Abstract
Background and Purpose
Total body irradiation (TBI) is a common component of hematopoietic cell transplantation (HCT) conditioning regimens. Preclinical studies suggest prolonged bone marrow (BM) injury after TBI could contribute to impaired engraftment and poor hematopoietic function.
Materials and Methods
We studied the longitudinal changes in the marrow environment in patients receiving allogeneic HCT with myeloablative (MA, n=42) and reduced intensity (RIC, n=56) doses of TBI from 2003-2013, including BM cellularity, histologic features of injury and repair, hematologic and immunologic recovery.
Results
Following MA conditioning, a 30% decrease in the marrow cellularity persisted at 1 year post-transplant (p=0.03). RIC HCT marrow cellularity transiently decreased but returned to baseline by 6 months even though the RIC group received mostly umbilical cord blood (UCB) grafts (82%, vs. 17% in the MA cohort, p<0.01). There was no evidence of persistent marrow vascular damage or inflammation. Recipients of more intensive conditioning did not show more persistent cytopenias with the exception of a tendency for minimal thrombocytopenia. Immune recovery was similar between MA and RIC.
Conclusions
These findings suggest that TBI associated with MA conditioning leads to prolonged reductions in marrow cellularity, but does not show additional histological evidence of long-term injury, which is further supported by similar peripheral counts and immunologic recovery.
Keywords: Bone marrow transplantation, total body irradiation, marrow cellularity
Introduction
Total body irradiation (TBI) is commonly used in the treatment of advanced or high-risk hematologic malignancies as a component of the preparative regimen prior to allogeneic hematopoietic cell transplantation (HCT). Historically, myeloablative (MA) transplant regimens have included high doses of cytotoxic chemotherapy and/or TBI with the goal of eradicating residual disease and achieving immunosuppression to allow for donor stem cell engraftment. Increasing the intensity of the conditioning regimen has been shown to reduce the risk of disease relapse albeit with a corresponding increase in the severity of the acute treatment-related toxicities [1].
Over the past two decades, reduced intensity conditioning (RIC) regimens have emerged as lower toxicity alternatives, especially in older patients or those with significant medical comorbidities. Reduced treatment-related mortality has followed HCT using these regimens [2-4]. In contrast, the development of novel targeted approaches concentrating the dose of radiation to the marrow (total marrow irradiation, or TMI) explored by our group and others are designed to maximize tumor killing while sparing more radiosensitive tissues that typically limit radiation dose [5-7]. Such novel approaches may extend the curative potential of allogeneic HCT to patients who may otherwise be at substantial relapse risk (e.g., high-risk myelodysplastic syndromes or acute leukemias with minimal residual disease prior to HCT). As the spectrum of treatment options expands from RIC to novel, dose-escalated, marrow-targeted therapeutic radiation protocols and both are further integrated with chemotherapy, it becomes critical to understand the long term impact of different treatments on hematopoietic competence and marrow environment health.
Pre-clinical studies reveal prolonged bone marrow injury after TBI [8]. Specifically, the bone marrow demonstrates an increase in the number of adipocytes and a corresponding decrease in hematopoietic precursors following exposure to chemotherapy or ionizing radiation [9-11]. Following HCT, expanded marrow adipocyte populations have also been demonstrated to negatively influence post-transplant hematopoietic engraftment in a murine model [12]. Conditioning-induced structural and functional marrow damage beyond the necessary immunosuppression for successful engraftment is not well understood in clinical HCT. While numerous studies have examined treatment responses in a variety of diseases utilizing either MA or RIC regimens [13-16], less is known regarding the bone marrow microenvironment, and the relative effect of the pre-transplant irradiation dose on hematopoietic engraftment and recovery [9-11]. Furthermore, it is unclear whether post-transplantation peripheral blood cell counts accurately reflect the extent of marrow damage. Several prior studies have shown that structural changes to the marrow environment may lead to hematopoietic dysfunction [17,18] as well as decreased bone mineralization and higher fracture risk [19-21].
We examined the features of marrow in response to TBI-containing MA and RIC regimens in a cohort of patients undergoing HCT for treatment of advanced or high-risk leukemia. We additionally explored the hematopoietic and immunological recovery to determine if there is a demonstrable effect of the conditioning regimen on the bone marrow histological structure and function over time. We correlated the degree of structural marrow change with hematopoietic function as demonstrated by peripheral blood cell production to better characterize marrow response and recovery following two commonly-utilized treatment regimens. This will inform clinical decision-making in the selection of appropriate conditioning protocols, including the optimization of novel TMI regimens.
Methods
Patients
Medical records from 98 patients (47 male, 51 female) receiving allogeneic HCT using either TBI-containing MA or RIC regimens at the University of Minnesota between January 2003 and June 2013 were reviewed. Patients were limited to age 45-55 at HCT to avoid confounding age-related differences in marrow cellularity [22,23].
Preparative Regimens
MA conditioning included cyclophosphamide 60 mg/kg on days -6 and -5 pre-HCT followed by 1320 cGy TBI delivered in 8 twice-daily fractions. Those receiving umbilical cord blood (UCB) grafts had the same MA conditioning, but with the addition of fludarabine 25 mg/m2/day x 3 days. Patients receiving RIC regimens received cyclophosphamide 50 mg/kg on day -6 in addition to fludarabine 40 mg/m2 on days -6 to -2 pre-transplant followed by TBI delivered in a single 200 cGy fraction on day -1. Total body radiotherapy for both MA and RIC groups was delivered via an opposed lateral technique [24]. Custom aluminum compensators for head/neck, lung and legs to account for the transverse tissue deficit were used to provide a uniform dose homogeneity within 10% of the prescribed dose.
Anti-thymocyte globulin was added to patients receiving UCB grafts without prior exposure to combination chemotherapy within 3 months prior to transplantation. Due to the retrospective nature of the study, there was heterogeneity in the patient populations receiving MA and RIC in addition to differences in the treatment protocols. We therefore analyzed the groups separately. The characteristics of MA and RIC patients including diagnosis, disease risk, prior autologous transplants, performance status and CMV serostatus were extracted from the medical record and reported.
Transplant procedures and engraftment
The graft and graft versus host disease (GVHD) prophylaxis protocols were extracted from the medical records for each group of patients. Factors impacting engraftment in each group were evaluated.
Bone Marrow Analysis
Iliac crest bone marrow specimens for each patient were examined at baseline prior to pre-transplant conditioning and at 21 days, 6 months and 1 year post-transplant according to the normal monitoring protocol for our institution. The average bone marrow cellularity for each specimen was determined from original clinical bone marrow biopsy reports. Data was censored for specimens that demonstrated evidence of recurrent leukemia or if the patient had undergone treatment for relapsed disease prior to specimen collection. The white blood count (WBC), absolute neutrophil count (ANC), hemoglobin and platelet count were also obtained for each patient on the same dates as the bone marrow collection. A subset of patients randomly selected from those having excellent quality archived bone marrow core biopsy slides available for review (i.e. large specimen, minimal artifacts that would interfere with interpretation). (10 MA, 10 RIC) underwent more comprehensive microscopic evaluation of the primary bone marrow biopsy samples. The goal was to elucidate changes in bone marrow environment not routinely characterized in detail during clinical evaluation of marrow sections that might be relevant to long term changes in the hematopoietic microenvironment. In addition to standard examination of the hematopoietic component, marrow samples were evaluated for vascular changes (edema, congestion, sinusoidal dilation, vasculitis), evidence of inflammation and necrosis, hemosiderosis, and fibrohistiocytic proliferation. For each characteristic, samples were scored as not present (0), mild (1), moderate (2), marked (3) or severe (4). All samples were evaluated by a single investigator (LS) and then independently verified by a second board certified hematopathologist (SY).
Immune Reconstitution
Samples were prospectively analyzed for the absolute numbers of lymphocytes, NK cell (CD3-CD56+), T cells (CD3+ lymphocytes), CD4+ T cells (CD3+CD4+ cells), CD8+ T cells (CD3+CD8+) and B cells (CD19+ lymphocytes) at 3, 6 and 12 months according to an institutional immune monitoring protocol. Comparison of analytes by conditioning at individual time-points was carried out by the non-parametric General-Wilcoxon test. Comparison of the overall average level of each cell type was evaluated at each time point among only those patients without non-relapse mortality prior to 1 year. Values for each cell type were transformed by the natural logarithm to yield a normal distribution assumption in the repeated measures analysis. Linear mixed models was employed to evaluate the repeated measures of each cell type using time from transplant as a continuous variable.
Definitions and Statistical Analysis
Neutrophil engraftment, defined as 3 consecutive days with ANC ≥ 0.5 × 109/L, was determined for each patient. In order to determine the influence of baseline bone marrow environment on hematopoietic recovery, we stratified the MA and RIC cohorts into quartiles based upon their pre-transplant marrow cellularity. Cumulative incidence treating non-event death as a competing risk was used to estimate engraftment and disease relapse [25]. Fine and Gray regression analysis was used to examine the independent effect of cellularity on engraftment [26]. For longitudinal analysis of cellularity, changes in the white blood count (WBC), hemoglobin (Hgb), absolute neutrophil count (ANC) and platelet count (Plt), were analyzed with repeated measures using general linear mixed models to test the hypothesis that measures change over time. The models assumed zero correlation between patients and constant correlation within subjects across time. The non-parametric Spearman’s rank correlation was used to measure the correlation between cellularity and other blood parameters within each time point. Overall significance of correlation over time between cellularity and the other blood parameters was tested using general linear mixed models. Statistical comparisons of histology at various time-points were evaluated using the sign-rank test.
Results
Table 1 describes the demographic and clinical characteristics of the patients in each cohort. The MA and RIC cohorts were similar with respect to diagnosis, disease risk, prior autologous transplants, performance status and CMV serostatus. There was a slight male predominance in the MA cohort (60% versus 40% in the RIC cohort; p=0.05). The majority (81%) of patients undergoing MA conditioning received hematopoietic stem cell grafts from matched sibling donors while most of the others (17%) received umbilical cord blood (UCB grafts). In contrast, most patients undergoing RIC regimens received UCB grafts (82%) versus matched sibling donors (13%). More patients in the MA cohort received calcineurin inhibitor plus methotrexate (MTX)-based GVHD prophylaxis, while in the RIC cohort, most received calcineurin inhibitor and mycophenylate mofetil (p=<0.01). This is expected based upon different standard of care approaches to GVHD prophylaxis with respect to the different conditioning intensities at our institution.
Table 1. Clinical characteristics for the patients undergoing either myeloablative (MA) or reduced intensity conditioning (RIC).
P-values for continuous variables were analyzed by the general Wilcoxon test while categorical variables were analyzed by chi-square. (URD = unrelated donor; UCB = umbilical cord blood; CsA = cyclosporine A; MTX = methotrexate; MMF = mycophenolate mofetil)
| Variable | MA | RIC | p-value | |
|---|---|---|---|---|
| Age | 40-50 | 25 (60%) | 31 (55%) | 0.68 |
| 51-59 | 17 (40%) | 25 (45%) | ||
| Age (Years) | N | 42 | 56 | 0.35 |
| Median | 49.7 | 50.4 | ||
| Min | 45 | 46 | ||
| Max | 55 | 55 | ||
| Gender | Male | 25 (60%) | 22 (39%) | 0.05 |
| Female | 17 (40%) | 34 (61%) | ||
| Diagnosis | ALL | 11 (26%) | 12 (21%) | 0.77 |
| AML | 26 (62%) | 35 (63%) | ||
| CML | 5 (12%) | 9 (16%) | ||
| Dx Risk | Standard Risk | 38 (91%) | 46 (82%) | 0.24 |
| High Risk | 4 (9%) | 10 (18%) | ||
| Donor Type | Matched Sibling | 34 (81%) | 7 (13%) | <0.01 |
| Matched URD | 1 (2%) | 3 (5%) | ||
| UCB | 7 (17%) | 46 (82%) | ||
| Year of TX | 2003-2008 | 25 (60%) | 44 (79%) | 0.04 |
| 2009-2013 | 17 (40%) | 12 (21%) | ||
| GvHD Prophy | MTX/CsA | 35 (83%) | <0.01 | |
| CsA/MMF | 7 (17%) | 54 (96%) | ||
| Sirolimus/MMF | 2 (4%) | |||
| Prior Auto HCT | No | 38 (91%) | 53 (95%) | 0.43 |
| Yes | 4 (9%) | 3 (5%) | ||
| Karnofsky | <90 | 3 (7%) | 9 (16%) | 0.18 |
| 90-100 | 39 (93%) | 47 (84%) | ||
|
Recipient CMV
Serostatus |
Negative | 21 (50%) | 21 (38%) | 0.22 |
| Positive | 21 (50%) | 35 (62%) |
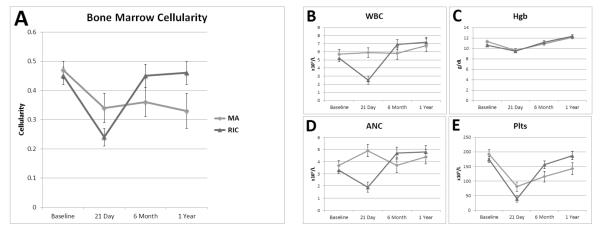
At baseline pre-HCT, the mean hematopoietic cellularity for MA and RIC cohorts was 47% and 45%, respectively. As expected, at day 21 following HCT, both cohorts demonstrated significant decreases in bone marrow cellularity (Figure 1a). However, recipients of MA conditioning demonstrated persistently decreased cellularity over time (least square means (standard error) of 47% (3%) at baseline, 36% (5%) at 6 months and 33% (6%) at 1 year; p = 0.04). In contrast, recipients of RIC conditioning had no statistically significant difference over time for marrow cellularity
Figure 1.
Quantitative analysis of the marrow cellularity (A) and peripheral blood components (B: white blood count, C: absolute neutrophil count, D: hemoglobin, E: platelets) for both cohorts undergoing MA or RIC conditioning. Both cohorts are represented in this figure to facilitate visual comparison of patterns of change over time, but direct comparison of groups at a given time point is not appropriate given heterogeneity between the study populations. The least square means of each component are shown at baseline prior to initiation of pre-transplant conditioning, 21 days, 6 months and 1 year post-transplant. (Error bars are SE).
There were no significant longitudinal changes in the total WBC (Figure 1b) or ANC (Figure 1c) in the MA cohort, with mean values remaining within reference intervals at each time point. In recipients of RIC conditioning, WBC and ANC were within reference intervals at baseline, but were significantly lower at day 21, with mean WBC falling below reference values (2.5 × 109/L, reference interval 4-11). Normalization of both the WBC and ANC was noted by the 6 month time point and remained stable at 1 year post-transplant in this patient demographic. In both MA and RIC, Hgb (Figure 1d) dropped significantly at day 21, but then increased significantly across each subsequent phase of the study. Platelets (Figure 1e) were within reference intervals (150,000-450,000/μl) at baseline and markedly dropped at day 21 under both MA and RIC conditioning. In recipients of MA conditioning, the platelets remained slightly below the reference interval at 6 months and 1 year while RIC patients had normalization of their platelet counts by 6 months and it additionally remained stable by 1 year.
In recipients of MA conditioning, the WBC (correlation = 0.59, p=0.01) and Plts (correlation = 0.51, p=0.03) were correlated with marrow cellularity at day 21 only, reflecting peripheral cytopenias associated with marrow cellularity (Table 2). Otherwise, there was no significant correlation of marrow cellularity and peripheral blood cell counts at any of the other examined time points in the MA cohort. In contrast, recipients of RIC showed significant correlation of WBC, ANC, and platelet count at baseline, with Hgb also demonstrating a trend (p=0.07). At day 21, only leukocytes and platelets were correlated with cellularity in the RIC group. Hgb was correlated at 6 months and there was a negative correlation observed between the ANC and marrow cellularity at one year, demonstrating decreasing impact of marrow cellularity on peripheral cell counts over time in RIC.
Table 2.
Correlation of marrow cellularity and peripheral blood counts within time points in recipients of (a) myeloablative and (b) reduced-intensity conditioning.
| A. Myeloablative | |||||
|---|---|---|---|---|---|
| Correlation of Cellularity by |
Baseline | D21 | 6 months | 1 year | Overall whether measures are correlated using repeated measures analysis |
| ANC: | NS | NS | NS | NS | p = 0.22 |
| WBC: | NS | 0.59 (p = 0.01) |
NS | NS | p = 0.05 |
| PLT: | NS | 0.51 (p = 0.03) |
NS | NS | p = 0.04 |
| HGB: | NS | NS | NS | NS | p = 0.71 |
| B. Reduced intensity | |||||
|---|---|---|---|---|---|
| Correlation of Cellularity by |
Baseline | D21 | 6 months | 1 year | Overall whether measures are correlated using repeated measures analysis |
| ANC: | 0.43 (p < 0.01) |
0.39 (p =0.01) |
NS | −0.44 (p =0.04) |
p < 0.01 |
| WBC: | 0.47 (p < 0.01) |
0.59 (p < 0.01) |
NS | NS | p < 0.01 |
| PLT: | 0.55 (p < 0.01) |
0.51 (p < 0.01) |
NS | NS | p < 0.01 |
| HGB: | NS | NS | 0.61 (p < 0.01) |
NS | p < 0.01 |
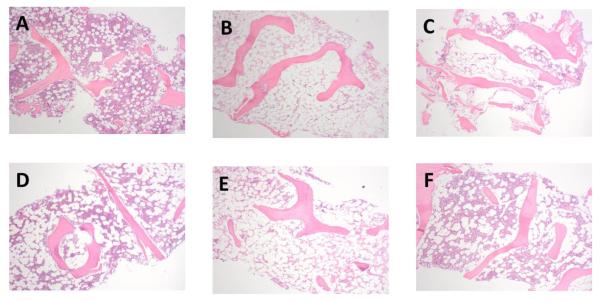
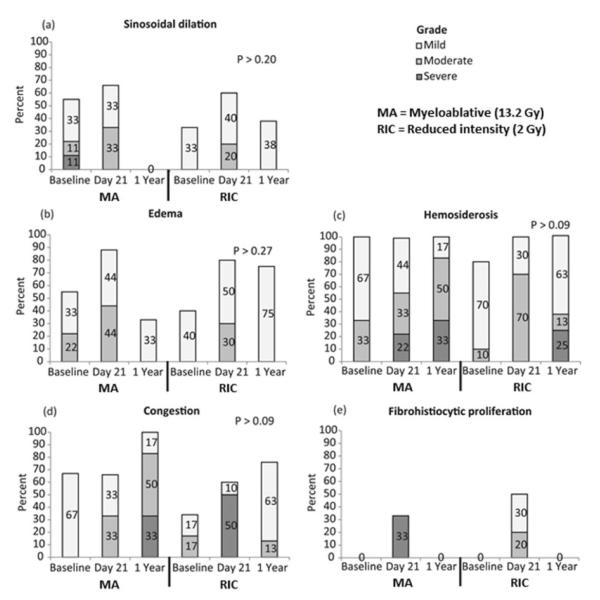
Representative micrographs depicting the marrow changes over time are shown in Figure 2. Detailed histologic analysis of the randomly selected MA and RIC subsets showed no inflammatory lesions in either group (Figure 3). The overall cellularity was lower at later time points in recipients of MA conditioning as described previously, however, there was no evidence of significant changes in vascular damage (sinusoidal dilation, edema, congestion), hemosiderosis, or fibrohistiocytic proliferation over time. In recipients of RIC conditioning, there was a trend toward increased edema and fibrohistiocytic proliferation at day 21 (p=0.06 for both) that resolved by 1 year post-transplant (Figure 3).
Figure 2.
MA Figure (A, B, C). Bone marrow biopsy sections demonstrating hematopoietic cellularity and fat over time in a patient treated with a myeloablative pre-conditioning regimen (H&E, 40X). (A) Baseline hematopoietic cellularity was normal at 50% of the area of the section. (B) By day 21 post-treatment, hematopoietic activity was barely perceptible at an estimated 2%, and (C) one year after treatment, hematopoietic activity was improved but still markedly low at 10%.
RIC Figure (D, E, F). Bone marrow biopsy sections demonstrating hematopoietic cellularity and fat over time in a patient treated with a reduced intensity conditioning regimen (H&E, 40X). (D) Baseline hematopoietic cellularity was normal to slightly low at 30% of the area of the section. (E) By day 21 post-treatment, hematopoietic activity was barely perceptible at an estimated 2%, and (F) one year after treatment, hematopoietic activity had recovered to above baseline at 40%, in contrast to the ablative patient above who continued to exhibit markedly suppressed hematopoietic activity).
Figure 3.
Histologic quantification of marrow vascular damage from 20 patients (10 MA, 10 RIC) selected from the study cohort. Both cohorts are represented in this figure to facilitate visual comparison of patterns of change over time, but direct comparison of groups at a given time point is not appropriate given heterogeneity between the study populations.
During the planning of this study, we reasoned that injury to the BM might also influence hematopoiesis including the recovery of lymphocyte subgroups after transplantation. Thus, we examined whether the MA and RIC groups showed different kinetics of NK cell (CD3-CD56+), T cell (CD3+, CD3+CD4+ and CD3+CD8+) or B cell (CD19+) recovery at 3, 6 or 12 months following HCT. We did not observe any significant difference in the absolute numbers of any of these subgroups with either MA or RIC conditioning (see supplemental data).
We did not observe any significant difference in either the 12-month relapse rate (29% vs 36%; p=0.56) or non-relapse mortality (19% vs 13%; p=0.40) in the MA or RIC patients, respectively. We also evaluated acute and chronic GVHD in both the acute and chronic setting as GVHD has been demonstrated to influence both marrow recovery and peripheral blood counts following transplant [27-29]. Among our cohort, the incidence of GVHD was broadly defined as involvement of any organ system, in either the acute (≤100 day post-transplant) or late stages following HCT. The RIC patients were noted to have slightly higher rates of moderate to severe acute GVHD compared to the MA group (55% vs 38%; p=0.13) while the MA cohort tended to have a higher rate of chronic GVHD compared to their RIC peers (33% vs 18%; p=0.09) although neither difference achieved statistical significance (see supplemental data).
Discussion
In this study, we analyzed the longitudinal impact of MA and RIC regimens on the BM environment and functional reconstitution post-HCT. Due to the fact that there was considerable variation in the conditioning regimen and graft source for the RIC and MA patients, we did not seek to directly compare the individual marrow cellularity measurements and peripheral blood counts at specified time points. Rather, we examined the changes over time compared with the pre-transplant baseline for each conditioning regimen. We observed that after RIC conditioning, the bone marrow cellularity and peripheral blood counts returned to pre-transplant baseline by 6 months following HCT, despite a predominant UCB graft source in this population, which has historically been associated with more prolonged hematopoietic recovery [30]. In contrast, a long-term decrease in cellularity was observed in recipients of MA conditioning. This observation suggests that prolonged injury to the marrow space with the MA regimen may have occurred but without major histologic differences noted on detailed analysis of the MA and RIC marrow specimens.
The observed modest discordance between rates of marrow and peripheral blood recovery may reflect baseline marrow reserve that, under the influence of homeostatic and pharmacologic stimuli with filgrastim, yield prompt recovery of peripheral counts despite a compromise in residual marrow reserve. In addition, bone marrow specimens obtained from the iliac crest may not be representative of the complete functional hematopoietic environment, which may be heterogeneously distributed throughout the skeleton, especially after chemotherapy and radiation [31,32]. Non-invasive imaging studies may help to further characterize the marrow distribution as our recent studies of water-fat MRI [32] and whole body MRI emphasize that the marrow structure is highly heterogeneous following exposure to chemotherapy and radiation.
Prior studies have suggested that host osteoblastic niches are responsible for the subsequent engraftment of donor HSCs following pre-transplant conditioning [11]. In our study, we observed that pre-transplant marrow cellularity did not significantly impact engraftment for either the patients receiving MA or RIC regimens (see supplemental material). The vascular niche may also play a role, and we identified a trend for increased interstitial edema and fibrohistiocytic proliferation in the short term (day 21) in RIC patients, suggesting that evaluation of vascular factors should be a potential consideration. Older age is an independent factor associated with increasing bone marrow adiposity and reduced hematopoietic cellularity [23,33]. RIC HCT is used for older patients or those with significant co-morbidities and thus we attempted to minimize this confounding factor by studying patients between the ages of 45-55.
GVHD has previously been demonstrated to influence the marrow environment as well as peripheral blood components [27-29]. In our study, we did not observe a significant difference in the rates of either acute or chronic GVHD stratified by either RIC or MA conditioning. While there is undoubtedly some influence on the marrow and peripheral blood counts by GVHD, we do not believe that the longitudinal differences in marrow recovery between these two groups can be solely attributed to this factor. Preclinical models have demonstrated that chemotherapy and radiation-induced damage can cause suppression of the hematopoietic component through an expansion of the adipocyte-niche within the marrow environment [9] and it is possible this effect is being observed in the present study with the more intensive MA conditioning regimen. Additional trials with detailed marrow histopathologic analysis performed in a prospective manner could potentially help to further clarify this finding.
Relapse of the underlying malignant disease is the most frequent cause of treatment failure following HCT. Typical MA conditioning regimens include up to 12 or 14 Gy, which has been considered to be the maximum tolerated TBI dose. While higher TBI doses than this have previously been tested and been shown to result in lower leukemia relapse, any benefit of disease control has been offset by increases in non-relapse mortality, resulting in equivalent disease free survival in retrospective analysis [1].
In addition to TBI, alternative radiotherapy techniques such as total lymphoid irradiation (TLI) or TMI have been utilized as part of the pre-transplant conditioning regimens. The advantage of these techniques is that they allow for immunomodulation and/or site-specific cytotoxic therapy to be delivered with relative sparing of non-involved critical organs. TMI, in particular, is currently being evaluated for its theoretic ability to permit dose escalation to the bone marrow which may harbor residual malignant progenitor cells while avoiding excess radiation-induced toxicities to critical structures that have limited dose escalation in the TBI setting. Improved knowledge of radiation induced injury to the bone marrow environment thus becomes critical to understanding the long-term impact on marrow function and hematopoiesis with these escalated marrow radiation doses. Although we did not specifically examine the effect of dose escalation on the marrow beyond conventional treatment paradigms, the evaluation of the longitudinal marrow response to two commonly utilized conditioning regimens in allogeneic HCT provide us with a basis on which to evaluate subsequent dose escalation/de-escalation studies. Each new approach poses novel challenges to long term bone marrow health and reconstitution and must be evaluated for impact in cancer survivors.
In conclusion, we observed decreased overall marrow hematopoietic cellularity over time associated with the MA conditioning regimen. This suppressed cellularity, although statistically significant, was not associated with clear differences in histologic marrow damage compared to RIC conditioning. Additionally, despite a predominance of UCB grafts in the RIC cohort, we observed a return to baseline marrow cellularity by 6 months that remained stable at 1 year post-transplant in this group of patients. Altogether these results provide insight into the structural and functional marrow changes following two common HCT conditioning regimens. Further prospective studies are necessary to further elucidate the marrow response with regards to treatment alterations in order to provide the optimal clinical outcomes – the eradication of residual disease while facilitating engraftment with minimal treatment-induced toxicity.
Supplementary Material
Acknowledgement
This work was supported by the National Institute of Health grants (1R01CA154491).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no conflict of interest.
References
- [1].Clift RA, Buckner CD, Appelbaum FR, et al. Allogeneic marrow transplantation in patients with acute myeloid leukemia in first remission: a randomized trial of two irradiation regimens. Blood. 1990;76:1867–1871. [PubMed] [Google Scholar]
- [2].Majhail NS, Brunstein CG, Tomblyn M, et al. Reduced-intensity allogeneic transplant in patients older than 55 years: unrelated umbilical cord blood is safe and effective for patients without a matched related donor. Biol Blood Marrow Transplant. 2008;14:282–289. doi: 10.1016/j.bbmt.2007.12.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ram R, Storb R, Sandmaier BM, et al. Non-myeloablative conditioning with allogeneic hematopoietic cell transplantation for the treatment of high-risk acute lymphoblastic leukemia. Haematologica. 2011;96:1113–1120. doi: 10.3324/haematol.2011.040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Slavin S, Nagler A, Naparstek E, et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood. 1998;91:756–763. [PubMed] [Google Scholar]
- [5].Hui SK, Kapatoes J, Fowler J, et al. Feasibility study of helical tomotherapy for total body or total marrow irradiation. Med Phys. 2005;32:3214–3224. doi: 10.1118/1.2044428. [DOI] [PubMed] [Google Scholar]
- [6].Wong JY, Liu A, Schultheiss T, et al. Targeted total marrow irradiation using three-dimensional image-guided tomographic intensity-modulated radiation therapy: an alternative to standard total body irradiation. Biol Blood Marrow Transplant. 2006;12:306–315. doi: 10.1016/j.bbmt.2005.10.026. [DOI] [PubMed] [Google Scholar]
- [7].Hui SK, Verneris MR, Higgins P, et al. Helical tomotherapy targeting total bone marrow - first clinical experience at the University of Minnesota. Acta Oncol. 2007;46:250–255. doi: 10.1080/02841860601042449. [DOI] [PubMed] [Google Scholar]
- [8].Wang Y, Liu L, Pazhanisamy SK, Li H, Meng A, Zhou D. Total body irradiation causes residual bone marrow injury by induction of persistent oxidative stress in murine hematopoietic stem cells. Free Radic Biol Med. 2010;48:348–356. doi: 10.1016/j.freeradbiomed.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Poncin G, Beaulieu A, Humblet C, et al. Characterization of spontaneous bone marrow recovery after sublethal total body irradiation: importance of the osteoblastic/adipocytic balance. PLoS One. 2012;7:e30818. doi: 10.1371/journal.pone.0030818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Banfi A, Bianchi G, Galotto M, Cancedda R, Quarto R. Bone marrow stromal damage after chemo/radiotherapy: occurrence, consequences and possibilities of treatment. Leuk Lymphoma. 2001;42:863–870. doi: 10.3109/10428190109097705. [DOI] [PubMed] [Google Scholar]
- [11].Dominici M, Rasini V, Bussolari R, et al. Restoration and reversible expansion of the osteoblastic hematopoietic stem cell niche after marrow radioablation. Blood. 2009;114:2333–2343. doi: 10.1182/blood-2008-10-183459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460:259–263. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Aoudjhane M, Labopin M, Gorin NC, et al. Comparative outcome of reduced intensity and myeloablative conditioning regimen in HLA identical sibling allogeneic haematopoietic stem cell transplantation for patients older than 50 years of age with acute myeloblastic leukaemia: a retrospective survey from the Acute Leukemia Working Party (ALWP) of the European group for Blood and Marrow Transplantation (EBMT) Leukemia. 2005;19:2304–2312. doi: 10.1038/sj.leu.2403967. [DOI] [PubMed] [Google Scholar]
- [14].Eom KS, Shin SH, Yoon JH, et al. Comparable long-term outcomes after reduced-intensity conditioning versus myeloablative conditioning allogeneic stem cell transplantation for adult high-risk acute lymphoblastic leukemia in complete remission. Am J Hematol. 2013;88:634–641. doi: 10.1002/ajh.23465. [DOI] [PubMed] [Google Scholar]
- [15].Mikell JL, Waller EK, Switchenko JM, et al. Similar Survival for Patients Undergoing Reduced-Intensity Total Body Irradiation (TBI) Versus Myeloablative TBI as Conditioning for Allogeneic Transplant in Acute Leukemia. Int J Radiat Oncol Biol Phys. 2014;89:360–369. doi: 10.1016/j.ijrobp.2014.02.032. [DOI] [PubMed] [Google Scholar]
- [16].Ringden O, Labopin M, Ehninger G, et al. Reduced intensity conditioning compared with myeloablative conditioning using unrelated donor transplants in patients with acute myeloid leukemia. J Clin Oncol. 2009;27:4570–4577. doi: 10.1200/JCO.2008.20.9692. [DOI] [PubMed] [Google Scholar]
- [17].Roy S, Javed S, Jain SK, Majumdar SS, Mukhopadhyay A. Donor hematopoietic stem cells confer long-term marrow reconstitution by self-renewal divisions exceeding to that of host cells. PLoS One. 2012;7:e50693. doi: 10.1371/journal.pone.0050693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Madhusudhan T, Majumdar SS, Mukhopadhyay A. Degeneration of stroma reduces retention of homed cells in bone marrow of lethally irradiated mice. Stem Cells Dev. 2004;13:173–182. doi: 10.1089/154732804323046774. [DOI] [PubMed] [Google Scholar]
- [19].Rosen CJ, Ackert-Bicknell C, Rodriguez JP, Pino AM. Marrow fat and the bone microenvironment: developmental, functional, and pathological implications. Crit Rev Eukaryot Gene Expr. 2009;19:109–124. doi: 10.1615/critreveukargeneexpr.v19.i2.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mostoufi-Moab S, Ginsberg JP, Bunin N, Zemel B, Shults J, Leonard MB. Bone density and structure in long-term survivors of pediatric allogeneic hematopoietic stem cell transplantation. J Bone Miner Res. 2012;27:760–769. doi: 10.1002/jbmr.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Savani BN, Donohue T, Kozanas E, et al. Increased risk of bone loss without fracture risk in long-term survivors after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13:517–520. doi: 10.1016/j.bbmt.2007.01.085. [DOI] [PubMed] [Google Scholar]
- [22].De Bisschop E, Luypaert R, Louis O, Osteaux M. Fat fraction of lumbar bone marrow using in vivo proton nuclear magnetic resonance spectroscopy. Bone. 1993;14:133–136. doi: 10.1016/8756-3282(93)90239-7. [DOI] [PubMed] [Google Scholar]
- [23].Georgiou KR, Hui SK, Xian CJ. Regulatory pathways associated with bone loss and bone marrow adiposity caused by aging, chemotherapy, glucocorticoid therapy and radiotherapy. Am J Stem Cells. 2012;1:205–224. [PMC free article] [PubMed] [Google Scholar]
- [24].Levitt and Tapley's technological basis of radiation therapy : clinical applications. Lippencott Williams & Wilkins; Philadelphia: 1999. Philadelphia. [Google Scholar]
- [25].Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med. 1997;16:901–910. doi: 10.1002/(sici)1097-0258(19970430)16:8<901::aid-sim543>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- [26].Fine J, Gray R, Fine J, Gray R. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- [27].Milone G, Camuglia MG, Avola G, et al. Acute GVHD after allogeneic hematopoietic transplantation affects early marrow reconstitution and speed of engraftment. Exp Hematol. 2015;43:430–8.e1. doi: 10.1016/j.exphem.2015.02.002. [DOI] [PubMed] [Google Scholar]
- [28].Shono Y, Ueha S, Wang Y, et al. Bone marrow graft-versus-host disease: early destruction of hematopoietic niche after MHC-mismatched hematopoietic stem cell transplantation. Blood. 2010;115:5401–5411. doi: 10.1182/blood-2009-11-253559. [DOI] [PubMed] [Google Scholar]
- [29].Shono Y, Shiratori S, Kosugi-Kanaya M, et al. Bone marrow graft-versus-host disease: evaluation of its clinical impact on disrupted hematopoiesis after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2014;20:495–500. doi: 10.1016/j.bbmt.2013.12.568. [DOI] [PubMed] [Google Scholar]
- [30].Solh M, Brunstein C, Morgan S, Weisdorf D. Platelet and red blood cell utilization and transfusion independence in umbilical cord blood and allogeneic peripheral blood hematopoietic cell transplants. Biol Blood Marrow Transplant. 2011;17:710–716. doi: 10.1016/j.bbmt.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Boyd AL, Bhatia M. Bone marrow localization and functional properties of human hematopoietic stem cells. Curr Opin Hematol. 2014 doi: 10.1097/MOH.0000000000000055. [DOI] [PubMed] [Google Scholar]
- [32].Bolan PJ, Arentsen L, Sueblinvong T, et al. Water-fat MRI for assessing changes in bone marrow composition due to radiation and chemotherapy in gynecologic cancer patients. J Magn Reson Imaging. 2013;38:1578–1584. doi: 10.1002/jmri.24071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Liu LF, Shen WJ, Ueno M, Patel S, Kraemer FB. Characterization of age-related gene expression profiling in bone marrow and epididymal adipocytes. BMC Genomics. 2011;12:212-2164–12-212. doi: 10.1186/1471-2164-12-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.