but because end-systolic pressure was higher in A57G-ELC vs. WT-ELC mice, tau was expected to be faster in the mutant mice. These changes were most likely associated with the type of cardiac remodeling observed in Tg-A57G animals demonstrated by eccentric hypertrophy and sphericalization of the LV. These changes were expected to contribute to systolic dysfunction in A57G-ELC mice (Kazmierczak et al. 2013).
Effect of phosphorylation on MLC mutant phenotype
Both MLCs contain phosphorylatable serine residues, Ser15 on the RLC (Chang et al. 2015; Scruggs and Solaro 2011; Warren et al. 2012; Yuan et al. 2015) and Ser195 on the ELC (Arrell et al. 2001; Meder et al. 2009) that can potentially serve as functional phosphorylation sites in both MLC proteins (Fig. 2). Phosphorylation of Ser15 in the myosin RLC has been widely recognized to play a functional role in cardiac muscle contraction in health and disease (Kamm and Stull 2011). The in vivo level of RLC phosphorylation in the healthy heart has been reported as ~0.4 mol phosphate/mole RLC (Chang et al. 2015). Complete dephosphorylation of the RLC was observed in the myocardium of HF patients (van der Velden et al. 2003a, b). Significantly reduced levels of RLC phosphorylation were also observed in RLC animal models of HCM compared to phosphorylation of WT-RLC mice (Abraham et al. 2009; Kerrick et al. 2009; Yuan et al. 2015). Interestingly, a slight increase in TnI phosphorylation in the myocardium of R58Q-RLC (Wang et al. 2006) and D166V-RLC (Kerrick et al. 2009) mice has been reported to occur concurrently with a decrease in RLC phosphorylation in these animal models of HCM. (Yuan et al. 2015). Both TnI and MyBP-C were seen to be associated with cardiac myopathies and changes in their phosphorylation are recognized to be indicative of cardiac disease (Kuster et al. 2014; Sadayappan et al. 2009; Wijnker et al. 2014). However, no statistically significant differences in phosphorylation of TnI or MyBP-C were observed in D166V-RLC animals or in the S15D-D166V rescue heart model of HCM designed to abrogate the consequences of D166V-RLC alone. In conclusion, our decade-long investigation of the RLC mutant induced pathology of the heart and results from other laboratories suggest that RLC phosphorylation has an important physiological role in the heart and may serve as a rescue tool to mitigate detrimental phenotypes associated with cardiomyopathy disease (Yuan et al. 2015).
We first tested whether phosphorylation of myosin RLC by Ca2+ calmodulin (CaM) activated MLCK can be affected by A13T-RLC, D94A-RLC and K104E-RLC mutations. In mice, K104E-RLC was shown to significantly decrease the endogenous RLC phosphorylation, but no effect was found in A13T-RLC mouse hearts (Huang et al. 2014; Kazmierczak et al. 2012). The lack of effect of A13T-RLC on myosin phosphorylation could be due to the low expression of A13T-RLC in mouse myocardium compared to the high (~100 %) expression of K104E-RLC in mice. No data on the effect of D94A-RLC on RLC phosphorylation in situ are available because of the lack of animal models of DCM-RLC available for research. Interestingly, treatment of K104E-RLC mouse fibers with Ca2+-CaM activated MLCK resulted in an increase in the I1,1/I1,0 equatorial reflections’ intensity ratio at both short and long sarcomere lengths, demonstrating sensitivity of K104E-RLC cross-bridges to phosphorylation-mediated structural changes (Huang 2015). In addition, the mutation increased IFS compared with WT-RLC mouse fibers, which was restored to the level of WT (at long sarcomere length) as a result of MLCK induced phosphorylation of K104E-RLC mouse papillary muscle fibers (Huang 2015). However, in functional studies, the steady state force and pCa50 were the same before and after K104E-RLC mouse fiber phosphorylation (Huang 2015).
In contrast to the myosin RLC, no kinase that specifically phosphorylates the ELC protein has been identified. Therefore, we generated Ser195 phosphomimetic ELC mutant proteins containing the S195D mutation in the background of either A57G- or M173V -ELC HCM-causing mutations. The recombinant proteins were exchanged for endogenous porcine cardiac ELC and mutant-exchanged cardiac muscle preparations were tested for the rescue effects of abnormal force generation and myofilament calcium sensitivity. The S195D-A57G phosphomimetic mutant of ELC was able to decrease an abnormally high calcium sensitivity of force rendered by the A57G-ELC mutation alone (Huang 2015). The same trend of S195D induced recovery of the Ca2+ sensitivity was observed in S195D-M173V versus M173V-ELC exchanged preparations (Huang 2015). The S195D-M173V-ELC phosphomimetic protein was also able to partially restore the low level of actin-activated myosin ATPase activity that was observed for M173V-ELC myosin. However, similar to the effect of phosphorylation of Ser15-RLC in skinned muscle fibers, there was no effect of S195D on force production in the ELC mutant-exchanged porcine papillary muscle strips (Huang 2015).
Potential mechanisms of action for RLC and ELC mutations
The important question addressed here pertains to the underlying causes of different disease mechanisms observed for the RLC versus ELC mutations in humans and in experiments utilizing animal models of human disease. The RLC and ELC mutations share common mechanisms by which they result in detrimental HCM/DCM phenotypes; however, due to the structural and functional differences between the RLC and ELC proteins and between the N- and C-terminal domains of the protein itself, distinct disease phenotypes among certain mutations are observed (Table 1).
For RLC, both A13T and K104E mutations cause HCM, while the D94A-RLC mutation leads to DCM in humans. In our studies, compared to control WT-RLC mice, the N-terminal A13T-RLC mutation increased maximal force generation, decreased maximal ATPase activity and did not affect endogenous RLC phosphorylation, while the C-terminal K104E-RLC mutation decreased maximal force generation, increased maximal ATPase activity, slowed down muscle relaxation and decreased endogenous RLC phosphorylation (Table 1). This lack of effect of A13T-RLC on the phosphorylation status in mouse myocardium was somewhat surprising considering the vicinity of A13T to S15 in the RLC molecule (Fig. 1b, left panel). One can speculate that perhaps the low (~10 %) level of mutant expression was not enough to impose changes in ~90 % of not mutated myosin heads in mice. However, the results collected for the N- and C-terminal RLC mutations suggested that they may lead to HCM through different mechanisms resulting in different manifestation of A13T-RLC vs. K104E-RLC phenotypes. The A13T-RLC phenotype was seen as early as in 6 month-old mice even though only 10 % transgenic protein was expressed. The significant functional changes observed, despite a low level of A13T mutant incorporation into myofilaments, suggested a ‘poison-peptide’ mechanism of disease (Kazmierczak et al. 2012). The disease phenotype of K104E-RLC was age dependent, and could be clearly manifested only in animals older than 13 months of age (Table 1). Thus, we conclude that compared to K104E-RLC, which was shown to cause a relatively benign phenotype, the A13T-RLC mutation has the potential to lead to a more severe HCM phenotype in humans.
For the DCM causing D94A-RLC mutation, most alterations were seen in the RLC structure. The D94A-RLC mutation changed the α-helical content and led to impaired MHC-RLC interactions. These results imply that subtle mutation-induced changes in the secondary structure of the RLC were most likely responsible for the inability of the mutant to stoichiometrically bind to RLC-depleted myosin and saturate the MHC binding sites to the level observed for WT-RLC (Huang et al. 2015). However, except for the elevated maximal ATPase activity, the functional defects were not clearly manifested in D94A-RLC reconstituted porcine cardiac muscle preparations (Table 1). The lack of visible phenotypes could be due to the D94A-RLC reconstitution protocol that was utilized in the experiments compared to the native transgenic mouse cardiac muscles studied for A13T-RLC and K104E-RLC. Tg-D94A mouse colonies have been successfully generated in the laboratory and the work in vivo and in vitro on disease phenotypes is in progress allowing for future conclusions on the D94A-induced DCM.
At the molecular level, all three RLC mutations altered the α-helical content of the protein in different ways implying different changes in the secondary structure of the RLC. These changes might be responsible for differently affected cross bridge cycling rates (ATPase activity) and altered interactions of mutant myosins with actin during force generation and muscle contraction (Table 1).
Differences in the ATPase and cross bridge cycling rates could also be seen at the myofilament level, in force generation experiments. The A13T-RLC mutation significantly increased maximal force (with lower cycling rates), while K104E-RLC reduced maximal tension (with higher cycling rates) (Table 1). These changes may further underlie different disease phenotypes observed at the heart level. Interestingly, the calcium sensitivity of force was not affected in any of the three studied RLC mutations (Table 1). This might be due to the absence of direct interactions between RLC and troponins or it is also possible that the direct calcium binding to the RLC is not being affected by these mutations. As shown earlier for two other HCM-RLC mutations, R58Q and D166V, they were able to cause delayed calcium and force transients (Kerrick et al. 2009; Wang et al. 2006) and significantly increased the Ca2+ sensitivity of contraction. These results suggested that the RLC can work as a delayed calcium buffer helping the sarcoplasmic reticulum to sequester calcium after contraction (Szczesna-Cordary et al. 2007). As reported earlier (Szczesna-Cordary et al. 2004), the calcium binding affinity of A13T-RLC was significantly lower than WT-RLC (higher Kd), but the calcium binding to RLC reconstituted myofibrils or fibers was similar between WT- and A13T- RLC. This suggested that alterations in calcium binding to A13T-RLC were not large enough to affect the overall calcium sensitivity of the fibers. The calcium binding properties of K104E and D94A RLCs are still under investigation.
For the ELC, the A57G-ELC mutation, similar to K104E-RLC, significantly decreased maximal force generation and increased passive tension. However, unlike RLC mutations, it significantly left shifted the calcium sensitivity of force, with no changes in the ATPase activity or muscle relaxation rates (Table 1). The in vivo studies using echocardiography and invasive hemodynamics showed a rare eccentric hypertrophy phenotype with signs of systolic dysfunction. These abnormalities were not observed in Tg-RLC animal models (Huang et al. 2014) (Table 1). More interestingly, the A57G-ELC mutation was reported to cause SCD in human patients while no cases of SCD were reported in either of studied RLC mutations (Andersen et al. 2001; Poetter et al. 1996). For the C-terminal ELC mutation M173V, similar to A57G-ELC, this mutation also showed a higher (left-shifted) calcium sensitivity of force compared with WT-ELC. In addition, the maximal ATPase activity was reduced (Table 1). It is worth mentioning that because the results on M173V-ELC were obtained in the mutant-exchanged porcine muscle preparations, any firm conclusion on this M173V-ELC mutation would require further in vivo structural and functional studies on M173V-ELC mice.
Differences between RLC and ELC phenotypes
Unlike RLC proteins, the ELC interacts with actin through its N-terminus (Sutoh 1982; Trayer and Trayer 1985; Winstanley et al. 1977), and this interaction is hypothesized to be the molecular target for the ELC disease causing mechanisms (Kazmierczak et al. 2009, 2014; Muthu et al. 2011). We have previously shown that the N-terminally truncated Δ43-ELC was able to shift the cross bridge mass distribution toward the thin filament. This result suggested that the N-terminus of ELC may work as a tether to regulate the thin and thick filament interaction (Muthu et al. 2011). Since RLC does not interact with the thin filaments, we suspect that it is the direct interaction between the N-terminus ELC and thin filaments that causes phenotypic differences between the RLC and ELC mutations. For example, the tethering effect of the N-terminus of ELC was manifested as increased rigor stiffness in Tg-A57G mice (Muthu et al. 2011). The increased rigor stiffness most likely reflected increased stiffness of the myosin head, and the lever arm domain in particular and further increased the unitary force produced by A57G-ELC myosin (Muthu et al. 2011). This together with decreased maximal force generation in fibers (Table 1) suggested that ELC mutations may lead to a reduced number of force generating myosin cross bridges. These phenotypes were not observed for the RLC mutants. One common denominator for both light chains could be the interaction between the ELC- and RLC-containing lever arm domain and actin via the cardiac MYBP-C, which was recently shown to span the neck domain of myosin head and actin with the C-terminal domains of cMyBP-C positioned along the thick filament surface, and the N-terminus of cMyBP-C extending toward neighboring thin filaments (Lee et al. 2015). Interestingly, even though the ELC does not bind calcium, A57G-ELC and M173V-ELC both left shifted the calcium sensitivity of force (Table 1), a hallmark of HCM disease, suggesting the possibility of the N-terminus ELC mediated cross talk between the thick and thin filaments and resultant alterations in pCa50. Alterations in calcium sensitivity were most likely induced by the changes of calcium binding to Troponin C, the major intracellular calcium buffer, e.g. increased the kon/koff ratio causing a leftward shift in the force-pCa relationship (Robinson et al. 2002). On the other hand, the binding of cMyBP-C to actin in situ, shown by Lee et al. (2015), implies that by the binding of the N-terminal domains of cMyBP-C to the thin filaments, cMyBP-C can potentially modulate the Ca2+ sensitivity of contraction that was altered by either RLC or ELC HCM/DCM causing mutations.
In conclusion, different phenotypes observed between myosin light chains’ mutations are most likely caused by different properties of the two light chains, RLC vs. ELC, and the differences between their N- and C-terminal domains. Studies with animal models for all discussed mutations in the RLC and ELC would have to be performed to conclude on the specific MLC rendered disease phenotype. It is also possible that there is a common disease mechanism for both RLC and ELC mutations that has not been tested in the reviewed studies. For example, the role of cMyBP-C in the disease mechanism should be further pursued as the information of the site of the mutation in both MLCs could be communicated to the thin filaments through cMyBP-C. More studies have to also be performed to conclude on myosin light chain phosphorylation potentially working as a common rescue mechanism for both RLC and ELC mutations linked to cardiomyopathies.
Acknowledgments
The authors thank Michelle Jones for critical reading of the manuscript. This work was supported in part by grants from the National Institutes of Health HL108343 and HL123255 (D.S-C.); and the American Heart Association 12PRE12030412 (W.H.). The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Center for Research Resources or the NIH.
Abbreviations
- A13T
Alanine-to-threonine mutation in myosin RLC
- A57G
Alanine-to-glycine mutation in myosin ELC
- ANP
Atrial natriuretic peptide
- BNP
Brain natriuretic peptide
- CaM
Calmodulin
- CD
Circular dichroism
- CO
Cardiac output
- D94A
Aspartic acid-to-alanine mutation in myosin RLC
- D166V
Aspartic acid-to-valine mutation in myosin RLC
- DCM
Dilated cardiomyopathy
- E/A
Doppler transmitral blood velocities, early (E)/ratio late (A) diastolic
- ECG
Echocardiography
- EF
Ejection fraction
- ELC
Essential light chain of myosin (MYL3 gene)
- HCM
Hypertrophic cardiomyopathy
- IFS
Interfilament lattice spacing
- IVS
Inter-ventricular septum
- H&E
Hematoxylin and eosin
- HF
Heart failure
- K104E
Lysine-to-glutamic acid mutation in myosin RLC
- LV
Left ventricle
- M173V
Methionine-to-valine mutation in myosin ELC
- MHC
Myosin heavy chain
- MLC
Myosin light chain
- MLCK
Myosin light chain kinase
- MyBP-C
Myosin binding protein-C
- NTg
Non-transgenic
- PV
Pressure volume
- R58Q
Arginine-to-glutamine mutation in myosin RLC
- RLC
Regulatory light chain of myosin (MYL2 gene)
- S15D
Serine-to-aspartic acid mutation to mimic phosphorylation
- SCD
Sudden cardiac death
- SR
Sarcoplasmic reticulum
- SW
Stroke work
- SV
Stroke volume
- Tg
Transgenic
- Tm
Tropomyosin
- Tn
Troponin
- WT
Wild-type
References
- Abraham TP, et al. Diastolic dysfunction in familial hyper-trophic cardiomyopathy transgenic model mice. Cardiovasc Res. 2009;82:84–92. doi: 10.1093/cvr/cvp016. doi:10.1093/cvr/cvp016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcalai R, Seidman JG, Seidman CE. Genetic basis of hypertrophic cardiomyopathy: from bench to the clinics. J Cardiovasc Electrophysiol. 2008;19:104–110. doi: 10.1111/j.1540-8167.2007.00965.x. [DOI] [PubMed] [Google Scholar]
- Alvarez-Acosta L, et al. Regulatory light chain (MYL2) mutations in familial hypertrophic cardiomyopathy. J Cardiovasc Dis. 2014;2:82–90. [Google Scholar]
- Andersen PS, et al. Myosin light chain mutations in familial hypertrophic cardiomyopathy: phenotypic presentation and frequency in Danish and South African populations. J Med Genet. 2001;38:e43. doi: 10.1136/jmg.38.12.e43. doi:10.1136/jmg.38.12.e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen PS, et al. Diagnostic yield, interpretation, and clinical utility of mutation screening of sarcomere encoding genes in Danish hypertrophic cardiomyopathy patients and relatives. Hum Mutat. 2009;30:363–370. doi: 10.1002/humu.20862. doi:10.1002/humu.20862. [DOI] [PubMed] [Google Scholar]
- Arrell DK, Neverova I, Fraser H, Marban E, Van Eyk JE. Proteomic analysis of pharmacologically preconditioned cardiomyocytes reveals novel phosphorylation of myosin light chain 1. Circ Res. 2001;89:480–487. doi: 10.1161/hh1801.097240. [DOI] [PubMed] [Google Scholar]
- Aydt EM, Wolff G, Morano I. Molecular modeling of the myosin-S1(A1) isoform. J Struct Biol. 2007;159:158–163. doi: 10.1016/j.jsb.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Chang AN, et al. Constitutive phosphorylation of cardiac myosin regulatory light chain in vivo. J Biol Chem. 2015 doi: 10.1074/jbc.M115.642165. doi:10.1074/jbc.M115.642165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellefave L, McNally EM. The genetics of dilated cardiomyopathy. Curr Opin Cardiol. 2011;25:198–204. doi: 10.1097/HCO.0b013e328337ba52. doi:10.1097/HCO.0b013e328337ba52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding P, Huang J, Battiprolu PK, Hill JA, Kamm KE, Stull JT. Cardiac myosin light chain kinase is necessary for myosin regulatory light chain phosphorylation and cardiac performance in vivo. J Biol Chem. 2010;285:40819–40829. doi: 10.1074/jbc.M110.160499. doi:10.1074/jbc.M110.160499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fewell JG, Osinska H, Klevitsky R, Ng W, Sfyris G, Bahrehmand F, Robbins J. A treadmill exercise regimen for identifying cardiovascular phenotypes in transgenic mice. Am J Physiol. 1997;273:H1595–H1605. doi: 10.1152/ajpheart.1997.273.3.H1595. [DOI] [PubMed] [Google Scholar]
- Garcia-Pavia P, et al. Genetic basis of end-stage hypertrophic cardiomyopathy. Eur J Heart Fail. 2011;13:1193–1201. doi: 10.1093/eurjhf/hfr110. doi:10.1093/eurjhf/hfr110. [DOI] [PubMed] [Google Scholar]
- Geeves MA. Molecular motors: stretching the lever-arm theory. Nature. 2002;415:129–131. doi: 10.1038/415129a. doi:10.1038/415129a. [DOI] [PubMed] [Google Scholar]
- Gomes AV, Venkatraman G, Potter JD. The miscommunicative cardiac cell: when good proteins go bad. Ann N Y Acad Sci. 2005;1047:30–37. doi: 10.1196/annals.1341.003. doi:10.47/1/30. [DOI] [PubMed] [Google Scholar]
- Hernandez OM, Jones M, Guzman G, Szczesna-Cordary D. Myosin essential light chain in health and disease. Am J Physiol Heart Circ Physiol. 2007;292:H1643–H1654. doi: 10.1152/ajpheart.00931.2006. doi:10.1152/ajpheart.00931.2006. [DOI] [PubMed] [Google Scholar]
- Hershberger RE, Hedges DJ, Morales A. Dilated cardiomyopathy: the complexity of a diverse genetic architecture. Nat Rev Cardiol. 2013;10:531–547. doi: 10.1038/nrcardio.2013.105. doi:10.1038/nrcardio.2013.105. [DOI] [PubMed] [Google Scholar]
- Hidalgo C, Saripalli C, Granzier HL. Effect of exercise training on post-translational and post-transcriptional regulation of titin stiffness in striated muscle of wild type and IG KO mice. Arch Biochem Biophys. 2014;552–553:100–107. doi: 10.1016/j.abb.2014.02.010. doi:10.1016/j.abb.2014.02.010. [DOI] [PubMed] [Google Scholar]
- Hougs L, et al. One third of Danish hypertrophic cardiomyopathy patients have mutations in MYH7 rod region. Eur J Hum Genet. 2005;13:161–165. doi: 10.1038/sj.ejhg.5201310. [DOI] [PubMed] [Google Scholar]
- Huang W. Molecular Mechanisms of Myosin Light Chain Mutation-Induced Cardiomyopathies Open Access Dissertations. 2015:1373. [Google Scholar]
- Huang J, Shelton JM, Richardson JA, Kamm KE, Stull JT. Myosin regulatory light chain phosphorylation attenuates cardiac hypertrophy. J Biol Chem. 2008;283:19748–19756. doi: 10.1074/jbc.M802605200. doi:10.1074/jbc. M802605200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, et al. Hypertrophic cardiomyopathy associated Lys104Glu mutation in the myosin regulatory light chain causes diastolic disturbance in mice. J Mol Cell Cardiol. 2014;74:318–329. doi: 10.1016/j.yjmcc.2014.06.011. doi:10.1016/j.yjmcc.2014.06.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, et al. Novel familial dilated cardiomyopathy mutation in MYL2 affects the structure and function of myosin regulatory light chain. FEBS J. 2015;282:2379–2393. doi: 10.1111/febs.13286. doi:10.1111/febs.13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamisago M, et al. Mutations in sarcomere protein genes as a cause of dilated cardiomyopathy. N Engl J Med. 2000;343:1688–1696. doi: 10.1056/NEJM200012073432304. doi:10.1056/nejm200012073432304. [DOI] [PubMed] [Google Scholar]
- Kamm KE, Stull JT. Signaling to myosin regulatory light chain in sarcomeres. J Biol Chem. 2011;286:9941–9947. doi: 10.1074/jbc.R110.198697. doi:10.1074/jbc.R110.198697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaski JP, et al. Prevalence of sarcomere protein gene mutations in preadolescent children with hypertrophic cardiomyopathy. Circ Cardiovasc Genet. 2009;2:436–441. doi: 10.1161/CIRCGENETICS.108.821314. [DOI] [PubMed] [Google Scholar]
- Kazmierczak K, Xu Y, Jones M, Guzman G, Hernandez OM, Kerrick WGL, Szczesna-Cordary D. The role of the N-terminus of the myosin essential light chain in cardiac muscle contraction. J Mol Biol. 2009;387:706–725. doi: 10.1016/j.jmb.2009.02.006. doi:10.1016/j.jmb.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierczak K, Muthu P, Huang W, Jones M, Wang Y, Szczesna-Cordary D. Myosin regulatory light chain mutation found in hypertrophic cardiomyopathy patients increases isometric force production in transgenic mice. Biochem J. 2012;442:95–103. doi: 10.1042/BJ20111145. doi:10.1042/BJ20111145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierczak K, et al. Discrete effects of A57G-myosin essential light chain mutation associated with familial hyper-trophic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2013;305:H575–H589. doi: 10.1152/ajpheart.00107.2013. doi:10.1152/ajpheart.00107.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierczak K, Yuan C-C, Liang J, Huang W, Rojas AI, Szczesna-Cordary D. Remodeling of the heart in hypertrophy in animal models with myosin essential light chain mutations. Front Physiol. 2014;5:353. doi: 10.3389/fphys.2014.00353. doi:10.3389/fphys.2014.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerrick WGL, Kazmierczak K, Xu Y, Wang Y, Szczesna-Cordary D. Malignant familial hypertrophic cardiomyopathy D166V mutation in the ventricular myosin regulatory light chain causes profound effects in skinned and intact papillary muscle fibers from transgenic. FASEB J. 2009;23:855–865. doi: 10.1096/fj.08-118182. doi:10.1096/fj.08-118182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuster DW, et al. Release kinetics of circulating cardiac myosin binding protein-C following cardiac injury. Am J Physiol Heart Circ Physiol. 2014;306:H547–H556. doi: 10.1152/ajpheart.00846.2013. doi:10.1152/ajpheart.00846.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, et al. Different expressivity of a ventricular essential myosin light chain gene Ala57Gly mutation in familial hyper-trophic cardiomyopathy. Am Heart J. 2001;141:184–189. doi: 10.1067/mhj.2001.112487. [DOI] [PubMed] [Google Scholar]
- Lee K, Harris SP, Sadayappan S, Craig R. Orientation of myosin binding protein c in the cardiac muscle sarcomere determined by domain-specific immuno-EM. J Mol Biol. 2015;427:274–286. doi: 10.1016/j.jmb.2014.10.023. doi:10.1016/j.jmb.2014.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lossie J, et al. Molecular mechanism regulating myosin and cardiac functions by ELC. Biochem Biophys Res Commun. 2014;450:464–469. doi: 10.1016/j.bbrc.2014.05.142. doi:10.1016/j.bbrc.2014.05.142. [DOI] [PubMed] [Google Scholar]
- Marian AJ, Roberts R. Molecular genetic basis of hypertrophic cardiomyopathy: genetic markers for sudden cardiac death. J Cardiovasc Electrophysiol. 1998;9:88–99. doi: 10.1111/j.1540-8167.1998.tb00871.x. [DOI] [PubMed] [Google Scholar]
- Maron BJ, Bonow RO, Seshagiri TNR, Roberts WC, Epstein SE. Hypertrophic cardiomyopathy with ventricular septal hypertrophy localized to the apical region of the left ventricle (apical hypertrophic cardiomyopathy). Am J Cardiol. 1982;49:1838–1848. doi: 10.1016/0002-9149(82)90200-4. [DOI] [PubMed] [Google Scholar]
- Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild DE. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation. 1995;92:785–789. doi: 10.1161/01.cir.92.4.785. [DOI] [PubMed] [Google Scholar]
- Marston SB. How do mutations in contractile proteins cause the primary familial cardiomyopathies? J Cardiovasc Transl Res. 2011;4:245–255. doi: 10.1007/s12265-011-9266-2. doi:10.1007/s12265-011-9266-2. [DOI] [PubMed] [Google Scholar]
- Meder B, et al. A single serine in the carboxyl terminus of cardiac essential myosin light chain-1 controls cardiomyocyte contractility in vivo. Circ Res. 2009;104:650–659. doi: 10.1161/CIRCRESAHA.108.186676. doi:10.1161/circresaha.108.186676. [DOI] [PubMed] [Google Scholar]
- Millat G, et al. Clinical and mutational spectrum in a cohort of 105 unrelated patients with dilated cardiomyopathy. Eur J Med Genet. 2011;54:e570–e575. doi: 10.1016/j.ejmg.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Morano I. Tuning the human heart molecular motors by myosin light chains. J Mol Med. 1999;77:544–555. doi: 10.1007/s001099900031. doi:10.1007/s001099900031. [DOI] [PubMed] [Google Scholar]
- Morita H, et al. Shared genetic causes of cardiac hypertrophy in children and adults. N Engl J Med. 2008;358:1899–1908. doi: 10.1056/NEJMoa075463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthu P, et al. Structural and functional aspects of the myosin essential light chain in cardiac muscle contraction FASEB journal : official publication of the Federation of American Societies for. Exp Biol. 2011;25:4394–4405. doi: 10.1096/fj.11-191973. doi:10.1096/fj.11-191973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthu P, Huang W, Kazmierczak K, Szczesna-Cordary D. Functional consequences of mutations in the myosin regulatory light chain associated with hypertrophic cardiomyopathy. In: Veselka J, editor. Cardiomyopathies—from basic research to clinical management. InTech; Croatia: 2012. pp. 383–408. chap. 17. doi:10.5772/29012. [Google Scholar]
- Olivotto I, et al. Myofilament protein gene mutation screening and outcome of patients with hypertrophic cardiomyopathy. Mayo Clin Proc. 2008;83:630–638. doi: 10.4065/83.6.630. [DOI] [PubMed] [Google Scholar]
- Olson TM, Karst ML, Whitby FG, Driscoll DJ. Myosin light chain mutation causes autosomal recessive cardiomyopathy with mid-cavitary hypertrophy and restrictive physiology. Circulation. 2002;105:2337–2340. doi: 10.1161/01.cir.0000018444.47798.94. [DOI] [PubMed] [Google Scholar]
- Perriard JC, Hirschy A, Ehler E. Dilated cardiomyopathy: a disease of the intercalated disc? Trends Cardiovasc Med. 2003;13:30–38. doi: 10.1016/s1050-1738(02)00209-8. [DOI] [PubMed] [Google Scholar]
- Petzhold D, Lossie J, Keller S, Werner S, Haase H, Morano I. Human essential myosin light chain isoforms revealed distinct myosin binding, sarcomeric sorting, and inotropic activity. Cardiovasc Res. 2011;90:513–520. doi: 10.1093/cvr/cvr026. doi:10.1093/cvr/cvr026. [DOI] [PubMed] [Google Scholar]
- Petzhold D, Simsek B, Meißner R, Mahmoodzadeh S, Morano I. Distinct interactions between actin and essential myosin light chain isoforms. Biochem Biophys Res Commun. 2014;449:284–288. doi: 10.1016/j.bbrc.2014.05.040. doi:10.1016/j.bbrc.2014.05.040. [DOI] [PubMed] [Google Scholar]
- Piran S, Liu P, Morales A, Hershberger RE. Where genome meets phenome: rationale for integrating genetic and protein biomarkers in the diagnosis and management of dilated cardiomyopathy and heart failure. J Am Coll Cardiol. 2012;60:283–289. doi: 10.1016/j.jacc.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Poetter K, et al. Mutations in either the essential or regulatory light chains of myosin are associated with a rare myopathy in human heart and skeletal muscle. Nat Genet. 1996;13:63–69. doi: 10.1038/ng0596-63. [DOI] [PubMed] [Google Scholar]
- Rayment I. The structural basis of the myosin ATPase activity. J Biol Chem. 1996;271:15850–15853. doi: 10.1074/jbc.271.27.15850. doi:10.1074/jbc.271.27.15850. [DOI] [PubMed] [Google Scholar]
- Rayment I, et al. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science. 1993;261:50–58. doi: 10.1126/science.8316857. doi:10. 1126/science.8316857. [DOI] [PubMed] [Google Scholar]
- Richard P, et al. Hypertrophic cardiomyopathy: Distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation. 2003;107:2227–2232. doi: 10.1161/01.CIR.0000066323.15244.54. and erratum (2004) 2109(2225):3258. [DOI] [PubMed] [Google Scholar]
- Robinson JM, Wang Y, Kerrick WG, Kawai R, Cheung HC. Activation of striated muscle: nearest-neighbor regulatory-unit and cross-bridge influence on myofilament kinetics. J Mol Biol. 2002;322:1065–1088. doi: 10.1016/s0022-2836(02)00855-0. [DOI] [PubMed] [Google Scholar]
- Sadayappan S, Gulick J, Klevitsky R, Lorenz JN, Sargent M, Molkentin JD, Robbins J. Cardiac myosin binding protein-C phosphorylation in a {beta}-myosin heavy chain background. Circulation. 2009;119:1253–1262. doi: 10.1161/CIRCULATIONAHA.108.798983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos S, et al. High Resolution Melting: improvements in the genetic diagnosis of Hypertrophic Cardiomyopathy in a Portuguese cohort. BMC Med Gen. 2012;13:17. doi: 10.1186/1471-2350-13-17. doi:10.1186/1471-2350-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub MC, Hefti MA, Zuellig RA, Morano I. Modulation of contractility in human cardiac hypertrophy by myosin essential light chain isoforms. Cardiovasc Res. 1998;37:381–404. doi: 10.1016/s0008-6363(97)00258-7. [DOI] [PubMed] [Google Scholar]
- Schonberger J, Seidman CE. Many roads lead to a broken heart: the genetics of dilated cardiomyopathy. Am J Hum Genet. 2001;69:249–260. doi: 10.1086/321978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scruggs SB, Solaro RJ. The significance of regulatory light chain phosphorylation in cardiac physiology. Arch Biochem Biophys. 2011;510:129–134. doi: 10.1016/j.abb.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scruggs SB, et al. Ablation of ventricular myosin regulatory light chain phosphorylation in mice causes cardiac dysfunction in situ and affects neighboring myofilament protein phosphorylation. J Biol Chem. 2009;284:5097–5106. doi: 10.1074/jbc.M807414200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh F, et al. Mouse and computational models link Mlc2v dephosphorylation to altered myosin kinetics in early cardiac disease. J Clin Invest. 2012;122:1209–1221. doi: 10.1172/JCI61134. doi:10.1172/JCI61134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutoh K. An actin-binding site on the 20 K fragment of myosin subfragment 1. Biochemistry. 1982;21:4800–4804. doi: 10.1021/bi00262a043. [DOI] [PubMed] [Google Scholar]
- Sweeney HL, Bowman BF, Stull JT. Myosin light chain phosphorylation in vertebrate striated muscle: regulation and function. Am J Physiol. 1993;264:C1085–C1095. doi: 10.1152/ajpcell.1993.264.5.C1085. [DOI] [PubMed] [Google Scholar]
- Szczesna D. Regulatory light chains of striated muscle myosin. Structure, function and malfunction. Curr Drug Targets Cardiovasc Haematol Disord. 2003;3:187–197. doi: 10.2174/1568006033481474. doi:10.2174/1568006033481474. [DOI] [PubMed] [Google Scholar]
- Szczesna D, et al. Familial hypertrophic cardiomyopathy mutations in the regulatory light chains of myosin affect their structure, Ca2? binding, and phosphorylation. J Biol Chem. 2001;276:7086–7092. doi: 10.1074/jbc.M009823200. doi:10.1074/jbc.M009823200. [DOI] [PubMed] [Google Scholar]
- Szczesna-Cordary D, Guzman G, Ng SS, Zhao J. Familial hypertrophic cardiomyopathy-linked alterations in Ca2? binding of human cardiac myosin regulatory light chain affect cardiac muscle contraction. J Biol Chem. 2004;279:3535–3542. doi: 10.1074/jbc.M307092200. [DOI] [PubMed] [Google Scholar]
- Szczesna-Cordary D, et al. Myosin regulatory light chain E22 K mutation results in decreased cardiac intracellular calcium and force transients FASEB journal : official publication of the Federation of American Societies for. Exp Biol. 2007;21:3974–3985. doi: 10.1096/fj.07-8630com. doi:10.1096/fj.07-8630com. [DOI] [PubMed] [Google Scholar]
- Szczesna-Cordary D, Morimoto S, Gomes AV, Moore JR. Cardiomyopathies: classification, clinical characterization, and functional phenotypes. Biochemistry research international. 2012;2012:870942. doi: 10.1155/2012/870942. doi:10.1155/2012/870942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timson DJ. Fine tuning the myosin motor: the role of the essential light chain in striated muscle myosin. Biochimie. 2003;85:639–645. doi: 10.1016/s0300-9084(03)00131-7. [DOI] [PubMed] [Google Scholar]
- Trayer HR, Trayer IP. Differential binding of rabbit fast muscle myosin light chain isoenzymes to regulated actin. FEBS Lett. 1985;180:170–173. doi: 10.1016/0014-5793(85)81065-6. [DOI] [PubMed] [Google Scholar]
- van der Velden J, et al. The effect of myosin light chain 2 dephosphorylation on Ca2?-sensitivity of force is enhanced in failing human hearts. Cardiovasc Res. 2003a;57:505–514. doi: 10.1016/s0008-6363(02)00662-4. doi:10.1016/s0008-6363(02)00662-4. [DOI] [PubMed] [Google Scholar]
- van der Velden J, et al. Increased Ca2+-sensitivity of the contractile apparatus in end-stage human heart failure results from altered phosphorylation of contractile proteins. Cardiovasc Res. 2003b;57:37–47. doi: 10.1016/s0008-6363(02)00606-5. doi:10.1016/s0008-6363(02)00606-5. [DOI] [PubMed] [Google Scholar]
- Wang Y, Xu Y, Kerrick WGL, Wang Y, Guzman G, Diaz-Perez Z, Szczesna-Cordary D. Prolonged Ca2+ and force transients in myosin RLC transgenic mouse fibers expressing malignant and benign FHC mutations. J Mol Biol. 2006;361:286–299. doi: 10.1016/j.jmb.2006.06.018. doi:10.1016/j.jmb.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Warren SA, et al. Myosin light chain phosphorylation is critical for adaptation to cardiac stress. Circulation. 2012;126:2575–2588. doi: 10.1161/CIRCULATIONAHA.112.116202. doi:10.1161/CIRCULATIONAHA.112.116202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnker PJ, Murphy AM, Stienen GJ, van der Velden J. Troponin I phosphorylation in human myocardium in health and disease. Neth Heart J. 2014;22:463–469. doi: 10.1007/s12471-014-0590-4. doi:10.1007/s12471-014-0590-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley MA, Trayer HR, Trayer IP. Role of the myosin light chains in binding to actin. FEBS Lett. 1977;77:239–242. doi: 10.1016/0014-5793(77)80242-1. [DOI] [PubMed] [Google Scholar]
- Xie X, Harrison DH, Schlichting I, Sweet RM, Kalabokis VN, Szent-Gyorgyi AG, Cohen C. Structure of the regulatory domain of scallop myosin at 2.8 A resolution. Nature. 1994;368:306–312. doi: 10.1038/368306a0. [DOI] [PubMed] [Google Scholar]
- Xu Q, Dewey S, Nguyen S, Gomes AV. Malignant and benign mutations in familial cardiomyopathies: insights into mutations linked to complex cardiovascular phenotypes. J Mol Cell Cardiol. 2010;48:899–909. doi: 10.1016/j.yjmcc.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Yuan CC, et al. Constitutive phosphorylation of cardiac myosin regulatory light chain prevents development of hypertrophic cardiomyopathy in mice. Proc Natl Acad Sci USA. 2015 doi: 10.1073/pnas.1505819112. doi:10.1073/pnas.1505819112. [DOI] [PMC free article] [PubMed] [Google Scholar]