Abstract
The protein-protein interaction of p53 and MDM2/X is a promising non genotoxic anticancer target. A rapid and efficient methodology was developed to synthesize the 2,3'-bis(1'H-indole) heterocyclic scaffold 2 as ester, acid and amide derivatives. Their binding affinity with MDM2 was evaluated using both fluorescence polarization (FP) assay and HSQC experiments, indicating good inhibition and a perfect starting point for further optimizations.
Keywords: p53, MDM2, MDMX, Protein-protein interaction, Cancer
Graphical abstract
The transcription factor and tumor suppressor p53 has multiple roles in stabilizing the genome by preventing mutations.1,2 However p53, our genome guardian, is the most mutated protein in human cancer with >50% of cancers not showing functional p53.3 The remainder group of cancers also exhibit reduced p53 pathway activity due to the negative regulation through the protein-protein interaction (PPI) with inhibitors as MDM2/X or viral proteins. MDM2 is a key regulator of p53 activity via a complex regulatory feedback system that involves all levels of expression control including transcription, mRNA translation and protein degradation. MDM2 inhibits the N-terminal trans-activation domain (TAD) of p53, and promotes p53 degradation through the ubiquitin-proteasome system (E3 ligase activity).4a,b Similarly to MDM2, the negative regulation of p53 of MDMX (90% homology with MDM2) is displayed via the inhibition of the TAD domain and the formation of heterodimers with MDM2 which can increase the rates of ubiquitinylation of p53 by MDM2.4c,d Therefore, blocking the interaction between wild-type p53 and its negative regulators MDM2 and MDMX has become an important target in oncology to restore the anti-tumor activity of p53.5–7
The discovery of novel p53-MDM2/X inhibitors was one of the highlights in anti-tumor agents.8 Since the disclosure of Nutlin-3,9 many scaffolds have been prepared and evaluated, including indo-imidazole, imidazoline, benzodiazepinedione and spirooxindole scaffolds.10 Thus, antagonizing the p53-MDM2 or/and MDMX interaction is a promising anticancer strategy and several compounds are presently undergoing early clinical evaluations.11–14 However, the discovery of new p53/MDM2/MDMX scaffolds is still of high interest due to low single agent activity currently seen in clinical trials and insufficient PKPD properties.
A three finger pharmacophore model is now widely accepted to be responsible for the binding of small molecules to the MDM2 and recently an extended four finger model was experimentally shown by co-crystallization.15,16 The corresponding amino acid residues in p53 are tryptophan (Trp23), leucine (Leu26) and phenylalanine (Phe19). Initially, indole fragment was taken as starting point to mimic tryptophan residue, where an important hydrogen bond was observed. Studies by Garcia-Echeverria et al. on a p53-derived linear octapeptide showed that a Trp23 to (6-Cl) Trp substitution gave rise to a 63-fold increase in affinity for MDM2.17 The starting point for our antagonist discoveries was the anchoring side chain of tryptophan embedded in a deep hydrophobic pocket formed by the residues Leu57, Phe86 and Ile99 using our pharmacophore based virtual screening platform ANCHOR.QUERY.7,15,18,25 This had led to several scaffolds potently antagonizing p53-MDM2. Amongst them we could also solve representative co-crystal structures of the imidazoloindole derivative 1 binding to MDM2 (PDB ID: 3LBK) and the close relative MDMX (PDB ID: 3LBJ) confirming the initial binding hypothesis.18 Based on these findings, a new class of 1,2,3-trisubstituted bis(indoles) heterocycles (Figure 1) was designed. In this paper, we report the novel synthesis and preliminary biophysical evaluation of this first of its kind antagonists bisindoles 2. It should be noted that the class of p53-MDM2 antagonistic imidazoloindole has also discovered independently by another group.18a
Figure 1.
Protein-protein interaction of p53 peptide (orange α-helix with sticks) with MDM2 (grey surface) (PDB ID 1YCR). Key amino acids Leu22 (marine sticks), Phe19 (green sticks), Trp23 (blue sticks) and Leu26 (red sticks) are mounted onto the orange p53 α-helix. Leu26 is embedded into the hydrophobic receptor amino acids Ile99, Ile103, Leu54 and Val93. The Trp23 pocket is formed by Leu57, Phe86 and Ile99. The Phe19 pocket consists of Val93, Gln72, Val75, Tyr67, Met62, Il261 and Gly58. Val93 undergoes hydrophobic interactions with p53Leu22.
Our hypothesis is that the additional phenyl annulated ring in scaffold 2 will make additional hydrophobic interactions with the mdm2Val93,10 mimicking p53Leu22 (Figure 2). To test this speculation we developed a short synthesis towards scaffold 2.
Figure 2.
Conversion of central imidazole scaffold 1 into indole scaffold 2 to address p53Leu22.
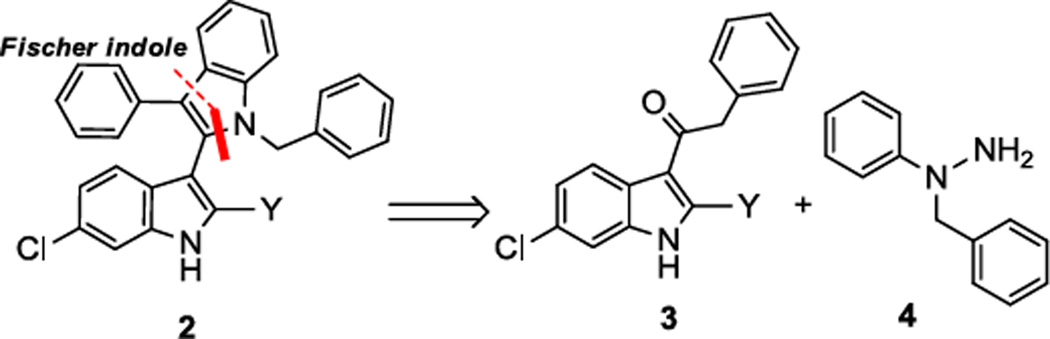
Our retrosynthetic plan of the designed derivatives 2 is based on the indole acetyl derivative 3 and hydrazine 4 which can react in a Fischer indole synthesis (Scheme 1).
Scheme 1.
Retrosynthesis of scaffold 2
After some optimization, derivative 3a was easily synthesized by selective acylation at the 3-position of the 6-chloroindole derivative 5a in high yields in the presence of Lewis acids, such as SnCl4 (Scheme 2).19–21 Compound 4a was derived by the direct alkylation of the secondary amine of the phenylhydrazine 7a, with no need of protecting groups.22,23 Finally, refluxing derivatives 3a and 4a in acetic acid, gave the targeted bisindole 2a in good to very good yields in a just 3-step sequence.
Scheme 2.
3-step sequence for preparing 2,3'-bis(1'H-indole) heterocycles.
The synthesis of bisindoles 2, offers many possibilities for SARs since both of the main components (3 and 4) could easily be modified (Figure 3). Different halogens as R1, R2 and R3 and different sizes in the linkers of the two aromatic groups were tested. Moreover, the importance of carboxylate moiety was taken into account, as found in previous MDM2 binders.15,16,18 Using the ethyl esters, the corresponding acids and various amides could be synthesized in order to optimize some physicochemical properties (Figure 3).
Figure 3.
SAR potential of bisindoles.
The 3-alkylation of the 6-chloroindole derivatives proceeded smoothly affording the corresponding compounds in excellent yields as shown in table 1.21 The crystal structure obtained of the indole derivative 3c, is described in supporting information (CCDC 981828).
Table 1.
3-Acylation of 6-chloroindole derivatives
The hydrazine derivatives 4a–c were synthesized by the regioselective alkylation of the secondary amine, thus different hydrazines and benzyl halides were utilized in order to initially explore the aforementioned interactions in the MDM2 pocket (Table 2).
Table 2.
Alkylation of the secondary amine of hydrazines 7.
Next, the synthesis of the desired bisindoles 2a–h was performed. Combining the two moieties, the indole derivatives 3 and hydrazines 4 into a Fischer indole synthesis produced a series of active compounds (Scheme 3). All the combinations and compounds that were synthesized are shown below.
Scheme 3.
Library of the indole derivatives 2
The structures of the above compounds were unambiguously characterized and confirmed by the crystal structures of the compounds 2a and 2h (SI, CCDCs 981829 and 981827 respectively). Due to the fact that ester groups are readily cleaved in cells by esterases and are sometimes metabolically unstable, we hydrolyzed, under basic conditions specific derivatives 2 into the corresponding acids 9 in order to compare and evaluate the activity. Additionally we know from our previous studies on scaffold with the indole anchor, that the free –COOH is always 1–2 orders of magnitude more potent towards MDM2 protein.7,15–17,24,25
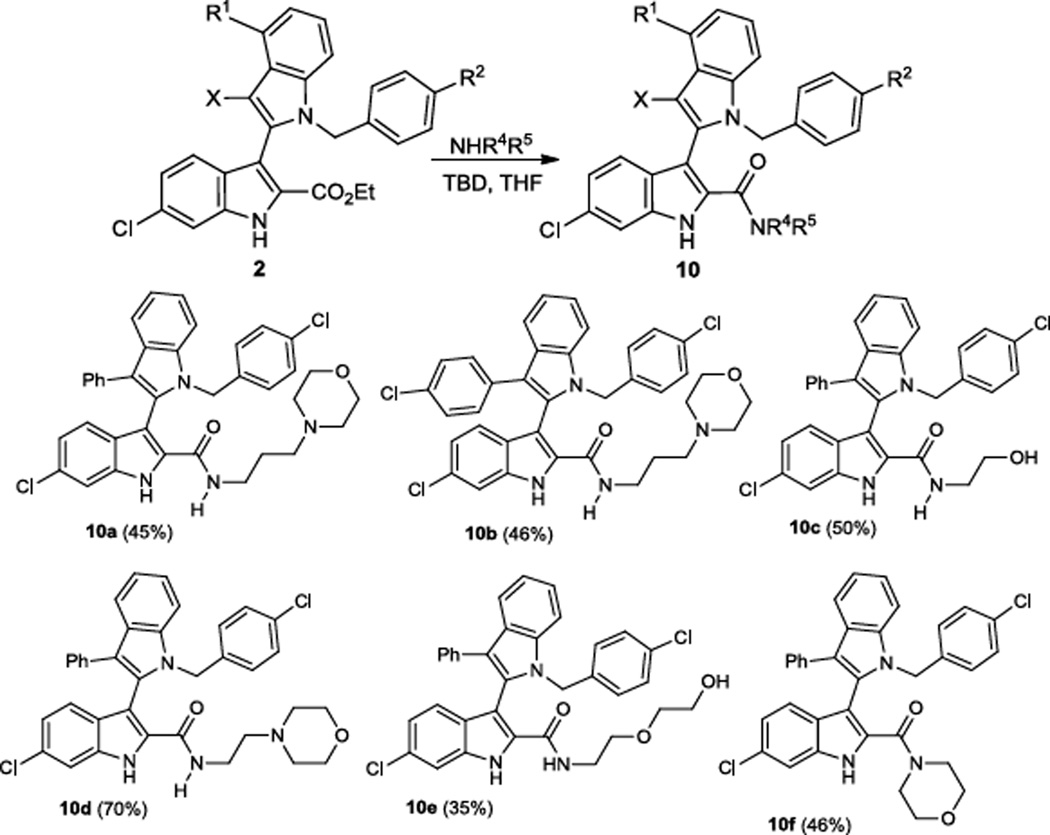
Apart from poor absorption-distribution-metabolism-excretion-toxicology (ADMET) properties, insufficiently water-soluble compounds often lead to poor reproducibility and unreliable results or even false positive hits during in vitro screening. In order to potentially improve the properties of our compounds, we converted the ester group of the derivatives 2 into the corresponding better water soluble amides 10 with a one-step TBD-catalyzed amidation procedure (Scheme 5).26 In our previous studies, amidation of the indole-2-carboxylate gave often improved water solubility.24
Scheme 5.
TBD-catalyzed amidation of the bisindoles 2
Binding affinities were determined by the fluorescence polarization method (FP) towards MDM2 and MDMX (SI).25 Representatives from each class compounds, esters, acids and an amide, were evaluated as inhibitors of the PPI. The results are summarized in the table 3. Noticeably, the acids 9a and 9d comparing with the corresponding esters 2a and 2e were found highly active towards both MDM2 and MDMX as previously reported by the indole anchor.7,15–17,24,25 In addition, compounds 9a,d provided some initial dual activity (MDM2/X) which is an interesting factor that most of the current inhibitors fail to combine.14 Moreover comparing the binding affinities of scaffold 1 (WK23)18 which was 0.9 µM and 36 µM for MDM2 and MDMX respectively, the novel scaffold 9, is more potent towards MDMX without significant loss of activity in MDM2 (9a) or even slight improvements (9d).
Table 3.
Results of the evaluation of inhibitory activity of compounds 2,3'-bis(1'H-indole) Heterocycles towards MDM2/MDMX as determined by FP.
| Ki (µM) | |||
|---|---|---|---|
| Compound | MDM2 | MDMX | |
| 2a | 17 | NA |  |
| 2c | 10 | NA | |
| 2d | >22 | NA | |
| 2e | 6.7 | 5.0 | |
| 2f | NA | NA | |
| 9a | 1.8 | 0.2 | |
| 9d | 0.7 | 1.5 | |
| 10d | NA | 6,09 | |
| WK23 (1) | 0.92 | 36 | |
NA: not active, Ki > 60 µM.
FP-based screening of protein-protein interactions often gives a high fraction of false positives especially with hydrophobic molecules and therefore it is advisable to run a second orthogonal biophysical assay. As a second, orthogonal screening system we performed the well-known heteronuclear single quantum coherence (HSQC) experiment where the 15N labeled MDM2 is titrated with compound 9d. The expected ligand-induced perturbations in NMR chemical shifts are indeed observed (Figure 4).27 Since all cross peaks in the MDM2 spectrum were assigned to particular amino acid residues before28,29 it is possible to analyze the way of interaction in the MDM2/9d complex. For example the interaction of 9d with the MDM2 tryptophan subpocket is expressed by the movement of the peak assigned to V93 (Figure 4). Chemical shift changes of T101 and M62 indicate interaction with the leucine and phenylalanine subpocket, respectively. Both the FP assay and HSQC test indicate that the acid derivatives are the active species as p53-MDM2/X inhibitors.
Figure 4.
Superposition of NMR HSQC spectra of the 15N-labeled MDM2 titrated against 9d. The spectrum of free MDM2 is shown in blue. The spectrum of 9d-MDM2 (ratio 1:1, respectively) is shown in red.
Our modelling (Figure 5) based on the HSQC binding data and known co-crystal structures and using MOLOC software30 revealed the nice alignment of the 6-chloro-indole moiety of compound 9d with the anchoring p53Trp23, whereas the two phenyl rings occupy the hydrophobic pockets mimicking p53Phe19 and p53Leu26. Moreover, the additionally introduced phenyl-annulated ring of the second indole moiety is predicted to cover mdm2Val93 through hydrophobic interactions. Then we rationalized the tight receptor ligand interaction using a small world network approach using Scorpion software (Figure 5).31 His96 is forming a pi-pi interaction with the p-Cl phenyl fragment and a hydrogen bond is formed between the Leu57 backbone carbonyl and the indole NH. Multiple van der Waals interactions can be used to rationalize the tight interactions. Amongst the major contributors of the interaction, shown as red balls, are F37-Tyr67, F27-Met62, C20-Met62, N7-Leu57. Cl10 contributes to an extended network including Phe86, Ile99, Ile103 and Leu57. Less strong contributors are C13-Val93, C3-, C1-, C6- of the buried indole moiety and C31-Leu57 and Cl36-Ile99. Interestingly small world network analysis of WK23 (PDB ID 3LBK) reveals no direct contribution of the scaffold imidazole to the tight interaction, whereas in 9d C13 interacts with Val93, supporting our hypothesis.
Figure 5.
above: Modeled binding pose of the most potent compound 9d (green sticks) in the MDM2 pocket (grey surface presentation, PDB ID 1YCR) and alignment with the hot-spot triade F19W23L26 (blue sticks). L57 and V93 are shown as grey sticks; below: Small network analysis of 9d in the MDM2 receptor. π-π, hydrogen bonding and van der Waals interactions are shown in red, blue and magenta dotted lines, respectively. Each ligand non-H atom is shown as colored ball according to its importance of contribution to the network (descending importance: red, purple, grey). Atom numbering of 9d is given in the right corner.
In conclusion, the designed 2,3'-bis(1'H-indole) scaffold 9 is active as dual action inhibitor of the p53-MDM2/X interactions with initial sub-µM affinities. Our hypothesis, that the extra phenyl ring in scaffold 2 makes additional hydrophobic interactions with the p53Val93 comparing with the derivatives 1, is suggested by 2D NMR and modeling studies. Further studies are ongoing to introduce more ‘drug-like’ properties into this scaffold and to investigate cellular mechanism-based anti-cancer behaviors.
Supplementary Material
Scheme 4.
Hydrolysis of derivatives 2 to the corresponding acids 9.
Acknowledgments
This research has been supported (to TAH) by a Marie Curie FP7-Reintegration-Grants within the 7th European Community Frame-work Programme and by a Project operated within the Foundation for Polish Science TEAM Programme, co-financed by the EU European Regional Development Fund. The research in the AD laboratory has been supported by the NIH (1R01GM097082) and by the Innovative Medicines Initiative (grant agreement n° 115489).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary data
Experimental procedures for the synthesis of compounds, characterization of compounds, crystal data, as well FP assay and NMR HSQC are provided in the supporting information.
References and notes
- 1.Lane DP. Nature. 1992;358:15. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- 2.Levine AJ. Cell. 1997;88:323. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 3.Römer L, Klein C, Dehner A, Kessler H, Buchner J. Angew. Chem. Int. Ed. 2006;45:6440. doi: 10.1002/anie.200600611. [DOI] [PubMed] [Google Scholar]
- 4.a) Haupt Y, Maya R, Kazaz A, Oren M. Nature. 1997;387:296. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]; b) Momand J, Wu HH, Dasgupta G. Gene. 2000;242:15. doi: 10.1016/s0378-1119(99)00487-4. [DOI] [PubMed] [Google Scholar]; c) Huang L, Yan Z, Liao X, Li Y, Yang J, Wang Z-G, Zuo Y, Kawai H, Shadfan M, Ganapathy S, Yuan Z-M. Proc. Natl. Acad. Sci. 2011 doi: 10.1073/pnas.1102309108. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Linares LK, Hengstermann A, Ciechanover A, Müller S, Scheffner M. Proc. Natl. Acad. Sci. U.S.A. 2003;100:12009. doi: 10.1073/pnas.2030930100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.a) Hainaut P, Hollstein M. Adv. Cancer Res. 2000;77:81. doi: 10.1016/s0065-230x(08)60785-x. [DOI] [PubMed] [Google Scholar]; b) Momand J, Wu HH, Dasgupta G. Gene. 2000;242:15. doi: 10.1016/s0378-1119(99)00487-4. [DOI] [PubMed] [Google Scholar]
- 6.Momand J, Zambetti GPJ. Cell. Biochem. 1997;64:343. [PubMed] [Google Scholar]
- 7.Czarna A, Beck B, Srivastava S, Popowicz GM, Wolf S, Huang Y, Bista M, Holak TA, Dömling A. Angew. Chem. Int. Ed. 2010;49:5352. doi: 10.1002/anie.201001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Popowicz GM, Dömling A, Holak TA. Angew. Chem. Int. Ed. 2011;50:2680. doi: 10.1002/anie.201003863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vassilev LT, Vu BT, Graves B, Carvajal D, Pod-laski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, Fotouhi N, Liu EA. Science. 2004;303:844. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 10.Khoury K, Holak TA, Dömling A. p53/MDM2 Antagonists: Towards Nongenotoxic Anticancer Treatments. In: Dömling A, editor. Protein-Protein Interactions in Drug Discovery. Wiley-VCH Verlag GmbH & Co. KGaA; 2013. pp. 129–163. [Google Scholar]
- 11.Zak K, Pecak A, Rys B, Wladyka B, Dömling A, Weber L, Holak TA, Dubin G. Expert Opin. Ther. Pat. 2013;23:425. doi: 10.1517/13543776.2013.765405. [DOI] [PubMed] [Google Scholar]
- 12.Wang S, Zhao Y, Bernard D, Aguilar A, Kumar S. Targeting the MDM2-p53 Protein-Protein Interaction for New Cancer Therapeutics. In: Wendt MD, editor. Protein-Protein Interactions. Springer Berlin Heidelberg: Topics in Medicinal Chemistry; 2012. pp. 57–79. [Google Scholar]
- 13.Zhao Y, Bernard D, Wang S. BioDiscovery. 2013;8:4. [Google Scholar]
- 14.Neochoritis C, Estrada-Ortiz N, Khoury K, Dömling A. Annual Reports in Medicinal Chemistry. 2014;49:167. [Google Scholar]
- 15.Bista M, Wolf S, Khoury K, Kowalska K, Huang Y, Wrona E, Arciniega M, Popowicz GM, Holak TA, Dömling A. Structure. 2013;21:2143. doi: 10.1016/j.str.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bauer MR, Boeckler FM. Structure. 2013;21:2095. doi: 10.1016/j.str.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 17.García-Echeverría C, Chène P, Blommers MJ, Furet P. J. Med. Chem. 2000;43:3205. doi: 10.1021/jm990966p. [DOI] [PubMed] [Google Scholar]
- 18.a) Boettcher A, Buschmann N, Furet P, Groell JM, Kallen J, Hergovich JL, Masuya K, Mayr L, Vaupel A. WO2008119741. 2008; b) Popowicz GM, Czarna A, Wolf S, Wang K, Wang W, Dömling A, Holak TA. Cell Cycle. 2010;9:1104. doi: 10.4161/cc.9.6.10956. [DOI] [PubMed] [Google Scholar]
- 19.Okauchi T, Itonaga M, Minami T, Owa T, Kitoh K, Yoshino H. Org. Lett. 2000;2:1485. doi: 10.1021/ol005841p. [DOI] [PubMed] [Google Scholar]
- 20.Ottoni O, Neder A, de VF, Dias AKB, Cruz RPA, Aquino LB. Org. Lett. 2001;3:1005. [PubMed] [Google Scholar]
- 21.Bharate SB, Yadav RR, Khan SI, Tekwani BL, Jacob MR, Khan IA, Vishwakarma RA. MedChemComm. 2013;4:1042. [Google Scholar]
- 22.Zhu M, Zheng N. Synthesis. 2011;14:2223. doi: 10.1055/s-0030-1260082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nara S, Sakamoto T, Miyazawa E, Kikugawa Y. Synth. Commun. 2003;33:87. [Google Scholar]
- 24.Srivastava S, Beck B, Wang W, Czarna A, Holak TA, Dömling J. Comb. Chem. 2009;11:631. doi: 10.1021/cc9000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.a) Furet P, Chène P, De Pover A, Valat TS, Lisztwan JH, Kallen J, Masuya K. Bioorg. Med. Chem. Lett. 2012;22:3498. doi: 10.1016/j.bmcl.2012.03.083. [DOI] [PubMed] [Google Scholar]; b) Ding K, Lu Y, Nikolovska-Coleska Z, Wang G, Qiu S, Shangary S, Gao W, Qin D, Stuckey J, Krajewski K, Roller PP, Wang S. J. Med. Chem. 2006;49:3432–3435. doi: 10.1021/jm051122a. [DOI] [PubMed] [Google Scholar]; c) Czarna A, Popowicz GM, Pecak A, Wolf S, Dubin G, Holak T. Cell Cycle. 2009;8:1176. doi: 10.4161/cc.8.8.8185. [DOI] [PubMed] [Google Scholar]
- 26.Kiesewetter MK, Scholten MD, Kirn N, Weber RL, Hedrick JL, Waymouth RM. J. Org. Chem. 2009;74:9490. doi: 10.1021/jo902369g. [DOI] [PubMed] [Google Scholar]
- 27.Shuker SB, Hajduk PJ, Meadows RP, Fesik SW. Science. 1996;274:1531. doi: 10.1126/science.274.5292.1531. [DOI] [PubMed] [Google Scholar]
- 28.Stoll R, Renner Ch, Hansen S, Palme S, Klein Ch, Belling A, Zeslawski W, Kamionka M, Rehm T, Muhlhahn P, Schumacher R, Hesse F, Kaluza B, Voelter W, Engh RA, Holak TA. Biochemistry. 2001;40:336. doi: 10.1021/bi000930v. [DOI] [PubMed] [Google Scholar]
- 29.a) Rehm T, Huber R, Holak TA. Structure. 2002;10:1613. doi: 10.1016/s0969-2126(02)00894-8. [DOI] [PubMed] [Google Scholar]; b) Wüthrich K. NMR of Proteins and Nucleic Acids. 1 edition. New York: Wiley-Interscience; 1986. [Google Scholar]
- 30.Gerber PR, Müller K. J. Comput. Aided Mol. Des. 1995;9:251. doi: 10.1007/BF00124456. [DOI] [PubMed] [Google Scholar]
- 31.Kuhn B, Fuchs JE, Reutlinger M, Stahl M, Tylor NRJ. Chem. Inf. Model. 2011;51:3180. doi: 10.1021/ci200319e. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.