Abstract
Background
Carotid intima-media thickness (cIMT) is associated with CV events in adults. Thicker cIMT is found in youth with CV risk factors including obesity. Which risk factors have the most effect upon cIMT in youth and whether obesity has direct or indirect effects is not known. We used structural equation modeling to elucidate direct and indirect pathways through which obesity and other risk factors were associated with cIMT.
Methods
We collected demographics, anthropometrics and laboratory data on 784 subjects age 10–24 years (mean 18.0 ± 3.3 years). Common, bulb and internal carotid cIMT were measured by ultrasound. Multivariable regression analysis was performed to assess independent determinants of cIMT. Analyses were repeated with structural equation modeling to determine direct and indirect effects.
Results
Multivariable regression models explained 11%–22% of variation of cIMT. Age, sex and systolic blood pressure (BP) z-score were significant determinants of all cIMT segments. Body mass index (BMI) z-score, race, presence of type 2 diabetes mellitus (T2DM), hemoglobin A1c (HbA1c) and non-HDL were significant for some segments (all p=0.05). The largest direct effect on cIMT was age (0.312) followed by BP (0.228), Blood glucose control (0.108) and non-HDL (0.134). BMI only had a significant indirect effect through blood glucose control, BP & non-HDL. High sensitivity C-reactive protein (CRP) had a small indirect effect through blood glucose control (all p=0.05).
Conclusions
Age and BP are the major factors with direct effect on cIMT. Glucose and non-HDL were also important in this cohort with a high prevalence of T2DM. BMI only has indirect effects, through other risk factors. Traditional CV risk factors have important direct effects on cIMT in the young, but adiposity exerts its influence only through other CV risk factors.
Keywords: Carotid arteries, obesity, pediatrics, risk factors, statistics
Introduction
Risk-factor related increase in carotid intima-media thickness (cIMT) is associated with cardiovascular (CV) events in adults. (1) Strong evidence links CV risk factors measured in childhood (12 years of age and younger) with increased cIMT in adulthood. (2) Emerging data suggest that carotid thickening occurs as early as adolescence in high risk youth. (3) Which risk factors have the strongest association with cIMT in children and adolescents is less clear, with some studies suggesting blood pressure (BP), (4) others obesity, (5) or lipids having the most influence. (6) In this study, we sought to examine the pathways by which risk factors influence cIMT in adolescents and young adults (older than 18 years). Due to the complexity of the relationships between these risk factors, we applied structural equation modeling to estimate biologically plausible pathways through which CV risk factors were associated with increased cIMT. Structural equation modeling (SEM) is an extension of the general linear model taking into account the modeling of independent and correlated errors. In SEM, ‘latent’ variables are derived by modelling groups of measured variables (i.e. ‘intelligence’ cannot be directly measured but might be inferred from a set of cognitive function tests). This is performed where direct measurement of the variable may be prone to error. SEM adjusts for the error in the latent variable resulting in more unbiased estimates for the relations between all the variables as compared to general linear models. In addition to assessing direct associations between dependent and independent variables, SEM also allows for assessment of mediation and moderation (indirect associations through other factors).
Methods
Population
The population was drawn from the baseline examination of an ongoing longitudinal study. This study was designed to compare and contrast the effects of obesity and type 2 diabetes mellitus (T2DM) on the CV system. (3) All subjects age 10–24 years with T2DM (N=253) were eligible. Subjects with T2DM were matched by age, race and sex to an obese control (BMI ≥95th percentile, N=256) proven non-diabetic by oral glucose tolerance test, and a lean control (BMI < 85th%, N=275). Mean age of the cohort was 18.0 ± 3.3 years. The diagnosis of type 2 diabetes was based on the American Diabetes Association criteria. (7, 8): the participants had fasting plasma glucose levels ≥126 mg/dl or symptoms of hyperglycemia and random plasma glucose ≥200 mg/dl, or 2-hr plasma glucose ≥200 mg/dl during an oral glucose tolerance test. Written informed consent was obtained from subjects ≥18 years old or the parent or guardian for subjects <18 years old according to Institutional Review Board at Cincinnati Children’s Hospital guidelines, in accordance with the Declaration of Helsinki.
Measurements
Demographics and anthropometric data were collected and fasting blood for glucose, insulin, lipids, high sensitivity C-reactive protein (CRP) and hemoglobin A1c (HbA1c) were drawn after a 10-hour overnight fast. The mean of 2 measures of height and weight were obtained with a calibrated stadiometer (Veeder-Rood, Elizabethtown, NC) and an electronic scale (Health-O-Meter, model 770; SECA, Hanover, MD). The mean of 2 measures of blood pressure was obtained with a mercury Sphygmomanometer (W. A. Baum Co., Inc., Copiague, NY) according to the standards of the Fourth Report on Blood Pressure Control in Children. (9) The fifth Korotokoff phase was designated as diastolic BP. Laboratory techniques and reproducibility data have been published previously. (3) Coefficient of variation for repeat measures of SBP were 1.9% and for DBP 4.1%. Assays of fasting plasma lipids (total cholesterol, low-density lipoprotein cholesterol concentration (LDL), high density lipoprotein concentration (HDL) and triglycerides) were carried out in a laboratory that is National Heart, Lung, and Blood Institute/Centers for Disease Control and Prevention standardized. LDL was calculated using the Friedewald equation. Non-HDL was calculated as total cholesterol– HDL.
Carotid ultrasound was performed using B-mode ultrasonography with a GE Vivid 7 ultrasound imaging system (GE Medical Systems, Wauwatosa, WI) with a high-resolution linear array transducer with at 7.5 MHz center frequency. The far wall of each carotid segment was examined independently from all angles to identify the thickest cIMT for bilateral common (1 cm proximal to bulb), bulb and internal carotid arteries. Multiple digital image loops were transmitted using the Camtronics Medical System (Hartland, WI) for offline reading which was performed at end diastole. A manual tracing technique was employed to measure the maximum carotid thickness from the leading edge (lumen-intima) to the leading edge (medial-adventitia). Due to the young age of the cohort, few plaques were noted. Readings were not performed at the site of plaque. All images were read by a single experienced and research-trained Registered Vascular Sonographer who was blinded to subject group. The mean cIMT measures of right and left carotid segments were used in analyses. Coefficient of variation for repeat measures of IMT with our more advanced reading techniques were 3.1% for the common and bulb and 3.7% for the internal carotid artery which are improved compared to previous work by our group. (3)
Statistical Analysis
SAS 9.3 and SAS Structural Equation Modeling for JMP were used for analyses (SAS Institute Inc., Cary, NC). A two sided p <0.05 was considered significant. Categorical variables were summarized as frequency and percentage. Continuous variables were summarized as mean and standard deviation. The proportion of racial and sex groups were compared using Pearson χ2 Test. Fasting insulin, glucose, HbA1C, high density lipoprotein cholesterol concentration (HDL), LDL, triglycerides, non-high density lipoprotein cholesterol concentration (non-HDL) and cIMT values were log transformed. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) z-scores were calculated according to the Fourth Report on Blood Pressure Control in Children (9). Body mass index (BMI) z-score was calculated according to the U.S. Centers for Disease Control and Prevention. (10) Bivariate relationships between CV risk factors and carotid segments were analyzed using Pearson correlation coefficients. Multivariable regression analysis was performed to assess independent determinants of cIMT and age-group interaction. In our previous work (3) we found an age by group interaction such that there was less of an effect of age on cIMT in T2DM. Therefore, we tested for an interaction between age and recruitment group (Lean, Obese, T2DM) in these analyses. We also tested for sex interactions but none were found. The full model included group, age, age-group interaction, sex, race, BMI z-score, SBP z-score, DBP z-score, CRP, fasting lipids, insulin, glucose and HbA1c. The variance inflation factor was <5 for the model therefore, significant collinearity among SBP and DBP, Glucose and HbA1c, or Group and BMI z-score was not present.
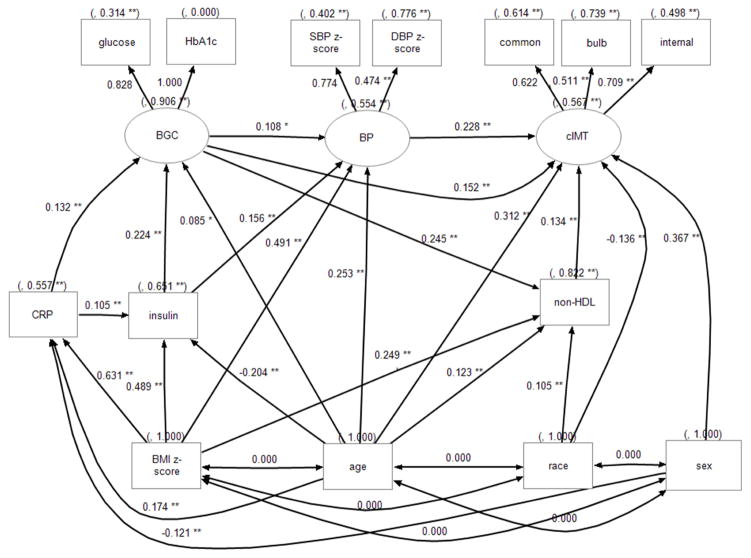
Structural equation models based on previous findings (11, 12) and theoretical rationales were tested to examine the relationships among CV risk factors (figure 1). Structural equation modeling allows measurement models and latent variables as a feature, where an outcome or factor could not be measured directly but though multiple indicators. We treated the individual segments of cIMT, BP z-scores, glucose and HbA1c as indicator (measured) variables, assuming each of them has measurement errors. Three latent variables or factors (cIMT, blood glucose control, and BP) were extracted from indicator variables to reduce error variances. For example, we can measure common, bulb and internal cIMT and obtain a mean and standard deviation (estimation of error). The SEM technique can then calculate a latent “cIMT” variable and estimate a value that explains more of the variance than explained in any of the 3 individual variables with less error. Latent variable “cIMT” was used to represent the 3 cIMT segments. Latent variable “BP” was extracted from SBP z-score and BP z-score. Latent variable “blood glucose control” was extracted from fasting glucose and HbA1c. Non-HDL was used because it consists of LDL and very low LDL which is part triglycerides. In the model structure, age, race, sex and BMI z-score were always independent variables. All other variables except indicator variables were regressed on age, race, sex and BMI z-score as well as variables lower in the pathway. A path from non-HDL to BP was proposed; however, it was removed along with all other non-significant paths in a stepwise fashion while maintaining acceptable fit comparable to the initial model. Models were also performed using full information maximum likelihood estimation, implementing regression-based imputation to take advantage of all subjects, including those with missing data. The model fit indices used for a good model included: 1) χ2/degrees of freedom<3; 2) non-normed index and comparative fit index >0.9; 3) root mean square error of approximation <0.05; 4) standardized root mean squares of residuals <0.08. Standardized effect sizes in figure 1 and table 3 were provided to allow the reader to better compare the magnitude of effect of variables that are measured with widely different units (for example a difference of 1 mmHg of BP is vastly different from 1 kg/m2 for BMI). The SEM was repeated with waist/height ratio as the measure of adiposity and no substantial differences were found (data not shown).
Figure 1.
Path diagram of cIMT and CV risk factors. Square objects indicate measured variables. Oval objects indicate latent variables. Numbers to the left of the paths are standardized beta coefficients indicating direct effect size (range −1.0 to 1.0). Numbers in parenthesis are variances. BGC = blood glucose control, Glucose = fasting glucose; Insulin = fasting insulin. *: p<0.05; ** p<0.01.
Table 3.
Standardized Direct, Indirect and Total Effects of Explanatory Variables on cIMT
| Direct effect | Indirect effect | Total effect | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Coeff | SE | P* | Coeff | SE | P* | Coeff | SE | P* | |
| (BP) | 0.2278 | 0.0514 | <.0001 | 0.2278 | 0.0514 | <.0001 | |||
| Non-HDL | 0.134 | 0.0427 | 0.0017 | 0.134 | 0.0427 | 0.0017 | |||
| (BGC) | 0.1523 | 0.043 | 0.0004 | 0.0574 | 0.0153 | 0.0002 | 0.2097 | 0.0404 | <.0001 |
| CRP | 0.0363 | 0.0108 | 0.0008 | 0.0363 | 0.0108 | 0.0008 | |||
| insulin | 0.0826 | 0.0176 | <.0001 | 0.0826 | 0.0176 | <.0001 | |||
| BMI z-score | 0.2086 | 0.0314 | <.0001 | 0.2086 | 0.0314 | <.0001 | |||
| Age | 0.3119 | 0.0405 | <.0001 | 0.0815 | 0.0173 | <.0001 | 0.3934 | 0.0386 | <.0001 |
| Race | −0.136 | 0.0396 | 0.0006 | 0.014 | 0.0064 | 0.0291 | −0.1219 | 0.0396 | 0.0021 |
| Sex | 0.3666 | 0.0379 | <.0001 | −0.0044 | 0.0017 | 0.0081 | 0.3622 | 0.038 | <.0001 |
2-sided P value. Latent Variables are in parentheses. BCG=Blood Glucose Control, The first set of columns provide the beta coefficients for direct effect from the measured or latent parameter on the latent cIMT variable (same numbers found in figure 1). The second column measures indirect effects with the final column listing the total sum of direct and indirect effects. *2-sided P value. Latent Variables are in parentheses. Only significant variables listed. BCG=Blood Glucose Control
Results
In our population (Table 1) there were more non-Caucasian and female participants. This ethnic distribution represents the demographics of subjects with T2DM in the Cincinnati area (predominantly African-American females). However, the proportion of male and female subjects did not differ by race. Pearson correlation coefficients revealed that all segments of cIMT were correlated with age, SBP and DBP z-scores, HDL (negative), LDL, non-HDL cholesterol, triglycerides, fasting glucose, and HbA1c. Bulb and internal cIMT segments were also positively correlated with BMI z-score and CRP (all p≤0.05, data not shown).
Table 1.
Description of the Study Population (N=784).
| Variables | Lean (N=275) | Obese (N=256) | T2DM (N=253) |
|---|---|---|---|
| Age (years) | 17.7 ± 3.5 | 18.1 ± 3.3 | 18.0 ± 3.2 |
| Race (Caucasian), N (%)* | 123 (45%) | 85 (33%) | 113 (45%) |
| Female, N (%) | 168 (61%) | 177 (69%) | 163 (64%) |
| Height (cm)* | 165.7 ± 11.1 | 166.6 ± 10.0 | 168.7 ± 10.1 |
| Weight (kg)* | 59.2 (50.9, 67.5) | 102.4 (89.5, 116.2) | 103.0 (84.1, 123.4) |
| BMI (kg/m2)* | 21.3 (19.6, 23.2) | 36.1 (32.0, 41.4) | 35.9 (31.4, 41.9) |
| BMI z score* | 0.045 ± 0.689 | 2.167 ± 0.349 | 2.094 ± 0.658 |
| Waist circumference (cm)* | 75.7 (71.6, 81.3) | 111.7 (102.4, 123.7) | 116.60 (102.5, 128.5) |
| Waist-height ratio* | 0.46 (0.43, 0.49) | 0.67 (0.61, 0.75) | 0.69 (0.61, 0.76) |
| Systolic blood pressure (mmHg)* | 108 (101, 115) | 117 (110, 123) | 121 (113, 129) |
| SBP z score* | −0.452 ± 0.889 | 0.365 ± 0.952 | 0.706 ± 1.075 |
| Diastolic blood pressure (mmHg)* | 61 (55, 67) | 66 (59, 75) | 67 (59, 76) |
| DBP z score* | −0.544 ± 1.055 | −0.012 ± 1.054 | 0.035 ± 1.150 |
| Total Cholesterol (mg/dL)* | 162 (141, 178) | 171 (149, 193) | 174 (150, 207) |
| LDL Cholesterol (mg/dL)* | 88 (72, 105) | 102 (85, 122) | 102 (83, 132) |
| Triglycerides (mg/dL)* | 65 (49, 88) | 86 (62, 118) | 115 (79, 168) |
| HDL Cholesterol (mg/dL)* | 56 (48, 63) | 46 (41, 52) | 42 (37, 51) |
| Fasting insulin (mIU/ml)* | 10 (8, 13) | 18 (14, 26) | 25 (15, 38) |
| Fasting glucose (mg/dl)* | 89 (85, 93) | 92 (88, 96) | 115 (93, 196) |
| HbA1c (%)* | 5.3 (5.1, 5.5) | 5.50 (5.2, 5.7) | 7.00 (5.8, 9.9) |
| C-Reactive Protein (mg/L)* | 0.4 (0.2, 0.9) | 2.75 (1.3, 6.4) | 3.50 (1.4, 7.2) |
| Common cIMT (mm)* | 0.49 (0.44, 0.56) | 0.49 (0.43, 0.54) | 0.52 (0.46, 0.59) |
| Bulb cIMT (mm)* | 0.47 (0.41, 0.54) | 0.49 (0.43, 0.56) | 0.52 (0.45, 0.60) |
| Internal cIMT (mm)* | 0.37 (0.33, 0.44) | 0.40 (0.34, 0.47) | 0.41 (0.36, 0.48) |
P ≤ 0.01 for group difference (ANOVA). Descriptive statistics are Mean ± SD or N (%) or Median (Q1, Q3).
Multivariable regression analysis
The multivariable regression models explained 19%, 11% and 22% of variation of common, bulb and internal cIMT, respectively (table 2). Age, sex and SBP z-score were significant determinants of all three cIMT segments. Non-Caucasians had thicker common and internal cIMT than Caucasians. Group (i.e. lean, obese or T2DM) was a significant determinant of common and bulb cIMT. BMI z-score was a significant determinant of bulb and internal cIMT. Group and age interaction was not significant for any cIMT segment. HbA1c was a significant determinant of common cIMT only. Non-HDL was a significant determinant of internal cIMT only (all p≤0.05). CRP was not a significant determinant of any cIMT segment.
Table 2.
Determinants of cIMT (log) Using Multivariable Regression Analysis (Beta ± SE).
| Parameter Estimate | Common | Bulb | Internal |
|---|---|---|---|
| Intercept | −0.9506 ± 0.0361‡ | −1.0057 ± 0.0426‡ | −1.3987 ± 0.0465‡ |
| Age (years) | 0.0099 ± 0.0017‡ | 0.0132 ± 0.0022‡ | 0.0170 ± 0.0022‡ |
| BMI z score | 0.0285 ± 0.0131* | 0.0186 ± 0.0075* | |
| SBP z score | 0.0191 ± 0.0059† | 0.0168 ± 0.0079* | 0.0212 ± 0.0078† |
| Non-HDL (mg/dl) | 0.0010 ± 0.0002‡ | ||
| HbA1c (%) | 0.0121 ± 0.0035‡ | ||
| Sex (Male) | 0.0957 ± 0.0120‡ | 0.0572 ± 0.0154‡ | 0.1220 ± 0.0153‡ |
| Race (Caucasian) | −0.0446 ± 0.0118‡ | −0.0484 ± 0.0150† | |
| Group Lean | Ref | Ref | |
| Group Obese | −0.0390 ± 0.0146† | −0.0368 ± 0.0324 | |
| Group T2DM | −0.0025 ± 0.0180 | 0.0160 ± 0.0319 | |
| R2 | 0.19 | 0.11 | 0.22 |
2-sided P<0.05.
p<0.01.
p<0.001. Only significant beta coefficients listed.
Structural Equation Modeling
After removing non-significant paths, structural equation modeling using maximal likelihood estimation resulted in a final model with good fit (χ2/df ratio 3.1; non-normed index =0.93; comparative fit index = 0.95; root mean square error of approximation = 0.058; standardized root mean squares of residuals = 0.045; Fig 1 and table 3).
The latent variable cIMT explained 39%, 26% and 50% of the variances of common, bulb and internal cIMT, respectively (figure shows path correlations which are squared to obtain r2 = variance). This indicates that our SEM provided a latent variable for cIMT that was highly correlated with the measured IMT variables (a robust model). Similarly, the latent variables provided by the SEM for BP, blood glucose were also highly significant signifying good model fit.
The SEM model explained 43% of variances of latent cIMT. This suggests that a greater proportion of the observed variability in IMT between subjects is accounted for by the CV risk factors included in our SEM model (43%) as compare to the general linear model (11 to 22%). The largest association with cIMT was age (total standardized effect of 0.39) mainly through direct association (0.312, table 3). Next was blood pressure control with direct association only (0.228), followed by blood glucose control with direct association and indirect association though blood pressure and non-HDL (total direct and indirect 0.210). Non-HDL had a small direct association with cIMT (o.134). There was also a sex and racial difference, with males and non-Caucasians demonstrating thicker cIMT. CRP did not have direct association with cIMT, but a small indirect association through blood glucose control. BMI only had a significant indirect association through glucose, BP and non-HDL (0.21). BMI was also associated with CRP, fasting insulin, blood glucose, non-HDL and blood pressure.
The structural equation modeling was repeated excluding subjects with T2DM. Results were similar, however the association of insulin and blood glucose with BP were not seen and there was no direct association of blood glucose with cIMT (data not shown).
Discussion
The purpose of these analyses was to evaluate independent pathways towards development of atherosclerosis in adolescent and young adults. We identified several relationships previously described in addition to novel biologically plausible pathways through which CV risk factors were associated with increased cIMT. Similar to traditional multivariable linear regression analyses performed in adults (13) and children and adolescents, (3) we found that age and BP were major factors with direct association with cIMT. We also found blood glucose had an independent direct association with cIMT in this population which was enriched with diabetic subjects. The importance of metabolic control on cIMT has been demonstrated in previous studies in adults (14) and children. (15)
However, in contrast to previous papers that employed regression modeling, our structural equation modeling approach revealed no direct association of adiposity with cIMT. BMI appears to exert its influence on cIMT through traditional CV risk factors. In addition, we found a direct association of lipids with cIMT using SEM, which has not always been seen in traditional multivariable regression. Finally, most (but not all (16)) adult studies suggest a strong association of inflammation with cIMT, (17, 18). However, most studies in pediatric populations including both children and adolescents, (3, 5, 19, 20) found no association and the CV Risk in Young Finns study showed childhood CRP did not enter traditional models exploring determinants of adult cIMT. (21) Our ability to show that inflammation has an association, although indirect, with carotid atherosclerosis may be attributed to the capability of structural equation modeling to identify complex relationships beyond those seen in traditional linear regression models.
Many adult studies have demonstrated a significant independent relationship between obesity and thicker cIMT (22, 23) with one study demonstrating that baseline BMI was an independent predictor of cIMT progression. (24) There are also data from large longitudinal cohorts demonstrating that childhood measures of adiposity predict cIMT measured in adulthood (Bogalusa Heart Study, (25) CV Risk in Young Finns Study). (21) Fewer data are available in relating adiposity to cIMT measured at a young age. In our previous study where 2/3 of the subjects with mean age 18 years were obese, we found that BMI was an independent predictor of carotid stiffness but not cIMT. (3) This lack of association of BMI with cIMT was also found in younger cohorts of children regardless of whether they were lean (26) or obese. (15, 20) A few studies found higher cIMT in obese compared to lean children and adolescents but this was only in subjects with obesity and high BP (27, 28) or the association was lost after adjustment for SBP (29) or for insulin resistance. (5) Other studies found higher cIMT in obese children and adolescents but did not perform adjustments for other CV risk factors to demonstrate an independent association. (19, 30) The few studies that found a direct, independent association of adiposity with cIMT in children and adolescents were performed in distinct ethnic populations (Chinese (31) and Mexican-American (32)) or did not include African-Americans. (33, 34) Our data suggest that in a mixed black-white population, BMI does not have a direct association with cIMT but operates through alterations in other CV risk factors. Although indirect, our result also suggest that the magnitude of the influence of BMI on carotid thickness is greater than non-HDL, and comparable to other risk factors including blood glucose control and blood pressure control.
Adult interventional trials have demonstrated that reduction of LDL-C can lead to regression of carotid atherosclerosis. (35) Conversely, higher levels of small dense LDL (36) and oxidized LDL (37) have been independently associated with progression of cIMT in adults. However, whether traditional measures of serum lipoprotein concentrations are independently associated with cIMT in adults is less clear with one study showing no correlation between cIMT and any lipid concentrations, (38) others showing negative relation to HDL-C (39, 40) and others showing positive association with LDL-C (41) or independent association only in a group with concurrent hypertension. (42) As with childhood measures of adiposity, children and adolescents with dyslipidemia have been shown to have higher cIMT as an adult in large longitudinal cohorts (43) and this seemed to be more severe if concomitant obesity is present. (44) Data where cIMT is measured in pediatric patients including both children and adolescents are fewer and these studies have found HDL-C, (26) total cholesterol (3) or triglycerides (31, 45) to be independent predictors of cIMT. Other studies of children and adolescents found no correlation between LDL-C or HDL-C and cIMT (20) even with obesity. (28) It is possible that a longer duration of exposure is needed, a more precise measure of atherogenic lipid particles is needed or advanced statistical modeling such as structural equation modeling employed in these analyses are needed to demonstrate a direct association of lipids with cIMT in young subjects.
There are limitations to our approach. For one, although BMI is an accepted and easy to measure parameter that reflects total body adiposity, Lear et al (46) found that in adults, visceral adipose tissue measured by CT scan was independently associated with cIMT. Therefore, we may have seen an independent relationship of adiposity on cIMT if we had access to a more precise measure of total fat and fat distribution. We also did not have direct measures of lipid particle size and number although we did see a direct relationship even without these parameters. Our results may also have differed if we had a direct measure of insulin sensitivity such as with glucose clamp, although insulin sensitivity (47) was not superior to HbA1c (48) in predicting carotid cIMT in adults. We would also like to acknowledge the cross-sectional nature of the study, and thus all pathways represent associations. However, our results support the previous findings in adults showing only indirect effect of fat mass on cIMT using advanced statistical approaches. (11) Finally, our sample was enriched with minorities with T2DM and therefore may provide results that are not generalizable to other populations.
In conclusion, we confirmed direct relationships between age, BP, glucose and carotid thickness in an adolescent and young adult cohort enriched for obesity and T2DM. Using the structural equation modeling technique, we also demonstrated a direct relationship between lipids and cIMT, which has not consistently demonstrated in pediatric studies using traditional statistical techniques. Most importantly, we showed that BMI affected cIMT not directly, but through other CV risk factors as one would expect from an understanding of the pathophysiology of development of atherosclerosis. We therefore recommend in future statistical evaluation of clinical study data, structural equation modeling should be added to better understand the relative importance of directly and indirectly related risk factors that play a causal role in pathways leading to sub-clinical atherosclerosis.
Highlights.
We used structural equation modeling to elucidate direct and indirect pathways through which obesity and other risk factors were associated with cIMT.
Age and BP are the major factors with direct effect on cIMT.
Glucose and non-HDL were also import in this cohort with a high prevalence of T2DM.
BMI only has indirect effects, through traditional CV risk factors.
Acknowledgments
This study was supported by NIH (NHLBI) R01 HL076269 & HL105591 and in part by US Public Health Service Grant #UL1 RR026314 from the National Center for Research Resources, NIH (CTSA grant).
We would like to thank the entire T2CVD team and the participants of the T2DVD study and their families, without whose support this study would not be possible.
Abbreviations
- BMI
Body mass index
- BP
blood pressure
- cIMT
carotid intima-media thickness
- CRP
high sensitivity C-reactive protein
- CV
cardiovascular
- DBP
diastolic blood pressure
- HbA1c
hemoglobin A1c
- HDL
high density lipoprotein cholesterol concentration
- LDL
Low-density lipoprotein cholesterol concentration
- Non-HDL
non- high density lipoprotein cholesterol concentration
- SEM
Structural equation modeling
- SBP
Systolic blood pressure
- T2DM
type 2 diabetes mellitus
Footnotes
Conflicts of interest: The authors have no other conflicts of interest or financial arrangements to disclose. The funding agencies were not involved in any aspect of planning study design, collection, analyses, and interpretation of the data or preparation of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baldassarre D, Veglia F, Hamsten A, Humphries SE, Rauramaa R, de Faire U, et al. Progression of Carotid Intima-Media Thickness as Predictor of Vascular Events: Results from the IMPROVE Study. Arteriosclerosis, Thrombosis, and Vascular Biology. 2013;33(9):2273–9. doi: 10.1161/ATVBAHA.113.301844. [DOI] [PubMed] [Google Scholar]
- 2.Juonala M, Magnussen CG, Venn A, Dwyer T, Burns TL, Davis PH, et al. Influence of age on associations between childhood risk factors and carotid intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study, the Childhood Determinants of Adult Health Study, the Bogalusa Heart Study, and the Muscatine Study for the International Childhood Cardiovascular Cohort (i3C) Consortium. Circulation. 2010;122(24):2514–20. doi: 10.1161/CIRCULATIONAHA.110.966465. [DOI] [PubMed] [Google Scholar]
- 3.Urbina EM, Kimball TR, McCoy CE, Khoury PR, Daniels SR, Dolan LM. Youth with obesity and obesity-related type 2 diabetes mellitus demonstrate abnormalities in carotid structure and function. Circulation. 2009;119(22):2913–9. doi: 10.1161/CIRCULATIONAHA.108.830380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sorof JM, Alexandrov AV, Garami Z, Turner JL, Grafe RE, Lai D, et al. Carotid ultrasonography for detection of vascular abnormalities in hypertensive children. Pediatric Nephrology. 2003;18(10):1020–4. doi: 10.1007/s00467-003-1187-0. [DOI] [PubMed] [Google Scholar]
- 5.Iannuzzi A, Licenziati MR, Acampora C, Salvatore V, Auriemma L, Romano ML, Panico S, Rubba P, Trevisan M. Increased carotid intima-media thickness and stiffness in obese children. Diabetes Care. 2004;27(10):2506–8. doi: 10.2337/diacare.27.10.2506. [DOI] [PubMed] [Google Scholar]
- 6.Shah AS, Urbina EM, Khoury PR, Kimball TR, Dolan LM. Lipids and lipoprotein ratios: Contribution to carotid intima media thickness in adolescents and young adults with type 2 diabetes mellitus. J Clin Lipidol. 2013;7(5):441–5. doi: 10.1016/j.jacl.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Standards of medical care in diabetes--2008. Diabetes Care. 2008;31(Suppl 1):S12–54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- 8.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2008;31(Suppl 1):S55–60. doi: 10.2337/dc08-S055. [DOI] [PubMed] [Google Scholar]
- 9.Foran TG, Sheahan NF, Cunningham C, Feely J. Pseudo-hypertension and arterial stiffness: a review. Physiol Meas. 2004;25(2):R21–33. doi: 10.1088/0967-3334/25/2/r02. [DOI] [PubMed] [Google Scholar]
- 10.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital and health statistics Series 11, Data from the national health survey. 2002;(246):1–190. [PubMed] [Google Scholar]
- 11.Kerkhof GF, Duivenvoorden HJ, Leunissen RW, Hokken-Koelega AC. Pathways leading to atherosclerosis: a structural equation modeling approach in young adults. Hypertension. 2011;57(2):255–60. doi: 10.1161/HYPERTENSIONAHA.110.163600. [DOI] [PubMed] [Google Scholar]
- 12.Chen W, Srinivasan SR, Berenson GS. Path analysis of metabolic syndrome components in black versus white children, adolescents, and adults: the Bogalusa Heart Study. Ann Epidemiol. 2008;18(2):85–91. doi: 10.1016/j.annepidem.2007.07.090. [DOI] [PubMed] [Google Scholar]
- 13.Psaty BM, Arnold AM, Olson J, Saad MF, Shea S, Post W, et al. Association between levels of blood pressure and measures of subclinical disease multi-ethnic study of atherosclerosis. American Journal of Hypertension. 2006;19(11):1110–7. doi: 10.1016/j.amjhyper.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Hedblad B, Nilsson P, Janzon L, Berglund G. Relation between insulin resistance and carotid intima-media thickness and stenosis in non-diabetic subjects. Results from a cross-sectional study in Malmo, Sweden. Diabetic Medicine. 2000;17(4):299–307. doi: 10.1046/j.1464-5491.2000.00280.x. [DOI] [PubMed] [Google Scholar]
- 15.Atabek ME, Pirgon O, Kivrak AS. Evidence for association between insulin resistance and premature carotid atherosclerosis in childhood obesity. Pediatric Research. 2007;61(3):345–9. doi: 10.1203/pdr.0b013e318030d206. [DOI] [PubMed] [Google Scholar]
- 16.Leinonen ES, Hiukka A, Hurt-Camejo E, Wiklund O, Sarna SS, Mattson Hulten L, Westerbacka J, Salonen RM, Salonen JT, Taskinen MR. Low-grade inflammation, endothelial activation and carotid intima-media thickness in type 2 diabetes. Journal of Internal Medicine. 2004;256(2):119–27. doi: 10.1111/j.1365-2796.2004.01350.x. [DOI] [PubMed] [Google Scholar]
- 17.Balletshofer BM, Haap M, Rittig K, Stock J, Lehn-Stefan A, Haring HU. Early carotid atherosclerosis in overweight non-diabetic individuals is associated with subclinical chronic inflammation independent of underlying insulin resistance. Hormone & Metabolic Research. 2005;37(5):331–5. doi: 10.1055/s-2005-861479. [DOI] [PubMed] [Google Scholar]
- 18.Blaha MJ, Rivera JJ, Budoff MJ, Blankstein R, Agatston A, O’Leary DH, et al. Association between obesity, high-sensitivity C-reactive protein >/=2 mg/L, and subclinical atherosclerosis: implications of JUPITER from the Multi-Ethnic Study of Atherosclerosis. Arteriosclerosis, Thrombosis & Vascular Biology. 2011;31(6):1430–8. doi: 10.1161/ATVBAHA.111.223768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyer AA, Kundt G, Steiner M, Schuff-Werner P, Kienast W. Impaired flow-mediated vasodilation, carotid artery intima-media thickening, and elevated endothelial plasma markers in obese children: the impact of cardiovascular risk factors. Pediatrics. 2006;117(5):1560–7. doi: 10.1542/peds.2005-2140. [DOI] [PubMed] [Google Scholar]
- 20.Vercoza AM, Baldisserotto M, de Los Santos CA, Poli-de-Figueiredo CE, d’Avila DO. Cardiovascular risk factors and carotid intima-media thickness in asymptomatic children. Pediatric Cardiology. 2009;30(8):1055–60. doi: 10.1007/s00246-009-9493-3. [DOI] [PubMed] [Google Scholar]
- 21.Juonala M, Raitakari MSA, Viikari J, Raitakari OT. Obesity in youth is not an independent predictor of carotid IMT in adulthood. The Cardiovascular Risk in Young Finns Study. Atherosclerosis. 2006;185(2):388–93. doi: 10.1016/j.atherosclerosis.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 22.Park J, Kim SH, Cho GY, Baik I, Kim NH, Lim HE, et al. Obesity phenotype and cardiovascular changes. Journal of Hypertension. 2011;29(9):1765–72. doi: 10.1097/HJH.0b013e32834a50f3. [DOI] [PubMed] [Google Scholar]
- 23.Blaha MJ, Rivera JJ, Budoff MJ, Blankstein R, Agatston A, O’Leary DH, et al. Association Between Obesity, High-Sensitivity C-Reactive Protein ≥2 mg/L, and Subclinical Atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2011;31(6):1430–8. doi: 10.1161/ATVBAHA.111.223768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reed D, Dwyer KM, Dwyer JH. Abdominal obesity and carotid artery wall thickness. The Los Angeles Atherosclerosis Study. International Journal of Obesity & Related Metabolic Disorders: Journal of the International Association for the Study of Obesity. 2003;27(12):1546–51. doi: 10.1038/sj.ijo.0802468. [DOI] [PubMed] [Google Scholar]
- 25.Freedman DS, Patel DA, Srinivasan SR, Chen W, Tang R, Bond MG, et al. The contribution of childhood obesity to adult carotid intima-media thickness: the Bogalusa Heart Study. International Journal of Obesity. 2008;32(5):749–56. doi: 10.1038/sj.ijo.0803798. [DOI] [PubMed] [Google Scholar]
- 26.Ayer JG, Harmer JA, Nakhla S, Xuan W, Ng MKC, Raitakari OT, et al. HDL-cholesterol, blood pressure, and asymmetric dimethylarginine are significantly associated with arterial wall thickness in children. Arteriosclerosis, Thrombosis & Vascular Biology. 2009;29(6):943–9. doi: 10.1161/ATVBAHA.109.184184. [DOI] [PubMed] [Google Scholar]
- 27.Stabouli S, Kotsis V, Papamichael C, Constantopoulos A, Zakopoulos N. Adolescent obesity is associated with high ambulatory blood pressure and increased carotid intimal-medial thickness. Journal of Pediatrics. 2005;147(5):651–6. doi: 10.1016/j.jpeds.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 28.Glowinska-Olszewska B, Tolwinska J, Urban M. Relationship between endothelial dysfunction, carotid artery intima media thickness and circulating markers of vascular inflammation in obese hypertensive children and adolescents. Journal of Pediatric Endocrinology. 2007;20(10):1125–36. [PubMed] [Google Scholar]
- 29.Geerts CC, Evelein AMV, Bots ML, van der Ent CK, Grobbee DE, Uiterwaal CSPM. Body fat distribution and early arterial changes in healthy 5-year-old children. Annals of Medicine. 2012;44(4):350–9. doi: 10.3109/07853890.2011.558520. [DOI] [PubMed] [Google Scholar]
- 30.Kotb NA, Gaber R, Salama M, Nagy HM, Elhendy A. Clinical and biochemical predictors of increased carotid intima-media thickness in overweight and obese adolescents with type 2 diabetes. Diabetes & Vascular Disease Research. 2012;9(1):35–41. doi: 10.1177/1479164111421804. [DOI] [PubMed] [Google Scholar]
- 31.Huang K, Zou CC, Yang XZ, Chen XQ, Liang L. Carotid intima-media thickness and serum endothelial marker levels in obese children with metabolic syndrome. Archives of Pediatrics & Adolescent Medicine. 2010;164(9):846–51. doi: 10.1001/archpediatrics.2010.160. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez-Enriquez GV, Rubio-Benitez MI, Garcia-Gallegos V, Portilla-de Buen E, Troyo-Sanroman R, Leal-Cortes CA. Contribution of TNF-308A and CCL2-2518A to carotid intima-media thickness in obese mexican children and adolescents. Archives of Medical Research. 2008;39(8):753–9. doi: 10.1016/j.arcmed.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 33.Reinehr T, Kiess W, de Sousa G, Stoffel-Wagner B, Wunsch R. Intima media thickness in childhood obesity: relations to inflammatory marker, glucose metabolism, and blood pressure. Metabolism: Clinical & Experimental. 2006;55(1):113–8. doi: 10.1016/j.metabol.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 34.Giannini C, de Giorgis T, Scarinci A, Ciampani M, Marcovecchio ML, Chiarelli F, Mohn A. Obese related effects of inflammatory markers and insulin resistance on increased carotid intima media thickness in pre-pubertal children. Atherosclerosis. 2008;197(1):448–56. doi: 10.1016/j.atherosclerosis.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 35.Taylor AJ, Kent SM, Flaherty PJ, Coyle LC, Markwood TT, Vernalis MN. ARBITER: Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol: a randomized trial comparing the effects of atorvastatin and pravastatin on carotid intima medial thickness. Circulation. 2002;106(16):2055–60. doi: 10.1161/01.cir.0000034508.55617.65. [DOI] [PubMed] [Google Scholar]
- 36.Norata GD, Raselli S, Grigore L, Garlaschelli K, Vianello D, Bertocco S, et al. Small dense LDL and VLDL predict common carotid artery IMT and elicit an inflammatory response in peripheral blood mononuclear and endothelial cells. Atherosclerosis. 2009;206(2):556–62. doi: 10.1016/j.atherosclerosis.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 37.Lopes-Virella MF, Hunt KJ, Baker NL, Lachin J, Nathan DM, Virella G. Levels of oxidized LDL and advanced glycation end products-modified LDL in circulating immune complexes are strongly associated with increased levels of carotid intima-media thickness and its progression in type 1 diabetes. Diabetes. 2011;60(2):582–9. doi: 10.2337/db10-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zanchetti A, Crepaldi G, Bond MG, Gallus GV, Veglia F, Ventura A, et al. Systolic and pulse blood pressures (but not diastolic blood pressure and serum cholesterol) are associated with alterations in carotid intima-media thickness in the moderately hypercholesterolaemic hypertensive patients of the Plaque Hypertension Lipid Lowering Italian Study. PHYLLIS study group. Journal of Hypertension. 2001;19(1):79–88. doi: 10.1097/00004872-200101000-00011. [DOI] [PubMed] [Google Scholar]
- 39.Fan AZ, Dwyer JH. Sex differences in the relation of HDL cholesterol to progression of carotid intima-media thickness: the Los Angeles Atherosclerosis Study. Atherosclerosis. 2007;195(1):e191–6. doi: 10.1016/j.atherosclerosis.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 40.Viscogliosi G, Andreozzi P, Marigliano V. Associations of plasma glucose levels and traits of metabolic syndrome with carotid intima media thickness in nondiabetic elderly subjects: are they mediated by insulin resistance? Metab Syndr Relat Disord. 2013;11(1):41–5. doi: 10.1089/met.2012.0087. [DOI] [PubMed] [Google Scholar]
- 41.Wang P-W, Liou C-W, Wang S-T, Eng H-L, Liu R-T, Tung S-C, et al. Relative impact of low-density lipoprotein-cholesterol concentration and insulin resistance on carotid wall thickening in nondiabetic, normotensive volunteers. Metabolism: Clinical & Experimental. 2002;51(2):255–9. doi: 10.1053/meta.2002.29998. [DOI] [PubMed] [Google Scholar]
- 42.Sun P, Dwyer KM, Merz CN, Sun W, Johnson CA, Shircore AM, et al. Blood pressure, LDL cholesterol, and intima-media thickness: a test of the “response to injury” hypothesis of atherosclerosis. Arteriosclerosis, Thrombosis & Vascular Biology. 2000;20(8):2005–10. doi: 10.1161/01.atv.20.8.2005. [DOI] [PubMed] [Google Scholar]
- 43.Juonala M, Viikari JS, Ronnemaa T, Marniemi J, Jula A, Loo BM, et al. Associations of dyslipidemias from childhood to adulthood with carotid intima-media thickness, elasticity, and brachial flow-mediated dilatation in adulthood: the Cardiovascular Risk in Young Finns Study. Arterioscler Thromb Vasc Biol. 2008;28(5):1012–7. doi: 10.1161/ATVBAHA.108.163329. [DOI] [PubMed] [Google Scholar]
- 44.Magnussen CG, Venn A, Thomson R, Juonala M, Srinivasan SR, Viikari JS, et al. The association of pediatric low- and high-density lipoprotein cholesterol dyslipidemia classifications and change in dyslipidemia status with carotid intima-media thickness in adulthood evidence from the cardiovascular risk in Young Finns study, the Bogalusa Heart study, and the CDAH (Childhood Determinants of Adult Health) study. J Am Coll Cardiol. 2009;53(10):860–9. doi: 10.1016/j.jacc.2008.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fang J, Zhang JP, Luo CX, Yu XM, Lv LQ. Carotid Intima-media thickness in childhood and adolescent obesity relations to abdominal obesity, high triglyceride level and insulin resistance. International Journal of Medical Sciences. 2010;7(5):278–83. doi: 10.7150/ijms.7.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lear SA, Humphries KH, Kohli S, Frohlich JJ, Birmingham CL, Mancini GBJ. Visceral adipose tissue, a potential risk factor for carotid atherosclerosis: results of the Multicultural Community Health Assessment Trial (M-CHAT) Stroke. 2007;38(9):2422–9. doi: 10.1161/STROKEAHA.107.484113. [DOI] [PubMed] [Google Scholar]
- 47.Larsson H, Berglund G, Ahren B. Insulin sensitivity, insulin secretion, and glucose tolerance versus intima-media thickness in nondiabetic postmenopausal women. Journal of Clinical Endocrinology & Metabolism. 2003;88(10):4791–7. doi: 10.1210/jc.2003-030329. [DOI] [PubMed] [Google Scholar]
- 48.Larsen JR, Brekke M, Bergengen L, Sandvik L, Arnesen H, Hanssen KF, et al. Mean HbA1c over 18 years predicts carotid intima media thickness in women with type 1 diabetes. Diabetologia. 2005;48(4):776–9. doi: 10.1007/s00125-005-1700-z. [DOI] [PubMed] [Google Scholar]