Abstract
Light-gated rhodopsin cation channels from chlorophyte algae have transformed neuroscience research through their use as membrane-depolarizing optogenetic tools for targeted photoactivation of neuron firing. Photosuppression of neuronal action potentials has been limited by the lack of equally efficient tools for membrane hyperpolarization. We describe anion channel rhodopsins (ACRs), a family of light-gated anion channels from cryptophyte algae that provide highly sensitive and efficient membrane hyperpolarization and neuronal silencing through light-gated chloride conduction. ACRs strictly conducted anions, completely excluding protons and larger cations, and hyperpolarized the membrane of cultured animal cells with much faster kinetics at less than one-thousandth of the light intensity required by the most efficient currently available optogenetic proteins. Natural ACRs provide optogenetic inhibition tools with unprecedented light sensitivity and temporal precision.
Microbial rhodopsins are functionally diverse (1, 2). Several are used as molecular tools for optogenetics to regulate cellular activity with light (3–5). Membrane-depolarizing phototaxis receptors from green (chlorophyte) flagellate algae (6), best known as channelrhodopsins (ChRs) function as millisecond–time scale light-gated cation channels (7, 8) and are widely used to depolarize genetically targeted populations of excitable cells. Hyperpolarizing rhodopsin ion pumps have been used to suppress neuron firing (9–13), but they transport only a single charge per captured photon and therefore have limited capacity. Recently, ChRs were engineered to conduct Cl−, but these optogenetic tools still retain some cation conductance and could be made highly light-sensitive only at the expense of slowing the channel kinetics with additional mutations (14, 15). Ideal for optogenetic hyperpolarization would be natural light-gated anion channels optimized by evolution to be strictly anion-selective and highly conductive with rapid kinetics.
Of the ∼50 known ChRs from chlorophytes, all that have been tested are exclusively cation channels (7, 8, 16–18). Photoreceptor currents similar to those mediated by ChRs in chlorophytes also occur in the phylogenetically distant cryptophyte algae (19). However, several rhodopsin proteins from genes cloned from these organisms did not exhibit channel activity (19, 20). The nuclear genome of the cryptophyte Guillardia theta has been completely sequenced (21). A BLAST search of model proteins identified 53 with sequence similarity to that of microbial (type I) rhodopsins. None showed high similarity to ChRs, but the models of one particular cluster (Fig. 1A and fig. S1) did contain some key residues characteristic of chlorophyte ChRs (Fig. 1B and fig. S2).
Fig. 1. Phylogeny and photoactivity of G. theta ACRs.
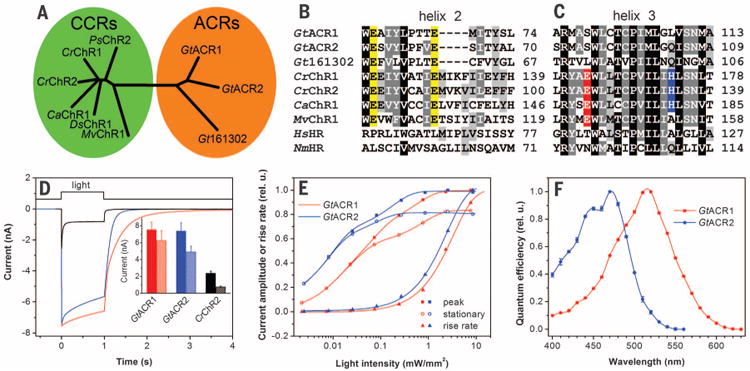
(A) Phylogenetic tree of CCRs and ACRs. (B and C) ClustalW alignments of transmembrane helices 2 (B) and 3 (C) Abbreviated organism names are: Gt Guillardia theta; Cr Chlamydomonas reinhardtii; Ca Chlamydomonas augustae; Mv Mesostigma viride; Hs, Halobacterium salinarum; Nm, Nonlabens marinus. The last residue numbers are shown on the right. Conserved Glu residues in helix 2 are highlighted in yellow, Glu residues in the position of bacteriorhodopsin Asp85 in red, and His residues corresponding to His134 of CrChR2 in blue. (D) Photocurrents of GtACR1, GtACR2, and CrChR2 in HEK293 cells in response to a saturating light pulse at −60 mV. (Inset) Mean amplitudes of peak (solid bars) and stationary (hatched bars) currents (n = 18 to 20 cells). (E) Dependence of the peak and stationary current amplitudes and rise rates on stimulus intensity. (F) Action spectra of photocurrents.
Gene fragments encoding seven transmembrane domains of G. theta proteins 111593,146828, and 161302 were well expressed in transfected human kidney embryonic (HEK293) cells. The first two constructs generated photocurrents, whereas the third did not. The first two functioned as light-gated anion channels; therefore we named them GtACR1 and GtACR2 (Guillardia theta anion channel rhodopsins 1 and 2).
With our standard solutions for electrophysiological recording (126 mM KCl in the pipette and 150 mM NaCl in the bath, pH 7.4; for other components see table S1), the currents generated by GtACR1 and GtACR2 were inward at the holding potential (Eh) of −60 mV (Fig. 1D). The mean plateau currents from GtACR1 and GtACR2 were, respectively, eight and six times larger than those from CrChR2 (Cr, Chlamydomonas reinhardtii), the most frequently used optogenetic tool, with a lesser degree of inactivation (Fig. 1, inset). The dependence of the current rise rate on the stimulus intensity exhibited a higher saturation level than the current amplitude (Fig. 1E) and therefore was used for construction of the action spectra. GtACR1 showed maximal sensitivity to 515-nm light, with a shoulder on the short-wavelength slope of the spectrum (Fig. 1F). The sensitivity of GtACR2 peaked at 470 nm, with additional bands at 445 and 415 nm (Fig. 1F).
The sign of GtACR1 and GtACR2 photocurrents reversed when the membrane potential was shifted to more positive values (Fig. 2, A and B, respectively). In the tested range from −60 to 60 mV, the current-voltage relationships (IE curves) were linear (Fig. 2C), unlike those for chlorophyte ChRs (22). To characterize the ion permeability of G. theta rhodopsins, we measured IE curves and determined the reversal potential (Erev) upon variation of the ionic composition of the bath solution. In contrast to chlorophyte ChRs, for which protons are the most highly permeable ions, Erev of the currents generated by GtACR1 and GtACR2 were not affected by pH (Fig. 2C). Moreover, no Erev shifts were observed when the large nonpermeable organic cation N-methyl-glucamine (NMG+) was replaced with Na+, K+, or Ca2+ (Fig. 2D). We conclude that GtACR1 and GtACR2 are not permeable by cations conducted by chlorophyte ChRs.
Fig. 2. ACRs do not conduct cations.
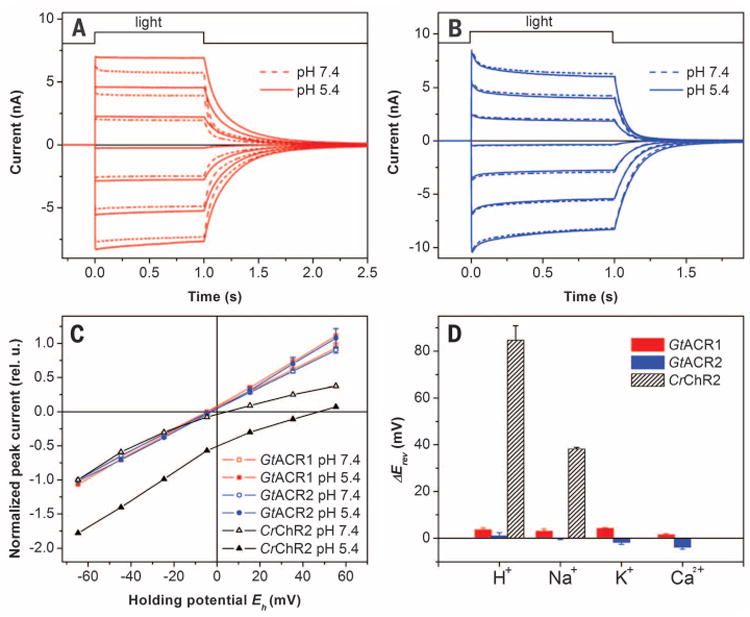
Photocurrents generated by GtACR1 (A) and GtACR2 (B) in HEK293 cells at the membrane potentials changed in 20-mV steps from −60 mV at the amplifier output (bottom to top). The pipette solution was standard, and the bath solution was as indicated. (C) IE relationships measured at various pH of the bath. The data (mean values ± SEM, n = 4 to 6 cells) were corrected for liquid junction potentials (table S1) and normalized to the value measured at −60 mV at pH 7.4. Representative data for CrChR2 are shown for comparison. (D) Erev shifts measured upon variation of the cation composition of the bath. The data are mean values ± SEM (n = 3 to 6 cells).
When most of the CI− in the bath was replaced with the large anion aspartate, yielding a Nernst equilibrium potential for CI− (ECl) of 81 mV, Erev shifted to 75 ± 2.4 and 80 ± 1.4 mV (mean ± SEM, n = 4 to 5 cells) for GtACR1 and GtACR2, respectively (Fig. 3C), as would be expected only if the currents were exclusively due to passive Cl− transport. We compared the permeability of various anions by substituting them for nonpermeable Asp− in the bath. For both G. theta ACRs, I−, NO3−, or Br− caused even greater Erev shifts than Cl−. F− caused a smaller shift, whereas SO42− was nonpermeable. The permeability sequence NO3− > I− > Br− > Cl− > F− > SO42− = Asp− determined for ACRs is in accord with the lyotropic series characteristic of many Cl− channels from animal cells (23).
Fig. 3. Anion selectivity of ACRs.
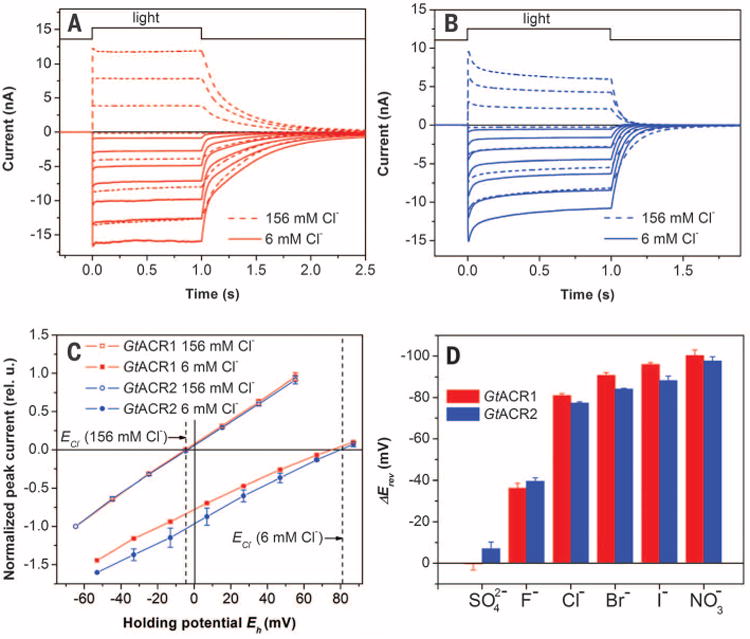
Photocurrents generated by GtACR1 (A) and GtACR2 (B) in HEK293 cells at the membrane potentials changed in 20-mV steps from −60 mVat the amplifier output (bottom to top). The pipette solution was standard, and the bath solution was as indicated. (C) IE relationships measured at various Cl− concentrations in the bath. The data (mean values ± SEM, n = 4 to 6 cells) were corrected for liquid junction potentials (table S1) and normalized to the value measured at −60 mV at 156 mM CI−. The dashed vertical lines show the Nernst equilibrium potential for Cl− at the bath concentrations used. (D) Erev shifts measured upon variation of the anion composition of the bath. The data are mean values ± SEM (n = 3 to 6 cells).
The cytoplasmic Cl− concentration in most animal cells, including neurons, is low (24). Under such conditions (5 mM Cl− in the pipette and 156 mM in the bath), G. theta ACRs generated hyperpolarizing currents in HEK293 cells at Eh above the Nernst equilibrium potential for Cl− (ECl) (fig. S3). The amplitude of GtACR2 photo-currents was similar, but the kinetics was faster than that of GtACR1 currents, which is advantageous for control of neuronal activity. Hyper-polarizing photocurrents generated by GtACR2 at the less-than-1000th lower light intensity were equal to the maximal currents generated by the proton pump archaerhodopsin-3 (Arch), a popular tool for optogenetic spike suppression (12), and by the recently reported slow ChloC mutant (14) (Fig. 4A, black arrow). The stimulus-response curve for the mutant was less steep than for GtACR2 because of the slower current kinetics of the latter (Fig. 4E). However, even at nonsaturating light intensities, GtACR2 remained more than 100 times more photosensitive than slow ChloC (Fig. 4A, red arrow). The larger amplitude of GtACR2 photocurrents was not due to its higher expression level, as assessed by measuring relative tag fluorescence (fig. S4). Higher unitary conductance of ACRs is shown by stationary noise analysis of macroscopic current fluctuations, which gave values for GtACR1 and GtACR2 more than 10 times higher than those for CrChR2 (fig. S5) (25).
Fig. 4. GtACR2 as a hyperpolarizing tool.
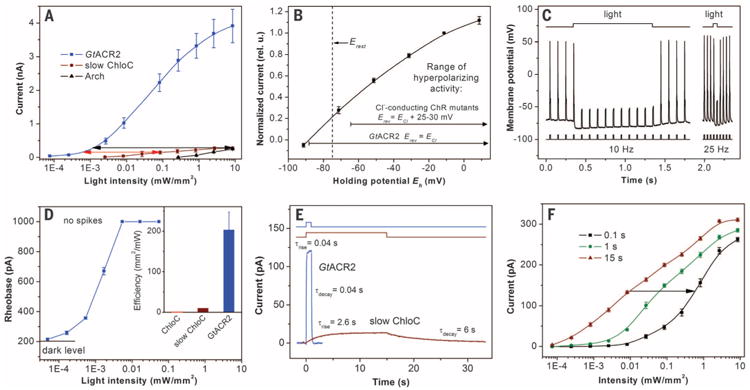
(A) Light-intensity dependence of photocurrents generated by GtACR2, slow ChloC, and Arch in HEK293 cells at 20 mV. The arrows show the difference in light sensitivity. (B) IE relationship for GtACR2 in neurons. The data (mean values ± SEM, n = 5 cells) were corrected for LJP (table S2). The dashed vertical line shows the resting potential (Erest). The ranges of activity for Cl−-conducting ChR mutants are from (14, 15). (C) Photoinhibition of spiking induced by pulsed current injection in a typical neuron expressing GtACR2. The light intensity was 0.026 mW/mm2. (D) The dependence of the rheobase of current ramp-evoked spikes on the light intensity in a typical neuron expressing GtACR2. The data are mean values ± SEM (n = 5 repetitions). Light was applied 0.1 s before the beginning of the current ramp. (Inset) Comparative efficiency of GtACR2 and the ChloC mutants represented as a reciprocal of the minimal light intensity sufficient to fully suppress spiking. The data for GtACR2 are the mean value ± SEM (n = 7 neurons). Data for the ChloC mutants under continuous illumination are from (14). (E) Kinetics of the photocurrents generated by GtACR2 in response to a 1-s light pulse and by slow ChloC in response to a 15-s light pulse (light intensity for both traces was 0.002 mW/mm2). The time constants (τ) were determined by single exponential fits of the recorded traces. The fitted curves are shown as thick lines of the same color as the data. (F) The light-intensity dependence of slow ChloC current amplitude measured at different times after the start of illumination. Data are mean values ± SEM (n = 5 cells). The arrow shows the increase in the light intensity necessary to reach the same current amplitude at 0.1 as at 15 s illumination.
In cultured rat pyramidal neurons, GtACR2 generated hyperpolarizing currents at Eh above -88 mV (Fig. 4B and fig. S6A). This value corresponds exactly to ECl under our conditions (table S2). This strict selectivity is a second advantage of ACRs over the previously reported Cl−-conducting ChR mutants, for which Erev is 25 to 30 mV more positive than ECl, due to residual cation permeability (14, 15). The range of potentials at which GtACR2 hyperpolarizes the membrane is therefore wider and extends through the values typical for resting potentials of neurons (Fig. 4B). In current clamp experiments, GtACR2 allowed precisely controlled optical silencing of spikes at frequencies up to at least 25 Hz (Fig. 4C and fig. S6B).
To compare the efficiency of GtACR2 with that of slow ChloC in neurons, we measured the rheobase of current ramp-evoked spikes at different light intensities using the same solutions and current injection protocol as in (14). In GtACR2-expressing neurons, full suppression of spiking was observed at 0.005 mW/mm2 (Fig. 4D). The fast ChloC mutant, comparable in its kinetics to GtACR2, could not fully suppress spiking even at 10 mW/mm2 of light, whereas a relatively higher efficiency of slow ChloC with full suppression at ∼0.1 mW/mm2 (the reciprocal of this value is plotted in the Fig. 4D, inset) was achieved only at the expense of a dramatically slower kinetics and the necessity to illuminate for at least 12 s (14) (Fig. 4E). As GtACR2-driven current reached its maximum within 0.1 s (Fig. 4E), its intensity dependence at any length of the light pulse above 0.1 s was identical to that shown in Fig. 4A In contrast, the rise of current generated by slow ChloC was 65 times slower (Fig. 4E). Taking into account the intensity dependence of the current amplitude measured with light stimuli of different duration (Fig. 4F), full suppression of spiking by slow ChloC with 0.1-s stimuli would occur at 7 mW/mm2, whereas by GtACR2 it would be reached at an intensity about three orders of magnitude lower.
The membrane potential, membrane resistance, and rheobase in the dark were not affected by GtACR2 expression in neurons, and the neuronal morphology of GtACR2-expressing neurons was also normal (fig. S7).
Phylogenetically and functionally, ACRs constitute a distinct family of rhodopsins that are fundamentally different from cation channel-rhodopsins (CCRs). As natural anion channels, ACRs provide hyperpolarizing optogenetic tools optimized by evolution for extremely high light sensitivity, absolute anion selectivity, and rapid kinetics.
Supplementary Material
Fig. S1: Phylogenetic tree of G. theta protein models. The models homologous to microbial rhodopsins were selected among those predicted by the Joint Genome Institute (JGI) sequencing project (http://genome.jgi.doe.gov/Guith1/Guith1.home.html) and aligned using ClustalW. The tree was constructed using the neighbor-joining method. GtR1, GtR2 and GtR3 are proteins identified previously. Eight models lack the conserved Lys residue in the seventh transmembrane helix that covalently links to retinal in known rhodopsins, and should therefore be considered opsin-related proteins. The models that show the highest homology to chlorophyte ChRs are highlighted. Out of five members in this cluster, the models 111593 and 141269 differ only in a 7 residue-stretch in the middle of the sequence, and the models 161302 and 150677 differ only in the length of their C-termini. Therefore, we limited our analysis to the models 111593, 146828 and 161302. In addition to 7TM domains, these G. theta proteins contain long (∼150 amino acid residues) C-terminal domains, as is also typical of chlorophyte ChRs.
Fig. S2: ClustalW alignments of transmembrane helices 1 and 7. Abbreviated organism names are: Gt, Guillardia theta; Cr, Chlamydomonas reinhardtii; Ca, Chlamydomonas augustae; Mv, Mesostigma viride. The last residue numbers are shown on the right. The residues corresponding to Ser-102 (63) (CrChR1/CrChR2 numbering) in helix 1 and Asn-297 (258) in helix 7 that together with Glu-129 (90) form the central gate in the crystallized CrChR1/CrChR2 chimera (27) are conserved in the two functional G. theta ACRs (highlighted cyan). However, the residues that form the inner gate in the chimera, Tyr-109 (70), His-173 (134) and His-304 (265), are not conserved in the two functional ACRs (the Tyr and one of the His residues are highlighted blue above and the second His residue highlighted blue in Fig. 1C in the main text). A conspicuous feature of ACRs is a non-carboxylic amino acid residue in the position of the proton acceptor Asp85 in bacteriorhodopsin, where nearly all cation-selective ChRs contain a Glu residue (highlighted red in Fig. 1C in the main text). A non-ionizable residue at the corresponding position is also typical of chloride-pumping rhodopsins from haloarchaea and marine flavobacteria, where the residue forms part of the chloride binding site in the unphotolyzed state as shown for haloarchaeal halorhodopsin (28). The mechanism of anion conduction in ACRs appears to be different from that of the Cl--conducting ChR mutants, as might be expected from their large difference in sequence. ACRs contain a Glu residue corresponding to Glu90 of the cation selectivity filter of CrChR2 (Fig. 1B in the main text) (22, 29). To confer Cl- permeability to this cation-conducting channel, Glu90 required replacement with an uncharged (Ser (15)) or cationic (Lys or Arg (14)) residue. However, the presence of the Glu90 homolog in ACRs shows it is not a barrier to anion permeation in the anion channels unlike in the cation channels.
Fig. S3: Photocurrents generated by GtACR1 (A) and GtACR2 (B) in HEK293 cells with low Cl- concentration in the pipette and high Cl- concentration in the bath. The membrane potentials were changed in 20-mV steps from -80 mV at the amplifier output (bottom to top).
Fig. S4: Maximal photocurrents (black bars, left axis) and relative EYFP tag fluorescence (green bars, right axis) of hyperpolarizing optogenetic tools. The data are mean values ± SEM (n = 10-20 cells) with fluorescence normalized to the values obtained for GtACR2.
Fig. S5: Stationary analysis of current noise generated by ACRs and slow ChloC. (A and B) Representative noise traces recorded in the dark and under illumination at the light intensity eliciting half-maximal currents from HEK293 cells transfected with GtACR1 (A) or slow ChloC (B). (C and D) Representative power spectra of the current noise recorded in the dark and under illumination for GtACR1 (C) and slow ChloC (D), as shown in A and B, respectively. The spectra were smoothed by adjacent averaging for presentation purposes. For slow ChloC, no difference was observed between the dark and light spectra, so its unitary conductance could not be determined. (E) The light minus dark difference spectrum for GtACR1 prior to smoothing (black line) and its computer approximation with a Lorentzian function (red line). Analysis of GtACR2-generated noise was carried out in a similar manner and is not shown. (F) The unitary conductance of ACRs calculated from the parameters of the Lorentzian fits in this study (mean values ± SEM; n = 8-11) and of wild-type CrChR2 assayed under the same conditions from (17).
Fig. S6: GtACR2 function in cultured pyramidal neurons. (A) Photocurrents generated by GtACR2 in cultured pyramidal neurons at low Cl-concentration in the pipette and high Cl- concentration in the bath. The membrane potentials were changed in 20-mV steps from -80 mV at the amplifier output (bottom to top). (B) Representative traces of spiking of a GtACR2-transfected neuron in response to a prolonged injection of a depolarizing current in the dark (left) and its silencing by a shorter illumination pulse (right). The injected current was 300 pA, the light intensity was 0.026 mW/mm2.
Fig. S7: Expression of GtACR2 does not affect morphology and electrical parameters of cultured neurons. (A) Representative images of GtACR2-expressing neurons (top row) demonstrate normal morphology when compared with control non-transfected neurons (bottom row). The neurons were infected with lentivirus one day after plating, fixed after 8 days in culture (7 days after infection) and labeled with antibodies against GFP (to label tagged GtACR2, green) and SV2 (a synaptic marker, red). Scale bar 20 μm. (B) The number of primary dendrites per neuron at a 50 μm radius around the center of the soma. (C) The number of synapses per neuron in a 50 μm circle around the center of the soma (D) The resting potential of neurons. (E) The membrane resistance of neurons. (F) Rheobase of ramp-evoked (1000 pA, 1 s) spiking in the dark. All data in panels (B-F) are mean values ± SEM (n = 5-7 cells). No statistically significant difference was detected between transfected and control non-transfected neurons (unpaired t test, two tailed).
Table S1: Composition of pipette and bath solutions and liquid junction potentials in experiments with HEK293 cells.
Table S2: Composition of pipette and bath solutions and liquid junction potentials in experiments with neurons.
Acknowledgments
E.G.G., O.A.S., J.L.S., and The University of Texas Health Science Center at Houston have filed a provisional patent application that relates to ACRs. We thank E. S. Boyden [Massachusetts Institute of Technology (MIT), Boston] for the archaeorhodopsin-3 expression construct and C. Lois (MIT) for the pCMV-VSVG and pΔ8.9 plasmids. This work was supported by NIH grants R01GM027750, R21MH098288, and S10RR022531, and a UTHealth BRAIN Initiative grant, the Hermann Eye Fund, and Endowed Chair AU-0009 from the Robert A. Welch Foundation.
Footnotes
References and Notes
- 1.Spudich JL, Sineshchekov OA, Govorunova EG. Biochim Biophys Acta. 2014;1837:546–552. doi: 10.1016/j.bbabio.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ernst OP, et al. Chem Rev. 2014;114:126–163. doi: 10.1021/cr4003769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deisseroth K. Nat Methods. 2011;8:26–29. doi: 10.1038/nmeth.f.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chow BY, Boyden ES. Sci Transl Med. 2013;5:177ps5. doi: 10.1126/scitranslmed.3003101. [DOI] [PubMed] [Google Scholar]
- 5.Lin JY, Knutsen PM, Muller A, Kleinfeld D, Tsien RY. Nat Neurosci. 2013;16:1499–1508. doi: 10.1038/nn.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sineshchekov OA, Jung KH, Spudich JL. Proc Natl Acad Sci USA. 2002;99:8689–8694. doi: 10.1073/pnas.122243399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagel G, et al. Science. 2002;296:2395–2398. doi: 10.1126/science.1072068. [DOI] [PubMed] [Google Scholar]
- 8.Nagel G, et al. Proc Natl Acad Sci USA. 2003;100:13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang F, et al. Nature. 2007;446:633–639. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
- 10.Han X, Boyden ES. PLOS ONE. 2007;2:e299. doi: 10.1371/journal.pone.0000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gradinaru V, Thompson KR, Deisseroth K. Brain Cell Biol. 2008;36:129–139. doi: 10.1007/s11068-008-9027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chow BY, et al. Nature. 2010;463:98–102. doi: 10.1038/nature08652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chuong AS, et al. Nat Neurosci. 2014;17:1123–1129. doi: 10.1038/nn.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wietek J, et al. Science. 2014;344:409–412. doi: 10.1126/science.1249375. [DOI] [PubMed] [Google Scholar]
- 15.Berndt A, Lee SY, Ramakrishnan C, Deisseroth K. Science. 2014;344:420–424. doi: 10.1126/science.1252367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang F, et al. Cell. 2011;147:1446–1457. doi: 10.1016/j.cell.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Govorunova EG, Sineshchekov OA, Li H, Janz R, Spudich JL. J Biol Chem. 2013;288:29911–29922. doi: 10.1074/jbc.M113.505495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klapoetke NC, et al. Nat Methods. 2014;11:338–346. doi: 10.1038/nmeth.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sineshchekov OA, et al. Biophys J. 2005;89:4310–4319. doi: 10.1529/biophysj.105.070920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gradinaru V, et al. Cell. 2010;141:154–165. doi: 10.1016/j.cell.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curtis BA, et al. Nature. 2012;492:59–65. doi: 10.1038/nature11681. [DOI] [PubMed] [Google Scholar]
- 22.Gradmann D, Berndt A, Schneider F, Hegemann P. Biophys J. 2011;101:1057–1068. doi: 10.1016/j.bpj.2011.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jentsch TJ, Stein V, Weinreich F, Zdebik AA. Physiol Rev. 2002;82:503–568. doi: 10.1152/physrev.00029.2001. [DOI] [PubMed] [Google Scholar]
- 24.Bregestovski P, Waseem T, Mukhtarov M. Front Mol Neurosci. 2009;2:15. doi: 10.3389/neuro.02.015.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feldbauer K, et al. Proc Natl Acad Sci USA. 2009;106:12317–12322. doi: 10.1073/pnas.0905852106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1: Phylogenetic tree of G. theta protein models. The models homologous to microbial rhodopsins were selected among those predicted by the Joint Genome Institute (JGI) sequencing project (http://genome.jgi.doe.gov/Guith1/Guith1.home.html) and aligned using ClustalW. The tree was constructed using the neighbor-joining method. GtR1, GtR2 and GtR3 are proteins identified previously. Eight models lack the conserved Lys residue in the seventh transmembrane helix that covalently links to retinal in known rhodopsins, and should therefore be considered opsin-related proteins. The models that show the highest homology to chlorophyte ChRs are highlighted. Out of five members in this cluster, the models 111593 and 141269 differ only in a 7 residue-stretch in the middle of the sequence, and the models 161302 and 150677 differ only in the length of their C-termini. Therefore, we limited our analysis to the models 111593, 146828 and 161302. In addition to 7TM domains, these G. theta proteins contain long (∼150 amino acid residues) C-terminal domains, as is also typical of chlorophyte ChRs.
Fig. S2: ClustalW alignments of transmembrane helices 1 and 7. Abbreviated organism names are: Gt, Guillardia theta; Cr, Chlamydomonas reinhardtii; Ca, Chlamydomonas augustae; Mv, Mesostigma viride. The last residue numbers are shown on the right. The residues corresponding to Ser-102 (63) (CrChR1/CrChR2 numbering) in helix 1 and Asn-297 (258) in helix 7 that together with Glu-129 (90) form the central gate in the crystallized CrChR1/CrChR2 chimera (27) are conserved in the two functional G. theta ACRs (highlighted cyan). However, the residues that form the inner gate in the chimera, Tyr-109 (70), His-173 (134) and His-304 (265), are not conserved in the two functional ACRs (the Tyr and one of the His residues are highlighted blue above and the second His residue highlighted blue in Fig. 1C in the main text). A conspicuous feature of ACRs is a non-carboxylic amino acid residue in the position of the proton acceptor Asp85 in bacteriorhodopsin, where nearly all cation-selective ChRs contain a Glu residue (highlighted red in Fig. 1C in the main text). A non-ionizable residue at the corresponding position is also typical of chloride-pumping rhodopsins from haloarchaea and marine flavobacteria, where the residue forms part of the chloride binding site in the unphotolyzed state as shown for haloarchaeal halorhodopsin (28). The mechanism of anion conduction in ACRs appears to be different from that of the Cl--conducting ChR mutants, as might be expected from their large difference in sequence. ACRs contain a Glu residue corresponding to Glu90 of the cation selectivity filter of CrChR2 (Fig. 1B in the main text) (22, 29). To confer Cl- permeability to this cation-conducting channel, Glu90 required replacement with an uncharged (Ser (15)) or cationic (Lys or Arg (14)) residue. However, the presence of the Glu90 homolog in ACRs shows it is not a barrier to anion permeation in the anion channels unlike in the cation channels.
Fig. S3: Photocurrents generated by GtACR1 (A) and GtACR2 (B) in HEK293 cells with low Cl- concentration in the pipette and high Cl- concentration in the bath. The membrane potentials were changed in 20-mV steps from -80 mV at the amplifier output (bottom to top).
Fig. S4: Maximal photocurrents (black bars, left axis) and relative EYFP tag fluorescence (green bars, right axis) of hyperpolarizing optogenetic tools. The data are mean values ± SEM (n = 10-20 cells) with fluorescence normalized to the values obtained for GtACR2.
Fig. S5: Stationary analysis of current noise generated by ACRs and slow ChloC. (A and B) Representative noise traces recorded in the dark and under illumination at the light intensity eliciting half-maximal currents from HEK293 cells transfected with GtACR1 (A) or slow ChloC (B). (C and D) Representative power spectra of the current noise recorded in the dark and under illumination for GtACR1 (C) and slow ChloC (D), as shown in A and B, respectively. The spectra were smoothed by adjacent averaging for presentation purposes. For slow ChloC, no difference was observed between the dark and light spectra, so its unitary conductance could not be determined. (E) The light minus dark difference spectrum for GtACR1 prior to smoothing (black line) and its computer approximation with a Lorentzian function (red line). Analysis of GtACR2-generated noise was carried out in a similar manner and is not shown. (F) The unitary conductance of ACRs calculated from the parameters of the Lorentzian fits in this study (mean values ± SEM; n = 8-11) and of wild-type CrChR2 assayed under the same conditions from (17).
Fig. S6: GtACR2 function in cultured pyramidal neurons. (A) Photocurrents generated by GtACR2 in cultured pyramidal neurons at low Cl-concentration in the pipette and high Cl- concentration in the bath. The membrane potentials were changed in 20-mV steps from -80 mV at the amplifier output (bottom to top). (B) Representative traces of spiking of a GtACR2-transfected neuron in response to a prolonged injection of a depolarizing current in the dark (left) and its silencing by a shorter illumination pulse (right). The injected current was 300 pA, the light intensity was 0.026 mW/mm2.
Fig. S7: Expression of GtACR2 does not affect morphology and electrical parameters of cultured neurons. (A) Representative images of GtACR2-expressing neurons (top row) demonstrate normal morphology when compared with control non-transfected neurons (bottom row). The neurons were infected with lentivirus one day after plating, fixed after 8 days in culture (7 days after infection) and labeled with antibodies against GFP (to label tagged GtACR2, green) and SV2 (a synaptic marker, red). Scale bar 20 μm. (B) The number of primary dendrites per neuron at a 50 μm radius around the center of the soma. (C) The number of synapses per neuron in a 50 μm circle around the center of the soma (D) The resting potential of neurons. (E) The membrane resistance of neurons. (F) Rheobase of ramp-evoked (1000 pA, 1 s) spiking in the dark. All data in panels (B-F) are mean values ± SEM (n = 5-7 cells). No statistically significant difference was detected between transfected and control non-transfected neurons (unpaired t test, two tailed).
Table S1: Composition of pipette and bath solutions and liquid junction potentials in experiments with HEK293 cells.
Table S2: Composition of pipette and bath solutions and liquid junction potentials in experiments with neurons.


