Abstract
Non-alcoholic steatohepatitis (NASH) affects 3–5% of the U. S. population having severe clinical complications to the development of fibrosis and end-stage liver diseases such as cirrhosis and hepatocellular carcinoma. A critical cause of NASH is chronic systemic inflammation promoted by innate immune cells such as liver macrophages (Mϕ) and natural killer (NK) cells. However, little is known about how the crosstalk between Mϕ and NK cells contributes to regulate NASH progression to fibrosis. In this report, we demonstrate that NKp46+ cells play an important role in preventing NASH progression to fibrosis by regulating M1/M2 polarization of liver Mϕ. Using a murine model of NASH, we demonstrate that DX5+NKp46+ NK cells are increased during disease and play a role in polarizing Mϕ toward M1-like phenotypes. This NK’s immunoregulatory function depends on the production of IFN-γ but not by granzyme-mediated cytolytic activity. Notably, depletion of NKp46+ cells promote the development of fibrosis with increased expression of profibrogenic genes as well as skewed M2 Mϕ phenotypes in hepatic tissues.
Conclusion
NK cell-derived IFN-γ may be essential for maintaining a balanced inflammatory environment that promotes tissue integrity and limiting NASH progression to fibrosis.
Keywords: NASH, fibrosis, inflammation, NK cells, macrophages, IFN-γ
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a major human health problem related to obesity, with complications of insulin resistance, type 2 diabetes, and cardiovascular disease. NAFLD affects about one third of the population in developed countries1. The hallmark of NAFLD is steatosis, the accumulation of lipids within hepatocytes, which can progress to nonalcoholic steatohepatitis (NASH). NASH is a chronic inflammatory disease leading to the development of fibrosis, cirrhosis and hepatocellular carcinoma2, 3. The inflammation is a key factor to facilitate the progression of the disease and is initiated by the release of damage-associated molecular patterns (DAMPs) by injured fatty hepatocytes4. Moreover, accumulation of DAMPs within the liver microenvironment promotes activation of tissue resident Mϕ, i.e. Kupffer cells, which in turn recruit inflammatory monocytes from the blood. Recruited CD11b+Ly6Chi monocytes into the fatty liver trigger local inflammatory responses including the production of TNF-α and depletion of Mϕ or silencing of myeloid cell-specific TNF-α expression prevents development of the disease in a murine NASH model5. Recruited monocytes are then further differentiated into M1 or M2 Mϕ under the inflammatory condition during liver injury: M1 Mϕ are activated by IFN-γ and/or microbial products whereas M2 Mϕ are induced by IL-4, IL-10, IL-13, or glucocorticoids6, 7. At the early of NASH, recruited monocytes are differentiated into pro-inflammatory Mϕ, but switch to an M2-like phenotype during the resolution of tissue damage and tissue repair8, 9. However, functional heterogeneity is a hallmark of Mϕ activation10–12 and Mϕ activation in vivo exhibits a wide range of complex phenotypes13–15. As a result, the fate and function of infiltrating monocytes as well as a balance between M1 and M2 Mϕ activation might be pivotal in regulating NASH pathogenesis and progression to fibrosis. However, the mechanisms underlying Mϕ activation during NASH are not well defined.
Natural killer (NK) cells are innate immune cells to detect infected, transformed or stressed cells, resulting in lysing cells via release of perforin, granzyme, or TRAIL, and regulating inflammatory responses by secretion of IFN-γ16–19. NK cells play a pivotal role in the liver, where they kill activated HSCs to curtail the development of fibrosis20 or regulate fibrosis by modulating the fibrogenic properties of other liver-resident immune cells19. NK cell activation is triggered by numerous ways including inflammatory cytokines such as IL-12, IL-15 and IL-1819. Then the intracellular signaling of NK cell activation occurs through integration of signaling from activating (e.g. NKp46, NKG2D) and inhibitory (e.g. NKG2A) receptors21. While the ligands of NKG2D are MICA/B and ULBP in humans and RAE-1 and MULTI in mice, universal ligands of NKp46 remain unknown22. For instance, NKp46 can recognize viral hemagglutinin on infected cells; however, its ligand on tumor cells is still unidentified23. A recent report showed that NK cells co-cultured with apoptotic debris upregulated the expression of NKp46, suggesting the expression of NKp46 ligand by dying cells24. Thus, apoptotic hepatocytes in NASH may activate NK cells via a potential interaction with NKp46, which in turn can shape the quality and quantity of the local inflammatory responses.
However, the immunoregulatory role of NK cells is complex as they can inhibit or enhance the magnitude of inflammation, and not fully understood during liver injury. This contradictory role may in part be explained by unique NK cell subsets present in the liver, which are known to be phenotypically and functionally different from splenic and peripheral blood NK cells17, 25. Recently, NK cells were designated as group 1 innate lymphoid cells (ILCs), which also include type 1 ILCs (ILC1s)26–28. The liver contains both ILC1s and conventional NK (cNK) cells that are distinguished by the expression of the integrins CD49a and DX5, respectively. ILC1s are NKp46+CD49a+DX5− cells, while cNK cells are NKp46+CD49a−DX5+ cells18. Until the report that ILC1s were a lineage distinct from cNK cells, they were characterized as liver-resident NK cells having more cytotoxic but less robust at cytokine production than NK cells found in the blood and spleen17. Importantly, peripheral and tissue resident NK cells have distinct functions during various stages of inflammation19. However, the respective contribution of liver-resident ILC1s and peripheral NK cells recruited to the liver during inflammation has not been determined in NASH. The understanding of NK-mediated immunoregulatory function may provide key insights into liver-specific cellular and molecular mechanisms for the liver disease progression.
In this report, we investigated the crosstalk between NKp46+ NK cells and Mϕ to understand how innate immune responses in the liver contribute to the pathogenesis of NASH. As Mϕ are the main producers of TGF-β in the liver, we hypothesized that NK cells regulate NASH to fibrosis progression by influencing Mϕ activation, leading to modulate the local TGF-β production for activating HSCs with collagen deposition. Here, using a murine model of NASH, we demonstrate that NKp46+DX5+ NK cells prevent fibrogenesis through the production of IFN-γ that differentiates Mϕ toward M1-like phenotypes and away from M2-like phenotypes.
Methods
Mice and diets
All experiments were performed on 6 to 12-week-old-female C57BL/6 mice (Taconic Farms). Mice were fed with a methionine and choline deficient (MCD) diet (TD.90262, Harlan laboratories) or a control diet (TD.94149). This murine model of NASH was previously characterized (Suppl. Fig. 1). Depletion of NKp46 cells was performed in NDE transgenic mice expressing the diphtheria toxin receptor (DTR) specifically in NK cells (gift from Dr. Eric Viver, CIML)29. Each DT injection depletes NK cells for 6 days. Therefore, NK cells were depleted by four injections of diphtheria toxin (DT, 0.5µg/mouse; Sigma-Aldrich) given 1 day before starting diet, on 1 day, 6 and 17 days of diet. To examine the off-target effects of DT, DT-injected C57BL/6 mice were used as controls. All mice were kept in a pathogen-free facility at the University of Virginia, and were handled according to mouse protocols approved by the UVA Animal Care and Use Committee.
Isolation of hepatic mononuclear cells
After measuring weight of PBS-flushed liver, tissues were collected for histology and gene expression. The remaining liver tissue was perfused with PBS/0.05% collagenase. Hepatic mononuclear leukocytes were prepared as previously described5. Mononuclear cells were washed and processed for FACS staining or for enrichment of DX5+ cells using anti-mouse DX5+ microbeads (Miltenyi, Biotec).
Mϕ polarization
264.1RAW cells were plated at 80,000 cells/ml overnight in 24-well plates. Mϕ were then differentiated for 2h either with IL-12/IL-18 (100ng/ml each), IFN-γ (10ng/ml), LPS (100ng/ml), LPS/ IFN-γ (100ng/ml/10ng/ml), or IL-4/IL-13 (20ng/ml/10ng/ml) in DMEM medium (10% FBS). After three PBS washes, RNA was extracted with RNeasy plus kit (Qiagen) and qPCR were performed.
Mϕ and DX5+ NK cells co-cultures
264.1RAW cells were plated at 80,000 cells/ml in 24-well plates overnight. On next day, DX5+ NK cells isolated from CT diet or MCD diet-fed mice were added to Mϕ at 1:1 ratio in presence of IL-12/IL-18 (100ng/ml each) in IMDM media (10% HyClone FBS, 2mM L-glutamine, 10U/ml Penicillin/streptomycin, 50µM β-mercaptoethanol). For in vitro IFN-γ blocking experiments, anti-IFN-γ antibodies were added to co-cultures at 2µg/ml, with rat IgG1,k as isotype control antibody. Positive controls for M2-polarized Mϕ were induced by the addition of IL-4/IL-13 (20ng/ml/10ng/ml). Positive controls for M1-like Mϕ were generated by the addition of LPS (100ng/ml). After 18h of co-culture, NK cells were removed by three PBS washes. Then, Mϕ’s RNA was prepared for qPCR of M1 and M2 Mϕ respective markers, nos2 and arg genes.
Flow cytometry and TNF-α, IFN-γ, and CD107a
Liver mononuclear cells were incubated with anti-CD16/CD32 (2.4G2; University of Virginia) to block Fc receptors, and then with specific antibodies against cell surface proteins: CD45 (30F11), NK1.1 (PK136), F4/80 (BM8) from eBioscience; CD11b (M1/70), CD3 (145-2C11), CD19 (MB19-1), Ly6C (AL-21) from BD Biosciences. For TNF-α and IFN-γ intracellular staining, mononuclear cells were incubated in presence of 1µl/ml GolgiStop/GolgiPlug at 37°C for 5h in presence of 10ng/ml of IL-12 and IL-18. Following surface staining (CD45, NKp46, CD3, and CD19), cells were then re-suspended into Cytofix/Cytoperm buffer (BD Biosciences), and stained for intracellular TNF-α (MP6-XT22, eBioscience), IFN-γ (XMG1.2, ebioscience) and isotype controls (Rat IgG1K). For CD107a/LAMP-1 staining, liver mononuclear cells were incubated with 10ng/ml of IL12/IL18 and 1µl/ml GolgiStop/GolgiPlug in presence of anti-mouse CD107a antibodies for 5h, and then surface stained for CD45, NKp46, CD3, and CD19. FACS compensations were done with BD Biosciences compensation-bead based single-colors. Isotype controls and FMOs (Fluorescence Minus One) were used for setting the gates. Cell number represents the cell number of the population of interest normalized to liver weight (g).
Statistics
Unpaired student’s t test (2 tailed) or Mann Whitney test were used for all analysis. p< 0.05 was considered statistically significant. *, **, and *** indicate p<0.05, p<0.01, and p<0.001, respectively.
Results
NKp46+DX5+ NK cells are selectively activated in the liver during NASH
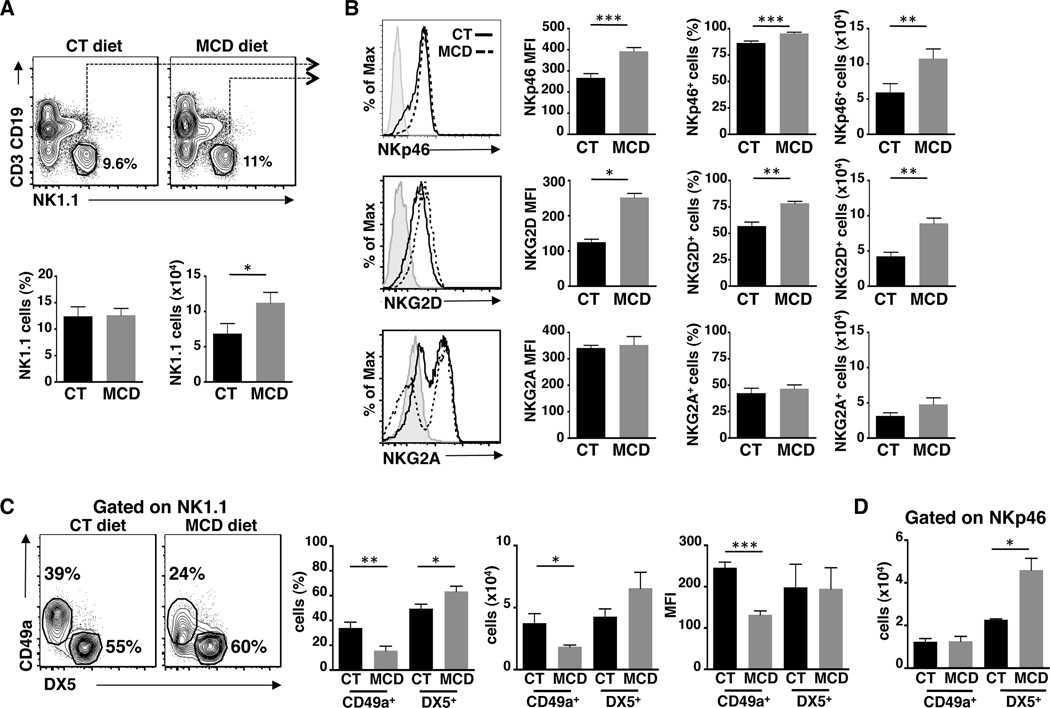
Metabolic insults in fatty liver disease induce chronic inflammatory responses that drive the development of fibrosis, often leading to hepatocellular carcinoma. NK cells represent the largest population of hepatic lymphocytes and are important sentinels, surveying damaged cells. As liver-resident Mϕ also contribute to the immunosurveillance of hepatic tissues, we hypothesized that crosstalk between NK cells and Mϕ might contribute to the pathogenesis of NASH. To this end, we employed a murine model of NASH, in which mice are fed a diet enriched in sucrose and fat, but deficient in methionine and choline30. We then examined changes in the number and frequency of NK1.1+ cells, which mark group 1 ILCs, including ILC1s and cNK cells. Although there were no differences in the frequency of NK1.1+ cells between mice fed with control or MCD diet, the absolute number of group 1 ILCs was slightly increased after 17 days on the MCD diet (Fig. 1A). In parallel, Mϕ cell number and frequency also increased over the time (D8, 10, 15, 20) in MCD-diet fed mice compared to control-diet fed mice (Suppl. Fig 2A and 2B). We next determined the activation status of group 1 ILCs, which is shaped by the complex integration of activating and inhibitory signals transduced by NK cell receptors. Expression of the activating receptors NKp46 and NKG2D was increased in NK1.1+ cells after 17 days of MCD diet compared to controls in intensity of expression (MFI), frequency, and cell number (Fig. 1B). In contrast, expression of the inhibitory NK cell receptor, NKG2A, was comparable between both groups of mice.
Figure 1. Increased activation of NK1.1 cells during NASH development.
(A) FACS plots, frequency and numbers of NK1.1+ cells within CD45+CD3−CD19− liver leukocytes isolated from C57BL/6 mice fed for 17 days of control (CT) or methionine and choline deficient (MCD) diet (total of n=4–5 mice per diet). (B) The intensity of expression (MFI), frequency and cells numbers of NK cells expressing of NK activating receptors (NKp46 and NKG2D) and NK inhibitory receptor (NKG2A/CD94) within NK1.1+CD3−CD19−CD45+ liver leukocytes (total of n=4–8 mice per diet). (C) FACS plots, frequency and numbers of liver tissue resident/ILC1 (CD49a+), and conventional (DX5+) NK cells within CD3−CD19−NK1.1+ cells. (D) Expression of DX5 within NKp46+CD19−CD3−CD45+ liver leukocytes. Black bars, CT diet; gray bars, MCD diet. *p<0.05, **p<0.01 and ***p<0.001 as determined by two-tailed t-test.
The liver contains two transcriptionally distinct group 1 ILCs: NK cells characterized by the expression of DX5 and ILC1s marked by CD49a expression. Importantly, DX5 expression is a marker of cNK cells and serves as marker for NK cells trafficked to the liver from the spleen and blood. To further characterize changes in group 1 ILC subsets in the liver during NASH, we evaluated the expression of CD49a and DX5 on NK1.1+ cells at D17 of MCD and control diets. Surprisingly, the MCD diet induced a loss of CD49a+ ILC1s with a concurrent increase of DX5+ cNK cells in frequency, cell number and MFI (Fig. 1C). These results indicate that conventional DX5+NK1.1+ cells are recruited to liver and possibly replace the function of CD49a+NK1.1+ ILC1s. Moreover, when we examined the expression of CD49a and DX5 on NKp46+ cells in NASH mice compared to control mice, the majority of NKp46+ cells were DX5+ cNK cells derived from the periphery (Fig. 1D). These data suggest that during NASH, inflammatory conditions in the liver play a pivotal role in recruiting and preferentially activating cNK cells.
NKp46+ NK cells increase the production of IFN-γ but not the cytolytic activity in NASH progression
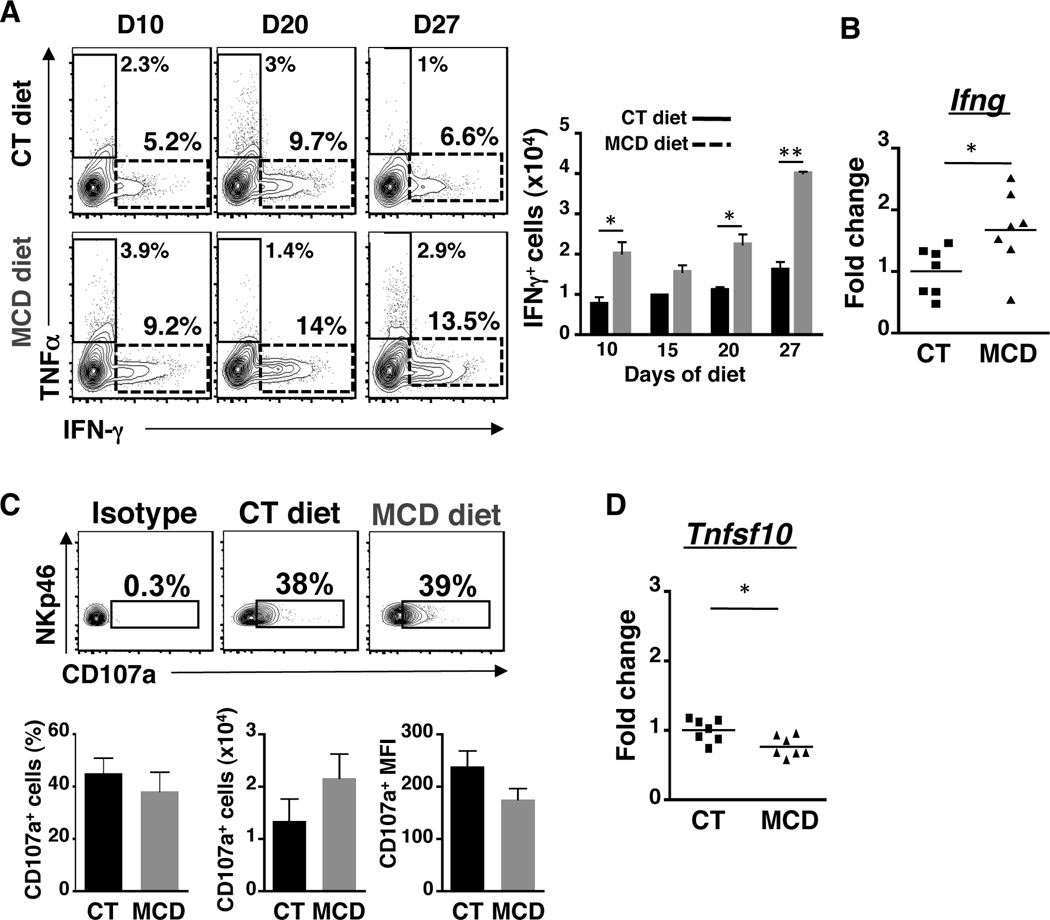
Effector functions of NK cells include cytolytic activity against target cells and production of inflammatory cytokines. We assessed these effector functions in NKp46+ cells that appear to be recruited to the inflamed liver during NASH and found that the number of IFN-γ-producing NKp46+ cells is increased during MCD diet (Fig. 2A). The increase in IFN-γ was not due to a universal increase in cytokines as production of TNF-α was minimal and comparable between MCD and control diet fed mice. The increase in IFN-γ production was confirmed at the transcriptional level as gene expression of Ifng was increased in DX5-enriched NK cells isolated from MCD diet-fed mice (Fig. 2B). However, there was no change in cytotoxicity by the NK cells during NASH progression as surface expression of the degranulation marker, CD107a, on CD3−CD19−NKp46+ cells was similar in frequency, number, and MFI between control and NASH mice (Fig. 2C). Moreover, we did not observe increased expression of Granzyme B on CD3−CD19−NKp46+ cells, or significant change in expression of hepatic Granzyme B and perforin genes (Suppl. Fig. 3A and 3B). Furthermore, expression of Tnfsf10 (TRAIL gene) in DX5-enriched NK cells was slightly reduced in NASH mice compared to controls (Fig. 2D). Taken together, these data demonstrate that NASH stimulates activated NKp46+ NK cells to produce IFN-γ without affecting their cytolytic function.
Figure 2. Increased IFN-γ production by NKp46+ NK cells in NASH.
(A) FACS plots of NKp46+ NK cells expressing TNF-α or IFN-γ within CD45+CD3−CD19− liver leukocytes isolated from mice fed for 10, 20 and 27 days of CT or MCD diet (total of n=4–5 mice per diet). Histogram showed the number of NKp46+ cells expressing IFN-γ from 0 to 27 days. (B) Gene expression of Ifng (IFN-γ) in DX5+ cells from mice fed with CT or MCD diet for 17 days. (C) FACS plots, frequency and number of NKp46+ cells expressing CD107a/LAMP-1 (D) Gene expression of Tnfsf10 (Trail) in DX5+ cells from mice fed with CT or MCD diet for 17 days. Black bars, CT diet; gray bars, MCD diet. *p<0.05, **p<0.01 and ***p<0.001 as determined by two-tailed t-test.
Depletion of NKp46+ NK cells exacerbates NASH to hepatic fibrosis progression
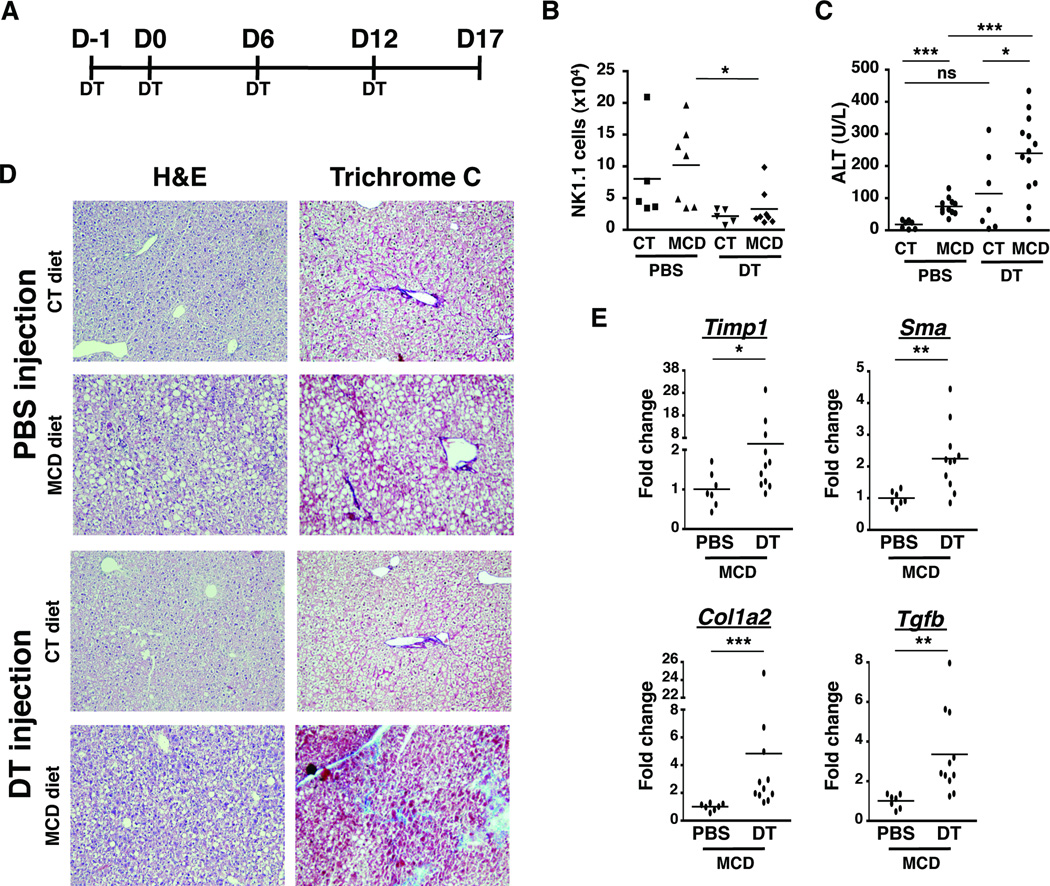
To address the function of IFN-γ+NKp46+ NK cells in the NASH development, we used NDE transgenic mice that express the diphtheria toxin (DT) receptor under the NKp46 promoter. Intraperitoneal injection of DT at D-1, 0, 6, and 12 of diet in NDE mice allowed us to specifically deplete NKp46+ cells, which include the IFN-γ-producing DX5+NK cells that infiltrate the liver during NASH (Fig. 3A). We confirmed that this regimen of DT treatment sufficiently depleted NK cells in both control and MCD-diet fed mice (Fig. 3B). Next, we assessed liver damage by measuring serum levels of the liver enzyme alanine transferase (ALT) in the blood of mice fed with control or MCD diet for 17 days and treated with PBS or DT. Serum ALT was increased in mice that developed NASH and was further exacerbated in the absence of NK cells (Fig. 3C). In order to visualize the increase in liver damage, we performed histological analysis by H&E and trichrome C staining of liver tissues. As expected, livers from MCD-diet fed mice showed evidence of steatosis characterized by areas of lipid accumulation (Fig. 3D). Importantly, NKp46-depleted MCD diet-fed mice had prominent areas of collagen deposition as detected by trichrome C staining. This early indication of fibrogenesis was accompanied by enhanced expression of profibrogenic genes such as timp-1, sma, col1a2, and tgfb (Fig. 3E). These results were not due to secondary effects of DT injections because increased liver damage, fibrogenic gene expression, and loss of NK cells were not observed in DT-treated non-transgenic mice fed with MCD diet for 17 days (Suppl. Fig. 4). Collectively, these data underscore the anti-fibrogenic function of NKp46+ NK cells in regulating the progression of disease in NASH.
Figure 3. Depletion of NKp46+ cells exacerbates NASH to hepatic fibrosis progression.
(A) NDE transgenic mice fed with CT or MCD diet for 17 days were treated either with 4 injections of PBS or Diphtheria Toxin (DT). (B) The depletion of NK1.1 cells was assessed by flow cytometry and the number of hepatic NK cells from CT or MCD diet fed-mice treated with PBS or DT was quantified. (C) Serum alanine aminotransferase (ALT) was measured. *p<0.05, **p<0.01 and ***p<0.001 as determined by two-tailed t-test. (D) H&E-stained and Trichrome C-stained tissue sections were examined under bright field microscope at 200X magnification. (E) Expression of fibrotic markers (Timp-1, Sma, Col1a2, and Tgf-b) was determined by real-time PCR in whole liver samples from MCD diet-fed mice injected with PBS or DT. The RNA level is expressed as fold induction compared to PBS-treated mice. *p<0.05, **p<0.01 and ***p<0.001 as determined by Mann Whitney test.
Loss of NKp46+ NK cells results in increased hepatic cell death, inflammation and expression of danger signals
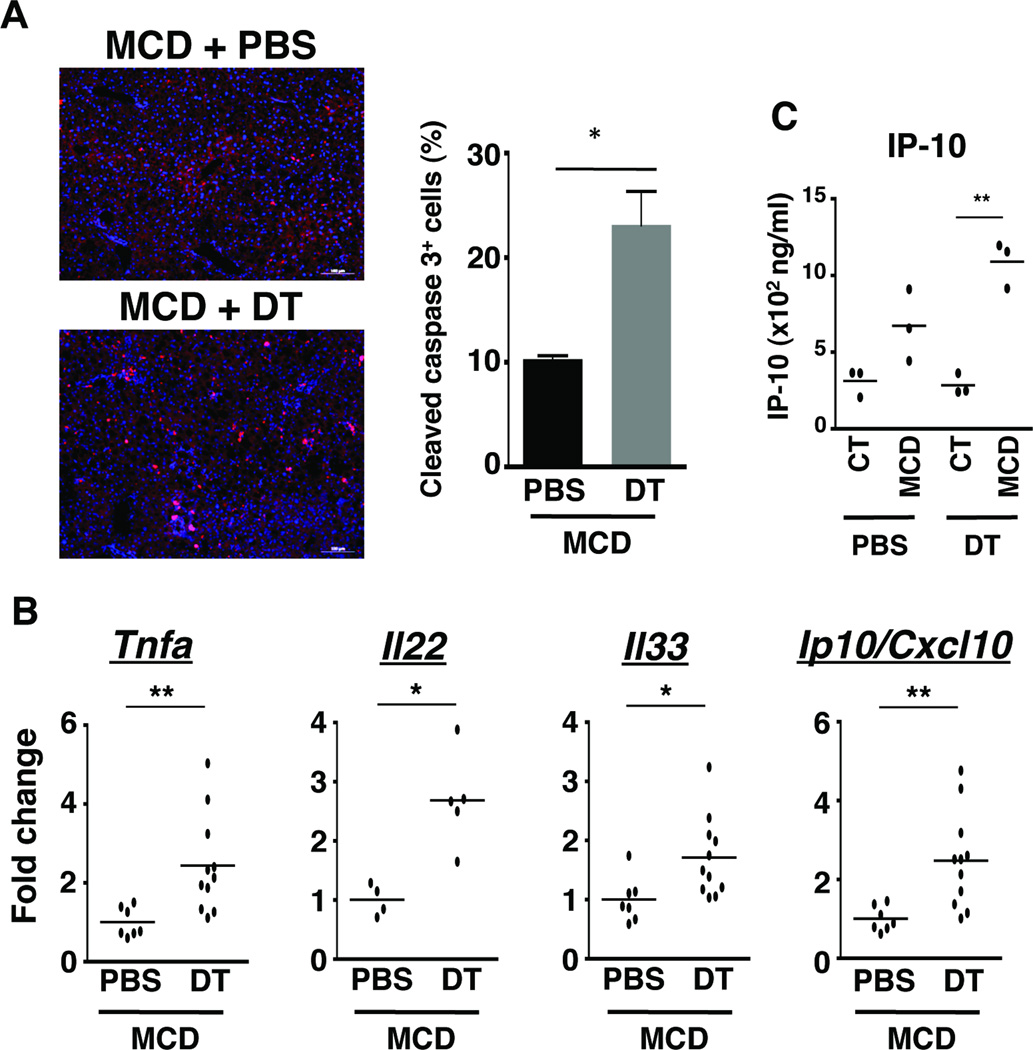
Fibrosis develops as a result of hepatocellular injury leading to cell death and release of DAMPs that trigger chronic inflammatory responses. Given that the loss of NKp46+ cells strongly enhanced the formation of fibrosis in mice fed with MCD diet (Fig. 3), we evaluated cell death and inflammation in liver tissue as additional signs of injury. Substantiating the elevated levels of serum ALT indicative of hepatocyte damage, the number of apoptotic cells was increased in liver tissues from NKp46-depleted MCD diet-fed mice (Fig. 4A). The increase in cell death was complemented by an increase in gene expression of the pro-inflammatory cytokine, TNF-α, upon depletion of NK cells (Fig. 4B). Moreover, transcription of Il22 was increased 2-fold in the absence of NK cells in NASH; IL-22 plays a protective role in liver inflammation as it limits damage and promotes tissue regeneration through hepatocyte proliferation (Fig. 4B). The cytokine IL-33 is classified as an alarmin, as it is released upon injury. IL-33 is a key mediator of fibrosis in a variety of tissues including the lung, liver, heart, kidneys, pancreas, and skin31. Hence, we examined whether the loss of NKp46+ cells might upregulate the expression of IL-33, which in turn, would accelerate the development of hepatic fibrosis. Indeed, we observed a significant increase in Il33 gene expression in livers isolated from MCD diet-fed mice after DT treatment (Fig. 4B). Finally, we determined the level of expression of IFN-γ-inducible protein Ip10/cxcl10, a chemokine that attracts immune cells including monocytes/macrophages and NK cells to site of inflammation, and is also positively associated with the severity of liver inflammation and fibrosis. Indeed, the expression of Ip10/cxcl10 gene was significantly increased at the mRNA (Fig. 4B) and protein level (Fig. 4C) in MCD diet-fed mice after NKp46 depletion when compared to PBS treated counterparts. NK cells thus function in a protective capacity during NASH as their loss leads to increased cell death and dysregulated inflammatory responses in the liver.
Figure 4. The loss of NKp46+ cells exacerbates cell death and inflammation in NASH.
(A) Liver sections from mice treated with PBS or DT and fed with MCD diet for 17 days were stained with DAPI (nucleus, blue) and with antibody against cleaved caspase 3 (red). Graph shows the percentage of cleaved caspase 3-positive cells (apoptotic cells). *p<0.05 as determined by two-tailed t-test. (B) Hepatic expression of transcripts of Tnfa, Il22, IL33 and Ip10/Cxcl10 were determined by real time PCR from liver samples of MCD diet-fed mice treated with PBS or DT. The RNA level is expressed as fold increase compared with control diet. *p<0.05, and **p<0.01 as determined by Mann Whitney test. (C) IP-10/CXCL10 from whole liver cell lysates of CT diet- and MCD diet-fed mice treated with PBS or DT. *p<0.05 as determined by two-tailed t-test.
Regulatory T cell frequency and numbers are not affected by the loss of NKp46+ NK cells
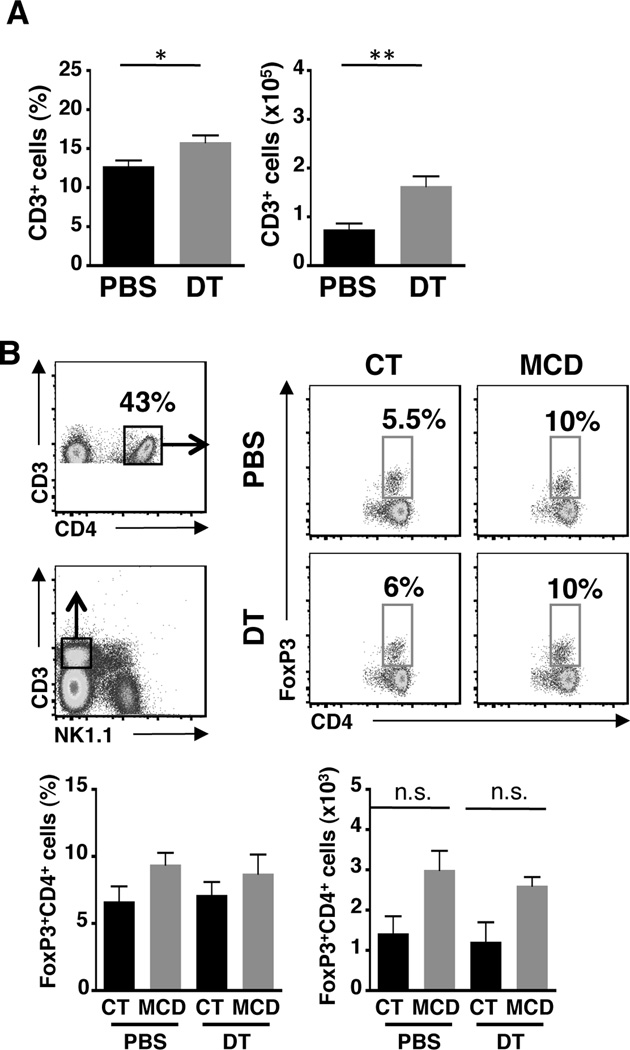
Regulatory T cells (Tregs) are known to play a protective role in chronic liver disease by subduing extensive inflammatory responses32. A recent study demonstrated that Tregs attenuate TGF-β-induced lung fibrosis33. In addition, dendritic cells also limit fibrosis-associated inflammation through the expansion of Tregs14. To determine whether fibrosis induced by the loss of NKp46+ cells in NASH mice was as a result of decreased Tregs, we first examined the changes in total T cells and found that both the frequency and absolute number of T cells were increased in DT-treated mice that developed NASH (Fig. 5A). This increase was reflected in the frequency and cell number of CD3+CD4+FoxP3+ Tregs with no statistical significance (Fig. 5B). Nonetheless, these data suggest that the development of fibrosis during NASH in absence of NKp46+ cells is not due to a loss of Tregs.
Figure 5. Tregs are unaffected by the loss of NKp46+ NK cells.
(A) Frequency and cell number of T cells in the liver of PBS or DT-treated mice fed with MCD diet for 17 days. (B) FACS plots show the gating strategy to identify Tregs (CD3+CD4+FoxP3+ cells). Graphs indicate frequency and cell number of hepatic Tregs from CT or MCD diet-fed mice treated with PBS or DT. *p<0.05 and ***p<0.001 as determined by two-tailed t-test.
NKp46+ NK cells polarize Mϕ toward an M1 phenotype in NASH
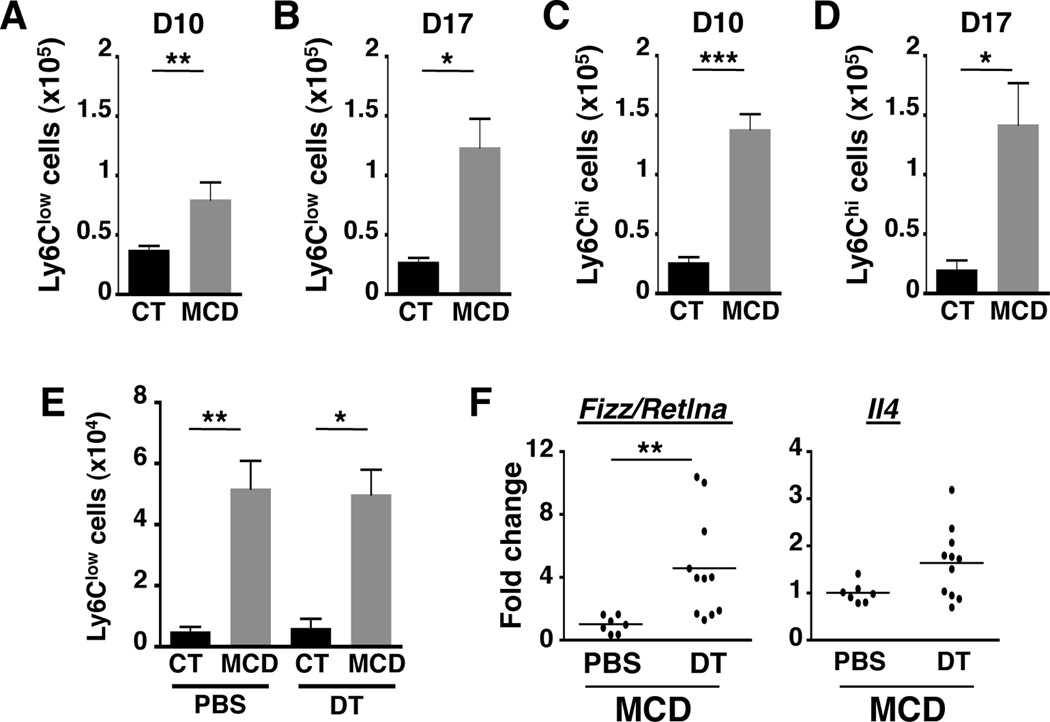
We next attempted to identify other cell types responsible for regulating NASH pathogenesis via crosstalk with NK cells. Proinflammatory M1 Mϕ have a prominent role in the initiation and development of NASH5. Alternatively activated Mϕ (M2 Mϕ) have been shown to secrete TGF-β, which accelerates the development of renal fibrosis34. Given that the loss of NKp46+ cells leads to increased fibrosis in NASH, we hypothesized that Mϕ polarization was skewed toward M2 Mϕ in the absence of NKp46+ NK cells. Previous studies have shown that pro-inflammatory liver Mϕ arise from infiltrating CD11b+F4/80lowCCR2+ monocytes and maintain a low-grade chronic inflammation in NASH livers5. These pro-inflammatory hepatic monocytes, also called M1-polarized Mϕ, are characterized by high expression of Ly6C and are able to produce large amounts of TNF-α. Our previous work demonstrated a significant infiltration of CD11b+Ly6ChiTNF-α+ monocytes into steatotic livers isolated from mice fed with MCD diet for 10 days. We confirmed that these pro-inflammatory monocytes continue to infiltrate into the liver during a later phase in disease progression (Suppl. Fig. 2A and 2B). Upon arrival in the liver, M1-polarized Mϕ may evolve into M2-like Mϕ that initiate a wide range of responses such as wound healing (i.e. collagen deposition), angiogenesis, dampening inflammation, or removing scar tissue. These M2-like Mϕ express low levels of Ly6C in contrast to Ly6ChiM1 Mϕ. When we compared the level of expression of Ly6C on liver Mϕ isolated from mouse fed with control or MCD diet for 10 and 17 days, we noticed a rise in the number of Ly6Clow liver Mϕ from D10 to D17 (Fig. 6A vs 6B). Indeed, at D17 of the MCD diet, the number of Ly6Clow Mϕ was comparable to the number of Ly6Chi Mϕ (Fig. 6B vs 6D), suggesting Ly6Clow Mϕ are either recruited from the blood or converted from infiltrating Ly6Chi Mϕ. In contrast, the number of Ly6Chi Mϕ from D10 to D17 remained constant (Fig. 6C vs 6D).
Figure 6. Increase in CD11b+F4/80lowLy6Clow Mϕ.
(A, B) Cell number of Ly6ClowCD11b+ and (C, D) Ly6ChiCD11b+ Mϕ in the liver of CT or MCD-fed mice for 10 and 17 days. (E) Number of Ly6ClowCD11b+F4/80low liver Mϕ from mice fed with CT and MCD diet for 17 days and treated with PBS or DT. *p<0.05 and **p<0.01 as determined by two-tailed t-test. (F) Transcripts of M2 Mϕ markers (retnla, IL-4) were quantified by qPCR from whole liver samples. RNA level is presented as fold-induction compared to PBS-treated mice fed with MCD diet for 17 days. **p<0.01 as determined by Mann Whitney test.
This accumulation of Ly6Clow Mϕ at D17 of MCD diet was also observed in the absence of NKp46+ NK cells (Fig. 6E). However, we investigated the possibility of functional differences despite comparable numbers of Ly6Clow Mϕ in the presence or absence of NKp46+ NK cells. We therefore evaluated the expression of M2-like Mϕ markers (i.e. Chi3l3/YM-1, Fizz/Retlna, and IL-4) in liver tissues isolated from mice fed with control or MCD diet for 17 days. MCD diet upregulated expression of Chi3l3/Ym-1, which was slightly enhanced upon depletion of NKp46+ cells (Suppl. Fig. 2C). The impact of the NK cell depletion was more pronounced in the increased expression of another alternatively activated Mϕ marker, Fizz/Retlna, in MCD diet-fed mice (Fig. 6F). Lastly, transcription of the cytokine IL-4, which polarizes Mϕ toward M2-like Mϕ, trended toward an increase, further indicating a net shift toward M2 Mϕ polarizing conditions in the absence of NK cells (Fig. 6F). These observations indicate that NASH triggers the influx of M1 Mϕ that differentiate into M2 Mϕ in the absence of NK cells, this process is exaggerated as polarization of M2 Mϕ is amplified.
IFN-γ from NASH NKp46+ NK cells polarizes Mϕ toward M1 phenotypes
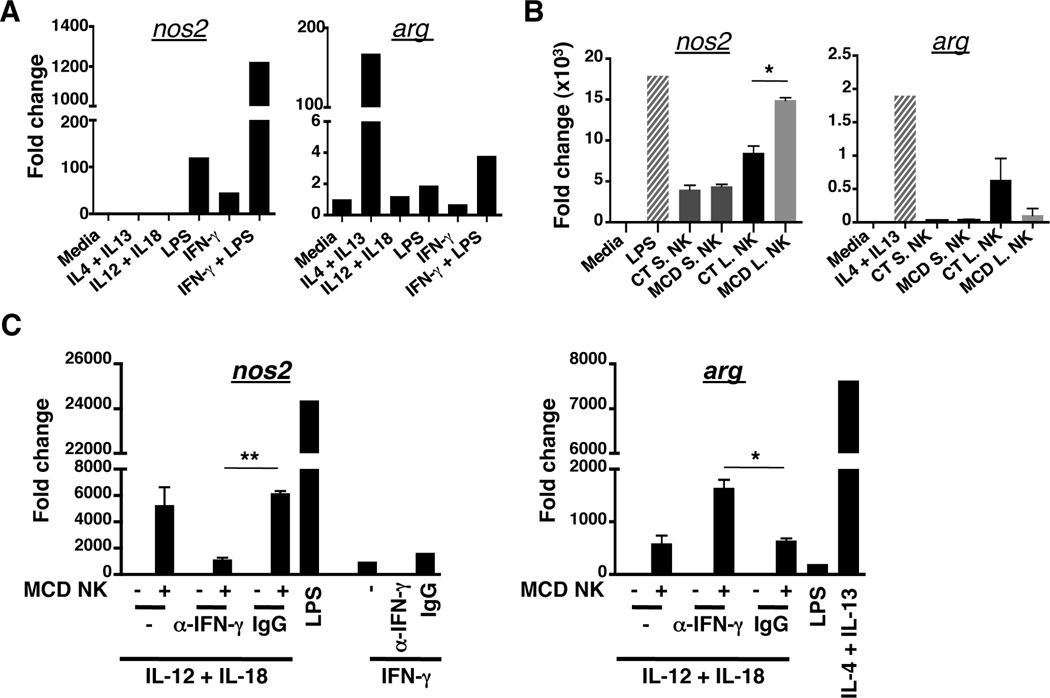
IFN-γ is a potent regulator of M1 Mϕ polarization. Given that the DX5+ NK cells produced IFN-γ in mice fed with the MCD diet, we speculated whether IFN-γ was necessary in inducing and/or maintaining M1 Mϕ in NASH livers. To test this hypothesis, we first confirmed that IFN-γ can polarize the Mϕ cell line, RAW264, into M1-like Mϕ RAW264 cells were cultured in the presence of a cocktail of cytokines to polarize them toward M1- or M2-like Mϕ. Polarization status was verified by gene expression of nos2 and arg, which mark M1 and M2 Mϕ, respectively. As seen in Fig. 7A, RAW264 cells polarized into nos2-expressing M1 Mϕ after incubation either with LPS or IFN-γ. Notably, addition of LPS and IFN-γ had a synergistic effect on nos2 expression. Several studies have reported elevated amounts of bacterial products in livers of NASH patients, which may be an indicator of increased gut permeability due to a disruption of the intestinal barrier. The synergy of M1 Mϕ polarization in the presence of both LPS and IFN-γ could therefore mimic the liver microenvironment in NASH patients. In contrast, M2 Mϕ polarization was potently induced by combined stimulation with IL-4 and IL-13, verifying that RAW264 cells were capable of being polarized into M1- or M2-like Mϕ. Lastly, the presence of IL-12 and IL-18, cytokines that activate NK cells, did not induce the expression of nos2 or arg genes.
Figure 7. NASH-conditioned NK cells polarize Mϕ toward an M1 phenotype.
(A) RAW264 Mϕ were cultured in the presence of various cytokines including IFN-γ alone or IFN-γ/LPS for 2hrs. nos2 (M1 marker) and arg (M2 marker) gene expression were analyzed by qPCR. (B) NK cells from liver (L) and spleen (S) of D17 MCD and CT diet-fed mice were co-cultured with RAW264 cells at a 1 to 1 ratio for 18hrs. After removing NK cells from the culture, nos2 and arg gene expression were analyzed in macrophages by qPCR. (C) Liver NK cells from MCD fed- mice were co-cultured with RAW264 cells at a 1 to 1 ratio for 18hrs in presence or absence of IFN-γ blocking antibody and IgG control antibody. After removing NK cells from culture, nos2 and arg gene expression were analyzed in macrophages by qPCR. *p<0.05, and **p<0.01 as determined by two-tailed t-test.
The importance of NK cell-derived IFN-γ in polarizing Mϕ was confirmed by ex vivo co-culture of RAW264 cells with IL-12/IL-18-activated spleen or liver NK cells isolated from mice fed with control or MCD diet. As expected, only activated-liver NK cells from MCD diet-fed mice were able to induce M1 polarization of RAW264 cells to levels comparable to stimulation with LPS (Fig. 7B). Conversely, liver NASH NK cells (MCD L. NK) were less efficient at stimulating arg expression in Mϕ compared to NK cells from CT diet-fed mice. To substantiate the role of NK cell-derived IFN-γ in polarizing M1 Mϕ during NASH, we assessed nos2 and arg expression in RAW264 cells co-cultured with IL-12/IL-18-stimulated NK cells from MCD diet-fed mice in the presence of IFN-γ-blocking antibodies. Expression of nos2 in Mϕ cultured with MCD liver NK cells was abrogated in presence of anti-IFN-γ antibody, but maintained in presence of isotype control (Fig. 7C). Remarkably, blocking IFN-γ increased expression of arg in RAW264 Mϕ, perhaps indicating that NK cell-derived IFN-γ not only polarizes Mϕ toward M1 activation, but also counters signals that promote M2 Mϕ polarization. IFN-γ producing-NKp46+ NK cells thus regulate M1/M2 Mϕ balance by polarizing Mϕ toward M1 phenotypes in NASH. In the absence of NKp46+ NK cells, M1/M2 balance is disrupted and skewed toward M2 Mϕ that drive hepatic fibrogenesis.
Discussion
In this report, we show that NKp46+ NK cells play an important role in preventing liver fibrosis during NASH by regulating M1/M2 Mϕ polarization in the liver microenvironment. In particular, IFN-γ-producing peripheral DX5+NKp46+ NK cells are increased in the livers of NASH mice, resulting in activating resident/infiltrating Mϕ into M1 Mϕ and deterring from fibrosis-inducing M2 Mϕ differentiation Consequently, loss of NKp46+ cells is associated with the upregulated expression of profibrogenic genes (i.e. Timp-1, Sma, Col1a2 and Tgfb) as well as M2 Mϕ marker genes (i.e. Retlna, Chi3l3 and IL-4) in hepatic tissues compared to wild type mice having intact NK cell. NKp46-deficient NASH mice show exacerbated liver damage accompanied by high ALT level, enhanced apoptotic cell death, and elevated expression of danger signal (Il33) and inflammatory cytokines (Tnfa, and Ip-10) compared to wild type mice. Notably, Il22 transcripts are also elevated in NKp46-deficient NASH mice. As both IL-22 and TNF-α stimulate hepatocyte proliferation, their concomitant increase in the absence of NK cells may be indicative of a compensatory mechanism to promote regeneration of damaged liver tissue, which accelerates ongoing fibrogenesis under continuous injury35, 36. Taken together, NK-derived IFN-γ plays a pivotal role in maintaining a balanced inflammatory environment and promoting tissue integrity during ongoing inflammation in NASH.
The prevalence of NAFLD is estimated to be ~30% of the population in Western countries37. Although NASH contributes to only 2–3% of this figure, it poses a significant health burden, as it is the precursor to fibrosis and cirrhosis of the liver37. As one of cellular and molecular events propelling NAFLD to NASH could be that widespread death of steatotic hepatocytes activates local immune cells including liver-resident Mϕ (Kupffer cells). Indeed, we and others have demonstrated that Kupffer cells activated during NASH secrete TNF-α and chemokines (MCP-1 and IP-10) to recruit proinflammatory CD11b+F4/80intLy6Chi monocytes into the liver5. The recruited monocytes perpetuate the inflammatory response through production of TNF-α and differentiate into M1-polarized Mϕ5. These TNF-α+ M1 Mϕ may promote activation of resident/infiltrating NK cells, increasing local bioavailability of IFN-γ, which in turn could reinforce M1 Mϕ polarization in a feed forward loop. Alternatively, infiltrating Ly6Chi monocytes could convert into Ly6Clow monocytes that give rise to other Mϕ subset involved in restoring tissue homeostasis38. These restorative Mϕ could promote fibrosis regression with the release of matrix metalloproteases (MMPs)9. Uncontrolled differentiation of these restorative Mϕ could propel the development of fibrosis through the release of extracellular matrix components and the expression of Timp-1, a MMP inhibitor. Our studies identify day 17 of the MCD diet as a key time point in this model of NASH at which the M1/M2 Mϕ balance determines the extent of fibrosis. More importantly, we demonstrate that NK cell-derived IFN-γ regulates the switch from M1 to M2 Mϕ in NASH livers. Given that the progression of NASH in humans spans several years, identifying changes in Mϕ phenotypes in NASH patients may reveal critical windows at which anti-fibrotic interventions may be effective.
Furthermore, the accelerated progression of fibrosis in the absence of NK cells can also be an inability to turn off danger signals. As shown in Figure 4B, gene expression of the alarmin IL-33 was increased in NKp46-depleted NASH mice. IL-33 is released by injured epithelial or endothelial cells that alerts adjacent immune cells of breaches in tissue integrity to initiate regenerative processes39. Under homeostatic conditions, the liver constitutively expresses IL-33 in hepatocytes and liver sinusoidal endothelial cells (LSECs)40. However, in fibrotic livers, expression of Il33 is elevated indicating an active role for IL-33 in the initiation of or response to fibrosis31. The local excess of IL-33 can be released into the blood of patients undergoing liver failure and has significant impact on liver-resident and distal immune cell populations as the receptor for IL-33, ST2, is predominantly expressed on Mϕ and Th2 cells40, 41. Consequently, IL-33 can potentiate Th2 Immune response and amplify the development of M2 Mϕ. Th2 cells are known to promote fibrosis by secretion of IL-4 and IL-1341. Interestingly, a recent study demonstrated that IL-33 functions in promoting fibrosis through the activation and accumulation of IL-13+ ILC2s, which are sufficient and required for hepatic fibrosis42 IL-13 could in turn contribute to the polarization and accumulation of M2-like Mϕ that promote liver fibrosis. Consequently, the loss of NKp46+ cells may polarize Mϕ failing to effectively clear damage cells and instead contribute to increased cell damage, initiating a dysfunctional inflammatory cascade that amplifies fibrogenesis.
While the effect of IL-33 on promoting fibrosis appears to be immune-mediated process, the secretion of IL-22 contributes to scar formation in the liver by acting on parenchymal cells. Various immune cells produce IL-22, but expression of its receptor is limited to non-immune cells such as hepatocytes, epithelia, and pancreatic cells43. Signaling through the IL-22 receptor enhances expression of antiapoptotic, antioxidant and proliferative genes44. While these programs may be beneficial in the acute setting, prolonged production of IL-22, as in existing hepatocellular carcinoma, can aggravate disease by promoting proliferation and inhibiting apoptosis36. Indeed, in our model of NASH, expression of IL-22 was increased in the absence of NKp46 (Figure 4B), perhaps indicating that IL-22 induces successive rounds of hepatocyte proliferation, which when combined with imbalances in matrix deposition, can exacerbate development of fibrosis. Moreover, in a murine model of hepatitis B virus infection, neutralization of IL-22 dampened Th17 cell responses that drive fibrogenesis45. The authors proposed a model wherein release of IL-22 by immune cells could stimulate IL-22R-expressing HSCs that in turn produce IP-10 to attract Th17 and other inflammatory cells into the liver. When considering this idea with our observations of increased IL-22 and IP-10 expression in the livers of NKp46-depleted mice, it is possible that skewing of additional immune subsets, namely T cells, may play a role in development of fibrosis in NASH. However, Tregs, which are known to attenuate fibrosis in the lung, were comparable in the presence or absence of NK cells in MCD-diet fed mice, indicating that T cell-mediated regulation of inflammation may not be causative in our experimental model.
In summary, our findings suggest that NKp46+ cells have a protective role in the development of fibrosis during NASH where they regulate the tight balance between liver inflammation and repair through Mϕ polarization. Mϕ have been considered as an attractive target for therapeutic approaches in liver fibrosis due to their ability to regulate fibrosis and to interact with various cells in the hepatic inflammatory environment. However, their highly pleiotropic and temporal roles and the lack of specific markers make it difficult to directly and specifically inactivate particular subsets of Mϕ in vivo. Thus, our study identifying NK cells as upstream regulators of Mϕ function would provide a new cellular target to modulate Mϕ-mediated inflammation in chronic liver diseases.
Supplementary Material
Acknowledgments
We thank the University of Virginia Flow Cytometry Core facility for excellent technical assistance with luminex assays. We thank Drs. P.C. Trampont and T.J. Braciale for apotome microscope and one-step-plus qPCR access, respectively. We thank members of Dr. Y.S. Hahn’s laboratory for their suggestions.
Financial Support: This work was supported by U19 AI083024 to Y.S.H. and R01 DK096076 to N.L.
List of abbreviations
- NASH
Non-alcoholic steatohepatitis
- MCD
Methionine-choline deficient
- DT
Diphtheria toxin
- IFN-γ
Interferon gamma
References
- 1.Wree A, Broderick L, Canbay A, et al. From NAFLD to NASH to cirrhosis-new insights into disease mechanisms. Nat Rev Gastroenterol Hepatol. 2013;10:627–636. doi: 10.1038/nrgastro.2013.149. [DOI] [PubMed] [Google Scholar]
- 2.Caldwell S, Hoehn KL, Hahn YS. The strange and critical intersection of hepatitis C and lipoprotein metabolism: "C-zing" the oil. Hepatology. 2013;57:1684–1687. doi: 10.1002/hep.26091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Minicis S, Day C, Svegliati-Baroni G. From NAFLD to NASH and HCC: pathogenetic mechanisms and therapeutic insights. Curr Pharm Des. 2013;19:5239–5249. [PubMed] [Google Scholar]
- 4.Meli R, Mattace Raso G, Calignano A. Role of innate immune response in non-alcoholic Fatty liver disease: metabolic complications and therapeutic tools. Front Immunol. 2014;5:177. doi: 10.3389/fimmu.2014.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tosello-Trampont AC, Landes SG, Nguyen V, et al. Kuppfer cells trigger nonalcoholic steatohepatitis development in diet-induced mouse model through tumor necrosis factor-alpha production. J Biol Chem. 2012;287:40161–40172. doi: 10.1074/jbc.M112.417014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishizuka EK, Ferreira MJ, Grund LZ, et al. Role of interplay between IL-4 and IFN-gamma in the in regulating M1 macrophage polarization induced by Nattectin. Int Immunopharmacol. 2012;14:513–522. doi: 10.1016/j.intimp.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Maina V, Sutti S, Locatelli I, et al. Bias in macrophage activation pattern influences non-alcoholic steatohepatitis (NASH) in mice. Clin Sci (Lond) 2012;122:545–553. doi: 10.1042/CS20110366. [DOI] [PubMed] [Google Scholar]
- 9.Ramachandran P, Pellicoro A, Vernon MA, et al. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci U S A. 2012;109:E3186–E3195. doi: 10.1073/pnas.1119964109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis MJ, Tsang TM, Qiu Y, et al. Macrophage M1/M2 polarization dynamically adapts to changes in cytokine microenvironments in Cryptococcus neoformans infection. MBio. 2013;4 doi: 10.1128/mBio.00264-13. e00264-00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goh C, Narayanan S, Hahn YS. Myeloid-derived suppressor cells: the dark knight or the joker in viral infections? Immunol Rev. 2013;255:210–221. doi: 10.1111/imr.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sica A, Invernizzi P, Mantovani A. Macrophage plasticity and polarization in liver homeostasis and pathology. Hepatology. 2014;59:2034–2042. doi: 10.1002/hep.26754. [DOI] [PubMed] [Google Scholar]
- 13.Chen CL, Tsukamoto H, Liu JC, et al. Reciprocal regulation by TLR4 and TGF-beta in tumor-initiating stem-like cells. J Clin Invest. 2013;123:2832–2849. doi: 10.1172/JCI65859. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Henning JR, Graffeo CS, Rehman A, et al. Dendritic cells limit fibroinflammatory injury in nonalcoholic steatohepatitis in mice. Hepatology. 2013;58:589–602. doi: 10.1002/hep.26267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Labonte AC, Tosello-Trampont AC, Hahn YS. The role of macrophage polarization in infectious and inflammatory diseases. Mol Cells. 2014;37:275–285. doi: 10.14348/molcells.2014.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krueger PD, Lassen MG, Qiao HH, Hahn YS. Regulation of NK Cell Repertoire and Function in the Liver. Critical Reviews in Immunology. 2011;31:43–52. doi: 10.1615/critrevimmunol.v31.i1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lassen MG, Lukens JR, Dolina JS, et al. Intrahepatic IL-10 Maintains NKG2A(+)Ly49(−) Liver NK Cells in a Functionally Hyporesponsive State. Journal of Immunology. 2010;184:2693–2701. doi: 10.4049/jimmunol.0901362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng H, Jiang X, Chen Y, et al. Liver-resident NK cells confer adaptive immunity in skin-contact inflammation. J Clin Invest. 2013;123:1444–1456. doi: 10.1172/JCI66381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi FD, Ljunggren HG, La Cava A, Van Kaer L. Organ-specific features of natural killer cells. Nat Rev Immunol. 2011;11:658–671. doi: 10.1038/nri3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao B, Radaeva S. Natural killer and natural killer T cells in liver fibrosis. Biochim Biophys Acta. 2013;1832:1061–1069. doi: 10.1016/j.bbadis.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watzl C, Long EO. Signal transduction during activation and inhibition of natural killer cells. Curr Protoc Immunol. 2010;Chapter 11(Unit 11):19B. doi: 10.1002/0471142735.im1109bs90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spear P, Wu MR, Sentman ML, Sentman CL. NKG2D ligands as therapeutic targets. Cancer Immun. 2013;13:8. [PMC free article] [PubMed] [Google Scholar]
- 23.Kruse PH, Matta J, Ugolini S, Vivier E. Natural cytotoxicity receptors and their ligands. Immunol Cell Biol. 2014;92:221–229. doi: 10.1038/icb.2013.98. [DOI] [PubMed] [Google Scholar]
- 24.Chong WP, Zhou J, Law HK, et al. Natural killer cells become tolerogenic after interaction with apoptotic cells. Eur J Immunol. 2010;40:1718–1727. doi: 10.1002/eji.200939768. [DOI] [PubMed] [Google Scholar]
- 25.Sojka DK, Tian Z, Yokoyama WM. Tissue-resident natural killer cells and their potential diversity. Semin Immunol. 2014;26:127–131. doi: 10.1016/j.smim.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klose CS, Flach M, Mohle L, et al. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell. 2014;157:340–356. doi: 10.1016/j.cell.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 27.Peng H, Tian Z. Re-examining the origin and function of liver-resident NK cells. Trends Immunol. 2015;36:293–299. doi: 10.1016/j.it.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 28.Serafini N, Vosshenrich CA, Di Santo JP. Transcriptional regulation of innate lymphoid cell fate. Nat Rev Immunol. 2015;15:415–428. doi: 10.1038/nri3855. [DOI] [PubMed] [Google Scholar]
- 29.Walzer T, Blery M, Chaix J, et al. Identification, activation, and selective in vivo ablation of mouse NK cells via NKp46. Proc Natl Acad Sci U S A. 2007;104:3384–3389. doi: 10.1073/pnas.0609692104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pickens MK, Yan JS, Ng RK, et al. Dietary sucrose is essential to the development of liver injury in the methionine-choline-deficient model of steatohepatitis. J Lipid Res. 2009;50:2072–2082. doi: 10.1194/jlr.M900022-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao Q, Li Y, Li M. The potential role of IL-33/ST2 signaling in fibrotic diseases. J Leukoc Biol. 2015 doi: 10.1189/jlb.3RU0115-012R. [DOI] [PubMed] [Google Scholar]
- 32.Oo YH, Sakaguchi S. Regulatory T-cell directed therapies in liver diseases. J Hepatol. 2013;59:1127–1134. doi: 10.1016/j.jhep.2013.05.034. [DOI] [PubMed] [Google Scholar]
- 33.Peng X, Moore MW, Peng H, et al. CD4+CD25+FoxP3+ Regulatory Tregs inhibit fibrocyte recruitment and fibrosis via suppression of FGF-9 production in the TGF-beta1 exposed murine lung. Front Pharmacol. 2014;5:80. doi: 10.3389/fphar.2014.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pan B, Liu G, Jiang Z, Zheng D. Regulation of renal fibrosis by macrophage polarization. Cell Physiol Biochem. 2015;35:1062–1069. doi: 10.1159/000373932. [DOI] [PubMed] [Google Scholar]
- 35.Cosgrove BD, Cheng C, Pritchard JR, et al. An inducible autocrine cascade regulates rat hepatocyte proliferation and apoptosis responses to tumor necrosis factor-alpha. Hepatology. 2008;48:276–288. doi: 10.1002/hep.22335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park O, Wang H, Weng H, et al. In vivo consequences of liver-specific interleukin-22 expression in mice: Implications for human liver disease progression. Hepatology. 2011;54:252–261. doi: 10.1002/hep.24339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig Dis. 2010;28:155–161. doi: 10.1159/000282080. [DOI] [PubMed] [Google Scholar]
- 38.Dal-Secco D, Wang J, Zeng Z, et al. A dynamic spectrum of monocytes arising from the in situ reprogramming of CCR2+ monocytes at a site of sterile injury. J Exp Med. 2015;212:447–456. doi: 10.1084/jem.20141539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arshad MI, Piquet-Pellorce C, Samson M. IL-33 and HMGB1 alarmins: sensors of cellular death and their involvement in liver pathology. Liver Int. 2012;32:1200–1210. doi: 10.1111/j.1478-3231.2012.02802.x. [DOI] [PubMed] [Google Scholar]
- 40.Milovanovic M, Volarevic V, Radosavljevic G, et al. IL-33/ST2 axis in inflammation and immunopathology. Immunol Res. 2012;52:89–99. doi: 10.1007/s12026-012-8283-9. [DOI] [PubMed] [Google Scholar]
- 41.Erhardt A, Tiegs G. IL-33--a cytokine which balances on a knife's edge? J Hepatol. 2012;56:7–10. doi: 10.1016/j.jhep.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 42.McHedlidze T, Waldner M, Zopf S, et al. Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis. Immunity. 2013;39:357–371. doi: 10.1016/j.immuni.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pan CX, Tang J, Wang XY, et al. Role of interleukin-22 in liver diseases. Inflamm Res. 2014;63:519–525. doi: 10.1007/s00011-014-0727-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao B. Hepatoprotective and anti-inflammatory cytokines in alcoholic liver disease. J Gastroenterol Hepatol. 2012;27(Suppl 2):89–93. doi: 10.1111/j.1440-1746.2011.07003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao J, Zhang Z, Luan Y, et al. Pathological functions of interleukin-22 in chronic liver inflammation and fibrosis with hepatitis B virus infection by promoting T helper 17 cell recruitment. Hepatology. 2014;59:1331–1342. doi: 10.1002/hep.26916. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.