Abstract
Objective
This multi-institutional phase I/II trial explored patient-assessed tolerance of increasingly hypofractionated (HPFX) radiation for low/intermediate risk prostate cancer.
Methods
347 patients enrolled from 2002–2010. Three increasing dose-per-fraction schedules of 64.7 Gy/22 fx, 58.08 Gy/16 fx and 51.6 Gy/12 fx were each designed to yield equivalent predicted late toxicity. Three quality of life (QoL) surveys were administered prior to treatment and annually up to 3 years.
Results
Bowel QoL data at 3 years revealed no significant difference among regimens (p=0.469). Bowel QoL for all regimens declined transiently, largely recovering by three years, with only the 22 fraction decrement reaching significance. Bladder outcomes at 3-years were comparable (p=0.343) although, for all patients combined, a significant decline was observed from baseline (p=0.008). Spitzer quality of life data revealed similarly excellent, 3-year means (p=0.188). International erectile function data also revealed no significant differences at 3 years although all measures except intercourse satisfaction worsened post-treatment.
Conclusions
Three-year QoL changes for bowel, bladder and SQLI were modest and similar for 3 HPFX regimens spanning 2.94–4.3 Gy per fraction. These favorable patient-scored outcomes demonstrate the safety and tolerability of such regimens and may be leveraged to support further implementation of mild to moderately hypofractionated radiotherapy in the setting of low and intermediate-risk prostate cancer
INTRODUCTION
As many patients with low and intermediate risk prostate cancer are expected to experience long-term survival, quality of life (QoL) outcomes are of substantial clinical significance. Emphasis on QoL endpoints has emerged as an important driving factor in trial design and individualized treatment selection. Currently, a number of patient reported QoL questionnaires are available to assess genitourinary, gastrointestinal and sexual endpoints and increasingly more quality of life data is becoming available.[1–3] While increased interest in hypofractionated treatment regimens for prostate cancer has led to trials testing efficacy, currently there is limited patient reported QoL data to support regimen safety.[1–7]
A number of phase I/II and III clinical studies have and are currently investigating safety, quality of life and biochemical control outcomes in the setting of hypofractionated treatment for prostate cancer.[8] While the likely low alpha/beta value has been a nidus for exploration of hypofractionation, technological advances have further supported the implementation of such schedules via improved conformality, image guidance and position reproducibility. In addition to a potentially improved therapeutic ratio, shortened treatments increase patient convenience, produce fewer disruptions of daily life and reduce the the financial and psychological burden of daily treatments of extended duration.
In an attempt to assess the patient tolerance of increasingly hypofractionated treatment schedules, we designed a phase I/II trial testing increasing dose-per-fraction regimens(NCT00214097). These fractionation schedules were designed to yield equivalent predicted late toxicity, with EQD2 doses of 75–77 Gy, assuming an alpha/beta ratio of 3 Gy for late effects. Here we report on quality of life (QoL) outcomes utilizing three validated questionnaires, Fox Chase Bowel/Bladder Toxicity, the Spitzer Quality of Life Index (SQLI) and the International Index of Erectile Function (IIEF). [9–11]
MATERIALS AND METHODS
From 2002 to 2010, 347 patients were enrolled across five institutions. Institutional IRB approval was obtained and all patients were appropriately consented. The design of this trial included a step-wise sequentially more hypofractionated treatment regimen implemented with rectal bleeding at 2 years as the rate-limiting step for fraction size escalation. A prorated design [12] assumed that 2 patients followed for one year was equivalent to one patient followed for 2 years, based upon an approximately linear cumulative incidence of rectal bleeding seen over the initial two years of post-treatment follow-up in other studies. [13] This reduced needed follow-up between escalation steps. There was also a nested fraction-per-week escalation (or deescalation) within each step in order to adjust as necessary based upon acute toxicities. Each hypofractionated level was initiated at four fractions-per-week and increased to five fractions-per-week only after thirty patients were found not to have experienced excess acute toxicity. Escalation was permitted only after fifty patient years of follow-up with acceptable rectal bleeding rates. The hypofractionation levels in this study were: level I, 64.7 Gy in 22 fractions of 2.94 Gy; level II, 58.08 Gy in 16 fractions of 3.63 Gy and level III, 51.6 Gy in 12 fractions of 4.3 Gy. Patients were treated to the prostate ± seminal vesicles utilizing intensity modulated radiotherapy (IMRT) with daily image guidance (see supplementary section for further details).
Statistical Analysis
QoL measures were obtained utilizing three separate instruments assessed at baseline, 12, 24 and 36 months after treatment. The Fox Chase Bowel/Bladder survey was divided into two sections: Bowel (questions 1 to 14, N=14) and Bladder (questions 19, and 21 to 30, N=11). To facilitate analysis, Bowel and Bladder scores for each section were rescaled to a total score of between 0 – 100, where higher scores indicated better QoL. The standard scoring mechanism was used for IIEF, where the QoL items corresponded to the following domains: erectile function (score range 1–30), orgasmic function (score range 0–10), sexual desire (score range 2–10), intercourse satisfaction (score range 0–15), and overall satisfaction (score range 2–10). Standard scoring was also used for the SQLI survey (score range 0–10). Unavailable QoL questionnaire rates ranged between 7–10%, 20–23% and 41–44% at 1, 2 and 3 years respectively for the IIEF, bowel/bladder and SQLI assessments and were similar for each of the 3 fractionation arms. All mean scores were adjusted for missing data. The missing data for questionnaires were handled by weighted mean imputation. Cases with more than half of the total questions missing were not included.
QoL scores were summarized using mean and standard deviation (SD) for the 22, 16, and 12 treatment hypofractionation levels at the different time periods. Analysis of covariance (ANCOVA) was used to study the changes in QoL scores from baseline to year 3 for each hypofractionation levels. This model evaluated whether the mean QoL scores at year 3 (dependent variable) were equal across the three treatment levels of the categorical variable hypofractionation (independent variable), while controlling for baseline scores (covariate).
All analyses were performed using the procedure PROC GLM from SAS/STAT software (ver 9.3) and plots were created using the ggplot2 package in R (ver 3.0.3).
RESULTS
Patient and Treatment Characteristics
This phase I/II trial included 347 men with low and intermediate risk (including 6 high risk) prostate cancer according to D’Amico classification.[14] The median age of men was 68 (range: 45 – 85 years). Median PSA was 6.3 ng/ml with Gleason scores ranging from 4–7 (table 1). The majority of patients classified as intermediate risk were due to Gleason 3 + 4 disease. Clinical stage ranged from T1c – T2c. Fifty-eight (17%) men received androgen deprivation therapy of 6 months duration (table 1). All patient characteristics and risk categories were evenly matched across fractionation groups (table 1). Treatment planning rectal dose constraints were adjusted by fractionation level (Table 2) and compliance rates in this prospective trial were very high at 94%, with only minor deviations in the remainder.
Table 1.
Patient characteristics
| Risk Factor | Level I | Level II | Level III | p – value | ||
|---|---|---|---|---|---|---|
| Number of patients | 101 | 111 | 135 | NA | ||
| Age | ||||||
| Median | 67 | 67 | 68 | 0.28 | ||
| Min-Max | 47–83 | 45–81 | 52–85 | |||
| Weight (lbs) | ||||||
| Median | 197 | 198 | 202 | 0.86 | ||
| Min-Max | 137–298 | 124–400 | 140–294 | |||
| Baseline IPSS | ||||||
| Median | 8 | 7 | 7 | 0.40 | ||
| Min-Max | 0–23 | 0–30 | 0–24 | |||
| Risk Group (NCC) | Number of Patients (I–III) | Frequency | ||||
| Low | 54 | 45 | 57 | 45 % | ||
| Intermediate | 45 | 66 | 74 | 53 % | ||
| High | 2 | 0 | 4 | 2 % | ||
| ADT (6 months) | Number of Patients (I–III) | Frequency | ||||
| Yes | 20 | 16 | 22 | 17% | ||
| No | 289 | 83% | ||||
Table 2.
Fractionation scheme and treatment planning rectal dose constraints
| Fractionation Level | I | II | III |
|---|---|---|---|
| Dose per Fraction (Gy) | 2.94 | 3.63 | 4.3 |
| Number of Fractions | 22 | 16 | 12 |
| Total Dose (Gy) | 64.7 | 58.1 | 51.6 |
| Tumor EQD2 (α/β 1.5) (Gy) | 82.6 | 85.1 | 85.5 |
| Late Normal Tissue EQD2 (α/β 3.0) (Gy) | 76.8 | 77.0 | 75.3 |
| Dose (Gy) | Rectal Percent Limits (%) | ||
| 30 | – | – | 40 |
| 35 | – | 38 | – |
| 40 | 37 | 32 | 27 |
| 50 | 25 | 19 | 12 |
| 55 | – | 12 | – |
| 60 | 13.5 | – | – |
Fox Chase Bowel/Bladder Toxicity Outcomes
Fox Chase Bowel/Bladder Toxicity questionnaire assessing bowel and bladder function were recorded at baseline and 1–3 years post treatment. Bowel quality of life outcomes across 22, 16, and 12 fraction schedules declined modestly and transiently and were comparable at 3 years with scores of 86.3, 87.7, and 85.4 respectively, out of a maximum 100 (p=0.469) (figure 1A; table 3). Similarly, bladder outcomes at 3-years were comparable at 79.5, 82.5 and 81.1 out of 100 (p=0.343) (figure 1B; table 3)). Differences in mean bowel/bladder function were compared at 3 years relative to baseline (table 3). Bowel QoL at three years was not significantly different from baseline among 16 and 12 fraction patients (p=0.622 and p=0.141 respectively), while a small but statistically significant QoL decrease was observed within the 22 fraction group relative to baseline (p=0.013) (table 3). For bladder QoL, small baseline to 3-year score decreases were similar and non significant across the 22, 16 and 12 fraction regimens (p=0.058, 0.337, 0.281, respectively) (table 3). When all bladder QoL arms were combined, however, a small but significant decline in QoL overall from baseline to 3 years was observed for bladder (table 3).
Figure 1.
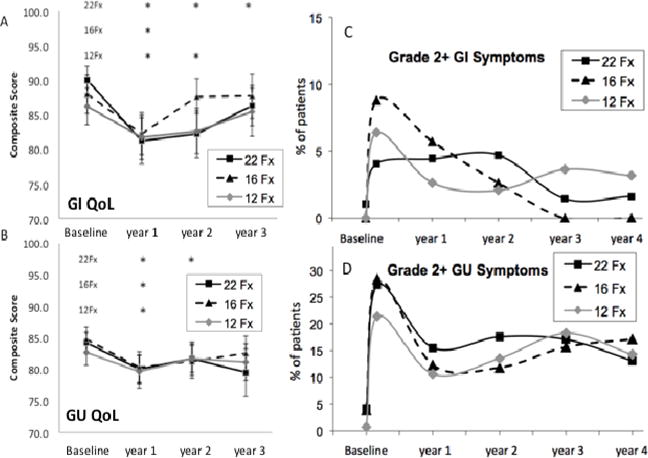
A, B: QoL metrics from baseline to year three. A. Bowel and B. Bladder
* Indicates statistically significant change from baseline.
C, D: Physician scored toxicities. C. Bowel and D. Bladder
Table 3.
Composite quality of life changes from baseline to year 3
| Domain | Treatment | Baseline Score: Mean ± SD |
Year 3 Score: Mean ± SD |
Change: Baseline to Year 3 (95% C.I.) |
p - value | Change: Baseline to Year 3 (95% C.I.) |
p - value |
|---|---|---|---|---|---|---|---|
| Bowel QoL Composite | 22 Fx | 90.0 ± 10.7 | 86.3 ± 11.1 | −4.3 (−7.7, −1.0) | 0.013 | −1.7 (−4.2, 0.7) | 0.169 |
| 16 Fx | 88.0 ± 14.6 | 87.7 ± 11.6 | 1.3 (−4.1, 6.7) | 0.622 | |||
| 12 Fx | 86.2 ± 14.6 | 85.4 ± 14.7 | −2.9 (−6.9, 1.0) | 0.141 | |||
| Bladder QoL Composite | 22 Fx | 84.2 ± 9.4 | 79.5 ± 13.5 | −4.8 (−9.8, 0.2) | 0.058 | −2.8 (−4.8, −0.7) | 0.008 |
| 16 Fx | 84.7 ± 10.1 | 82.5 ± 10.1 | −2.0 (−6.0, 2.1) | 0.337 | |||
| 12 Fx | 82.7 ± 11.3 | 81.1 ± 12.2 | −2.0 (−5.6, 1.6) | 0.281 | |||
| Erectile Function | 22 Fx | 15.6 ± 11.7 | 10.4 ± 10.6 | −6.1 (−9.3, −3.0) | <0.001 | −3.0 (−4.8, −1.1) | 0.002 |
| 16 Fx | 17.6 ± 11.7 | 13.6 ± 11.7 | −1.8 (−5.0, 1.5) | 0.28 | |||
| 12 Fx | 15.1 ± 11.1 | 9.6 ± 8.9 | −4.2 (−7.2, −1.2) | 0.007 |
Physician scored GI and GU toxicities were also not significantly different for the 3 fractionation levels at 3 years (figure 1C and D) p = 0.380 (GI tox) and p= 0.974 (GU tox). However, while comparison of 3-year versus baseline GI toxicity scores (using a Fox Chase-LENT rectal toxicity scale) revealed no significant change (figure 1C) (p = 1.0; NA and 0.055) for the 22,16 and 12 fraction patients, consistent with the patient-ecorded QoL results (figure 1A), this was not the case however for physician-scored grade 2+ GU toxicities, which were scored higher at 3 years than at pretreatment baseline (figure 1D) (p =0.007, 0.011 and <0.001), in contrast to the similar 3-year and baseline outcomes found for GU QoL (figure 1B). Inspection of the physician-scored data reveals a likely explanation, in that 16.7% of the all-patient average 17.2% GU grade 2+ toxicity was at the grade 2 level and 8.2% out of this 16.7% was related to continued alpha blocker use at 3 years, with alpha blocker use scored as a grade 2 toxicity in the modified CTCAE v3 GU scoring system that was used. Thus, the relatively low level of GU QoL issues reported by patients at 3 years may at least in part relate to the effectiveness of alpha blockers for symptom management.
A detailed analysis of changes in response over time to each individual bowel and bladder QoL question is presented in supplementary tables 1 and 3. These tables are included to provide more detail on the data collected, with no adjustments made for multiple comparisons.
International Index of Erectile Function
The international index of erectile function (IIEF) is composed of 15 questions utilizing a numerical scale of 1–5 and was administered at baseline, and 1–3 years following the completion of therapy. Comparison of 3-year adjusted scores for erectile function (p=0.07), orgasmic function (p=0.078), sexual desire (p=0.231), intercourse satisfaction (p=0.354) and overall satisfaction (p=0.191) were not significantly different between fractionation regimens (supplementary table 2). Overall, when combined, the total group experienced a decline in erectile function from baseline to year three (table 3). Also, all IIEF measures except intercourse satisfaction did decline post-treatment when individually compared to baseline (erectile function: p=0.002, orgasmic function: p=0.002, sexual desire: p=0.011, intercourse satisfaction: p=0.191, and overall satisfaction: <0.0001) (supplementary table 4). A detailed listing of changes in responses over time to individual IIEF questions is presented in supplementary table 5.
Spitzer Quality of Life Outcomes
The Spitzer quality of life index (SQLI) is composed of five items (activity, daily living, health, support, outlook) scored utilizing a numerical scale of 0–2 and was recorded at baseline and 1–3 years following the completion of therapy. The numerical range for responses per time point evaluated were 0 – 10, with 10 demonstrating no appreciable deficits in any of the 5 categories assessed. The reported SQLI scores at baseline were, 9.7 ± 0.7, 9.4 ± 1.2 and 9.5 ± 1.4 in the 22, 16 and 12 fraction arms respectively. At three years of follow-up the SQLI scores were not different, being 9.5 ± 1.4, 9.8 ± 0.4 and 9.5 ± 1.4 in the 22 (p=0.081), 16 (p=0.482), and 12 (p=0.080) fraction arms respectively. Comparison of baseline values between fractionation schedules and at 3 years follow-up among treatment arms demonstrated no significant difference in SQLI outcomes (p=0.188) (figure 2). Differences in mean SQLI between treatment arms were also compared at 3 years with normalization for baseline. The differences between 3 year mean recorded scores were compared with no significant difference between fractionation regimens (table 4).
Figure 2.
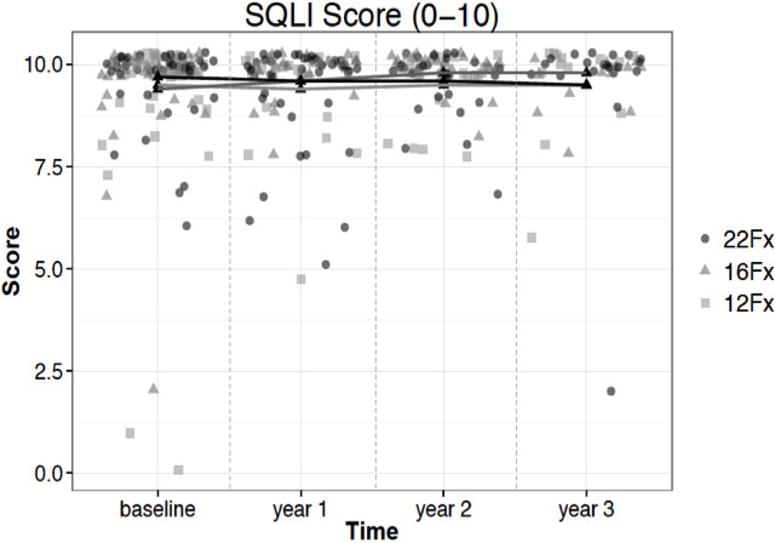
Spitzer Quality of Life change from baseline to year three
Table 4.
Spitzer Quality of Life Index statistics at baseline and year 3
| Domain | Treatment | Baseline Score Mean ± SD | Year 3 Mean ± SD | p – value |
|---|---|---|---|---|
| SQLI | 22 Fx | 9.7 ± 0.7 | 9.5 ± 1.4 | 0.188 |
| 16 Fx | 9.4 ± 1.2 | 9.8 ± 0.4 | ||
| 12 Fx | 9.5 ± 1.4 | 9.5 ± 1.4 | ||
| SQLI (all pts.) |
Change: Baseline – Year 3 (95% CI) −0.03 (−0.25, 0.18) |
0.750 | ||
DISCUSSION
Initially spurred by estimates of Hall and Brenner suggesting that prostate cancer possessed an alpha/beta value of around 1.5, hypofractionation has become and continues to be a subject of investigation.[15] Most subsequent studies have estimated alpha/beta values to be in the range of 1.2 – 4.0 with much data supporting values close to 1.5, although with considerable associated uncertainty.[16–20] In contrast, linear quadratic modeling studies for rectal and other late normal tissue toxicities, have suggested higher alpha/beta ratios generally in the 2–4 range, implying the potential for therapeutic ratio gain from larger fraction sizes.[21, 22] This potential for therapeutic gain, coupled with the convenience and probable cost benefits of hypofractionation, have led to numerous hypofractionation studies.[6, 23–26] A number of such studies have investigated a range of 5–28 fractions delivered over 2 – 5.5 wks.
Data has become increasingly available regarding biochemical control and physician scored toxicities. [4, 6, 23–26], indicating acceptable tumor control and generally minimal differences in long-term physician-scored toxicity between conventionally fractionated and hypofractionated regimens. [27–30]
However, in contrast, mature patient-reported outcomes (PROs) for moderate hypofractionation remains more limited. Certainly, ongoing phase III randomized control studies including RTOG 0415 (NCT00331773), the Medical Research Council study by Dearnaley et al. [31] and the Ontario Clinical Oncology Group’s PROFIT Trial (NCT00304759) will in the future contribute substantial, high quality QoL information, but are not yet available in published form. Intermediate and long-term quality of life outcome assessments are particularly essential in prostate cancer patients, given the expectation that many will experience extended survival.
Among studies that are available, Norkus et al reported on very short term QoL outcomes from 124 patients treated with a moderately hypofractionated regimen of 63 Gy in 20 fractions of 3.15 Gy. EPIC questionnaires were utilized to assess bowel and bladder outcomes and demonstrated no significant differences among mean values at 3 months.[4] Note was made of initial QoL decline which was greater in the hypofractionated arm at 1 month, with however more rapid recovery by month three.
Jereczek-Fossa et al reported on longer-term QoL data from a prospective cohort of 337 patients treated with a moderately hypofractionated regimen of 70.2 Gy in 26 fractions of 2.7 Gy.[5] Here erectile function, urinary, bowel and overall QoL were assessed through utilization of the IIEF-5, International Prostate Symptom Score and EORTC cancer specific QLQ-PR25 and QLQ-C30 at baseline, 6, 12, and 24 months. No significant changes were noted with regard to urinary or prostate symptom score QoL metrics. Slight deterioration in bowel QoL was noted (p=0.02) as well as in erectile function in those patients not receiving androgen deprivation therapy (p=0.002) at last follow-up. Further data from Pervez et al [2] reported on outcomes from 60 patients with high-risk prostate cancer treated with 68 Gy in 2.72 Gy fractions to the prostate and 45 Gy in 1.8 Gy fractions delivered to the whole pelvis, all administered with androgen suppression. Here the authors demonstrated a significant decline in bowel and sexual QoL metrics at 6 months, which did improve but not return to baseline at 24 months. Urinary QoL was not statistically affected at last follow-up.
Focusing on randomized trials, Yeoh et al [30], in the Adelaide trial, have demonstrated the tolerability of 55 Gy in 20 fractions of 2.75 Gy (EQD2 = 63.2 Gy in comparison to that of conventionally fractionated 64 Gy in 32 fractions of 2 Gy. This study compared these fractionation regimens in 217 individuals and found similar GI/GU toxicity using the LENT-SOMA and a GI questionnaire at 60 months of follow-up, consistent with the similar effective doses in the two arms.
The CHHiP trial [31], which compared 74 Gy in 37 fractions to either 57 or 60 Gy in 19 or 20 fractions, has now reported early PRO data [32]. The included PRO substudy enrolled 2100 patients and included bowel, bladder and sexual QoL instruments, using UCLA-PCI, including Short Form (SF)-36 and either FACT-P) or EPIC and SF-12 quality-of-life questionnaires completed pre-radiotherapy and at 2.5, 6, 12, 18, and 24 months post-radiotherapy. There were no significant differences between the conventional and the two hypofractionation arms for any QoL metrics over time, with all showing early QoL decreases but full or nearly full recovery with the exception of sexual function. Comparison of the average EQD2 for the hypofractionated versus the conventional arms, 70.2 versus 74 Gy, respectively, demonstrates the experimental arms to be slightly gentler. This may explain why recovery of patients reported GI and GU QoL decrements were more rapid in the CHHiP study at 6 months than in our own (with its higher EQD2 range of 75–77 Gy), where recovery to baseline was not realized until year 2 or 3.
The Fox Chase randomized, phase 3 trial [29] enrolled 303 men with low to high risk cancer to 76 Gy in 38 fractions versus 70.2 Gy in 26 fractions at 2.7 Gy per fraction and the results of the PRO subsection of the trial have been presented.[33] EPIC and International Prostate Symptom Score (IPSS) questionnaires were used. Overall, while mean score changes for the EPIC bowel, sexual, hormonal, or urinary irritative/obstructive domains displayed similar decline and recovery patterns over time, the decreases in both the EPIC urinary incontinence domain and in IPSS score were greater at 3 years in the hypofractionated than the conventional arm (p=0.03 and 0.06, respectively), although these differences diminished with further follow up. The EQD2 for the hypofractionation arm for this trial was the highest of all discussed here and was in fact about 5% higher than the conventional arm, at 80 versus 76 Gy, perhaps explaining the lessened QoL metrics at 3 years.
Favorable outcomes with moderately hypofractionated regimens have now led to investigation of extreme hypofractionation in the form of stereotactic body radiotherapy (SBRT).[7, 34–36] QoL metrics in the setting of prostate SBRT have now begun to accumulate in the literature and appear initially favorable.[35] Additional ongoing trials to include RTOG 0938 are also positioned to contribute further biochemical control, toxicity and QoL outcomes in the SBRT setting.
Here we have presented quality of life data from a prospective, multi-institutional trial delivering progressively increasing dose-per-fraction regimens. These data provide quantifiable outcomes with regard to patient reported QOL measures. Bowel and bladder quality of life reported here were comparable at 3 years across all treatment arms. Bowel QoL was transiently depressed in all treatment arms but returned to or near to baseline by 3 years post treatment. Bladder function decline was also modest and non-significant at each fractionation level, and again showed recovery by 3 years. Overall, these findings demonstrated similar and generally mild detriments in patient-perceived bowel and bladder toxicity after 3-year follow up, supporting the tolerability and safety of these hypofractionation schedules.
Sexual outcomes as assessed by IIEF at 3 years, when adjusted for baseline differences, also revealed no significant outcome differences among fractionation regimens. However, patients in all regimens did show overall decline in most IIEF sub scores with time.
Lastly, SQLI data was acquired and reviewed to gage overall daily quality of life following hypofractionated prostate radiation per this trial. At three years of follow-up the SQLI scores did not statistically differ among the 22, 16, and 12 fraction stratification arms. Importantly, these reported SQLI outcomes showed no significant decrease over the 3 year follow up relative to baseline values (p=0.188, Fig 2). These findings support the absence of a statistically significant detriment as perceived by patients with regard to overall daily quality of life. Thus, this study, which utilized linear quadratic modeling to design three fractionation schedules with similar predicted late toxicity risks, ultimately produced patient-derived QoL metrics that were correspondingly similar across the three schedules.
In conclusion, this study reports on the 3-year follow up of localized prostate cancer patients treated prospectively in a multi-institutional study with increasingly hypofractionated regimens. These data represent the first QoL information from a trial spanning the 2.9 – 4.3 Gy per fraction range. In fact, the 12 fraction, 4.3 Gy arm of this trial reports on the highest non-SBRT fraction size for which QoL information is now available. Preliminary, encouraging treatment efficacy data for these fractionation schedules has been presented [37] but final analysis awaits additional time to assure a minimum targeted median follow-up of 7 years for all of the sequentially accrued fractionation arms.
While this was a prospective trial with well-defined eligibility rules, central registration, review of plans and data management as well as remote site audits, the limitations of a sequential cohort design, which include the potential for undetected treatment technique or patient selection migration, should be acknowledged. In addition, while the bowel and bladder QoL instruments were detailed, comprehensive and were tailored toward prostate treatment, more standardized, validated instruments are now commonly in use and it is not possible to directly assess the extent to which our outcomes would differ if other instruments were used.
Overall, given the rapidly expanding body of data suggesting excellent tumor control and acceptable conventionally scored toxicity with hypofractionated regimens, results as presented here further support Hypofractionation, including a 4.3 Gy per fraction regimen, as an acceptable form of treatment for prostate cancer. RTOG 0938, a randomized phase II trial that compared the 4.3 Gy, 12 fraction regimen from our study to an SBRT regimen and that includes QoL measures, may potentially provide even further such validation.
Supplementary Material
Supplementary table 1: International Index of Erectile Function (IIEF) Summary Statistics at baseline and 3 years.
Supplementary table 2: Breakdown of Bowel Responses by Question.
Supplementary table 3: Breakdown of Bladder Responses by Question.
Supplementary table 4: International Index of Erectile Function Individual Component Statistics.
Supplementary table 5: Breakdown of IIEF Responses by Question.
Acknowledgments
Funding: R01CA106835 and P01 CA88960
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest Notification: Conflict of Interest: none
References
- 1.Quon H, Cheung PC, Loblaw DA, Morton G, Pang G, Szumacher E, et al. Quality of life after hypofractionated concomitant intensity-modulated radiotherapy boost for high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2012;83:617–623. doi: 10.1016/j.ijrobp.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Pervez N, Krauze AV, Yee D, Parliament M, Mihai A, Ghosh S, et al. Quality-of-life outcomes in high-risk prostate cancer patients treated with helical tomotherapy in a hypofractionated radiation schedule with long-term androgen suppression. Curr Oncol. 2012;19:e201–210. doi: 10.3747/co.19.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katz A, Ferrer M, Suarez JF, Multicentric Spanish Group of Clinically Localized Prostate C Comparison of quality of life after stereotactic body radiotherapy and surgery for early-stage prostate cancer. Radiat Oncol. 2012;7:194. doi: 10.1186/1748-717X-7-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norkus D, Karklelyte A, Engels B, Versmessen H, Griskevicius R, De Ridder M, et al. A randomized hypofractionation dose escalation trial for high risk prostate cancer patients: interim analysis of acute toxicity and quality of life in 124 patients. Radiat Oncol. 2013;8:206. doi: 10.1186/1748-717X-8-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jereczek-Fossa BA, Santoro L, Zerini D, Fodor C, Vischioni B, Dispinzieri M, et al. Image guided hypofractionated radiotherapy and quality of life for localized prostate cancer: prospective longitudinal study in 337 patients. J Urol. 2013;189:2099–2103. doi: 10.1016/j.juro.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Pollack A, Hanlon AL, Horwitz EM, Feigenberg SJ, Konski AA, Movsas B, et al. Dosimetry and preliminary acute toxicity in the first 100 men treated for prostate cancer on a randomized hypofractionation dose escalation trial. Int J Radiat Oncol Biol Phys. 2006;64:518–526. doi: 10.1016/j.ijrobp.2005.07.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King CR, Freeman D, Kaplan I, Fuller D, Bolzicco G, Collins S, et al. Stereotactic body radiotherapy for localized prostate cancer: pooled analysis from a multi-institutional consortium of prospective phase II trials. Radiother Oncol. 2013;109:217–221. doi: 10.1016/j.radonc.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 8.Cabrera AR, Lee WR. Hypofractionation for clinically localized prostate cancer. Semin Radiat Oncol. 2013;23:191–197. doi: 10.1016/j.semradonc.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Hanlon AL, Watkins Bruner D, Peter R, Hanks GE. Quality of life study in prostate cancer patients treated with three-dimensional conformal radiation therapy: comparing late bowel and bladder quality of life symptoms to that of the normal population. Int J Radiat Oncol Biol Phys. 2001;49:51–59. doi: 10.1016/s0360-3016(00)01365-1. [DOI] [PubMed] [Google Scholar]
- 10.Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–830. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 11.Spitzer WO, Dobson AJ, Hall J, Chesterman E, Levi J, Shepherd R, et al. Measuring the quality of life of cancer patients: a concise QL-index for use by physicians. J Chronic Dis. 1981;34:585–597. doi: 10.1016/0021-9681(81)90058-8. [DOI] [PubMed] [Google Scholar]
- 12.Cheung YK, Chappell R. Sequential designs for phase I clinical trials with lateonset toxicities. Biometrics. 2000;56:1177–1182. doi: 10.1111/j.0006-341x.2000.01177.x. [DOI] [PubMed] [Google Scholar]
- 13.Teshima T, Hanks GE, Hanlon AL, Peter RS, Schultheiss TE. Rectal bleeding after conformal 3D treatment of prostate cancer: time to occurrence, response to treatment and duration of morbidity. Int J Radiat Oncol Biol Phys. 1997;39:77–83. doi: 10.1016/s0360-3016(97)00301-5. [DOI] [PubMed] [Google Scholar]
- 14.D’Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 15.Brenner DJ, Hall EJ. Fractionation and protraction for radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys. 1999;43:1095–1101. doi: 10.1016/s0360-3016(98)00438-6. [DOI] [PubMed] [Google Scholar]
- 16.King CR, Mayo CS. Is the prostrate alpha/beta ratio of 1.5 from Brenner & Hall a modeling artifact. Int J Radiat Oncol Biol Phys. 2000;47:536–539. doi: 10.1016/s0360-3016(00)00442-9. [DOI] [PubMed] [Google Scholar]
- 17.D’Souza WD, Thames HD. Is the alpha/beta ratio for prostate cancer low? Int J Radiat Oncol Biol Phys. 2001;51:1–3. doi: 10.1016/s0360-3016(01)01650-9. [DOI] [PubMed] [Google Scholar]
- 18.Fowler J, Chappell R, Ritter M. Is alpha/beta for prostate tumors really low? Int J Radiat Oncol Biol Phys. 2001;50:1021–1031. doi: 10.1016/s0360-3016(01)01607-8. [DOI] [PubMed] [Google Scholar]
- 19.King CR, Fowler JF. A simple analytic derivation suggests that prostate cancer alpha/beta ratio is low. Int J Radiat Oncol Biol Phys. 2001;51:213–214. doi: 10.1016/s0360-3016(01)01651-0. [DOI] [PubMed] [Google Scholar]
- 20.Brenner DJ, Martinez AA, Edmundson GK, Mitchell C, Thames HD, Armour EP. Direct evidence that prostate tumors show high sensitivity to fractionation (low alpha/beta ratio), similar to late-responding normal tissue. Int J Radiat Oncol Biol Phys. 2002;52:6–13. doi: 10.1016/s0360-3016(01)02664-5. [DOI] [PubMed] [Google Scholar]
- 21.Brenner DJ. Fractionation and late rectal toxicity. Int J Radiat Oncol Biol Phys. 2004;60:1013–1015. doi: 10.1016/j.ijrobp.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 22.Marzi S, Saracino B, Petrongari MG, Arcangeli S, Gomellini S, Arcangeli G, et al. Modeling of alpha/beta for late rectal toxicity from a randomized phase II study: conventional versus hypofractionated scheme for localized prostate cancer. J Exp Clin Cancer Res. 2009;28:117. doi: 10.1186/1756-9966-28-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kupelian PA, Willoughby TR, Reddy CA, Klein EA, Mahadevan A. Hypofractionated intensity-modulated radiotherapy (70 Gy at 2.5 Gy per fraction) for localized prostate cancer: Cleveland Clinic experience. Int J Radiat Oncol Biol Phys. 2007;68:1424–1430. doi: 10.1016/j.ijrobp.2007.01.067. [DOI] [PubMed] [Google Scholar]
- 24.Tsuji H, Yanagi T, Ishikawa H, Kamada T, Mizoe JE, Kanai T, et al. Hypofractionated radiotherapy with carbon ion beams for prostate cancer. Int J Radiat Oncol Biol Phys. 2005;63:1153–1160. doi: 10.1016/j.ijrobp.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 25.Soete G, Arcangeli S, De Meerleer G, Landoni V, Fonteyne V, Arcangeli G, et al. Phase II study of a four-week hypofractionated external beam radiotherapy regimen for prostate cancer: report on acute toxicity. Radiother Oncol. 2006;80:78–81. doi: 10.1016/j.radonc.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Martin JM, Rosewall T, Bayley A, Bristow R, Chung P, Crook J, et al. Phase II trial of hypofractionated image-guided intensity-modulated radiotherapy for localized prostate adenocarcinoma. Int J Radiat Oncol Biol Phys. 2007;69:1084–1089. doi: 10.1016/j.ijrobp.2007.04.049. [DOI] [PubMed] [Google Scholar]
- 27.Koontz BF, Bossi A, Cozzarini C, Wiegel T, D’Amico A. A systematic review of hypofractionation for primary management of prostate cancer. Eur Urol. 2015;68:683–691. doi: 10.1016/j.eururo.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 28.Arcangeli G, Fowler J, Gomellini S, Arcangeli S, Saracino B, Petrongari MG, et al. Acute and late toxicity in a randomized trial of conventional versus hypofractionated three-dimensional conformal radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2011;79:1013–1021. doi: 10.1016/j.ijrobp.2009.12.045. [DOI] [PubMed] [Google Scholar]
- 29.Pollack A, Walker G, Horwitz EM, Price R, Feigenberg S, Konski AA, et al. Randomized trial of hypofractionated external-beam radiotherapy for prostate cancer. J Clin Oncol. 2013;31:3860–3868. doi: 10.1200/JCO.2013.51.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeoh EE, Botten RJ, Butters J, Di Matteo AC, Holloway RH, Fowler J. Hypofractionated versus conventionally fractionated radiotherapy for prostate carcinoma: final results of phase III randomized trial. Int J Radiat Oncol Biol Phys. 2011;81:1271–1278. doi: 10.1016/j.ijrobp.2010.07.1984. [DOI] [PubMed] [Google Scholar]
- 31.Dearnaley D, Syndikus I, Sumo G, Bidmead M, Bloomfield D, Clark C, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: preliminary safety results from the CHHiP randomised controlled trial. Lancet Oncol. 2012;13:43–54. doi: 10.1016/S1470-2045(11)70293-5. [DOI] [PubMed] [Google Scholar]
- 32.Wilkins A, Mossop H, Syndikus I, Khoo V, Bloomfield D, Parker C, et al. Hypofractionated radiotherapy versus conventionally fractionated radiotherapy for patients with intermediate-risk localised prostate cancer: 2-year patient-reported outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2015 doi: 10.1016/S1470-2045(15)00280-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaikh T, Li T, Johnson ME, Wang L, Hallman MA, Greenberg RE, et al. Long-term Patient Reported Outcomes From a Phase 3 Randomized Prospective Trial of Conventional Versus Hypofractionated IMRT Radiation Therapy for Localized Prostate Cancer. International Journal of Radiation Oncology • Biology • Physics. 2015;93:S34–S35. doi: 10.1016/j.ijrobp.2016.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.King CR, Brooks JD, Gill H, Presti JC., Jr Long-term outcomes from a prospective trial of stereotactic body radiotherapy for low-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2012;82:877–882. doi: 10.1016/j.ijrobp.2010.11.054. [DOI] [PubMed] [Google Scholar]
- 35.Katz AJ, Santoro M, Diblasio F, Ashley R. Stereotactic body radiotherapy for localized prostate cancer: disease control and quality of life at 6 years. Radiat Oncol. 2013;8:118. doi: 10.1186/1748-717X-8-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Freeman DE, King CR. Stereotactic body radiotherapy for low-risk prostate cancer: five-year outcomes. Radiat Oncol. 2011;6:3. doi: 10.1186/1748-717X-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ritter MAFJ, Kupelian PA, Petereit DG, LAwton CA, Chappell RJ, Tome WA. Five-year Efficacy and Toxicity Outcomes from a Phase I/II Trial of Increasingly Hypofractionated Radiation Therapy for Prostate Cancer. International Journal of Radiation Oncology Biology Physics. 2011;81:S99. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary table 1: International Index of Erectile Function (IIEF) Summary Statistics at baseline and 3 years.
Supplementary table 2: Breakdown of Bowel Responses by Question.
Supplementary table 3: Breakdown of Bladder Responses by Question.
Supplementary table 4: International Index of Erectile Function Individual Component Statistics.
Supplementary table 5: Breakdown of IIEF Responses by Question.


