Abstract
AMP kinase (AMPK) is an evolutionarily conserved enzyme required for adaptive responses to various physiological and pathological conditions. AMPK executes numerous cellular functions, some of which are often perceived at odds with each other. While AMPK is essential for embryonic growth and development, its full impact in adult tissues is revealed under stressful situations that organisms face in the real world. Conflicting reports about its cellular functions, particularly in cancer, are intriguing and a growing number of AMPK activators are being developed to treat human diseases such as cancer and diabetes. Whether these drugs will have only context-specific benefits or detrimental effects in the treatment of human cancer will be a subject of intense research. Here we review the current state of AMPK research with an emphasis on cancer and discuss the yet unresolved context-dependent functions of AMPK in human cancer.
Descriptive Words: AMPK, Cancer, mTOR, LKB1, Metformin, Oncogenic stress
AMPK subunit structure: unanswered questions and the brewing controversy
Few other molecules in modern medicine have earned a status as controversial as AMP activated protein kinase (AMPK). The reasons, although less discussed, are apparent. AMPK is not a single-peptide enzyme; rather it is a complex of three subunits [1–3] that are chosen in a given cell from an available pool of seven subunits. Therefore, theoretically, twelve types of AMPK complexes can form in a cell, although specific complexes are preferentially formed in different tissues (see below). How each of the twelve possible trimeric complexes preferentially form and function under different contexts is mostly unknown. Little is known about whether each complex localizes to specific subcellular locale and serves distinct situation-dependent functions targeting specific set of substrates, and if the same AMPK complex functions differently in the diseased state compared to normal tissue. Therefore, a complex scenario has emerged where overly simplistic conclusions drawn from conflicting studies have in some cases caused significant confusion. In this review, we will briefly discuss AMPK structure and function in eukaryotes and elaborate on the current status of AMPK research in cancer and the complex issues that need to be addressed in the next few decades. A detailed description of the mechanisms of AMPK activation, AMPK structure and function in normal physiology and in other human diseases including diabetes, cardiovascular and neurological diseases can be found in several excellent reviews [1–5].
The AMPK heterotrimer: known and unknown
AMPK is a multisubstrate serine/threonine kinase. Purified AMPK was found to exist as a heterotrimer of catalytic α and regulatory β and γ subunits [6,7]. In mammals, there are two genes encoding the catalytic α1 and α2 subunits (called PRKAA1 and PRKAA2), two genes encoding the β1 and β2 subunits (PRKAB1 and PRKAB2), and three genes encoding the γ1, γ2 and γ3 subunits (PRKAG1, PRKAG2 and PRKAG3) [1–3]. The rooted trees of the α and β subunits suggest that vertebrate PRKAA1/A2 and PRKAB1/B2 genes arose by duplications of ancestral genes in lower eukaryotes. The evolutionary trees also show a higher mutation rate of β (1.8183 substitutions per site) compared to α (1.1024 substitutions per site) indicating that the β subunit evolved 1.65 times faster than the α subunit [8]. The N-terminus of the α subunit contains the catalytic domain as well as a phosphorylation site for upstream kinases that regulate its activity [9]. The γ subunits are nucleotide binding regulatory subunits that bind AMP/ADP. The conserved C-terminus of the β subunit interacts with both the α and γ subunits and plays an obligatory role in AMPK complex formation [10,11]. In addition, the β subunits contain a conserved carbohydrate binding domain that perhaps allows AMPK to function as a glycogen sensor [12,13].While the AMPK subunits are expressed more or less ubiquitously and the αβγ AMPK complex can come in twelve different flavors, the individual AMPK subunits in humans and mice display considerable variation in tissue-specific expression [14,15], subunit association, and subcellular localization.
Earlier studies demonstrated that the α2 catalytic subunit associates exclusively with β1 in slow twitch (type I) soleus muscle fibers, whereas in fast twitch (type II) extensor digitorium longus muscle fibers, α2 associates with both β1 and β2 subunits [16,17]. It is important to note that the preferred combination in a given tissue (or cell type within a tissue) is not static and can shift towards a different combination in response to a change in cellular physiology. The majority of these observations came from classic studies in skeletal muscle. For example, in human skeletal muscle three complexes are expressed in a preferred order (α2β2γ1>>α2β2γ3=α1β2γ1) and the preferred complex shifts depending on the intensity and duration of exercise [18–20]. The α2 AMPK complex while essential for basal AMPK function in skeletal muscle (and this function is not compensated by α1), is not required during adaptive response (e.g., exercise) during which the α1 AMPK complex can compensate for loss of α2 [21]. To add to this complexity is the recent finding that while the predominant AMPK complex in the human liver is α1βγ1, it is α1β1γ1 and α2β1γ1 in the dog and rat liver, respectively [22]. These interspecies differences could potentially complicate data interpretation when drawn from one species and applied to another. In mouse neural stem/progenitor cells, the AMPK complex containing the β1 subunit is predominant [10], yet the complex shifts to an equal β1 and β2-containing complex in differentiated neural cells like neurons and astrocytes (our unpublished data). Similar shift in AMPK subunit expression was also reported in myoblasts and myotubes [23] If cancer is a disease of undifferentiated stem/progenitor-like cells or de-differentiated cells, it is worth noting that in human glioblastoma cell lines the ratio of β1 and β2 is reminiscent of neural stem/progenitor cells [24]. Little is known about the regulation of AMPK subunits and the significance of AMPK complex switch during progenitor cell proliferation and differentiation. The subcellular localization of the AMPK subunits also varies. For example, in skeletal muscle, the α2, but not the α1 subunit translocates to the nucleus in response to exercise [25]. The β1 subunit is enriched in the nucleus of neurons [26] and neural stem cells [10], whereas β2 is predominantly cytoplasmic. The subcellular localization of β subunits is crucial for AMPK function, as studies in yeast have shown that they direct the localization of the catalytic α subunit and thus dictate the subset of substrates that can be phosphorylated in a given context [27]. In a recent study, compartmentalized AMPK signaling in subcellular regions has been demonstrated by the use of novel molecular sensors [28].
There are at least two other levels of complexity about AMPK heterotrimer formation and function that need to be addressed. First, we do not know much about the isoform (transcript variant) expression of the AMPK subunits in normal and diseased tissue. Our published [15] and unpublished results from germline β1 and β2 knockout mice revealed that at least three organs – the liver, kidney and skeletal muscle express multiple isoforms of β1 and β2 subunits. These isoforms were detected by protein analysis and these or additional isoforms may be expressed in other tissues at lower levels that remained undetected by our analysis. Whether AMPK isoforms are differentially expressed under various physiological or pathological conditions and whether this impacts AMPK complex structure, subcellular localization and substrate choice are unknown.
Second, results from our and other laboratories indicate that as yet unknown tissue-specific factors regulate AMPK complex formation and activity. This is because when compensatory increase in expression of one subunit occurs due to knockout of another subunit, AMPK complex formation and activity still remains uncompensated. For example, in the α2 knockout skeletal muscle, a significant compensatory increase of α1 protein occurs but AMPK activity remains downregulated [21, 29]. Similarly, in the β2 knockout muscle, the β1 subunit is upregulated several fold but AMPK activity remains ~70% below normal [15, 30]. Even the stability of the α subunits (that require β subunits for stability) remains affected in the presence of upregulated β1, suggesting unknown factors that regulate tissue-specific AMPK heterotrimer formation. These results tell us that much remains unknown about the tissue-specific regulation of AMPK subunits and activity in normal physiology and how AMPK complex formation, activity and substrate phosphorylation is impacted in a complex disease like cancer. Interpretation of the many conflicting results on AMPK function in healthy and diseased tissue may necessitate deeper understanding of these complex issues.
Is AMPK simply an energy Sensor?
AMPK regulation
AMPK was originally identified in a rat liver preparation as a contaminant of its now well-known substrate acetyl Co-A carboxylase (ACC), the rate limiting enzyme in fatty acid synthesis [31–33]. The enzyme was purified [34] and was found to greatly accelerate phosphorylation and inactivation of ACC in the presence of AMP, while reducing ACC phosphorylation to activate it at high ATP concentration. The enzyme was also found to inhibit HMG-CoA reductase, the rate limiting enzyme in cholesterol biosynthesis [35]. Therefore, this enzyme that was inhibited by ATP and activated by AMP and by phosphorylation, and was named AMP-activated protein kinase or AMPK [36]. It is important to note that AMPK is not the only enzyme regulated by ATP/AMP. Several metabolic enzymes (e.g., the glycolytic enzyme phosphofructokinase, the citric acid cycle enzyme citrate synthase and the glycogen metabolism enzyme glycogen phosphorylase) are sensitive to ATP/AMP ratio. However, a change in nucleotide ratio is probably one of the many ways by which AMPK is regulated (see Figure 1).
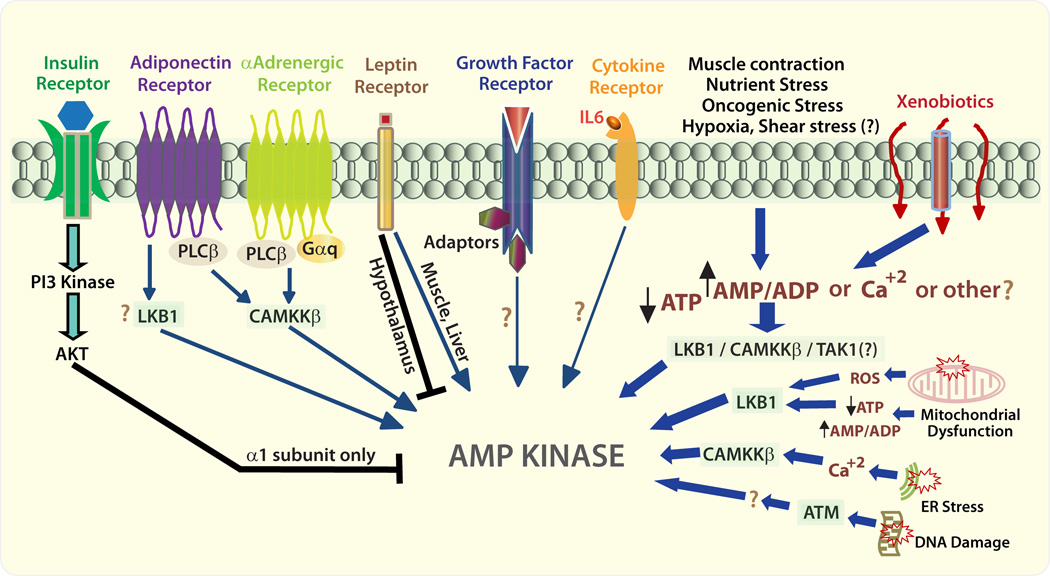
Figure 1. Modes of AMPK Activation.
Signaling through various cell surface receptors influence AMPK activity. In some contexts, AKT can inhibit AMPKα1 by phosphorylation; α2 is a poor AKT substrate. Much is unknown about the mechanisms by which AMPK is activated by these various upstream signals, particularly the AMPKK’s (LKB1 or CAMKKβ or TAK1) that mediate AMPK activation. The metabolic hormone leptin activates AMPK in the liver and muscle but inhibits hypothamic AMPK in the brain by unknown mechanisms. Metabolic stress activates AMPK by various mechanisms: some are mediated by LKB1; others go through CAMKKβ and in some case AMPK activation by one AMPKK may be intensified by another AMPKK. Some xenobiotics may diffuse through the plasma membrane bilayer while others may use channels and the mechanism by which the myriad xenobiotics activate AMPK is largely unknown. The question marks indicate pathways that need to be independently validated and require further investigation. PI3K: Phophatidylinositol-3-kinase; PLC: Phospholipase C; Gαq: G protein class αq that activates PLCβ; LKB1: Liver kinase B1; CAMKKβ: Calcium calmodulin kinase kinase β; TAK1: TGFβ activated kinase 1; ROS: reactive oxygen species; ER: endoplasmic reticulum.
To date, activation by AMP:ATP ratio and Ca+2 are the best studied mechanisms of AMPK activation. When the cellular AMP:ATP ratio increases, AMP/ADP binds to the γ subunits. This interaction causes (a) allosteric activation, (b) a conformational change in the catalytic α subunits that enables activating phosphorylation at T172 in α1 and/or α2 by upstream kinases, and (c) inhibition of T172 dephosphorylation by phosphatases [37–39]. It was later found that binding of ADP can also activate AMPK [40–42]. There are at least three (probably more) kinases that can phosphorylate AMPK at T172 – Liver kinase B1 (LKB1) which is also known as Serine/threonine kinase 11 (STK11), Calcium/calmodulin-dependent kinase kinase (CaMKKβ) and TGFβ-activated kinase (TAK1) [43–49]. Of these LKB1 and CaMKKβ are the best studied, and they are the major upstream kinases for AMPK. LKB1 activates AMPK in response to AMP/ADP, while CaMKKβ activates AMPK in response to Ca+2, and both pathways can act independently or synergistically [50]. G-protein coupled receptor (GPCR) activation and T cell receptor ligation to CD3 increase cellular Ca+2 causing AMPK activation. Likewise, the calcium ionophore ionomycin also activates AMPK in a CAMKKβ-dependent manner [51]. The AMPK holoenzyme can also be activated by allosteric regulation of the β subunits with or without phosphorylation of T172 of the α subunits; however, this requires autophosphorylation at S108 in the β subunits (specifically the β1 subunit) [52]. AMPK activation without T172 phosphorylation was also observed in vivo [53, 54]. Therefore, for a better understanding of AMPK function in a given context, both T172 phosphorylation and phosphorylated levels of its substrate/s relevant to the specific context should be carefully considered.
What does AMPK sense?
It is important to note that AMPK senses cellular energy (and Ca+2) and not nutrient levels per se. One possible exception is glycogen that binds to the β subunits [55, 56], but the implications of such an interaction are not fully understood. AMPK has evolved to sense energy stress and to allow organisms to survive sudden periods of acute stress or longer lean periods when food is scarce. These episodes of environmental stress that organisms face in the wild are difficult, if not impossible, to recreate in the laboratory where animals are maintained in a constant environment, and therefore, the full gamut of AMPK function will likely remain unknown. Some examples include AMPK activation in overwintering/hibernating species, diapausing embryos, during drop in O2 in the local environment (estivating fish) and acute hypoxia [57–64]. It is important to note that AMPK is also a sharp sensor of cellular osmotic pressure and cellular entry of a host of xenobiotics that includes synthetic small molecules, drugs, pro-drugs, and natural products [1, 65, 66].
There are reports that some xenobiotics either inhibited mitochondrial function (thus possibly increased the AMP:ATP ratio) or elevated intracellular Ca+2 [65], and these were proposed as the mechanisms of AMPK activation by xenobiotics. One should note that multiple studies including ours [22, 67] found that the antidiabetic drug and mitochondrial Complex I inhibitor metformin (an indirect AMPK activator) did not change nucleotide ratio, but still activated AMPK, and in contrast to the observation of Hawley et al., (65) in HEK293T cells, we found no change in AMP:ATP ratio in resveratrol-induced AMPK activation in Neuro2A cells [68]. While most cancer cells efficiently compensate for mitochondrial ATP loss by augmenting glycolysis as we found in glioma cells [24] which may mask any chronic change in nucleotide ratio, other mechanisms including an AMP/ADP-independent function of AMPK as a sensor of cellular insult or simply an altered physiological state [62] cannot be ruled out. Xenobiotic sensing occurs through the P450 system that includes several genes transcribed by the nuclear receptors CAR and PXR [69]. AMPK was found to be necessary for xenobiotics-induced transcription [70–72] and an important unanswered question is whether xenobiotic-sensing of AMPK is an evolutionarily conserved function that occurs through AMPK-dependent CAR and PXR regulation, independent of its energy or Ca+2 sensing functions. It is noteworthy that cellular pathology (e.g., cancer) exhibits various stresses including oncogenic, nutrient, energy, O2, osmotic and shear stress along with chemotherapy and radiotherapy-induced stress [73–77], and it is possible that active AMPK provides stress-resistance in human cancers. Physiological, pathological and drug-induced AMPK activation likely occurs through different mechanisms with unique context-dependent consequences and a crucial question that warrants investigation is the role of different AMPK complexes that contribute to AMPK activity under these myriad contexts.
The Many functions of AMPK
AMPK function in normal growth and development
The AMPK heterotrimer is ubiquitously expressed. The canonical function of AMPK is to inhibit anabolism and activate catabolism (see Figure 2). Embryonic lethality of AMPK null lower eukaryotes and mice [11, 78–81] suggests that AMPK is indispensable for early development. Genetic studies in model organisms confirmed that loss of AMPK causes various metabolic phenotypes in mice [15, 27, 81, 82], and defects in cell polarity, growth, development and longevity of both plants and animals [11, 78, 79, 81, 83–87]. However, the most enigmatic functions of AMPK are in mitosis and cell proliferation. Active AMPK localizes to the mitotic spindle of mammalian cells [88] and AMPK was shown to phosphorylate 28 novel proteins involved in chromosomal segregation, mitosis, cytokinesis and cytoskeletal reorganization [89]. The physiological implications of these observations are unclear and warrant further investigation. The critical requirement of AMPK for normal cell cycle progression is also found in mammalian and Drosophila cells [78, 90–92] and in a whole genome RNAi library screen of mammalian cells [93]. We earlier discovered that AMPK phosphorylates the retinoblastoma (RB) protein to allow cell cycle progression of neural progenitor cells [10] and a strong correlation between AMPK and RB phosphorylation was recently confirmed by another group [94]. In an unbiased proteomic screen of phospho-proteins enriched in mitosis, high levels of phosphorylated AMPK α2 was found, and importantly, this phosphorylation was maintained at high ATP levels [95]. Whether AMPK’s role in mitosis is uncoupled from or connected to its energy sensing function is unclear. A low energy level (when AMPK is known to be activated) is understandably a ‘Stop’ signal for cells to enter energy-intensive cell cycle. Yet, as noted above, active AMPK is found in dividing cells.
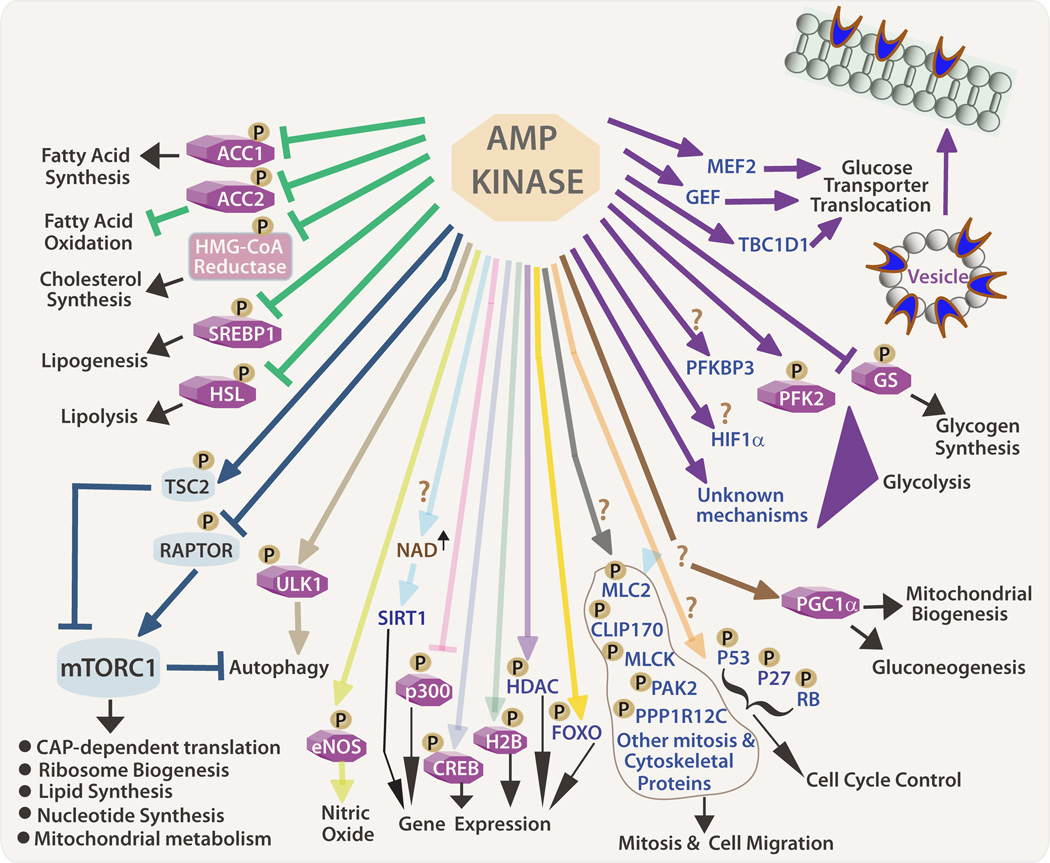
Figure 2. AMPK Downstream Targets.
AMPK directly or indirectly phosphorylates multiple substrates to increase glucose transporter translocation from cytoplasmic vesicles to plasma membrane and promote glycolysis. Through inhibitory phosphorylation, AMPK is a negative regulator of lipogenesis (green lines) and inhibits mTORC1 by phosphorylating (activating) TSC2 or inhibiting RAPTOR, the mTORC1 binding partner. Both mTOR and AMPK phosphorylates ULK1 at different sites to inhibit and activate autophagy, respectively. The question marks indicate substrates/pathways that require independent validation and further investigation. ACC: Acetyl-CoA carboxylase; HMG-CoA: 3-hydroxy-3-methylglutaryl CoA; SREBP1: Sterol regulatory element-binding protein 1; HSL: Hormone sensitive lipase; TSC2: Tuberous Sclerosis Complex 2; ULK1: Unc-51-like kinase 1; eNOS: endothelial nitric oxide synthase; CREB: cAMP response element-binding protein; H2B: Histone 2B; MLC2: Myosin light chain 2; CLIP170: Cytoplasmic linker protein 170; MLCK: Non-myosin light chain kinase; PAK2: p21-activated kinase 2; PPP1R12C: Protein phosphatase 1, regulatory subunit 12C; RB: Retinoblastoma; PGC1α: Peroxisome proliferator-activated receptor gamma coactivator 1-α; HIF1α: Hypoxia-inducible factor 1α; PFK2: Phosphofructokinase 2; PFKBP3: 6-Phospho-2-kinase/fructose-2–6 bisphosphatase; GS: Glycogen synthase; TBC1D1: Tre-2/USP6, BUB2, cdc16 domain family member 1; MEF2: Myocyte enhancer factor 2; GEF: GLUT4 enhancer factor.
How can we reconcile these novel findings with the canonical role of AMPK as a low energy sensor? A decision to enter cell cycle is dictated by growth factor signaling, and we and others have observed a correlation between growth factor stimulation and AMPK activation that occurs possibly through the interaction of AMPK with growth factor adaptor proteins such as Src and Grb2 [10,96–101]. Should these observations be considered outliers in the backdrop of the overwhelming literature about AMPK’s role in growth suppression? Could it be that specific subcellular pools of AMPK heterotrimers are activated in an energy-rich context, independent of AMP/ATP ratio to coordinate proper cell division, while other AMPK complexes simultaneously perform the canonical functions of AMPK? Further research is needed to explain these seemingly discordant roles of AMPK in normal physiology.
The controversy about AMPK function in cancer
It is fair to state that there is no single unifying role of AMPK in cancer. The debate as to whether AMPK promotes or suppresses tumor growth stems from (a) the use of non-specific and promiscuous pharmacological agents that inhibit or indirectly activate AMPK and therefore create a discrepancy between pharmacological and genetic studies, (b) our limited understanding of context-dependent and cell type-dependent AMPK heterotrimer composition, subcellular localization and substrate selectivity, (c) oversimplification of AMPK-regulated pathways, which often fails to discriminate between signaling in normal cells and anomalous signaling in cancer cells, and (d) the absence of genetic models to rigorously examine the role of AMPK role in different stages of tumor development. The most prominent of the pharmacological agents in this context are the anti-diabetic drug metformin, the AMP mimetic AICAR and the multi-kinase inhibitor Compound C. A cause and effect relationship was initially drawn between AMPK activation by AICAR, metformin (and other synthetic compounds and natural products) and inhibition of cancer cell proliferation [102–107]. We and others have now convincingly demonstrated that AICAR, metformin and Compound C [22,108–111] are highly non-specific, and all three agents inhibit proliferation through AMPK-independent mechanisms. AMPK activation by xenobiotics per se is not a cause of cell death; instead AMPK activation occurs as a response mechanism to protect cells from the toxicity of these agents. This proposal is consistent with the increased vulnerability of AMPK null hepatocytes [112], LKB1 mutant lung cancer cells [113] and AMPK null glioma and stromal cells [24] to AICAR or metformin.
Contrary to the above pharmacological studies, experiments in human cancer cells using genetic tools demonstrated that AMPK supports cancer cell survival, proliferation and migration through redox homeostasis, by cooperating with oncogenes (Myc), augmenting cellular transformation and metabolic reprograming, regulating microtubule dynamics, providing protection from chemotherapy and radiation, and through unknown mechanisms [114–128]. The caveat is that most of these are in vitro studies that need to be tested in vivo. A few in vivo studies that indicate that AMPK has a contrasting role in cancer includes its tumor promoting role in RAS-transformed fibroblasts, astrocytic and breast tumors [119,120,125], and its tumor suppressive role in a mouse model of Myc-induced lymphoma [129]. Recently, in vitro genetic studies also showed a growth suppressive action of AMPK through its regulation of the Hippo pathway [130–132]. Examining the functions of a stress sensor such as AMPK in cell lines adapted in culture for decades that are grown under the artificial and most favorable cell culture conditions (e.g., 25 mM glucose and ambient air) is not the best approach. For mechanistic studies, all attempts should be made to include primary cancer lines freshly established from patient tumors that have not been passaged for years. Another option for in vitro mechanistic studies would be organoid cultures or other 3-D culture systems maintained at physiological nutrient and oxygen levels – these systems could potentially allow formation of nutrient and oxygen gradients mimicking solid tumors and would be clinically more relevant for studying functions of stress sensors like AMPK. However, although significantly more challenging for mechanistic studies, the true functions of AMPK in cancer initiation, promotion or suppression will be revealed only by mouse models and genetic patient-derived xenograft (PDX) models.
The AMPK pathway in the human cancer genome
The rationale that supports a tumor suppressive role for AMPK was based on the discovery of the tumor suppressor LKB1 (STK11) as the upstream AMPKK that activates AMPK [44]. Germline mutations in LKB1 causes benign hamartomas in the Peutz-Jeghers cancer syndrome (PJS), and PJS patients have a higher risk of developing mainly intestinal and breast cancer [133–135]. A distinction should however be drawn between LKB1 and other tumor suppressors (TS) that are frequently inactivated in human cancer. The mutational landscape of major human cancers revealed that unlike the two frequently mutated TS genes - TP53 (mutation frequency range: 2–94%; pan-cancer frequency: 42%) and PTEN (mutation frequency range: 2–63%, 94%; pan-cancer frequency: 9.7%), pan-cancer frequency of LKB1 mutation is one of the lowest among TS genes (0.9%) and except for lung adenocarcinoma and uterine cancer, where LKB1 mutation frequency is around 10% and 1.7%, respectively, LKB1 is rarely mutated in human cancer (mutation frequency range: 0–0.4%) [136–138]. One can argue that in the tug of war between the throttle and gas pedal (keeping LKB1 may slow down proliferation but increase survival in the tumor milieu), most tumors may have evolved to retain rather than mutate LKB1. Indeed, contrary to other TS genes that suppress transformation, both LKB1 and AMPK are required for oncogene-driven transformation of fibroblasts, and LKB1 null tumor cells are preferentially killed by stress-inducing drugs [44,113,119].
Two other important aspects about the LKB1-AMPK axis in cancer that need to be highlighted is that first, LKB1 has at least 13 targets other than AMPK [139] and some of LKB1’s effects are likely mediated by substrates other than AMPK. Indeed, expression of a hypomorphic LKB1 allele in mouse caused AMPK-dependent branching defects of the developing lung, while pancreatic cyst formation was AMPK-independent [140]. Second, the other major AMPKK, CAMKKβ is not a TS gene in human cancer [136–138]. In fact, CAMKKβ is overexpressed up to 7-fold in 92/98 (94%) of gastric cancer [141], and played an AMPK-mediated role in metabolic reprograming, migration and invasion in prostate and colon cancer [126,128,142,143]. It is reasonable to predict that oncogene-induced ER stress and calcium disequilibrium can activate a specific pool of the AMPK heterotrimer through CAMKKβ.
Analysis of AMPK mutation data in human cancer [144,145] shows interesting results. The AMPK α2 subunit is mutated at a frequency of 0.2 – 10% across all human cancers (highest in small cell lung cancer and melanoma, but under 4% in all other cancers). The only cancer where α2 is amplified is sarcoma (~6%). On the contrary, the AMPK α1 subunit is rarely mutated but instead amplified at a frequency of 1 – 15% (15% in lung squamous cell and adenocarcinoma, and between 5–10% in sarcoma and cancers of the stomach, bladder, esophagus, head and neck, uterus and ovary). Is the AMPKα1 complex oncogenic and the AMPKα2 complex growth suppressive in a context-dependent manner? Supporting the above argument, deletion of α2 enhanced while deletion of α1 suppressed growth of RAS-transformed murine fibroblasts [146]. However, opposing the argument are reports from in vitro studies that AKT activation specifically reduces AMPKα1 activity by phosphorylating α1 at S487, while having no effect on α2 which is a poor ATK substrate [147–149]. The significance of these findings remains to be tested in vivo. The frequency of β1 subunit mutation is low (<2%), except nerve sheath tumors where it is deleted (~13%). However, it is amplified in sarcoma, prostate, ovarian and stomach cancer (3–7%). In sharp contrast, the β2 subunit is amplified across nearly all cancers (4–16%). Among the γ subunits, the only cancer where γ1 is significantly deleted (16%) is nerve sheath tumors, while the only cancers where data on the γ2 subunit are consistent are ovarian cancer, where it is amplified (~12%), and AML where it is deleted in about 4% of cases. Remarkably, despite overexpression in gastric cancer, CAMKKβ is deleted in about 13% of nerve sheath tumors and ~4% of melanoma. Experimental validation studies will be required to test the functional impact of these mutations. Besides these genetic changes, epigenetic mechanisms significantly influence gene expression and much work is needed to understand the regulation of AMPK subunits, LKB1 and CAMKKβ in human cancer.
The AMPK - lipogenesis axis in cancer
AMPK phosphorylates ACC to inhibit its activity (Figure 2). A distinction between the consequences of inhibition of ACCA versus ACCB that is often overlooked and requires particular attention in cancer is that ACCA inhibition blocks de novo fatty acid (FA) synthesis, while ACCB inhibition by AMPK increases FA oxidation [150]. One overwhelming argument is that AMPK activation would inhibit ACCA, block lipogenesis and reduce tumor growth. Indeed, the high expression of FASN (the FA synthesizing gene) is correlated with poor prognosis in many cancers, and FASN inhibition caused apoptosis in vitro and in vivo [151]. However, it remains unclear if the main cause of cancer cell death is inhibition of FA synthesis per se or cytotoxic effects of accumulated malonyl-Co-A that occurs after FASN inhibition [152]. This is an important point to ponder because FASN or ACCA knockdown does not kill lung, glioma or breast cancer cells [24,114,153]. In fact, ACCA or ACCB knockdown or inhibition by AMPK is required to maintain NADPH levels and in vivo growth of lung and breast tumor xenografts [114]. We reported that expression of an ACCA mutant that cannot be phosphorylated by AMPK had no effect on glioma cell proliferation. However, reducing exogenous lipids alone inhibited proliferation [24] suggesting that dietary lipids could be crucial for certain cancers.
Given that the flow of glucose to the FA precursor citrate is limited due to the Warburg effect (preferential glycolytic metabolism in the presence of oxygen), and citrate production by reductive decarboxylation of glutamine-derived α ketoglutarate may not be sufficient to compensate for the Warburg effect, some cancers may partially uncouple the negative regulation of AMPK on ACCA (and thus de novo lipogenesis), and become more reliant on dietary lipids. Recent studies underscore the importance of macromolecule scavenging by cancer cells [154,155] and dietary FAs could be scavenged or transported through FA transporters to support tumor growth. Interestingly, FASN and ACCA are not greatly amplified in human cancer (range – 3–9% and 0.5–5, respectively), while ACCB is mutated in a wide range of cancers (2–13%), and at least some genes in the FA oxidation and transport pathway (CPT1A, SLC27A1, SLC27A3) are amplified in some cancers (range 3–30%; 2–12% and 2–15%, respectively) [141,142]. To target de novo lipogenesis, ACCA inhibition by specific inhibitors rather than by AMPK activation should be a better strategy to avoid unintended consequences of AMPK activation like tumor survival through autophagy, enhanced glycolysis, macromolecule import and redox homeostasis [111,122,156–158] and other pro-tumor functions of AMPK.
The AMPK - mTOR axis in cancer
AMPK phosphorylates the tumor suppressor TSC2 and the mTORC1 partner RAPTOR to inhibit mTOR (Figure 2) [159–160]. While there is no question this regulatory mechanism exists, an oversimplified linear view of this pathway together with drawing a parallelism between normal and cancer cells has led to apparently incompatible results from different laboratories. Although AMPK activation during glucose stress represses mTOR in vitro to increase cell survival in some but not all cases [114,161], we found that AMPK silencing in glioma cells did not do the opposite, i.e., activate mTOR [24], suggesting either the existence of other ways of controlling mTOR or interference between AMPK and mTOR due to the complex rewiring of signaling networks in cancer cells. Indeed, mTOR regulation is highly context-specific and can be controlled independent of AMPK. For example, we earlier reported that in skeletal muscle, AMPKβ2 knockout caused an expected basal mTOR activation that was greatly amplified during exercise when AMPK activation suppressed mTOR in wildtype mice [15]. However, nutrient stress in vivo (fasting) inhibited mTOR independent of AMPK [15]. In fact, mTOR inhibition can occur through REDD1 and p38 independent of TSC2 in a context-dependent manner [162–164]. Therefore, AMPK is not absolutely necessary for mTOR inhibition suggesting that in certain contexts, activated AMPK and mTOR may co-exist. Indeed, this is what we observed in glioblastoma suggesting that basally active AMPK (and possibly only specific AMPK heterotrimers) may not be sufficient to put a brake on mTOR [24]However, in-depth analysis of tumor tissue by immunohistochemistry and flow cytometry is required to examine if the two signals exist in same or distinct cells.
Global activation of alternative splicing, including aberrant splicing of genes, occurs in human cancers in response to oncogene activation, tumor suppressor loss, tumor hypoxia and other changes in the tumor microenvironment [165–167]. Many of these splice variants play roles during development but are reactivated in cancer to evade growth suppressors, apoptosis, immune detection and to engage in tumor metabolic reprograming. In prostate cancer, androgens drive alternative splicing of TSC2, resulting in the dominant expression of a splice variant that lacks TSC1 and RHEB interacting domains leading to constitutive mTOR activation [168] and potentially de-linking AMPK and mTORC1. If proven true in other contexts and tumors, this is a fascinating mechanism of “having the cake and eating it too” that may allow simultaneous AMPK and mTOR activation.
A third and potentially interesting mechanism by which cancer cells may evade AMPK’s inhibition of mTOR-regulated protein translation is by switching from CAP to IRES (Internal Ribosomal Entry Site) - dependent translation that often occurs during oncogenesis [169–171]. AMPK is activated while mTOR is inhibited during nutrient stress and hypoxia that are common features in advanced tumors [172,173]. In stressed areas of tumors, a switch to IRES-mediated, mTOR-independent translation allows synthesis of crucial proteins involved in growth, proliferation, angiogenesis, survival, and resistance to chemotherapy and radiation. Some examples include cMyc, VEGF, XIAP, APAF1, FGF2, TrkB and Aurora kinase [174–177]. Therefore, it is conceivable that in a heterogeneous tumor environment, high AMPK and high mTOR signaling can co-exist in regions of high and low stress, respectively, and AMPK and mTOR signaling could be in dynamic flux between the different cell populations. Indeed, coordinated regulation of AMPK and mTOR signaling occurs during human mesenchymal stem cell differentiation [178]. An interesting question is whether certain AMPK subunits are also translated by the IRES-mediated mechanism in high stress areas of solid tumors.
Concluding Remarks
A renewed interest in examining mitochondrial function in human cancer has led to the discovery of multiple aspects of tumorigenesis that are regulated by mitochondria. These include metabolic reprograming like reductive carboxylation of α-ketoglutarate, ROS production, metastasis, epithelial-mesenchymal transition (EMT) and drug resistance among others [179–185]. AMPK plays an important role in promoting mitochondrial biogenesis and function in normal tissue and cancer [116,126,186,187]. Therefore, mitochondria-dependent functions during tumor evolution may be favored by active AMPK in certain tumors. A hallmark of cancer metabolic reprograming is aerobic glycolysis (Warburg effect). Several studies now show that active AMPK supports glycolysis in human tumors [116,125,156,188,189], although it countered the Warburg effect in a mouse model of lymphoma [129], reflecting a possible tumor or species-specific role of AMPK in glycolysis. Is AMPK activated or inhibited during transformation to cope with transformation-associated metabolic reprograming? It is unclear whether all cells in a solid tumor switch to glycolytic metabolism or if genetic and intratumoral environmental heterogeneity require some cells to rely more on mitochondrial oxidation of glucose, fatty acids and other substrates (see Outstanding Questions).
Cells with a proliferative advantage in nutrient-rich environments may initially suppress AMPK to boost biosynthetic processes. Indeed, the AMPKα2 transcript is suppressed in Stages I &II but upregulated in grades III and IV of human gastric cancer [190]. As tumor cells undergo clonal expansion with accumulating mutations, and the tumor grows in volume, oncogenic stress, fluctuating nutrient and oxygen levels, malformed neovasculature and harsh acidic environment may force tumor cells to depend on AMPK and other stress enzymes to support their altered metabolism and survival (Figure 3). When AMPK suppresses proliferation by inhibiting mTOR while supports tumor energy balance and reprogramed metabolism, what is the rationale to activate AMPK to inhibit mTOR when specific mTOR inhibitors are available? A similar logic applies to inhibition of lipogenesis in cancer. Considering the potential risk of enhancing the diverse pro-tumor effects of AMPK, including the risk of relieving S6K suppression of IRS1 and mTORC2 activation during mTORC1 inhibition [191,192] by AMPK activators, a combined inhibition of mTOR and AMPK may prove to be a more effective strategy for certain advanced tumors. However, in cancers in which the net role effect of AMPK is growth suppression, specific and direct AMPK activators could be therapeutically more beneficial. In this regard, in a recent report, AMPK activation by relatively specific AMPK activators killed AML cells only with hyperactivated mTORC1 [193]. Interestingly, similar to our previous results [26], this study also found a disconnect between AMPK activation and mTOR suppression since AMPK activation did not inhibit mTORC1 in at least three AML cell lines and primary AML samples. With the caveat that human tumors are distinct from mouse tumors in many aspects, rigorous and parallel studies in genetically engineered mouse models and PDX models using controlled and tunable AMPK expression and inhibition will reveal the tissue specific effect of AMPK on tumorigenesis and progression.
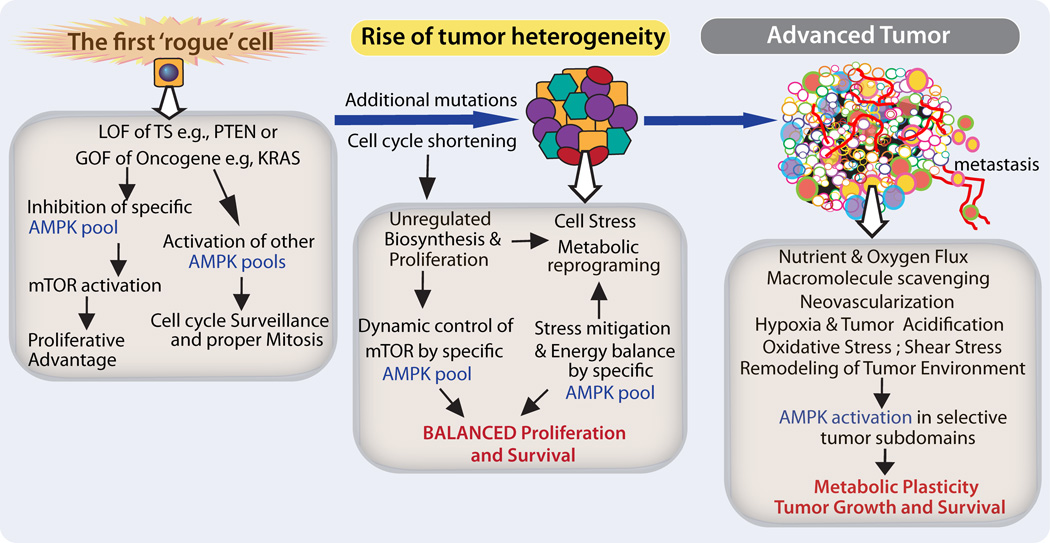
Figure 3. Context-dependent role of AMPK in Cancer.
The cartoon shows probable roles of AMPK during the evolution of solid tumors. On one hand, specific genetic changes in cells may up-or downregulate AMPK activity, while on the other hand, the consequence of AMPK up-or downregulation may be diverse in cells of different genetic backgrounds and tissues. The cartoon is just one example in which loss of function (LOF), or gain of function (GOF) of tumor suppressors (TS) and oncogenes, respectively, may initially downregulate cytoplasmic AMPK activity to relieve its control on mTOR rendering a proliferative advantage to cells. Simultaneously, an active nuclear pool of AMPK may survey and ensure proper division of transformed cells. As tumor cells multiply, individual cells accumulate additional stochastic mutations and epigenetic changes leading to rise of clones, clonal competition, expansion and tumor heterogeneity. Each of these clones continues to evolve as the tumor itself evolves into an organized structure. These clonal subsets may conform to the principle of ‘division of labor’ such that subsets with high levels of either AMPK or mTOR may serve different functions and support each other to sustain overall tumor growth. In these subsets of cells, mTOR and AMPK signature may not be static but could be could be dynamically regulated. Active AMPK likely mitigates oncogenic stress and regulates metabolic reprograming. Finally, the hostile environment of solid tumors, particularly the specific subdomains of overgrowth, poor supply of nutrients and O2, exert considerable strain on tumor growth. In these regions, AMPK may allow metabolic plasticity and survival and potentially metastasis by regulating anchorage-independent survival and migration through blood vessels and normal parenchyma.
TRENDS BOX.
AMP activated protein kinase (AMPK) is an evolutionarily conserved cellular energy sensor
There are many recognized and unrecognized roles of AMPK in normal and pathophysiology
Some functions of AMPK are likely beyond energy sensing
The multisubunit structure and sensitivity to various stresses confer complexity to AMPK function
Appropriate genetic models are desperately needed to understand the context-dependent role of AMPK in metabolic diseases including cancer
Outstanding Questions.
How do individual AMPK complexes preferentially form under different contexts?
Are there complex-specific and context-specific preferred AMPK substrates?
Can we conclude on the net effect of AMPK activation from a snap shot of phospho-signal on only one of its substrates (e.g., ACC)?
Can the same complex function differently in diseased versus normal cells?
Does the myriad stimuli that activate AMPK (physiological, pathological and drugs) require different AMPK regulatory (β/γ) subunits?
How is tissue-specific expression of AMPK subunits regulated at the genetic and epigenetic level? Are there tissue-specific factors that allow formation of certain AMPK complexes only?
Are AMPK transcript variants differentially expressed under normal physiology versus pathology and do they regulate AMPK biology?
How are the AMPK subunits regulated during development, and what is the significance of subunit switch?
Is xenobiotic sensing a conserved function of AMPK that occurs through mechanisms independent of energy or Ca+2 sensing?
Is AMPK signaling binary/digital (on or off) or analog (continuous gradient)? Are there ‘better’ AMPK substrates (that can get phosphorylated at low AMPK activity), and ‘poor’ AMPK substrates?
Does AMPK mediate spindle formation, chromosomal segregation, cytokinesis and cytoskeletal reorganization? Are AMPK null cells error-prone during DNA replication and susceptible to aneuploidy? Is AMPK’s role in mitosis uncoupled from its energy sensing function? Could it be that specific subcellular pools of AMPK heterotrimers are activated in an energy-rich context, independent of AMP/ATP ratio to coordinate proper cell division?
Is AMPK activated or inhibited during transformation to cope with transformation-associated metabolic reprograming? What factors dictate tissue or even species-specific role of AMPK in tumor suppression or promotion?
ACKNOWLEDGEMENTS
We thank Jane Anderson for proofreading the manuscript. This work was supported by the National Institute of Health (1R01NS075291-01A1) to B.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Hardie DG. AMP-activated protein kinase: maintaining energy homeostasis at the cellular and whole-body levels. Annu Rev Nutr. 2014;34:31–55. doi: 10.1146/annurev-nutr-071812-161148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carling D, et al. AMP-activated protein kinase: new regulation, new roles? Biochem J. 2012;445:11–27. doi: 10.1042/BJ20120546. [DOI] [PubMed] [Google Scholar]
- 3.Viollet B, et al. AMPK inhibition in health and disease. Crit Rev Biochem Mol Biol. 2010;45:276–295. doi: 10.3109/10409238.2010.488215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinberg GR, Kemp BE. AMPK in Health and Disease. Physiol Rev. 2009;89:1025–1078. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- 5.Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13:1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies SP, et al. Purification of the AMP-activated protein kinase on ATP-gamma-sepharose and analysis of its subunit structure. Eur J Biochem. 1994;223:351–357. doi: 10.1111/j.1432-1033.1994.tb19001.x. [DOI] [PubMed] [Google Scholar]
- 7.Mitchelhill KI, et al. Mammalian AMP-activated protein kinase shares structural and functional homology with the catalytic domain of yeast Snf1 protein kinase. J Biol Chem. 1994;269:2361–2364. [PubMed] [Google Scholar]
- 8.Rider MH. Role of AMP-activated protein kinase in metabolic depression in animals. J Comp Physiol B. 2015 doi: 10.1007/s00360-015-0920-x. [DOI] [PubMed] [Google Scholar]
- 9.Crute BE, et al. Functional domains of the alpha1 catalytic subunit of the AMP-activated protein kinase. J Biol Chem. 1998;273:35347–35354. doi: 10.1074/jbc.273.52.35347. [DOI] [PubMed] [Google Scholar]
- 10.Dasgupta B, Milbrandt J. AMP-activated protein kinase phosphorylates retinoblastoma protein to control mammalian brain development. Dev Cell. 2009;16:256–270. doi: 10.1016/j.devcel.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spasic MR, et al. Drosophila alicorn is a neuronal maintenance factor protecting against activity-induced retinal degeneration. J Neurosci. 2008;28:6419–6429. doi: 10.1523/JNEUROSCI.1646-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McBride A, Hardie DG. AMP-activated protein kinase -a sensor of glycogen as well as AMP and ATP? Acta Physiol (Oxf) 2009;196:99–113. doi: 10.1111/j.1748-1716.2009.01975.x. [DOI] [PubMed] [Google Scholar]
- 13.Polekhina G, et al. AMPK beta subunit targets metabolic stress sensing to glycogen. Curr Biol. 2003;13:867–871. doi: 10.1016/s0960-9822(03)00292-6. [DOI] [PubMed] [Google Scholar]
- 14.Quentin T, et al. Different expression of the catalytic alpha subunits of the AMP activated protein kinase--an immunohistochemical study in human tissue. Histol Histopathol. 2011;26:589–596. doi: 10.14670/HH-26.589. [DOI] [PubMed] [Google Scholar]
- 15.Dasgupta B, et al. The AMPK beta2 subunit is required for energy homeostasis during metabolic stress. Mol Cell Biol. 2012;32:2837–2848. doi: 10.1128/MCB.05853-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Z, et al. Expression of the AMP-activated protein kinase beta1 and beta2 subunits in skeletal muscle. FEBS Lett. 1999;460:343–348. doi: 10.1016/s0014-5793(99)01371-x. [DOI] [PubMed] [Google Scholar]
- 17.Treebak JT, et al. Identification of a novel phosphorylation site on TBC1D4 regulated by AMP-activated protein kinase in skeletal muscle. Am J Physiol Cell Physiol. 2010;298:C377–C385. doi: 10.1152/ajpcell.00297.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wojtaszewski JF, et al. 5'AMP activated protein kinase expression in human skeletal muscle: effects of strength training and type 2 diabetes. J Physiol. 2005;564:563–573. doi: 10.1113/jphysiol.2005.082669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Birk JB, Wojtaszewski JF. Predominant alpha2/beta2/gamma3 AMPK activation during exercise in human skeletal muscle. J Physiol. 2006;577:1021–1032. doi: 10.1113/jphysiol.2006.120972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Treebak JT, et al. AS160 phosphorylation is associated with activation of alpha2beta2gamma1- but not alpha2beta2gamma3-AMPK trimeric complex in skeletal muscle during exercise in humans. Am J Physiol Endocrinol Metab. 2007;292:E715–E722. doi: 10.1152/ajpendo.00380.2006. [DOI] [PubMed] [Google Scholar]
- 21.Jorgensen SB, et al. Effects of alpha-AMPK knockout on exercise-induced gene activation in mouse skeletal muscle. FASEB J. 2005;19:1146–1148. doi: 10.1096/fj.04-3144fje. [DOI] [PubMed] [Google Scholar]
- 22.Wu J, et al. Chemoproteomic analysis of intertissue and interspecies isoform diversity of AMP-activated protein kinase (AMPK) J Biol Chem. 2013;288:35904–35912. doi: 10.1074/jbc.M113.508747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niesler CU, et al. The changing AMPK expression profile in differentiating mouse skeletal muscle myoblast cells helps confer increasing resistance to apoptosis. Exp Physiol. 2007;92:207–217. doi: 10.1113/expphysiol.2006.034736. [DOI] [PubMed] [Google Scholar]
- 24.Liu X, et al. Discrete mechanisms of mTOR and cell cycle regulation by AMPK agonists independent of AMPK. Proc Natl Acad Sci U S A. 2014b;111:E435–E444. doi: 10.1073/pnas.1311121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGee SL, et al. Exercise inc reases nuclear AMPK alpha2 in human skeletal muscle. Diabetes. 2003;52:926–928. doi: 10.2337/diabetes.52.4.926. [DOI] [PubMed] [Google Scholar]
- 26.Turnley AM, et al. Cellular distribution and developmental expression of AMP-activated protein kinase isoforms in mouse central nervous system. J Neurochem. 1999;72:1707–1716. doi: 10.1046/j.1471-4159.1999.721707.x. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt MC, McCartney RR. Beta-subunits of Snf1 kinase are required for kinase function and substrate definition. EMBO J. 2000;19:4936–4943. doi: 10.1093/emboj/19.18.4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyamoto T, et al. Compartmentalized AMPK signaling illuminated by genetically encoded molecular sensors and actuators. Cell Rep. 2015;11:657–670. doi: 10.1016/j.celrep.2015.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Viollet B, et al. The AMP-activated protein kinase alpha2 catalytic subunit controls whole-body insulin sensitivity. J Clin Invest. 2003;111:91–98. doi: 10.1172/JCI16567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steinberg GR, et al. Whole body deletion of AMP-activated protein kinase {beta}2 reduces muscle AMPK activity and exercise capacity. J Biol Chem. 2010;285:37198–37209. doi: 10.1074/jbc.M110.102434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carlson CA, Kim KH. Regulation of hepatic acetyl coenzyme A carboxylase by phosphorylation and dephosphorylation. J Biol Chem. 1973;248:378–380. [PubMed] [Google Scholar]
- 32.Yeh LA, et al. Regulation of rat liver acetyl-CoA carboxylase. Regulation of phosphorylation and inactivation of acetyl-CoA carboxylase by the adenylate energy charge. J Biol Chem. 1980;255:2308–2314. [PubMed] [Google Scholar]
- 33.Ly S, Kim KH. Inactivation of hepatic acetyl-CoA carboxylase by catecholamine and its agonists through the alpha-adrenergic receptors. J Biol Chem. 1981;256:11585–11590. [PubMed] [Google Scholar]
- 34.Lent B, Kim KH. Purification and properties of a kinase which phosphorylates and inactivates acetyl-CoA carboxylase. J Biol Chem. 1982;257:1897–1901. [PubMed] [Google Scholar]
- 35.Carling D, et al. A common bicyclic protein kinase cascade inactivates the regulatory enzymes of fatty acid and cholesterol biosynthesis. FEBS Lett. 1987;223:217–222. doi: 10.1016/0014-5793(87)80292-2. [DOI] [PubMed] [Google Scholar]
- 36.Munday MR, et al. Identification by amino acid sequencing of three major regulatory phosphorylation sites on rat acetyl-CoA carboxylase. Eur J Biochem. 1988;175:331–338. doi: 10.1111/j.1432-1033.1988.tb14201.x. [DOI] [PubMed] [Google Scholar]
- 37.Gowans GJ, et al. AMP is a true physiological regulator of AMP-activated protein kinase by both allosteric activation and enhancing net phosphorylation. Cell Metab. 2013;18:556–566. doi: 10.1016/j.cmet.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davies SP, et al. 5'-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2C alpha and native bovine protein phosphatase-2AC. FEBS Lett. 1995;377:421–425. doi: 10.1016/0014-5793(95)01368-7. [DOI] [PubMed] [Google Scholar]
- 39.Hawley SA, et al. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem. 1996;271:27879–27887. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- 40.Xiao B, et al. Structure of mammalian AMPK and its regulation by ADP. Nature. 2011;472:230–233. doi: 10.1038/nature09932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oakhill JS, et al. beta-Subunit myristoylation is the gatekeeper for initiating metabolic stress sensing by AMP-activated protein kinase (AMPK) Proc Natl Acad Sci U S A. 2010;107:19237–19241. doi: 10.1073/pnas.1009705107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oakhill JS, et al. AMPK is a direct ad enylate charge-regulated protein kinase. Science. 2011;332:1433–1435. doi: 10.1126/science.1200094. [DOI] [PubMed] [Google Scholar]
- 43.Hawley SA, et al. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shaw RJ, et al. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hawley SA, et al. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 46.Hurley RL, et al. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem. 2005;280:29060–29066. doi: 10.1074/jbc.M503824200. [DOI] [PubMed] [Google Scholar]
- 47.Woods A, et al. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 48.Momcilovic M, et al. Mammalian TAK1 activates Snf1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro. J Biol Chem. 2006;281:25336–25343. doi: 10.1074/jbc.M604399200. [DOI] [PubMed] [Google Scholar]
- 49.Xie M, et al. A pivotal role for endogenous TGF-beta-activated kinase-1 in the LKB1/AMP-activated protein kinase energy-sensor pathway. Proc Natl Acad Sci U S A. 2006;103:17378–17383. doi: 10.1073/pnas.0604708103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fogarty S, et al. Calmodulin-dependent protein kinase kinase-beta activates AMPK without forming a stable complex: synergistic effects of Ca2+ and AMP. Biochem J. 2010;426:109–118. doi: 10.1042/BJ20091372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tamas P, et al. Regulation of the energy sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T lymphocytes. J Exp Med. 2006;203:1665–1670. doi: 10.1084/jem.20052469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scott JW, et al. Small molecule drug A-769662 and AMP synergistically activate naive AMPK independent of upstream kinase signaling. Chem Biol. 2014;21:619–627. doi: 10.1016/j.chembiol.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 53.Dagon Y, et al. p70S6 kinase phosphorylates AMPK on serine 491 to mediate leptin's effect on food intake. Cell Metab. 2012;16:104–112. doi: 10.1016/j.cmet.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pulinilkunnil T, et al. Adrenergic regulation of AMP-activated protein kinase in brown adipose tissue in vivo. J Biol Chem. 2011;286:8798–8809. doi: 10.1074/jbc.M111.218719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hudson ER, et al. A novel domain in AMP-activated protein kinase causes glycogen storage bodies similar to those seen in hereditary cardiac arrhythmias. Curr Biol. 2003;13:861–866. doi: 10.1016/s0960-9822(03)00249-5. [DOI] [PubMed] [Google Scholar]
- 56.McBride A, et al. The glycogen-binding domain on the AMPK beta subunit allows the kinase to act as a glycogen sensor. Cell Metab. 2009;9:23–34. doi: 10.1016/j.cmet.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Horman S, et al. Evaluation of the role of AMP-activated protein kinase and its downstream targets in mammalian hibernation. Comp Biochem Physiol B Biochem Mol Biol. 2005;142:374–382. doi: 10.1016/j.cbpb.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 58.Rider MH, et al. AMP-activated protein kinase and metabolic regulation in cold-hardy insects. J Insect Physiol. 2011;57:1453–1462. doi: 10.1016/j.jinsphys.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 59.Podrabsky JE, Hand SC. Depression of protein synthesis during diapause in embryos of the annual killifish Austrofundulus limnaeus. Physiol Biochem Zool. 2000;73:799–808. doi: 10.1086/318106. [DOI] [PubMed] [Google Scholar]
- 60.Mendelsohn BA, et al. The zebrafish embryo as a dynamic model of anoxia tolerance. Dev Dyn. 2008;237:1780–1788. doi: 10.1002/dvdy.21581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hand SC, et al. Metabolic restructuring during energy-limited states: insights from Artemia franciscana embryos and other animals. J Insect Physiol. 2011;57:584–594. doi: 10.1016/j.jinsphys.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu XJ, et al. Activation of an AMP-activated protein kinase is involved in post-diapause development of Artemia franciscana encysted embryos. BMC Dev Biol. 2009;9:21. doi: 10.1186/1471-213X-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jibb LA, Richards JG. AMP-activated protein kinase activity during metabolic rate depression in the hypoxic goldfish, Carassius auratus. J Exp Biol. 2008;211:3111–3122. doi: 10.1242/jeb.019117. [DOI] [PubMed] [Google Scholar]
- 64.Healy JE, et al. AMPK and ACC change with fasting and physiological condition in euthermic and hibernating golden-mantled ground squirrels (Callospermophilus lateralis) Comp Biochem Physiol A Mol Integr Physiol. 2011;159:322–331. doi: 10.1016/j.cbpa.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hawley SA, et al. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 2010;11:554–565. doi: 10.1016/j.cmet.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hardie DG. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25:1895–1908. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hawley SA, et al. The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism. Diabetes. 2002;51:2420–2425. doi: 10.2337/diabetes.51.8.2420. [DOI] [PubMed] [Google Scholar]
- 68.Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci U S A. 2007;104:7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang YM, et al. Role of CAR and PXR in xenobiotic sensing and metabolism. Expert Opin Drug Metab Toxicol. 2012;8:803–817. doi: 10.1517/17425255.2012.685237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rencurel F, et al. Stimulation of AMP-activated protein kinase is essential for the induction of drug metabolizing enzymes by phenobarbital in human and mouse liver. Mol Pharmacol. 2006;70:1925–1934. doi: 10.1124/mol.106.029421. [DOI] [PubMed] [Google Scholar]
- 71.Rencurel F, et al. AMP-activated protein kinase mediates phenobarbital induction of CYP2B gene expression in hepatocytes and a newly derived human hepatoma cell line. J Biol Chem. 2005;280:4367–4373. doi: 10.1074/jbc.M412711200. [DOI] [PubMed] [Google Scholar]
- 72.Blattler SM, et al. In the regulation of cytochrome P450 genes, phenobarbital targets LKB1 for necessary activation of AMP-activated protein kinase. Proc Natl Acad Sci U S A. 2007;104:1045–1050. doi: 10.1073/pnas.0610216104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koumenis C, et al. Tumor microenvironment and cellular stress: signaling, metabolism, imaging, and therapeutic targets. Preface. Adv Exp Med Biol. 2014;772:v–viii. doi: 10.1007/978-1-4614-5915-6. [DOI] [PubMed] [Google Scholar]
- 74.Neelsen KJ, et al. Oncogenes induce genotoxic stress by mitotic processing of unusual replication intermediates. J Cell Biol. 2013;200:699–708. doi: 10.1083/jcb.201212058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bartkova J, et al. Replication stress and oxidative damage contribute to aberrant constitutive activation of DNA damage signaling in human gliomas. Oncogene. 2010;29:5095–5102. doi: 10.1038/onc.2010.249. [DOI] [PubMed] [Google Scholar]
- 76.Wang M, Kaufman RJ. The impact of the endoplasmic reticulum protein-folding environment on cancer development. Nat Rev Cancer. 2014;14:581–597. doi: 10.1038/nrc3800. [DOI] [PubMed] [Google Scholar]
- 77.Huang LE. Biochemistry. How HIF-1alpha handles stress. Science. 2013;339:1285–1286. doi: 10.1126/science.1236966. [DOI] [PubMed] [Google Scholar]
- 78.Lee JH, et al. Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature. 2007;447:1017–1020. doi: 10.1038/nature05828. [DOI] [PubMed] [Google Scholar]
- 79.Baena-Gonzalez E, et al. A central integrator of transcription networks in plant stress and energy signaling. Nature. 2007;448:938–942. doi: 10.1038/nature06069. [DOI] [PubMed] [Google Scholar]
- 80.Mirouse V, et al. LKB1 and AMPK maintain epithelial cell polarity under energetic stress. J Cell Biol. 2007;177:387–392. doi: 10.1083/jcb.200702053. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 81.Viollet B, et al. AMPK: Lessons from transgenic and knockout animals. Front Biosci (Landmark Ed) 2009;14:19–44. doi: 10.2741/3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dzamko N, et al. AMPK beta1 deletion reduces appetite, preventing obesity and hepatic insulin resistance. J Biol Chem. 2010;285:115–122. doi: 10.1074/jbc.M109.056762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cove D, et al. Mosses as model systems for the study of metabolism and development. Annu Rev Plant Biol. 2006;57:497–520. doi: 10.1146/annurev.arplant.57.032905.105338. [DOI] [PubMed] [Google Scholar]
- 84.Greer EL, et al. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr Biol. 2007;17:1646–1656. doi: 10.1016/j.cub.2007.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mair W, et al. Lifespan extension induced by AMPK and calcineurin is mediated by CRTC-1 and CREB. Nature. 2011;470:404–408. doi: 10.1038/nature09706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stenesen D, et al. Adenosine nucleotide biosynthesis and AMPK regulate adult life span and mediate the longevity benefit of caloric restriction in flies. Cell Metab. 2013;17:101–112. doi: 10.1016/j.cmet.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lu JY, et al. Acetylation of yeast AMPK controls intrinsic aging independently of caloric restriction. Cell. 2011;146:969–979. doi: 10.1016/j.cell.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vazquez-Martin A, et al. The active form of the metabolic sensor: AMP-activated protein kinase (AMPK) directly binds the mitotic apparatus and travels from centrosomes to the spindle midzone during mitosis and cytokinesis. Cell Cycle. 2009;8:2385–2398. doi: 10.4161/cc.8.15.9082. [DOI] [PubMed] [Google Scholar]
- 89.Banko MR, et al. Chemical genetic screen for AMPKalpha2 substrates uncovers a network of proteins involved in mitosis. Mol Cell. 2011;44:878–892. doi: 10.1016/j.molcel.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fukuyama Y, et al. Hypoxia induces expression and activation of AMPK in rat dental pulp cells. J Dent Res. 2007;86:903–907. doi: 10.1177/154405910708600919. [DOI] [PubMed] [Google Scholar]
- 91.Merlen G, et al. AMPKalpha1 controls hepatocyte proliferation independently of energy balance by regulating Cyclin A2 expression. J Hepatol. 2014;60:152–159. doi: 10.1016/j.jhep.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 92.Bettencourt-Dias M, et al. Genome-wide survey of protein kinases required for cell cycle progression. Nature. 2004;432:980–987. doi: 10.1038/nature03160. [DOI] [PubMed] [Google Scholar]
- 93.Moffat J, et al. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell. 2006;124:1283–1298. doi: 10.1016/j.cell.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 94.Rios M, et al. AMPK activation by oncogenesis is required to maintain cancer cell proliferation in astrocytic tumors. Cancer Res. 2013;73:2628–2638. doi: 10.1158/0008-5472.CAN-12-0861. [DOI] [PubMed] [Google Scholar]
- 95.Daub H, et al. Kinase-selective enrichment enables quantitative phosphoproteomics of the kinome across the cell cycle. Mol Cell. 2008;31:438–448. doi: 10.1016/j.molcel.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 96.Levine YC, et al. Agonist-modulated regulation of AMP-activated protein kinase (AMPK) in endothelial cells. Evidence for an AMPK ->Rac1 ->Akt ->endothelial nitric-oxide synthase pathway. J Biol Chem. 2007;282:20351–20364. doi: 10.1074/jbc.M702182200. [DOI] [PubMed] [Google Scholar]
- 97.Reihill JA, et al. AMP-activated protein kinase mediates VEGF-stimulated endothelial NO production. Biochem Biophys Res Commun. 2007;354:1084–1088. doi: 10.1016/j.bbrc.2007.01.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zou MH, et al. Activation of 5'-AMP-activated kinase is mediated through c-Src and phosphoinositide 3-kinase activity during hypoxia-reoxygenation of bovine aortic endothelial cells. Role of peroxynitrite. J Biol Chem. 2003;278:34003–34010. doi: 10.1074/jbc.M300215200. [DOI] [PubMed] [Google Scholar]
- 99.Chau MD, et al. Fibroblast growth f actor 21 regulates energy metabolism by activating the AMPK-SIRT1-PGC-1alpha pathway. Proc Natl Acad Sci U S A. 2010;107:12553–12558. doi: 10.1073/pnas.1006962107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pan Z, et al. The function study on the interaction between Grb2 and AMPK. Mol Cell Biochem. 2008;307:121–127. doi: 10.1007/s11010-007-9591-6. [DOI] [PubMed] [Google Scholar]
- 101.Suzuki A, et al. IGF-1 phosphorylates AMPK-alpha subunit in ATM-dependent and LKB1-independent manner. Biochem Biophys Res Commun. 2004;324:986–992. doi: 10.1016/j.bbrc.2004.09.145. [DOI] [PubMed] [Google Scholar]
- 102.Guo D, et al. The AMPK agonist AICAR inhibits the growth of EGFRvIII-expressing glioblastomas by inhibiting lipogenesis. Proc Natl Acad Sci U S A. 2009;106:12932–12937. doi: 10.1073/pnas.0906606106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Swinnen JV, et al. Mimicry of a cellular low energy status blocks tumor cell anabolism and suppresses the malignant phenotype. Cancer Res. 2005;65:2441–2448. doi: 10.1158/0008-5472.CAN-04-3025. [DOI] [PubMed] [Google Scholar]
- 104.Tang YC, et al. Identification of aneuploidy-selective antiproliferation compounds. Cell. 2011;144:499–512. doi: 10.1016/j.cell.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dowling RJ, et al. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007;67:10804–10812. doi: 10.1158/0008-5472.CAN-07-2310. [DOI] [PubMed] [Google Scholar]
- 106.Huang X, et al. Important role of the LKB1-AMPK pathway in suppressing tumorigenesis in PTEN-deficient mice. Biochem J. 2008;412:211–221. doi: 10.1042/BJ20080557. [DOI] [PubMed] [Google Scholar]
- 107.Guigas B, et al. Metformin inhibits mitochondrial permeability transition and cell death: a pharmacological in vitro study. Biochem J. 2004;382:877–884. doi: 10.1042/BJ20040885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu X, et al. The AMPK inhibitor compound C is a potent AMPK-independent antiglioma agent. Mol Cancer Ther. 2014a;13:596–605. doi: 10.1158/1535-7163.MCT-13-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Santidrian AF, et al. AICAR induces apoptosis independently of AMPK and p53 through up-regulation of the BH3-only proteins BIM and NOXA in chronic lymphocytic leukemia cells. Blood. 2010;116:3023–3032. doi: 10.1182/blood-2010-05-283960. [DOI] [PubMed] [Google Scholar]
- 110.Memmott RM, et al. Metformin prevents tobacco carcinogen--induced lung tumorigenesis. Cancer Prev Res (Phila) 2010;3:1066–1076. doi: 10.1158/1940-6207.CAPR-10-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bain J, et al. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Foretz M, et al. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest. 2010;120:2355–2369. doi: 10.1172/JCI40671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shackelford DB, et al. LKB1 inactivation dictates therapeutic response of non-small cell lung cancer to the metabolism drug phenformin. Cancer Cell. 2013;23:143–158. doi: 10.1016/j.ccr.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jeon SM, et al. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature. 2012;485:661–665. doi: 10.1038/nature11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu L, et al. Deregulated MYC expression induces dependence upon AMPK-related kinase 5. Nature. 2012;483:608–612. doi: 10.1038/nature10927. [DOI] [PubMed] [Google Scholar]
- 116.Yan M, et al. The tumor suppressor folliculin regulates AMPK-dependent metabolic transformation. J Clin Invest. 2014;124:2640–2650. doi: 10.1172/JCI71749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fernandez MR, et al. Kinase suppressor of Ras 2 (KSR2) regulates tumor cell transformation via AMPK. Mol Cell Biol. 2012;32:3718–3731. doi: 10.1128/MCB.06754-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ros S, et al. Functional metabolic screen identifies 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4 as an important regulator of prostate cancer cell survival. Cancer Discov. 2012;2:328–343. doi: 10.1158/2159-8290.CD-11-0234. [DOI] [PubMed] [Google Scholar]
- 119.Laderoute KR, et al. 5'-AMP-activated protein kinase (AMPK) is induced by low-oxygen and glucose deprivation conditions found in solid-tumor microenvironments. Mol Cell Biol. 2006;26:5336–5347. doi: 10.1128/MCB.00166-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rios M, et al. Lipoprotein internalisation induced by oncogenic AMPK activation is essential to maintain glioblastoma cell growth. Eur J Cancer. 2014;50:3187–3197. doi: 10.1016/j.ejca.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 121.Kim HS, et al. Inhibition of AMP-activated protein kinase sensitizes cancer cells to cisplatin-induced apoptosis via hyper-induction of p53. J Biol Chem. 2008;283:3731–3742. doi: 10.1074/jbc.M704432200. [DOI] [PubMed] [Google Scholar]
- 122.Chhipa RR, et al. Survival advantage of AMPK activation to androgen-independent prostate cancer cells during energy stress. Cell Signal. 2010;22:1554–1561. doi: 10.1016/j.cellsig.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zannella VE, et al. AMPK regulates metabolism and survival in response to ionizing radiation. Radiother Oncol. 2011;99:293–299. doi: 10.1016/j.radonc.2011.05.049. [DOI] [PubMed] [Google Scholar]
- 124.Kato K, et al. Critical roles of AMP-activated protein kinase in constitutive tolerance of cancer cells to nutrient deprivation and tumor formation. Oncogene. 2002;21:6082–6090. doi: 10.1038/sj.onc.1205737. [DOI] [PubMed] [Google Scholar]
- 125.Laderoute KR, et al. 5'-AMP-activated protein kinase (AMPK) supports the growth of aggressive experimental human breast cancer tumors. J Biol Chem. 2014;289:22850–22864. doi: 10.1074/jbc.M114.576371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tennakoon JB, et al. Androgens regulate prostate cancer cell growth via an AMPK-PGC-1alpha-mediated metabolic switch. Oncogene. 2014;33:5251–5261. doi: 10.1038/onc.2013.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nakano A, et al. AMPK controls the speed of microtubule polymerization and directional cell migration through CLIP-170 phosphorylation. Nat Cell Biol. 2010;12:583–590. doi: 10.1038/ncb2060. [DOI] [PubMed] [Google Scholar]
- 128.Frigo DE, et al. CaM kin ase kinase beta-mediated activation of the growth regulatory kinase AMPK is required for androgen-dependent migration of prostate cancer cells. Cancer Res. 2011;71:528–537. doi: 10.1158/0008-5472.CAN-10-2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Faubert B, et al. AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Cell Metab. 2013;17:113–124. doi: 10.1016/j.cmet.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.DeRan M, et al. Energy stress regulates hippo-YAP signaling involving AMPK-mediated regulation of angiomotin-like 1 protein. Cell Rep. 2014;9:495–503. doi: 10.1016/j.celrep.2014.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mo JS, et al. Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway. Nat Cell Biol. 2015;17:500–510. doi: 10.1038/ncb3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang W, et al. AMPK modulates Hippo pathway activity to regulate energy homeostasis. Nat Cell Biol. 2015;17:490–499. doi: 10.1038/ncb3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hemminki A, et al. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 1998;391:184–187. doi: 10.1038/34432. [DOI] [PubMed] [Google Scholar]
- 134.Jenne DE, et al. Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat Genet. 1998;18:38–43. doi: 10.1038/ng0198-38. [DOI] [PubMed] [Google Scholar]
- 135.Alessi DR, et al. LKB1-dependent signaling pathways. Annu Rev Biochem. 2006;75:137–163. doi: 10.1146/annurev.biochem.75.103004.142702. [DOI] [PubMed] [Google Scholar]
- 136.Kandoth C, et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502:333–339. doi: 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lawrence MS, et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature. 2014;505:495–501. doi: 10.1038/nature12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tamborero D, et al. Comprehensive identification of mutational cancer driver genes across 12 tumor types. Sci Rep. 2013;3:2650. doi: 10.1038/srep02650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lizcano JM, et al. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 2004;23:833–843. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lo B, et al. Lkb1 regulates organogenesis and early oncogenesis along AMPK-dependent and -independent pathways. J Cell Biol. 2012;199:1117–1130. doi: 10.1083/jcb.201208080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Subbannayya Y, et al. Calcium calmodulin dependent kinase kinase 2 - a novel therapeutic target for gastric adenocarcinoma. Cancer Biol Ther. 2015;16:336–345. doi: 10.4161/15384047.2014.972264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Massie CE, et al. The androgen receptor fuels prostate cancer by regulating central metabolism and biosynthesis. EMBO J. 2011;30:2719–2733. doi: 10.1038/emboj.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Martinez-Reyes I, et al. AMPK and GCN2-ATF4 signal the repression of mitochondria in colon cancer cells. Biochem J. 2012;444:249–259. doi: 10.1042/BJ20111829. [DOI] [PubMed] [Google Scholar]
- 144.Gao J, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cerami E, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Phoenix KN, et al. AMPKalpha2 Suppresses Murine Embryonic Fibroblast Transformation and Tumorigenesis. Genes Cancer. 2012;3:51–62. doi: 10.1177/1947601912452883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kovacic S, et al. Akt activity negatively regulates phosphorylation of AMP-activated protein kinase in the heart. J Biol Chem. 2003;278:39422–39427. doi: 10.1074/jbc.M305371200. [DOI] [PubMed] [Google Scholar]
- 148.Horman S, et al. Insulin antagonizes ischemia-induced Thr172 phosphorylation of AMP-activated protein kinase alpha-subunits in heart via hierarchical phosphorylation of Ser485/491. J Biol Chem. 2006;281:5335–5340. doi: 10.1074/jbc.M506850200. [DOI] [PubMed] [Google Scholar]
- 149.Hawley SA, et al. Phosphorylation by Akt within the ST loop of AMPK-alpha1 down-regulates its activation in tumour cells. Biochem J. 2014;459:275–287. doi: 10.1042/BJ20131344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hardie DG, Pan DA. Regulation of fatty acid synthesis and oxidation by the AMP-activated protein kinase. Biochem Soc Trans. 2002;30:1064–1070. doi: 10.1042/bst0301064. [DOI] [PubMed] [Google Scholar]
- 151.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 152.Pizer ES, et al. Malonyl-coenzyme-A is a potential mediator of cytotoxicity induced by fatty-acid synthase inhibition in human breast cancer cells and xenografts. Cancer Res. 2000;60:213–218. [PubMed] [Google Scholar]
- 153.Kuhajda FP. Fatty acid synthase and cancer: new application of an old pathway. Cancer Res. 2006;66:5977–5980. doi: 10.1158/0008-5472.CAN-05-4673. [DOI] [PubMed] [Google Scholar]
- 154.Commisso C, et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 2013;497:633–637. doi: 10.1038/nature12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sheen JH, et al. Defective regulation of autophagy upon leucine deprivation reveals a targetable liability of human melanoma cells in vitro and in vivo. Cancer Cell. 2011;19:613–628. doi: 10.1016/j.ccr.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Domenech E, et al. AMPK and PFKFB3 mediate glycolysis and survival in response to mitophagy during mitotic arrest. Nat Cell Biol. 2015 doi: 10.1038/ncb3231. [DOI] [PubMed] [Google Scholar]
- 157.Rios M, et al. Lipoprotein internalisation induced by oncogenic AMPK activation is essential to maintain glioblastoma cell growth. Eur J Cancer. 2014;50:3187–3197. doi: 10.1016/j.ejca.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 158.Harhaji-Trajkovic L, et al. AMPK-mediated autophagy inhibits apoptosis in cisplatin-treated tumour cells. J Cell Mol Med. 2009;13:3644–3654. doi: 10.1111/j.1582-4934.2009.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Inoki K, et al. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 160.Gwinn DM, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Choo AY, et al. Glucose addiction of TSC null cells is caused by failed mTORC1-dependent balancing of metabolic demand with supply. Mol Cell. 2010;38:487–499. doi: 10.1016/j.molcel.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ben Sahra I, et al. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res. 2011;71:4366–4372. doi: 10.1158/0008-5472.CAN-10-1769. [DOI] [PubMed] [Google Scholar]
- 163.Wolff NC, et al. Cell-type-dependent regulation of mTORC1 by REDD1 and the tumor suppressors TSC1/TSC2 and LKB1 in response to hypoxia. Mol Cell Biol. 2011;31:1870–1884. doi: 10.1128/MCB.01393-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zheng M, et al. Inactivation of Rheb by PRAK-mediated phosphorylation is essential for energy-depletion-induced suppression of mTORC1. Nat Cell Biol. 2011;13:263–272. doi: 10.1038/ncb2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bonomi S, et al. Oncogenic alternative splicing switches: role in cancer progression and prospects for therapy. Int J Cell Biol. 2013;2013:962038. doi: 10.1155/2013/962038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.David CJ, Manley JL. Alternative pre-mRNA splicing regulation in cancer: pathways and programs unhinged. Genes Dev. 2010;24:2343–2364. doi: 10.1101/gad.1973010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Oltean S, Bates DO. Hallmarks of alternative splicing in cancer. Oncogene. 2014;33:5311–5318. doi: 10.1038/onc.2013.533. [DOI] [PubMed] [Google Scholar]
- 168.Munkley J, et al. A novel androgen-regulated isoform of the TSC2 tumour suppressor gene increases cell proliferation. Oncotarget. 2014;5:131–139. doi: 10.18632/oncotarget.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Silvera D, et al. Translational c ontrol in cancer. Nat Rev Cancer. 2010;10:254–266. doi: 10.1038/nrc2824. [DOI] [PubMed] [Google Scholar]
- 170.Braunstein S, et al. A hypoxia-controlled cap-dependent to cap-independent translation switch in breast cancer. Mol Cell. 2007;28:501–512. doi: 10.1016/j.molcel.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 171.Ron DHH. In: Translational Control in Biology and Medicine. Mathews MB, Hershey NSJWB, editors. Cold Spring Harbor Laboratory Press; 2007. pp. 345–368. [Google Scholar]
- 172.Holcik M, Sonenberg N. Translational control in stress and apoptosis. Nat Rev Mol Cell Biol. 2005;6:318–327. doi: 10.1038/nrm1618. [DOI] [PubMed] [Google Scholar]
- 173.Connolly E, et al. Hypoxia inhibits protein synthesis through a 4E-BP1 and elongation factor 2 kinase pathway controlled by mTOR and uncoupled in breast cancer cells. Mol Cell Biol. 2006;26:3955–3965. doi: 10.1128/MCB.26.10.3955-3965.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Thakor N, Holcik M. IRES-mediated translation of cellular messenger RNA operates in eIF2alpha- independent manner during stress. Nucleic Acids Res. 2012;40:541–552. doi: 10.1093/nar/gkr701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Stoneley M, et al. C-Myc 5' untranslated region contains an internal ribosome entry segment. Oncogene. 1998;16:423–428. doi: 10.1038/sj.onc.1201763. [DOI] [PubMed] [Google Scholar]
- 176.Creancier L, et al. Fibroblast growth factor 2 internal ribosome entry site (IRES) activity ex vivo and in transgenic mice reveals a stringent tissue-specific regulation. J Cell Biol. 2000;150:275–281. doi: 10.1083/jcb.150.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Dobson T, et al. Dysregulating IRES-dependent translation contributes to overexpression of oncogenic Aurora A Kinase. Mol Cancer Res. 2013;11:887–900. doi: 10.1158/1541-7786.MCR-12-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Pantovic A, et al. Coordinated time-dependent modulation of AMPK/Akt/mTOR signaling and autophagy controls osteogenic differentiation of human mesenchymal stem cells. Bone. 2013;52:524–531. doi: 10.1016/j.bone.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 179.Maher EA, et al. Metabolism of [U-13 C]glucose in human brain tumors in vivo. NMR Biomed. 2012;25:1234–1244. doi: 10.1002/nbm.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mullen AR, et al. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature. 2012;481:385–388. doi: 10.1038/nature10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Viale A, et al. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature. 2014;514:628–632. doi: 10.1038/nature13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Weinberg F, et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci U S A. 2010;107:8788–8793. doi: 10.1073/pnas.1003428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Roesch A, et al. Overcoming intrinsic multidrug resistance in melanoma by blocking the mitochondrial respiratory chain of slow-cycling JARID1B(high) cells. Cancer Cell. 2013;23:811–825. doi: 10.1016/j.ccr.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Porporato PE, et al. A mitochondrial switch promotes tumor metastasis. Cell Rep. 2014;8:754–766. doi: 10.1016/j.celrep.2014.06.043. [DOI] [PubMed] [Google Scholar]
- 185.LeBleu VS, et al. PGC-1alpha mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol. 2014;16:992–1003. 1001–1015. doi: 10.1038/ncb3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bergeron R, et al. Chronic activation of AMP kinase results in NRF-1 activation and mitochondrial biogenesis. Am J Physiol Endocrinol Metab. 2001;281:E1340–E1346. doi: 10.1152/ajpendo.2001.281.6.E1340. [DOI] [PubMed] [Google Scholar]
- 187.Huang B, et al. Adiponectin promotes pa ncreatic cancer progression by inhibiting apoptosis via the activation of AMPK/Sirt1/PGC-1alpha signaling. Oncotarget. 2014;5:4732–4745. doi: 10.18632/oncotarget.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hart PC, et al. MnSOD upregulation sustains the Warburg effect via mitochondrial ROS and AMPK-dependent signalling in cancer. Nat Commun. 2015;6:6053. doi: 10.1038/ncomms7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wu SB, Wei YH. AMPK-mediated increase of glycolysis as an adaptive response to oxidative stress in human cells: implication of the cell survival in mitochondrial diseases. Biochim Biophys Acta. 2012;1822:233–247. doi: 10.1016/j.bbadis.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 190.Kim YH, et al. AMPKalpha modulation in cancer progression: multilayer integrative analysis of the whole transcriptome in Asian gastric cancer. Cancer Res. 2012;72:2512–2521. doi: 10.1158/0008-5472.CAN-11-3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.O'Reilly KE, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Tremblay F, et al. Identification of IRS-1 Ser-1101 as a target of S6K1 in nutrient- and obesity-induced insulin resistance. Proc Natl Acad Sci U S A. 2007;104:14056–14061. doi: 10.1073/pnas.0706517104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sujobert P, et al. Co-activation of AMPK and mTORC1 Induces Cytotoxicity in Acute Myeloid Leukemia. Cell Rep. 2015;11:1446–1457. doi: 10.1016/j.celrep.2015.04.063. [DOI] [PubMed] [Google Scholar]