Summary
Stored platelets undergo biochemical, structural and functional changes that lead to decreased efficacy and safety of platelet transfusions. Not only do platelets acquire markers of activation during storage, but they also fail to respond normally to agonists post-storage. We hypothesized that resveratrol, a cardioprotective antioxidant, could act as a novel platelet storage additive to safely prevent unwanted platelet activation during storage, while simultaneously preserving normal haemostatic function. Human platelets treated with resveratrol and stored for five days released less thromboxane B2 and prostaglandin E2 compared to control platelets. Resveratrol preserved the ability of platelets to aggregate, spread and respond to thrombin, suggesting an improved ability to activate post-storage. Utilizing an in vitro model of transfusion and thromboelastography, clot strength was improved with resveratrol treatment compared to conventionally stored platelets. The mechanism of resveratrol’s beneficial actions on stored platelets was partly mediated through decreased platelet apoptosis in storage, resulting in a longer half-life following transfusion. Lastly, an in vivo mouse model of transfusion demonstrated that stored platelets are prothrombotic and that resveratrol delayed vessel occlusion time to a level similar to transfusion with fresh platelets. We show resveratrol has a dual ability to reduce unwanted platelet activation during storage, while preserving critical haemostatic function.
Keywords: haemostasis, platelet transfusion, resveratrol, thrombosis, platelet function, apoptosis
Introduction
Platelet transfusion is a routine medical practice for the treatment or prevention of bleeding. In the United States, platelets concentrates (PC) are prepared by apheresis or from whole blood and are stored for up to five days. Almost uniformly, PC are transfused between three and five days of storage to allow for pathogen testing (local experience, Strong Memorial Hospital, Rochester, NY). The intent of platelet transfusion is to stop or prevent bleeding due to thrombocytopenia by providing patients with sufficient donor platelets to maintain basal haemostatic functions (Cauwenberghs, et al 2007). Ideally, transfused platelets will lack markers of activation that could lead to their destruction or clearance, and remain quiescent until needed at sites of vascular injury (Weyrich and Zimmerman 2004). Upon exposure to vascular damage, platelets should activate to form a haemostatic plug (Hawiger 1987). However, stored platelets undergo extensive biochemical, structural and functional changes, termed the platelet storage lesion, which can lead to decreased efficacy and safety of platelet transfusions (Cauwenberghs, et al 2007, Ohto and Nollet 2011, Springer, et al 2009). Following transfusion, these unwanted changes in platelet function can contribute to platelet-related complications, ranging from mild to moderately severe adverse reactions (rigors, fever, inflammation) to life-threatening events (thrombosis, stroke, transfusion-related acute lung injury (TRALI)) (Heal, et al 2009, Slichter 2007, Tormey and Stack 2009).
During storage, platelets become activated and release prothrombotic and proinflammatory mediators, which may contribute to adverse transfusion reactions (Blumberg, et al 2006, Kaufman, et al 2007). However, platelets are hypo-responsive to agonists post-storage, probably compromising the haemostatic benefit of supplementing thrombocytopenic patients with donor platelets (Curvers, et al 2004). Alternative storage methods that seek to preserve platelet function during storage are under investigation, including platelet storage solutions, storage bags with increased gas permeability and cold storage (Cookson, et al 2012, Reddoch, et al 2013, Skripchenko, et al 2011, Slichter, et al 2014). While many studies demonstrate improved metabolic markers (pH, lactate, glucose) and parameters of platelet quality (mean platelet volume, “swirling” reaction, shape change), these may not be predictive of in vivo platelet clinical efficacy and safety (clot formation, prothrombotic potential, in vivo survival) (Rinder, et al 2003). Additionally, several reports suggest that the lifespan of platelets in storage is regulated by apoptosis. Although anucleate, platelets undergo apoptosis in response to stress and in vitro storage, resulting in mitochondrial membrane depolarization, caspase activation and phosphatidylserine exposure (Leytin 2012). Our study focuses on addition of the natural antioxidant, resveratrol, as a novel method to inhibit platelet activation during storage, while preserving the ability of platelets to maintain basal physiological functions upon transfusion.
Resveratrol (trans-3,5,4′-trihydroxystilbene) is a naturally occurring polyphenolic compound abundantly found in grapes, peanuts, and red wine (Bhat, et al 2001). It is well known for its cardioprotective, anti-inflammatory and anti-oxidant properties, and is currently under investigation in 75 clinical trials for its beneficial biological actions in patients with Alzheimer’s disease, obesity, type II diabetes and other disorders (Baur and Sinclair 2006; https://clinicaltrials.gov/ct2/results?term=resveratrol&Search=Search). Additionally, resveratrol has the ability to extend the lifespan of model organisms, including Saccharmomyces cerevisiae, C. elegans, Drosophila melanogaster and mice (Bauer, et al 2004, Baur, et al 2006, Howitz, et al 2003, Strong, et al 2013, Viswanathan, et al 2005, Wang, et al 2013, Wood, et al 2004). It is a naturally occurring product with little to no toxicity, even at high doses (Boocock, et al 2007, Juan, et al 2002, Poulsen, et al 2013). Resveratrol dampens platelet aggregation and thromboxane production in vitro in the micromolar range (Dobrydneva, et al 1999, Pace-Asciak, et al 1995, Sobotková, et al 2009), but no data are available on the effects of resveratrol on stored platelets.
Resveratrol has been studied in humans and animal models, but a major knowledge gap lies in its potential to preserve normal platelet function during storage. There are currently no ideal options for platelet storage, including the use of additive solutions. An ideal storage solution should simultaneously prevent unwanted platelet activation during storage, while preserving normal platelet haemostatic function. In the present study, we investigated the potential of resveratrol to preserve platelet function during storage.
Methods
Platelet storage
Healthy human donors who had not taken anti-platelet drugs for two weeks prior to donation were consented in accordance with the Declaration of Helsinki under an approved University of Rochester institutional review board protocol. PC were generated using the platelet rich plasma (PRP) method via standard blood banking procedures (Fung, et al 2014). Briefly, 550 ml whole blood was collected into CP2D/AS-3 triple blood bag units (Pall Medical, Covina, CA) and centrifuged at 250 × g for 15 min at 20°C. PRP was immediately expressed through an attached leucoreduction filter and centrifuged at 3800 × g for 10 min. Platelet poor plasma (PPP) was removed, leaving 100 ml of PRP. Platelets were rested for 30 min then resuspended with gentle agitation for 1 h at 20°C. PC were split into custom-made 15 ml platelet storage bags (Blood Cell Storgage, Inc., Seattle, WA) or attached 500 ml platelet storage satellite bags using a sterile technique. No significant differences were observed between platelets stored in satellite bags or mini storage bags.
Vehicle (0.1% dimethyl sulfoxide [DMSO]), 10 μM resveratrol or trans-trimethoxy-resveratrol (dissolved in DMSO; Cayman Chemical, Ann Arbor, MI) were resuspended in 1 ml PPP and injected into the platelet storage bags. Aspirin (acetylsalicylic acid [ASA]; Sigma-Aldrich, St Louis, MO) was prepared in ethanol and normal saline (50:50) and used at 5 μM (1% final ethanol) by resuspension in 1 ml PPP and injected into the platelet storage bags prior to storage. PC were stored for five days in a 20°C incubator on a Belly Dancer orbital shaker (Sigma-Aldrich, St Louis, MO). Bags were sampled immediately after preparation (day 1) or days 3 or 5 post-preparation by removal of 1.5 ml PC using a sterile technique. No significant differences were observed between 0.1% DMSO vehicle treatment compared to no treatment (data not shown).
Washed platelets
For platelet spreading and thrombin stimulation assays, platelets were washed prior to activation to prevent clot formation upon activation. Platelets were pelleted by centrifugation of PC at 1000 × g for 10 min in the presence of 1 μg/ml prostacyclin (Cayman Chemical, Ann Arbor, MI), gently resuspended in Tyrode’s (Sigma-Aldrich, St Louis, MO) acid-citrate-dextrose (ACD, 25/3, vol/vol) solution containing 0.1 μg/ml prostacyclin, then centrifuged at 1000 × g for 10 min. Platelets were resuspended in Tyrode’s buffer and used within 3 h.
Platelet spreading
Washed platelets (1 × 107 platelets) were spread on fibrinogen-coated coverslips (100 μg/ml; Sigma-Aldrich, St Louis, MO) for 45 min at 37°C, washed with phosphate-buffered saline, and fixed with 4% paraformaldehyde. Spreading was visualized by differential interference contrast (DIC) optics using an Olympus BX51 microscope (Olympus, Melville, NY) at 100X. The percentage of fully spread platelets was determined by manually counting four fields of view.
Immunoassays
Thromboxane B2 (TXB2) and prostaglandin E2 (PGE2) were assayed by enzyme immunoassay (EIA; Cayman Chemical, Ann Arbor, MI). Platelet factor 4 (PF4) was assayed by enzyme linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN). Soluble CD40L (sCD40L) was assayed by a previously published ELISA (Kaufman, et al 2007).
Flow cytometry
Platelets (1 × 109 platelets/l) were blocked with human Fc Receptor blocking reagent (Miltenyi Biotech, Bergisch Gladbach, Germany) for 15 min. Antibodies were purchased from BD Biosciences (Franklin Lakes, NJ). Platelets were stained with mouse anti-human CD61-alexa fluor 488 and mouse anti-human CD62P-alexa fluor 647 or mouse anti-human CD61-alexa fluor 647 and mouse anti-human annexin V-fluorescein isothiocyanate (FITC) for 30 min at 20°C. Platelets were identified by forward and side scatter and CD61 positivity on an Accuri flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA). Platelet microparticles (PMPs) were enumerated by gating CD61 positive events between 0.1 μM and 1.0 μM, determined by megamix sizing beads (Biocytex, Marseille, France).
Reactive oxygen species
Reactive oxygen species (ROS) were quantified by incubation of washed platelets with 5 μmol/l dichlorodihydrofluorescein diacetate (H2DCFDA; C400, Molecular Probes, Eugene, OR) and multimode reader (Varioskan Flash, Thermo Scientific, Waltham, MA). The background fluorescence of resveratrol in Tyrode’s buffer was subtracted from resveratrol-treated platelets to account for effects of resveratrol directly on the dye.
Platelet Activation
Washed platelets (1 × 108 platelets) were activated with 0.2 u/ml thrombin or left unactivated for 15 min at 37°C. Supernatants were generated by centrifugation of washed platelets at 1200 × g for 15 min and analysed for mediator release as described above.
Aggregometry
Platelet aggregation was performed by the turbidimetric method using a Chrono-log Lumi-Aggregometer with AGGRO/LINK software (Chrono-Log Corp., Havertown, PA). PRP (500 μl) was placed in a silicone-coated cuvette with constant stirring at 1200 rpm using a siliconized stir bar. The PPP from each sample was used as the reference sample, denoting 100% light transmission. Aggregation was initiated using the following agonists and concentrations: 10 μM ADP, 10 μg/ml collagen, or a combination of 5 μM ADP and 5 μg/ml collagen.
Thromboelastography (TEG)
Thrombocytopenic samples were generated by centrifugation of citrated whole blood at 180 × g for 12 min at 20°C. PRP was replaced by an equal amount of autologous PPP obtained from a different tube by centrifugation at 1900 × g for 12 min at 20°C. The platelet count of thrombocytopenic samples was 13.0 +/− 2.0 × 109/l. Platelets were mixed at a 1:4 ratio with autologous fresh thrombocytopenic whole blood. Kaolin was used to initiate clot formation. TEG was performed using a TEG haemostasis system (Haemoscope Corp,, Niles, IL) within 2 h of collection (Refaai, et al 2013).
Mitochondrial membrane potential
Mitochondrial membrane potential was measured using JC-1 dye (Molecular Probes, Eugene, OR). The ratio of red/green fluorescence (monomers/aggregates) was determined by incubation of platelets (1 × 109 platelets/l) with 2.0 μg/ml JC-1 for 30 min. Resveratrol stored in normal saline in platelet storage bags was used as a negative control for the effects of resveratrol directly on JC-1 dye. Because resveratrol alone in normal saline decreased red fluorescence, these background values were subtracted from resveratrol-treated PC.
Western Blotting
Total protein was quantified by bicinchoninic acid assay (Thermo-Scientific, Waltham, MA) and 10 μg was separated using sodium dodecyl sulphate-ployacrylamide gel electrophoresis, transferred onto a polyvinylidene difluoride membrane, blocked with 5% bovine serum albumin, and probed with rabbit anti-human cleaved caspase-3 (Cell Signaling, Danvers, MA), rabbit anti-human Bcl-XL (also termed BCL2L1) (Santa Cruz Biotechnology, Dallas, TX), rabbit anti-human Bak (also termed BAK1) (Cell Signaling), or mouse anti-human actin. Densitometry was performed using ImageJ software (National Institutes of Health, Bethesda, MD.).
In vivo studies
Mouse experiments were performed in accordance with a University Committee on Animal Resources (UCAR) protocol, approved by the University of Rochester. Whole blood was collected by terminal retro-orbital bleeds from isoflurane-anesthetized 8- to 10-week-old C57Bl/6 male mice into citric acid. Whole blood from 30 mice was centrifuged at 250 × g for 15 min at room temperature and the top 2/3 of PRP was removed, avoiding red blood cell (RBC) and white blood cell (WBC) contamination. A complete blood count was performed using a HemaTrue Haematology Analyser (Heska, Loveland, CO) to obtain platelet counts and ensure no RBC or WBC contamination. PRP was split into two equal samples and treated with vehicle (0.1% DMSO) or 10 μM resveratrol, then stored in an upright 10 ml cell culture flask at room temperature with gentle agitation.
Platelet recovery
Mouse platelets (prepared above) were labelled with carboxyfluorescein succinimidyl ester (CFSE; LifeTechnologies, Eugene OR), washed with Tyrode’s buffer, then 5 × 107 platelets were transfused by retro-orbital injection into 8-week-old male mice. Mice were serially bled and the percentage of CFSE-positive platelets was enumerated by flow cytometry.
Ferric chloride thrombosis
Platelets were labelled with 10 μM calcein-AM (Molecular Probes, Eugene OR) by retro-orbital injection. PRP (200 μl at 1 × 1011 platelets/l) was injected intravenously into 4-week-old male mice anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) via intramuscular injection. The mesentery was externalized and thrombosis was initiated by application of 5 mm2 Whatmann’s paper soaked in 10% ferric chloride to the vessel surface for 45 s. Platelet accumulation was recorded with a digital imaging camera in real-time until stable vessel occlusion occurred (Nikon Ti-S inverted microscope and high speed digital imaging camera with NIS Elements image analysis software; Nikon, Tokyo, Japan).
Results
Resveratrol improves platelet spreading after storage
Although platelets spontaneously activate during storage, they do not function normally in response to a single agonist post-storage (Shapira, et al 2000). Platelet spreading is a haemostatic event crucial for wound healing and closure of broken vasculature (Hawiger 1987). However, the ability of stored platelets to spread has not been investigated. An initial screen of cardioprotective molecules was performed to identify a compound that could mitigate, but not abrogate, spontaneous platelet activation (data not shown). Based on this screen and dose-response studies, 10 μM resveratrol was selected for further evaulation. Immediately after preparation, PC were treated with resveratrol and stored for up to five days. Neither platelet counts nor mean platelet volume were affected by resveratrol treatment (Supplemental Fig 1). We demonstrate that platelets lost their ability to spread on fibrinogen post-storage (Fig 1A). Resveratrol significantly restored the ability of platelets to spread by approximately two-fold at three and five days post-storage (Fig 1B–C). Similar results were observed when unwashed stored platelets were spread on fibrinogen post-storage, suggesting that platelet washing did not deleteriously affect platelet spreading (data not shown).
Figure 1. Resveratrol improves platelet spreading after storage.
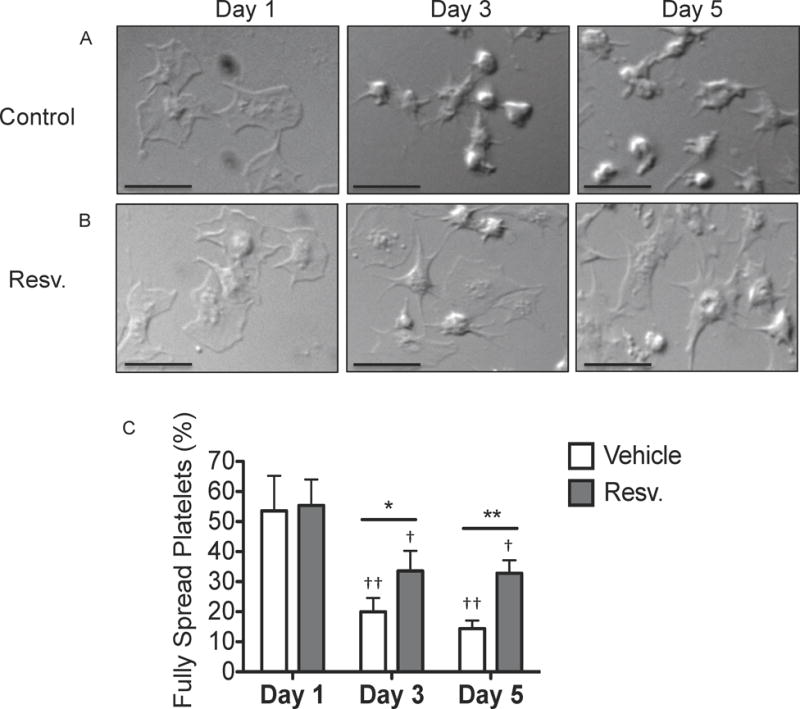
Human platelets were collected from healthy human donors and platelet concentrates (PC) were prepared according to standard blood banking procedures. PC were treated with vehicle or 10 μM resveratrol (Resv.) prior to storage and stored for up to 5 days at room temperature with agitation. After 1, 3 or 5 days of storage, platelets were washed and allowed to spread on fibrinogen-coated coverslips for 45 min. Coverslips were fixed and visualized by differential interference contrast (DIC) optics using an Olympus BX51 microscope at 100X and SPOT computer software. Scale bars = 10 μm. One representative donor of 3 is shown (A–B). The percentage of fully spread platelets per field of view was determined by manually counting four fields of view for 5 individual donors (C). Mean +/− SEM. n=5 Statistical significance determined by Two-Way RM ANOVA with Dunnett’s post-test. † p<0.05, †† p<0.01 compared to Day 1. *p<0.05, **p<0.01 compared to vehicle.
Resveratrol prevents accumulation of proinflammatory mediators during platelet storage
Next, the ability of resveratrol to inhibit the spontaneous activation of platelets during storage was investigated. Supernatants were sampled for inflammatory mediator accumulation on days 1, 3, and 5 of storage. Levels of TXB2, PGE2, PF4 and sCD40L increased over time in control stored PC and expression of CD62P on the platelet surface similarly increased (Fig 2A–E). Accumulation of TXB2, the stable metabolite of thromboxane A2, in resveratrol-treated platelets was significantly less than control on day 3 of storage (Fig 2A). Resveratrol ablated PGE2 accumulation at day 3 and was still significantly reduced at day 5 (Fig 2B). PF4 and sCD40L levels were not affected by resveratrol, nor was expression of CD62P on the platelet surface (Fig 2C–E). Consistent with previous reports, reactive oxygen species (ROS) in stored platelets increased over time (Perales Villarroel, et al 2013) and here we show that resveratrol prevented this accumulation, probably due to its antioxidant properties (Fig 2F). Despite the resveratrol-mediated decrease in ROS during storage, pH levels in PC was not affected (Supplemental Fig 2A). Additionally, glucose levels in stored PC decreased over time, probably due to metabolic consumption as evidenced by platelet activation, but resveratrol did not significantly alter glucose consumption over time, despite its ability to reduce markers of platelet activation (Supplemental Fig 2B).
Figure 2. Resveratrol prevents accumulation of proinflammatory mediators during storage.
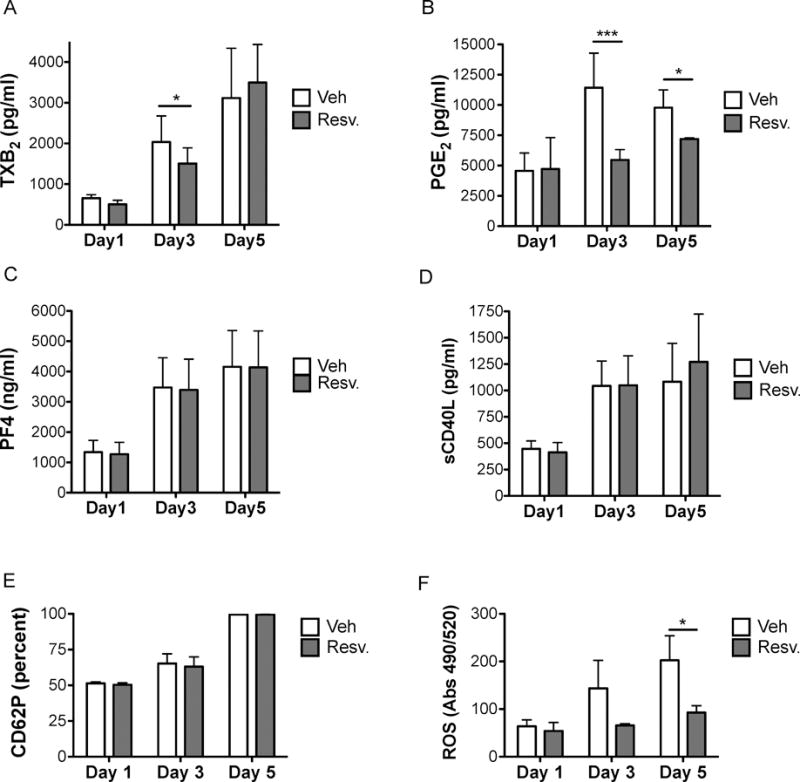
Human platelets were collected from healthy human donors and platelet concentrates (PC) were prepared according to standard blood banking procedures. PC were treated with vehicle (Veh) or 10 μM resveratrol (Resv.) prior to storage and stored for up to 5 days at room temperature with agitation. Supernatant mediator levels in platelet poor plasma were analysed by enzyme immunoassy (A–B) or enzyme-linked immunosorbent assay (C–D). Membrane-bound CD62P expression was evaluated on washed platelets by flow cytometry (E). Reactive oxygen species (ROS) production was measured in platelets using H2DCFDA dye (F). Mean +/− SEM. n=3 Statistical significance was determined by Two-Way RM ANOVA with Dunnett’s post-test. *p<0.05, ***p<0.001
Resveratrol preserves the ability of human platelets to activate post-storage
Stored platelets are less responsive to agonist post-storage (Shapira, et al 2000). We confirmed this finding and newly demonstrate that resveratrol-treated stored platelets improved platelet response to agonist stimulation. Freshly prepared platelets (day 1) produced TXB2 and PGE2 in response to thrombin treatment while resveratrol significantly decreased TXB2 and PGE2 production at day 1 (Fig 3A–B). On the other hand, platelets stored for 3 or more days produced significantly less TXB2 and PGE2 in response to thrombin stimulation. At day 5 of storage, resveratrol-treated platelets produced significantly more TXB2 and PGE2 in response to thrombin stimulation compared to control platelets. These new data show that while resveratrol initially inhibited platelet activation, it preserved the ability of platelets to activate post-storage. Furthermore and importantly, these new findings show that the inhibitory actions of resveratrol are reversible, unlike inhibition by ASA, which irreversibly prevented TXB2 and PGE2 release.
Figure 3. Resveratrol preserves the ability of platelets to activate post-storage.
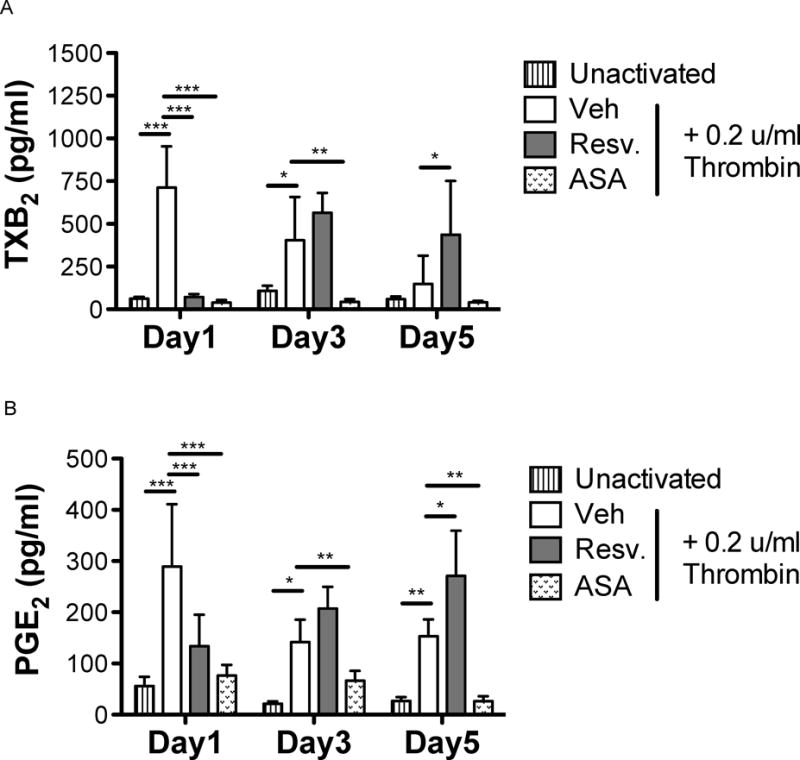
Human platelets were collected from healthy human donors and platelet concentrates (PC) were prepared according to standard blood banking procedures. PC were treated with vehicle (Veh), 10 μM resveratrol (Resv.), or 5 μM aspirin (ASA) prior to storage and stored for up to 5 days at room temperature with agitation. Platelets (1 × 108 platelets/ml) were washed and either left unactivated or activated with 0.2 u/ml thrombin for 15 min. Supernatants were collected and thromboxane B2 (TXB2) and prostaglandin E2 (PGE2) were assayed by enzyme immunoassy. Mean +/− SEM. n = 4 Statistical significance was determined by Two-Way RM ANOVA with Bonferroni post-test. *p<0.05, *p<0.01, ***p<0.001
Resveratrol preserves the ability of stored platelets to aggregate
In addition to losing the ability to release TXB2 and PGE2 post-storage, platelets require two agonists to aggregate post-storage. Platelets that had been stored for 5 days aggregated approximately 5-fold less than fresh platelets in response to ADP and approximately 4-fold less in response to collagen (Fig 4). Resveratrol restored the ability of platelets to aggregate after 5 days of storage in response to ADP and collagen by 3- and 2-fold, respectively (Fig 4A–D). Although resveratrol restored the ability of stored platelets to aggregate in response to ADP (compared to no aggregation in control platelets), secondary aggregation was not achieved and platelets readily disaggregated. Additionally, the ability to aggregate in response to dual agonist stimulation was improved with resveratrol treatment, which was only investigated at 5 days post-storage (Fig 4E–F). On the other hand, ASA-treated platelets were unable to aggregate after 5 days of storage.
Figure 4. Resveratrol preserves the ability of platelets to aggregate post-storage.
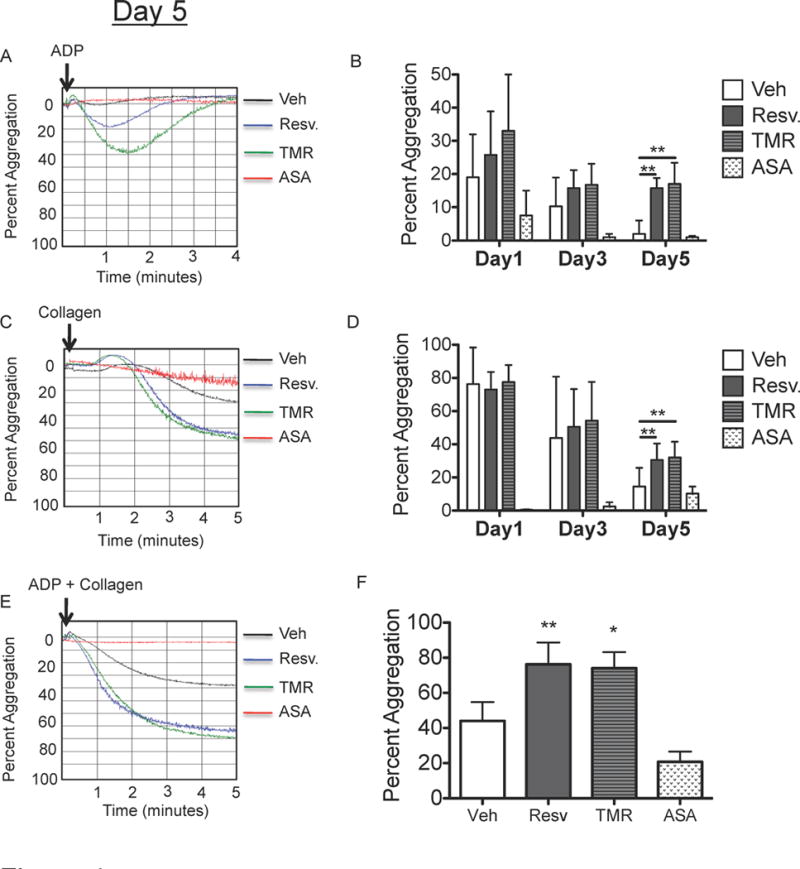
Human platelets were collected from healthy human donors and platelet concentrates (PC) were prepared according to standard blood banking procedures. PC were treated with vehicle (Veh), 10 μM resveratrol (Resv.), 10 μM trans-trimethoxy-resveratrol (TMR) or 5 μM aspirin (ASA) prior to storage and stored for up to 5 days at room temperature with agitation. Platelet poor plasma was generated from each sample and used as a reference denoting 100% light transmittance. Aggregation was initiated by addition of 10 μM ADP (A–B), 10 μg/ml collagen (C–D) or 5μM ADP + 5 μg/ml collagen (E–F). Representative traces from one donor at day 5 are shown (A, C, E). Dual agonist stimulation was only performed at day 5 post-storage (F). Mean +/− SEM. n = 4 Statistical significance was determined by one-way (F) or two-way (B, D) RM ANOVA with Dunnett’s post-test. **p<0.01
Resveratrol preserves the ability of stored platelets to aggregate independent of COX1 inhibition
Resveratrol is a reversible inhibitor of cyclooxygenase-1 (COX1) and its inhibitory action is dependent on the m-hydroquinone moiety (3,5-di-OH group) (Szewczuk, et al 2004). To determine whether the ability of resveratrol to preserve platelet function during storage was due to COX1 inhibition early in storage, platelets were stored with the resveratrol structural analogue, trans-trimethoxy-resveratrol (TMR). TMR exhibits comparable in vitro and in vivo activities to resveratrol, (Kim, et al 2012, Scherzberg, et al 2015) but is unable to inactivate COX1 due to the absence of the m-hydroquinone moiety (Szewczuk, et al 2004). We confirmed that TMR was unable to inhibit COX1 in human platelets by showing that while resveratrol inhibited thrombin-induced TXB2 release, TMR had no effect on TXB2 release. (Supplemental Fig 3). However, TMR preserved the ability of platelets to aggregate at 5 days post-storage to a similar degree as resveratrol (Fig 4), suggesting that the mechanism by which resveratrol preserved platelet function was independent of COX1 inactivation early in storage.
Resveratrol improves the function of stored human platelets in an in vitro model of transfusion
To assess whether resveratrol-treated PC might be superior to standard treatment in a transfusion setting, an in vitro model of autologous transfusion was utilized. Thrombocytopenic whole blood was prepared by platelet-depleting fresh whole blood to mimic a thrombocytopenic patient. Thrombocytopenic whole blood was “transfused” at a ratio of 4:1 (thrombocytopenic whole blood:platelets) with autologous fresh or stored platelets and thromboelastography (TEG) was performed to assess clot formation. The maximum amplitude (MA), a TEG parameter that measures clot strength and considered the best indicator of platelet function, was evaluated (Bowbrick, et al 2003). The decrease in MA from fresh whole blood (58.1+/−2.5 mm) to fresh thrombocytopenic whole blood (28.2+/−1.7 mm) is probably due to the decreased platelet count (Fig 5A–B), as MA is approximately 80% dependent on platelet function (Wiinberg, et al 2005). Transfusion of either fresh control or resveratrol-treated platelets (day 1) to thrombocytopenic whole blood improved clot strength by approximately 60% (47.0+/−3.8 mm and 48.2+/−1.6 mm, respectively; Fig 5C–E). After five days of storage, transfusion of stored control platelets did not significantly improve clot strength (35.7+/−1.7 mm; Fig 5C and E), while stored platelets treated with resveratrol were able to maintain clot strength upon transfusion, similar to days 1 and 3 (44.4+/−2.1 mm; Figs.5D–E). No differences were observed between platelet counts following transfusion (Fig 5F), suggesting that treatment of PC with resveratrol prior to storage could enhance the ability of platelets to function upon transfusion.
Figure 5. Resveratrol improves the function of stored human platelets in an in vitro model of transfusion.
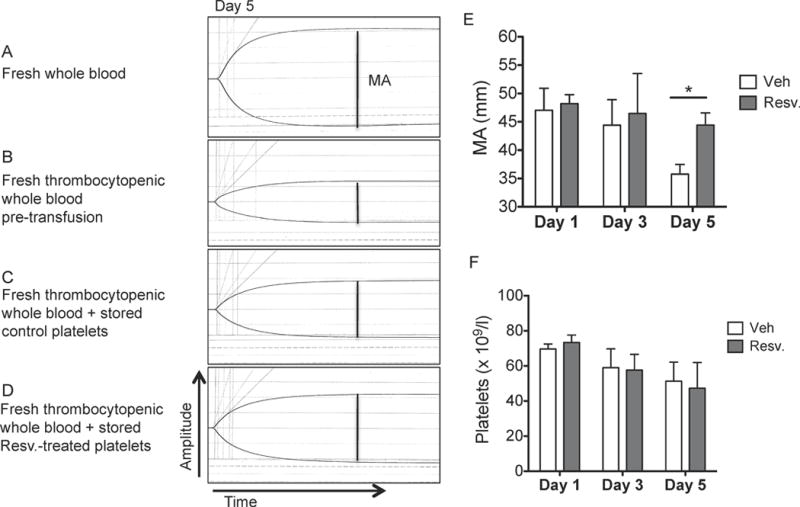
Human platelets were collected from healthy donors and processed according to standard blood banking procedures. Platelet concentrates were treated with vehicle (Veh) or 10 μM resveratrol (Resv.) prior to storage and stored for up to 5 days at room temperature with agitation. Thrombocytopenic whole blood (B) was generated from fresh whole blood (A). Stored control platelets (C) or stored resveratrol-treated platelets (D) (20 × 109/l) were mixed at a 1:4 ratio with autologous fresh thrombocytopenic whole blood. Thromboelastography was measured within 2 h of collection. The maximum amplitude (MA; mm; indicated by dotted line) was measured for each sample. One representative donor from day 5 post-storage (A–D) and quantification of 3 independent donors (E) are shown. The platelet counts following transfusion were measured (F). Mean +/− SEM. n=3 Statistical significance was determined by Two-Way RM ANOVA with Dunnett’s post-test. *p<0.05
Resveratrol mitigates apoptosis in stored human platelets
The lifespan of platelets during storage is thought to be regulated by apoptosis, and stored platelets express apoptotic markers over time (Gyulkhandanyan, et al 2012, Leytin 2012). Here, we show that stored platelets have reduced mitochondrial membrane potential after 5 days of storage, while resveratrol treatment prevented mitochondrial membrane depolarization (Fig 6A). Similarly, the ratio of the pro-survival protein Bcl-xl (BCL2L1) to the pro-apoptotic protein Bak (BAK1) is thought to be a main regulator of platelet apoptosis (Leytin 2012). Resveratrol improved the ratio of Bcl-xl/Bak at 5 days post-storage (Fig 6 B–C). Storage also induced a time-dependent increase in cleaved caspase-3 in human platelets, while resveratrol-treated platelets had significantly lower levels of active caspase-3 at day 5 (Fig 6 B, D). Platelets expose phosphatidylserine (PS) in response to activation or apoptosis in addition to making platelet microparticles (PMPs). Here, we show that stored platelets increase PS exposure and PMP production over time, both of which were dampened by resveratrol (Fig 6E–F). Taken together, these data support a role for resveratrol in mitigating apoptosis during platelet storage.
Figure 6. Resveratrol mitigates apoptosis that occurs during platelet storage.
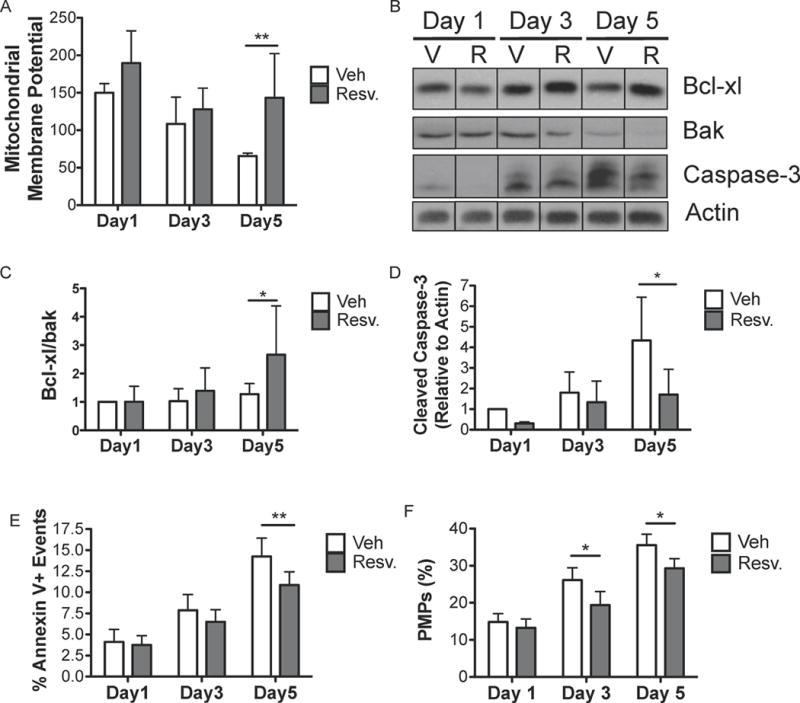
Human platelets were collected from healthy donors and processed according to standard blood banking procedures. Platelet concentrates (PC) were treated with vehicle (Veh; V) or 10 μM resveratrol (Resv.; R) prior to storage and stored for up to 5 days at room temperature with agitation. Mitochondrial membrane potential was measured using JC-1 dye on washed platelets generated at each time point (A). Washed platelets were lysed and immunoblotted for Bcl-xl, Bak, active caspase-3 and actin (B). Densitometry was performed for three independent human donors (C–D). The percentage of annexin V positive platelets and the percentage of platelet microparticles (PMPs) relative to platelets were determined by flow cytometry (E–F). Mean +/− SEM. n=4 (A–D), n=6 (E–F) Statistical significance was determined by Two-Way RM ANOVA with Dunnett’s post-test. *p<0.05, **p<0.01.
Trans-resveratrol-treated platelets have a longer half-life in vivo post-transfusion
In order to determine whether resveratrol-treated platelets had enhanced survival in vivo, we utilized a mouse model of transfusion. Mouse platelets were treated with resveratrol and stored for 24 h, then labelled with CFSE and transfused into autologous recipients. There were no differences in recovery at 4 h post-transfusion between control and resveratrol-treated platelets (Fig 7A). However, resveratrol-treated platelets had an enhanced recovery at 24 and 48 h post-transfusion, suggesting that resveratrol-treated platelets have a higher survival than control platelets in vivo.
Figure 7. Trans-resveratrol-treated platelets have a longer half-life and decreased thrombosis in vivo post-transfusion.
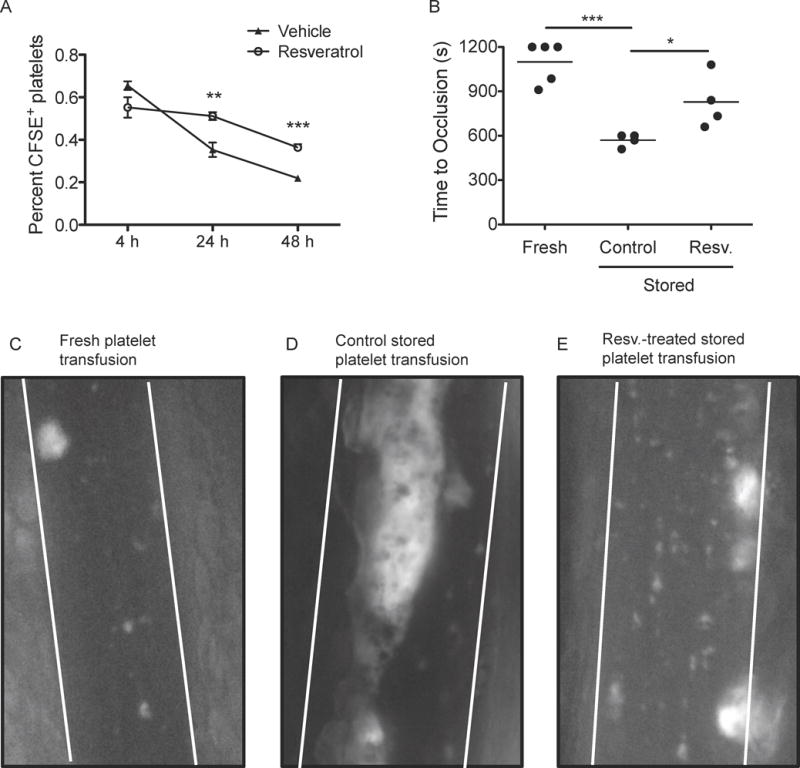
Blood was collected via retro-orbital bleeds from 30 C57Bl/6 mice, and platelet rich plasma (PRP) was generated via centrifugation. PRP was treated with vehicle (0.1% DMSO) or 10 μM trans-resveratrol and stored for 24 h at room temperature with gentle agitation. (A) Platelets were fluorescently labelled with CFSE and transfused into 8-week-old C57Bl/6 autologous recipients retro-orbitally. Blood was collected via retro-orbital bleed after 4, 24 or 48 h and the percentage of CFSE-positive platelets was determined by flow cytometry. n=12. Mean+/− SEM. Statistical significance was determined by Two-Way ANOVA with Bonferroni post-test. **p<0.01, ***p<0.001. (B) Fresh (C), control stored platelets (D), or resveratrol-treated stored platelets (10 μM; E) were fluorescently labelled with calcein-AM and transfused into 4-week-old C57Bl/6 autologous recipients retro-orbitally. 10% ferric chloride was applied to a mesenteric arteriole to initiate thrombosis. Blood flow was visualized by intravital microscopy; dotted lines indicate vessel wall location. Images from 650 s are shown for one representative animal (C–E) and the time to full vessel occlusion was measured (B). Statistical significance was determined by One-Way ANOVA with Tukey post-test. *p<0.05, ***p<0.001
Resveratrol-treated platelets are less prothrombotic than control platelets after transfusion
A major goal of our study was to preserve normal platelet haemostatic function without generating prothrombotic levels of activation. To assess the function of stored platelets in vivo, autologous mouse transfusions with fresh, control stored, or resveratrol-treated stored platelets were performed. Thrombosis was initiated using a ferric chloride injury model and the time to vessel occlusion was measured (Fig 7B). Transfusion of fresh platelets led to an occlusion time of approximately 1100 s (Fig 7C), comparable to non-transfused mice (data not shown). Transfusion of control platelets stored for 1 day resulted in vessel occlusion occurring in half the time (570 s) of fresh platelets (Fig 7D). Resveratrol-treated stored platelets had less thrombotic potential upon transfusion, partially returning occlusion time to that of fresh platelets (830 s; Fig 7E). These data demonstrate that resveratrol-treated stored platelets are less thrombotic than standard stored platelets in an in vivo model of transfusion.
Discussion
Our novel findings demonstrate that, unlike other traditional agents that inhibit platelet function, resveratrol (a plant-derived anti-oxidant compound) uniquely mitigates unwanted platelet activation, while preserving the ability of stored platelets to function post-storage. Not only do platelets acquire markers of activation during storage, but they also fail to respond normally to agonists post-storage (Shapira, et al 2000). This leads to a dual dysfunction, whereby patients may be receiving platelets that have a reduced capacity to perform normal haemostatic functions, yet constitute an increased proinflammatory and prothrombotic risk. Clearly, platelet transfusions are a life-saving practice in thrombocytopenic patients with bleeding. However, there is room to improve the efficacy and safety of these transfusions, which are crucial for patient populations at high risk for bleeding, such as patients with haematological malignancies, transplant, surgical and trauma patients. Our goal was to identify a compound, with few to no known toxicities that could mitigate the platelet storage lesion. We initially screened several compounds to identify a lead candidate that could dampen, but not abrogate platelet activation (data not shown). Based on this screen, resveratrol was evaluated for its ability to prevent spontaneous activation of stored platelets.
In this study, we demonstrate, using both in vitro assays and in a preclinical mouse model, that resveratrol mitigates platelet activation associated with the platelet storage lesion, thus maintaining platelet function in a closer to normal state during storage for transfusion. Stored platelets largely lose their ability to respond to physiological agonists over time, which may be due to their partial activation during preparation and storage, as evidenced by the release of proinflammatory and prothrombotic bioactive mediators (TXB2, PGE2). We confirm here that stored platelets do not fully aggregate in response to ADP or collagen alone (Supplemental Fig 2) and newly show that stored platelets lose their ability to spread on fibrinogen. Resveratrol treatment, however, greatly improved the ability of stored platelets to aggregate (Fig 4) and spread (Fig 1). Platelets undergo severe stress during preparation for storage, which can adversely affect their ability to function normally, as evidenced by their reduced ability to aggregate in response to ADP. Freshly prepared platelets only maximally aggregated to approximately 20% in response to high dose ADP. In contrast, platelets collected in vacutainer tubes and not subjected to the harsh conditions of leucoreduction and platelet pelleting typically maximally aggregate 80–90% in response to ADP (data not shown). Although resveratrol preserved the ability of platelets to aggregate post-storage, addition of resveratrol prior to leucoreduction and serial centrifugation could further improve platelet quality during storage. Interestingly, although resveratrol preserved the ability of stored platelets to aggregate in response to ADP, sustained aggregation was not achieved and the platelets readily disaggregated. Disaggregation occurs in the absence of secondary signals from dense granule release, suggesting that resveratrol selectively affects platelet signalling.
While mitigating the platelet storage lesion and inappropriate activation is a crucial first step toward safer and effective transfusions, platelets must also function normally following transfusion. Addition of stored platelets to thrombocytopenic whole blood to mimic transfusion decreased clot strength, measured by TEG. Resveratrol improved clot strength more than two-fold over that of conventionally stored platelets. No other TEG parameters were affected by resveratrol exposure, suggesting that these beneficial effects were platelet-specific and providing evidence that resveratrol treatment may provide improved haemostatic response following transfusion.
Some clinical studies suggest that transfusion of stored platelets can have prothrombotic side effects, probably due to platelet activation and the accumulation of prothrombotic and proinflammatory mediators over time (Khorana, et al 2008, Sahler, et al 2011). We used an in vivo mouse model of thrombosis to show that autologous transfusion of conventionally stored mouse platelets into naïve recipients accelerated thrombosis. This prothrombotic phenotype was ameliorated following transfusion of resveratrol-treated stored platelets, supporting our hypothesis that resveratrol-stored platelets more closely resemble fresh, unstored platelets. As donor platelets represent a small fraction of the total platelets after transfusion in this model, accelerated thrombosis is probably a consequence of activation of endogenous platelets and other vascular cells by proinflammatory mediators in the PC supernatants (Cognasse, et al 2006, Cook, et al 2005, Khorana, et al 2008, Spiess 2004, Spiess, et al 2004), and not solely from the infused stored platelets. We have previously reported that stored PC accumulate high levels of bioactive proinflammatory mediators, such as sCD40L (Kaufman, et al 2007), which contribute to transfusion-related adverse outcomes, such as TRALI (Khan, et al 2006) and fever (Phipps, et al 2001). Additionally, apoptotic platelets and PMPs are known to be prothrombotic and probably contribute to the accelerated thrombosis in this model (Jackson and Schoenwaelder 2010, Leytin and Freedman 2003). These two factors are potentially key mechanisms by which vasculature is primed to inflammatory insults, resulting in accelerated thrombosis. Many patients receiving platelet transfusions have underlying inflammatory conditions, which could synergize with inflammatory mediators from stored blood products, leading to adverse outcomes, such as thrombosis (Sahler, et al 2011). Therefore, the ability of resveratrol to dampen the accumulation of potentially harmful mediators could, speculatively, lead to a reduced incidence of adverse transfusion reactions.
Our innovative investigations of resveratrol effects during platelet storage support a mechanism whereby resveratrol maintains platelet integrity to improve haemostasis following transfusion. However, the mechanism of action of resveratrol on human platelets is not well understood. Resveratrol is reported to inhibit the synthesis of prostaglandins through COX1 inactivation in nucleated cells (Szewczuk, et al 2004). We show for the first time that stored platelet release of bioactive mediators was attenuated by resveratrol. Importantly, this inhibition was reversible, as platelets stored with resveratrol maintained their ability to make TXB2 and PGE2 in response to thrombin stimulation. This was in contrast to ASA, which irreversibly inhibits platelet activation. Interestingly, the ability of resveratrol to preserve platelet function during storage was independent of its ability to inhibit COX1 activity early in storage, as the resveratrol analogue lacking COX1 inhibitory functions, TMR, was still able to improve platelet function post-storage (Fig 4). We propose that resveratrol exerts its beneficial actions through multiple mechanisms. Early in storage, resveratrol reversibly inhibited COX1 activity, leading to reduced accumulation of TXB2 and PGE2 over time. This beneficial action would probably reduce the proinflammatory impact of stored PC on patients receiving transfusions. More studies are needed to determine at what point during the first three days of storage platelets regain their ability to produce prostaglandins in response to stimulation, as transfusion of resveratrol-inactivated platelets (e.g., day 1 freshly prepared platelets) could impair in vivo haemostatic functions.
Although both TXB2 and PGE2 accumulated during storage, resveratrol affected PGE2 accumulation to a greater degree. These data may suggest an alternative mechanism of inhibition by resveratrol downstream of COX1. Furthermore, the effect of resveratrol on release of other mediators was not as pronounced as the effect on prostaglandins. Resveratrol reduced sCD40L and PF4 release from freshly isolated platelets (data not shown), but no differences were observed during platelet storage. It is possible that resveratrol, probably due to its ability to inhibit COX1, selectively mitigates prostaglandin synthesis, and any inhibition of PF4 and sCD40L release in freshly isolated platelets was a result of impaired autocrine activation signals from PGE2 and TXB2.
Additionally, our data demonstrate that resveratrol reduced apoptosis during storage. The pro-survival effects of resveratrol are clearest at 5 days post-storage, which could be attributed to low levels of apoptosis early in platelet storage. However, resveratrol may be able to extend the lifespan of platelets during storage, although this has not been investigated yet. Importantly, we demonstrate that resveratrol-treated platelets had enhanced in vivo survival after transfusion in a mouse model. This may be due to the decreased activation status of the stored platelets, reducing their clearance from the circulation. As no differences in recovery were observed at 4 h post-transfusion, it is possible that the enhanced survival of resveratrol-treated platelets was due to reduced apoptosis during storage. The mechanism by which resveratrol preserves platelet function during storage is likely to be multifactorial and complex. However, our new data suggest that it is independent of reversible inhibition of COX1, but instead involves reduced platelet apoptosis leading to improved function and survival post-storage. However, reductions in apoptosis are probably not solely responsible for resveratrol’s ability to preserve platelet function, as resveratrol only slightly affected apoptosis in this study. Resveratrol has numerous molecular targets and further studies should comprehensively evaluate resveratrol’s mechanism of action in stored platelets.
We propose that an ideal or at least improved platelet storage additive would reduce platelet activation during storage, but allow normal haemostatic function post-transfusion. An additional benefit of using resveratrol as a novel storage additive is that resveratrol persists in plasma stored at room temperature for one month (Iranshahy, et al 2013). However, intravenous infusion of resveratrol results in rapid metabolism by the liver (Muzzio, et al 2012, Walle, et al 2004). Therefore, resveratrol remains stable during platelet storage, and is rapidly cleared post-transfusion. An added benefit of resveratrol is that it is a naturally occurring plant product that has minimal toxicity at high doses (Boocock, et al 2007, Dash, et al 2013, Juan, et al 2002, la Porte, et al 2010, Poulsen, et al 2013). The dosage used in our platelet storage studies are within the levels used pharmacologically or in nutritional supplements (Boocock, et al 2007, la Porte, et al 2010, Muzzio, et al 2012).
One limitation of our study was the sole use of platelets generated via the PRP method. Studies evaluating the potential of resveratrol to mitigate the platelet storage lesion in apheresis platelets are warranted. As resveratrol was added to platelets after leucoreduction and preparation for storage, similar results are likely to be seen in apheresis platelets. Furthermore, addition of resveratrol prior to leucoreduction and centrifugation has the potential to improve platelet function to a greater degree due to its ability to prevent activation of platelets immediately after treatment.
We provide additional evidence for the potential health benefits of resveratrol, a naturally occurring anti-oxidant compound. Our novel findings on resveratrol treatment of platelets demonstrate that: (1) spontaneous platelet activation was decreased, (2) platelets have an improved ability to function normally post-storage, and (3) the thrombotic potential of stored platelets was markedly decreased following transfusion (probably due to the mitigation of platelet activation and apoptosis during storage). The crucial and innovative findings from these studies is that resveratrol has a dual ability to decrease inflammatory mediator release during storage, while preserving platelet function leading to enhanced in vivo survival. Our data support resveratrol, or perhaps similar, more stable and bioactive chemical analogues, as a promising new platelet storage additive. Future studies will be necessary to fully investigate the mechanisms by which resveratrol acts on stored platelets, and to determine the efficacy and safety of this approach in clinical applications.
Supplementary Material
Acknowledgments
The authors wish to thank Grace Conley for help with TEG experiments, and the Strong Blood Bank and Ann Casey for phlebotomy. This work was supported in part by ES001247, HL095467, T32-AI007285, T90DE021985, a University of Rochester grant from Howard Hughes Medical Institute through the Med into Grad Initiative, and UL1RR024160 and UL1TR000042 from the National Center For Research Resources (NCRR). The content is solely the responsibility of the authors and does not necessarily represent the views of NCRR or NIH.
Footnotes
Author contributions
K.L. Lannan designed and performed research, analysed data and wrote the manuscript. S.L. Spinelli, N. Blumberg, and R.P. Phipps contributed to study design, supervised the project and revised the manuscript. M. Refaai contributed to TEG experiments and revised the manuscript. C.N. Morrell and S.K. Ture contributed to the in vivo transfusion experiments and revised the manuscript.
Conflict of interest
N. Blumberg has received lecture honoraria and consulting fees from Antek, Inc., Fenwal, Pall BioMedical, Biomet (Citra Labs) and Caridian (Terumo), manufacturers of leucoreduction filters, blood component storage devices, and cell washing devices. The other authors have nothing to disclose.
References
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer JH, Goupil S, Garber GB, Helfand SL. An accelerated assay for the identification of lifespan-extending interventions in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2004;101:12980–12985. doi: 10.1073/pnas.0403493101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat KPL, Kosmeder JW, Pezzuto JM. Biological effects of resveratrol. Antioxid Redox Signal. 2001;3:1041–1064. doi: 10.1089/152308601317203567. [DOI] [PubMed] [Google Scholar]
- Blumberg N, Gettings KF, Turner C, Heal JM, Phipps RP. An association of soluble CD40 ligand (CD154) with adverse reactions to platelet transfusions. Transfusion. 2006;46:1813–1821. doi: 10.1111/j.1537-2995.2006.00979.x. [DOI] [PubMed] [Google Scholar]
- Boocock DJ, Faust GE, Patel KR, Schinas AM, Brown VA, Ducharme MP, Booth TD, Crowell JA, Perloff M, Gescher AJ, Steward WP, Brenner DE. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers Prev. 2007;16:1246–1252. doi: 10.1158/1055-9965.EPI-07-0022. [DOI] [PubMed] [Google Scholar]
- Bowbrick VA, Mikhailidis DP, Stansby G. Influence of platelet count and activity on thromboelastography parameters. Platelets. 2003;14:219–224. doi: 10.1080/0953710031000118849. [DOI] [PubMed] [Google Scholar]
- Cauwenberghs S, van Pampus E, Curvers J, Akkerman JW, Heemskerk JW. Hemostatic and signaling functions of transfused platelets. Transfus Med Rev. 2007;21:287–294. doi: 10.1016/j.tmrv.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Cognasse F, Boussoulade F, Chavarin P, Acquart S, Fabrigli P, Lamy B, Garraud O. Release of potential immunomodulatory factors during platelet storage. Transfusion. 2006;46:1184–1189. doi: 10.1111/j.1537-2995.2006.00869.x. [DOI] [PubMed] [Google Scholar]
- Cook D, Crowther M, Meade M, Rabbat C, Griffith L, Schiff D, Geerts W, Guyatt G. Deep venous thrombosis in medical-surgical critically ill patients: prevalence, incidence, and risk factors. Crit Care Med. 2005;33:1565–1571. doi: 10.1097/01.ccm.0000171207.95319.b2. [DOI] [PubMed] [Google Scholar]
- Cookson P, Thomas S, Marschner S, Goodrich R, Cardigan R. In vitro quality of single-donor platelets treated with riboflavin and ultraviolet light and stored in platelet storage medium for up to 8 days. Transfusion. 2012;52:983–994. doi: 10.1111/j.1537-2995.2011.03388.x. [DOI] [PubMed] [Google Scholar]
- Curvers J, van Pampus EC, Feijge MA, Rombout-Sestrienkova E, Giesen PL, Heemskerk JW. Decreased responsiveness and development of activation markers of PLTs stored in plasma. Transfusion. 2004;44:49–58. doi: 10.1111/j.0041-1132.2004.00628.x. [DOI] [PubMed] [Google Scholar]
- Dash S, Xiao C, Morgantini C, Szeto L, Lewis GF. High-dose resveratrol treatment for 2 weeks inhibits intestinal and hepatic lipoprotein production in overweight/obese men. Arterioscler Thromb Vasc Biol. 2013;33:2895–2901. doi: 10.1161/ATVBAHA.113.302342. [DOI] [PubMed] [Google Scholar]
- Dobrydneva Y, Williams RL, Blackmore PF. trans-Resveratrol inhibits calcium influx in thrombin-stimulated human platelets. Br J Pharmacol. 1999;128:149–157. doi: 10.1038/sj.bjp.0702749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung MK, Grossman BJ, Hillyer CD, Westhoff CM. Technical Manual of the American Association of Blood Banks. American Association of Blood Banks (AABB); Bethesda, MD: 2014. [Google Scholar]
- Gyulkhandanyan AV, Mutlu A, Freedman J, Leytin V. Markers of platelet apoptosis: methodology and applications. J Thromb Thrombolysis. 2012;33:397–411. doi: 10.1007/s11239-012-0688-8. [DOI] [PubMed] [Google Scholar]
- Hawiger J. Formation and regulation of platelet and fibrin hemostatic plug. Hum Pathol. 1987;18:111–122. doi: 10.1016/s0046-8177(87)80330-1. [DOI] [PubMed] [Google Scholar]
- Heal JM, Phipps RP, Blumberg N. One big unhappy family: transfusion alloimmunization, thrombosis, and immune modulation/inflammation. Transfusion. 2009;49:1032–1036. doi: 10.1111/j.1537-2995.2009.02182.x. [DOI] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Iranshahy M, Mohammadpoor AH, Hassanzadeh-Khayyat M, Iranshahi M. Method Validation for the One-Month Stability Study of trans-Resveratrol in Human Plasma. Jundishapur J Nat Pharm Prod. 2013;8:65–69. [PMC free article] [PubMed] [Google Scholar]
- Jackson SP, Schoenwaelder SM. Procoagulant platelets: are they necrotic? Blood. 2010;116:2011–2018. doi: 10.1182/blood-2010-01-261669. [DOI] [PubMed] [Google Scholar]
- Juan ME, Vinardell MP, Planas JM. The daily oral administration of high doses of trans-resveratrol to rats for 28 days is not harmful. J Nutr. 2002;132:257–260. doi: 10.1093/jn/132.2.257. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Spinelli SL, Schultz E, Blumberg N, Phipps RP. Release of biologically active CD154 during collection and storage of platelet concentrates prepared for transfusion. J Thromb Haemost. 2007;5:788–796. doi: 10.1111/j.1538-7836.2007.02412.x. [DOI] [PubMed] [Google Scholar]
- Khan SY, Kelher MR, Heal JM, Blumberg N, Boshkov LK, Phipps R, Gettings KF, McLaughlin NJ, Silliman CC. Soluble CD40 ligand accumulates in stored blood components, primes neutrophils through CD40, and is a potential cofactor in the development of transfusion-related acute lung injury. Blood. 2006;108:2455–2462. doi: 10.1182/blood-2006-04-017251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorana AA, Francis CW, Blumberg N, Culakova E, Refaai MA, Lyman GH. Blood transfusions, thrombosis, and mortality in hospitalized patients with cancer. Arch Intern Med. 2008;168:2377–2381. doi: 10.1001/archinte.168.21.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DW, Kim YM, Kang SD, Han YM, Pae HO. Effects of Resveratrol and trans-3,5,4′-Trimethoxystilbene on Glutamate-Induced Cytotoxicity, Heme Oxygenase-1, and Sirtuin 1 in HT22 Neuronal Cells. Biomol Ther (Seoul) 2012;20:306–312. doi: 10.4062/biomolther.2012.20.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- la Porte C, Voduc N, Zhang G, Seguin I, Tardiff D, Singhal N, Cameron DW. Steady-State pharmacokinetics and tolerability of trans-resveratrol 2000 mg twice daily with food, quercetin and alcohol (ethanol) in healthy human subjects. Clin Pharmacokinet. 2010;49:449–454. doi: 10.2165/11531820-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Leytin V. Apoptosis in the anucleate platelet. Blood Rev. 2012;26:51–63. doi: 10.1016/j.blre.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Leytin V, Freedman J. Platelet apoptosis in stored platelet concentrates and other models. Transfus Apher Sci. 2003;28:285–295. doi: 10.1016/S1473-0502(03)00048-X. [DOI] [PubMed] [Google Scholar]
- Muzzio M, Huang Z, Hu SC, Johnson WD, McCormick DL, Kapetanovic IM. Determination of resveratrol and its sulfate and glucuronide metabolites in plasma by LC-MS/MS and their pharmacokinetics in dogs. J Pharm Biomed Anal. 2012;59:201–208. doi: 10.1016/j.jpba.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohto H, Nollet KE. Overview on platelet preservation: better controls over storage lesion. Transfus Apher Sci. 2011;44:321–325. doi: 10.1016/j.transci.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Pace-Asciak CR, Hahn S, Diamandis EP, Soleas G, Goldberg DM. The red wine phenolics trans-resveratrol and quercetin block human platelet aggregation and eicosanoid synthesis: implications for protection against coronary heart disease. Clin Chim Acta. 1995;235:207–219. doi: 10.1016/0009-8981(95)06045-1. [DOI] [PubMed] [Google Scholar]
- Perales Villarroel JP, Figueredo R, Guan Y, Tomaiuolo M, Karamercan MA, Welsh J, Selak MA, Becker LB, Sims C. Increased platelet storage time is associated with mitochondrial dysfunction and impaired platelet function. J Surg Res. 2013;184:422–429. doi: 10.1016/j.jss.2013.05.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phipps RP, Kaufman J, Blumberg N. Platelet derived CD154 (CD40 ligand) and febrile responses to transfusion. Lancet. 2001;357:2023–2024. doi: 10.1016/s0140-6736(00)05108-4. [DOI] [PubMed] [Google Scholar]
- Poulsen MM, Vestergaard PF, Clasen BF, Radko Y, Christensen LP, Stødkilde-Jørgensen H, Møller N, Jessen N, Pedersen SB, Jørgensen JO. High-dose resveratrol supplementation in obese men: an investigator-initiated, randomized, placebo-controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition. Diabetes. 2013;62:1186–1195. doi: 10.2337/db12-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddoch KM, Pidcoke HF, Montgomery RK, Fedyk C, Aden JK, Ramasubramanian AK, Cap AP. Hemostatic function of apheresis platelets stored at 4 °C and 22 °C. Shock. 2013;41(Suppl 1):54–61. doi: 10.1097/SHK.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refaai MA, Carter J, Henrichs KF, Davidson DC, Pollock SJ, Casey AE, Spinelli SL, Phipps RP, Francis CW, Blumberg N. Alterations of platelet function and clot formation kinetics after in vitro exposure to anti-A and -B. Transfusion. 2013;53:382–393. doi: 10.1111/j.1537-2995.2012.03718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinder HM, Snyder EL, Tracey JB, Dincecco D, Wang C, Baril L, Rinder CS, Smith BR. Reversibility of severe metabolic stress in stored platelets after in vitro plasma rescue or in vivo transfusion: restoration of secretory function and maintenance of platelet survival. Transfusion. 2003;43:1230–1237. doi: 10.1046/j.1537-2995.2003.00484.x. [DOI] [PubMed] [Google Scholar]
- Sahler J, Grimshaw K, Spinelli SL, Refaai MA, Phipps RP, Blumberg N. Platelet storage and transfusions: new concerns associated with an old therapy. Drug Discov Today Dis Mech. 2011;8:e9–e14. doi: 10.1016/j.ddmec.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherzberg MC, Kiehl A, Zivkovic A, Stark H, Stein J, Fürst R, Steinhilber D, Ulrich-Rückert S. Structural modification of resveratrol leads to increased anti-tumor activity, but causes profound changes in the mode of action. Toxicol Appl Pharmacol. 2015;287:67–76. doi: 10.1016/j.taap.2015.05.020. [DOI] [PubMed] [Google Scholar]
- Shapira S, Friedman Z, Shapiro H, Presseizen K, Radnay J, Ellis MH. The effect of storage on the expression of platelet membrane phosphatidylserine and the subsequent impacton the coagulant function of stored platelets. Transfusion. 2000;40:1257–1263. doi: 10.1046/j.1537-2995.2000.40101257.x. [DOI] [PubMed] [Google Scholar]
- Skripchenko A, Myrup A, Thompson-Montgomery D, Awatefe H, Wagner SJ. Mitochondrial dysfunction of platelets stored in first- and second-generation containers is, in part, associated with elevated carbon dioxide levels. Transfusion. 2011;51:371–379. doi: 10.1111/j.1537-2995.2010.02829.x. [DOI] [PubMed] [Google Scholar]
- Slichter SJ. Platelet transfusion therapy. Hematol Oncol Clin North Am. 2007;21:697–729. vii. doi: 10.1016/j.hoc.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Slichter SJ, Corson J, Jones MK, Christoffel T, Pellham E, Bailey SL, Bolgiano D. Exploratory studies of extended storage of apheresis platelets in a platelet additive solution (PAS) Blood. 2014;123:271–280. doi: 10.1182/blood-2013-05-501247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobotková A, Másová-Chrastinová L, Suttnar J, Stikarová J, Májek P, Reicheltová Z, Kotlín R, Weisel JW, Malý M, Dyr JE. Antioxidants change platelet responses to various stimulating events. Free Radic Biol Med. 2009;47:1707–1714. doi: 10.1016/j.freeradbiomed.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiess BD. Risks of transfusion: outcome focus. Transfusion. 2004;44:4S–14S. doi: 10.1111/j.0041-1132.2004.04244.x. [DOI] [PubMed] [Google Scholar]
- Spiess BD, Royston D, Levy JH, Fitch J, Dietrich W, Body S, Murkin J, Nadel A. Platelet transfusions during coronary artery bypass graft surgery are associated with serious adverse outcomes. Transfusion. 2004;44:1143–1148. doi: 10.1111/j.1537-2995.2004.03322.x. [DOI] [PubMed] [Google Scholar]
- Springer DL, Miller JH, Spinelli SL, Pasa-Tolic L, Purvine SO, Daly DS, Zangar RC, Jin S, Blumberg N, Francis CW, Taubman MB, Casey AE, Wittlin SD, Phipps RP. Platelet proteome changes associated with diabetes and during platelet storage for transfusion. J Proteome Res. 2009;8:2261–2272. doi: 10.1021/pr800885j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong R, Miller RA, Astle CM, Baur JA, de Cabo R, Fernandez E, Guo W, Javors M, Kirkland JL, Nelson JF, Sinclair DA, Teter B, Williams D, Zaveri N, Nadon NL, Harrison DE. Evaluation of resveratrol, green tea extract, curcumin, oxaloacetic acid, and medium-chain triglyceride oil on life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2013;68:6–16. doi: 10.1093/gerona/gls070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szewczuk LM, Forti L, Stivala LA, Penning TM. Resveratrol is a peroxidase-mediated inactivator of COX-1 but not COX-2: a mechanistic approach to the design of COX-1 selective agents. J Biol Chem. 2004;279:22727–22737. doi: 10.1074/jbc.M314302200. [DOI] [PubMed] [Google Scholar]
- Tormey CA, Stack G. Immunogenicity of blood group antigens: a mathematical model corrected for antibody evanescence with exclusion of naturally occurring and pregnancy-related antibodies. Blood. 2009;114:4279–4282. doi: 10.1182/blood-2009-06-227793. [DOI] [PubMed] [Google Scholar]
- Viswanathan M, Kim SK, Berdichevsky A, Guarente L. A role for SIR-2.1 regulation of ER stress response genes in determining C. elegans life span. Dev Cell. 2005;9:605–615. doi: 10.1016/j.devcel.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Walle T, Hsieh F, DeLegge MH, Oatis JE, Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32:1377–1382. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- Wang C, Wheeler CT, Alberico T, Sun X, Seeberger J, Laslo M, Spangler E, Kern B, de Cabo R, Zou S. The effect of resveratrol on lifespan depends on both gender and dietary nutrient composition in Drosophila melanogaster. Age (Dordr) 2013;35:69–81. doi: 10.1007/s11357-011-9332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyrich AS, Zimmerman GA. Platelets: signaling cells in the immune continuum. Trends Immunol. 2004;25:489–495. doi: 10.1016/j.it.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Wiinberg B, Jensen AL, Rojkjaer R, Johansson P, Kjelgaard-Hansen M, Kristensen AT. Validation of human recombinant tissue factor-activated thromboelastography on citrated whole blood from clinically healthy dogs. Vet Clin Pathol. 2005;34:389–393. doi: 10.1111/j.1939-165x.2005.tb00066.x. [DOI] [PubMed] [Google Scholar]
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


