Abstract
For adults with Philadelphia chromosome negative (Ph-) acute lymphoblastic leukemia (ALL) in first complete remission (CR1), allogeneic hematopoietic cell transplantation (HCT) is an established curative strategy. However, pediatric-inspired chemotherapy may also offer durable leukemia free survival in the absence of HCT. We compared 422 HCT recipients aged 18-50 years with Ph-ALL in CR1 reported to the CIBMTR with an age-matched concurrent cohort of 108 Ph- ALL CR1 patients who received a Dana-Farber Consortium pediatric-inspired non-HCT regimen. At four years follow-up, incidence of relapse after HCT was 24% [95% C.I. 19-28] vs. 23% [95% C.I. 15-32] for the non-HCT (“chemo”) cohort (p=0.97). Treatment-related mortality (TRM) was higher in the HCT cohort (HCT 37% [95% C.I. 31-42] vs. chemo 6% [95% C.I. 3 – 12], p<0.0001). DFS in the HCT cohort was 40% [95% C.I. 35-45] vs. 71% [95% C.I. 60-79] for chemo, p<0.0001. Similarly, OS favored chemo (HCT 45% [95% C.I. 40-50]) vs. chemo 73% [95% C.I. 63-81], p<0.0001). In multivariable analysis, the sole factor predictive of shorter OS was the administration of HCT (HR 3.12 [1.99 – 4.90], p<0.0001). For younger adults with Ph- ALL, pediatric-inspired chemotherapy had lower TRM, no increase in relapse, and superior overall survival compared to HCT.
Keywords: Leukemia, Acute Lymphoblastic, Chemotherapy, Hematopoietic Stem Cell Transplantation, Clinical Trials
Introduction
In Philadelphia chromosome negative (Ph-) Acute Lymphoblastic Leukemia (ALL), multi-agent chemotherapy protocols result in high complete remission rates, but for adults long-term disease-free survival (DFS) can be maintained in only about 30-45% of patients.1-3 These results are in contrast to the gratifying outcomes seen in children and adolescents treated within pediatric clinical trials consortia.4,5
In younger adults who achieve 1st complete remission (CR1), two major therapeutic approaches have been administered to maintain CR and improve long-term survival. First, allogeneic hematopoietic cell transplantation (HCT) may be offered. Donor-versus no-donor trials, meta-analyses and clinical practice guidelines conclude that related donor HCT is a more efficacious therapy compared to conventional chemotherapy or autologous HCT.3,6-8 Although the major published phase III trials were restricted to related donor (RD) alloHCT, recent observational data suggest that unrelated donor (URD) HCT produces similar outcomes to RD HCT.9,10 Thus, HCT in CR1 has become a popular post-remission modality regardless of whether suitably matched RDs or URDs are used7.
The second post-remission approach for adult ALL patients is the administration of “pediatric-inspired” chemotherapy protocols. These regimens are more dose-intensive than standard chemotherapy approaches for adults, particularly with respect to non-myelosuppressive agents such as corticosteroids, vincristine, and L-Asparaginase. Single arm clinical trials and cohort studies suggest that pediatric-inspired regimens offer excellent outcomes in younger adults, with long-term survival in 60-68% of patients.11-15 It is plausible that pediatric-inspired chemotherapy regimens are at least as effective, and potentially superior to HCT for adults with Ph-ALL in CR1. However, these two post-remission approaches have never been directly compared. In the current study we compared outcomes in a cohort of younger adults with Ph- ALL who received HCT in CR1 to a similar population of younger adults with ALL who received one of two pediatric-inspired regimens, while adjusting for important patient-, disease-, and transplant-related variables that may affect outcomes.
Subjects and Methods
Inclusion Criteria
We analyzed two cohorts of contemporaneously treated Ph- ALL patients in CR1: (1) Patients who underwent a first bone marrow or peripheral blood allogeneic HLA-identical RD or URD HCT for Ph- ALL in the US or Canada, reported to the CIBMTR; (2) Patients who received pediatric-inspired chemotherapy regimens for Ph- ALL as part of the Dana-Farber Cancer Institute (DFCI) ALL consortium. Patients from both cohorts were aged 18 to 50 years and received their post-remission therapy between June 1, 2002 and December 31, 2011.
The CIBMTR cohort came from 97 reporting centers in the U.S. and Canada. We excluded patients who received HCT from RDs who were not fully matched (N=27), and URDs who were matched at <7/8 HLA loci (N=15) or whose level of HLA match was not reported (N=65). We also excluded patients without day-100 comprehensive research forms (N=13), no informed consent for research studies (N=6), and patients in whom the date of CR1 was unknown (14). This left 422 HCT recipients for further analysis. Completeness of follow-up was 93% at 5 years.
The chemotherapy cohort consisted of Ph- patients enrolled in two consecutively conducted DFCI ALL consortium phase II clinical trials between June 1, 2002 and December 31, 2011 (DFCI 01-0175 and 06-254).11,16 There were 13 participating consortium centers in the U.S and Canada. Details regarding eligibility and treatment characteristics of DFCI 01-0175 been published elsewhere.11,16 The successor protocol (DFCI 06-254) was a modified version of DFCI 01-0175, wherein the formulation of L-Asparaginase was switched from an E. Coli to a pegylated product, and 3 additional cycles of intensification chemotherapy were added after the CNS prophylaxis phase.16 In the DFCI 01-0175 study (June 2002 - February 2008), 74 Ph- patients were enrolled. Of these, only the 61 patients who met criteria for CR1 within 6 weeks of treatment initiation were included in the current analysis. For the DFCI 06-254 study (March 2007 – December 2011), the acceptable time to achieve CR1 was extended from 6 to 8 weeks, based on previous studies that suggest that a dose intensive second induction cycle may permit a larger proportion of patients to achieve CR1, thus increasing the potential benefit of receiving pediatric-inspired post-remission therapy.7 For the DFCI 06-254 trial, 52 Ph- patients were enrolled. Of these, 47 met criteria for CR1 within 8 weeks of treatment initiation. Combining the two trials a total of 108 patients were eligible for initial analysis. However, 1 of these patients was lost to follow-up on the day that CR1 was documented, leaving 107 chemotherapy patients for subsequent analyses.
Endpoints
Overall survival (OS) was defined as time from CR1 to death. Disease-free survival (DFS) was defined as time from CR to treatment failure (death or relapse). Treatment-related mortality (TRM) was defined as time to death without evidence of leukemia. Relapse was defined as time to marrow or extramedullary leukemia recurrence. Patients were censored at time of last follow-up.
Variables analyzed included age at CR1 (by decades); T-cell vs. B-cell vs. unclassified; diagnostic white cell count (WBC) ×109/L: <30 vs. 30-100 vs. >100; time to achieve CR1: <4 weeks vs. 4-8 weeks vs. >8 weeks; extramedullary disease at diagnosis: CNS involved (yes/no); cytogenetic abnormalities, according to CIBMTR risk categories: High risk [t(4;11), t(14;18), hypodiploidy (<46), complex] vs. standard (all others).
Variables specific to the HCT cohort included months from diagnosis to transplant: <5 vs. >5; from CR1 to HCT: <3 vs. >3; conditioning regimen: total body irradiation (TBI); yes vs. no; type of donor: HLA-identical sibling vs. 8/8 HLA allele matched URD vs. 7/8 matched URD; donor-recipient gender match: F-M vs. others; donor-recipient CMV serostatus: -/- vs. others; graft source: bone marrow vs. peripheral blood stem cells; year of transplant: 2002-2007 vs. 2008-2011; GVHD prophylaxis: ex vivo T cell depletion vs. cyclosporine A and methotrexate vs. tacrolimus and methotrexate vs. mycophenolate mofetil and cyclosporine A or tacrolimus vs. sirolimus; anti-thymocyte globulin (ATG) or alemtuzumab: yes vs. no. Before study entry, all patients signed informed consent documents that were approved by the Institutional Review Board at each institution for treatment and subsequent data analysis.
Statistical analyses
Study time for both cohorts began at CR1. Patients in the DFCI chemotherapy cohort who received HCT in CR1 remained in the chemotherapy cohort for this analysis, but were censored at time of HCT (n=10). If patients in the chemotherapy cohort relapsed and received HCT beyond CR1 (n=4), the relapse was counted as the event date for chemo outcomes including relapse and treatment failure.
Patient-, disease- and transplant- related factors were compared between treatment groups using the Chi-square test for categorical variables and the Wilcoxon two-sample test for continuous variables. Events were summarized by the cumulative incidence estimate with TRM as a competing risk for relapse and the converse. Comparing outcomes in the HCT and non-HCT groups required adjustment for differences in time to treatment using left-truncated analysis starting from CR117 and differences in baseline characteristics. Probabilities of disease-free and overall survival were calculated using the left-truncated Kaplan‐Meier estimator.18 Probabilities of other major endpoints included were generated using left-truncated cumulative incidence estimates to account for competing risks. To adjust for differences in baseline characteristics, Cox proportional hazards regression was used to compare the two treatment groups. For multivariable analysis, the following variables were considered: age at CR1, by decades; T-cell vs. B-cell vs. unclassified; diagnostic WBC,×109/L: <30 vs. 30-100 vs. >100; time to achieve CR1: <4 weeks vs. 4-8 weeks vs. >8 weeks; extramedullary disease at diagnosis: CNS involved (yes/no); cytogenetic abnormalities: complex vs. standard.
The assumption of proportional hazards for each factor in the Cox model was tested and as needed non-proportional hazards were constructed using the maximized partial likelihood method to find the most appropriate breakpoint. A backward stepwise model selection approach was used to identify all significant risk factors. Each step of model building contained the main effect for treatment groups. Factors significant at a 5% level were kept in the final model. The potential interactions between main effect and all significant risk factors were tested. P values of <0.05 were considered statistically significant. All statistical analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC).
Results
The characteristics of the 422 HCT patients and 108 chemotherapy patients are summarized in Table 1. Median follow-up time of living patients was 65 months (range 6-130) in the HCT group and 48 months (<1-94) in the chemotherapy group. The HCT group was characterized by a higher median age (34 vs.30years, p=0.001) and lower proportion of T-cell ALL (14% vs. 22%, p=0.03). CNS disease at diagnosis was less frequent in the HCT cohort (HCT 6% vs. chemo 11%, p<0.001). Cytogenetic risk groups were similar between the cohorts. Minimal residual disease testing was not reported in the majority of patients in both cohorts, and was thus not analyzed.
Table 1. Characteristics of study population.
| Variable | HCT | Chemo | p-value |
|---|---|---|---|
| Number of patients | 422 | 108d | |
| Number of centers | 97 | 13 | |
| Age in decades | 0.05 | ||
| Median (range) | 34 (18-50) | 30 (18-50) | 0.001 |
| 18-29 | 161 (38) | 54 (50) | |
| 30-39 | 126 (30) | 30 (27) | |
| 40-50 | 135 (32) | 24 (23) | |
| Gender | 0.67 | ||
| Male | 253 (60) | 68 (62) | |
| Female | 169 (40) | 40 (38) | |
| Immunophenoype | 0.03 | ||
| T-cell | 61 (14) | 24 (22) | |
| B-cell | 343 (81) | 84 (78) | |
| Unspecified | 18 (4) | 0 | |
| WBC at diagnosis (×109/L) | <0.001 | ||
| Median (range) | 12 (<1-515) | 8 (1-1424) | 0.30 |
| <= 30 | 238 (56) | 80 (74) | |
| 30 – 100 | 67 (16) | 13 (13) | |
| > 100 | 53 (13) | 14 (13) | |
| Missing | 64 (15) | 1 (<1) | |
| CNS involvement at diagnosis | <0.001 | ||
| No | 392 (93) | 90 (83) | |
| Yes | 27 (6) | 12 (11) | |
| Missing | 3 (<1) | 6 (6) | |
| Cytogenetic abnormalities | 0.30 | ||
| t(4;11) or MLL rearranged | 34 (8) | 11 (10) | |
| t(1;19) | 7 (2) | 4(4) | |
| Othersa | 268 (63) | 71 (66) | |
| Missing | 113 (27) | 22 (20) | |
| Cytogenetic risk group | 0.01 | ||
| High risk (t(4;11)/t(14;18)/hypodiploidy/complex) | 48 (12) | 24 (22) | |
| Standard | 261 (62) | 62 (57) | |
| Missing | 113 (27) | 22 (20) | |
| Time to achieve CR1 (weeks) | <0.001 | ||
| Median (interquartile range) | 8 (4-14) | 4 (4-5) | <0.001 |
| <4 weeks | 48 (11) | 8 (7) | |
| 4-8 weeks | 169 (39) | 99 (92) | |
| >=8 weeks | 205 (47) | 1 (<1) | |
| Time from diagnosis to HCT (months) | N/A | ||
| Median (range) | 5 (2-31) | ||
| <5 months | 164 (39) | ||
| >=5 months | 258 (61) | ||
| Time from CR1 to HCT (months) | N/A | ||
| Median (range) | 3 (<1-30) | ||
| <3 months | 201 (47) | ||
| >=3 months | 223 (53) | ||
| KPS (%) pre HCT | |||
| <80 | 25 (6) | ||
| >=80 | 372 (88) | ||
| Missing | 25 (6) | ||
| Conditioning intensity | |||
| Myeloablative | 396 (94) | N/A | |
| RIC/NMA | 17 (4) | ||
| Missing | 8 (2) | ||
| Total body irradiation | N/A | ||
| No | 58 (14) | ||
| Yes | 364 (86) | ||
| Type of donor | N/A | ||
| HLA-identical sibling | 176 (42) | ||
| Unrelated HLA 8/8 allele matched | 168 (40) | ||
| Unrelated HLA 7/8 allele matched | 78 (18) | ||
| HLA-identical sib donor age | N/A | ||
| Median (range) | 35 (13-61) | ||
| Unrelated donor age | |||
| Median (range) | 35 (19-61) | ||
| Donor/Recipient sex match | N/A | ||
| M/M | 168 (40) | ||
| M/F | 87 (21) | ||
| F/M | 84 (20) | ||
| F/F | 81 (19) | ||
| Missing | 2 (<1) | ||
| Donor/Recipient CMV serostatus | N/A | ||
| D+/R+ | 113 (27) | ||
| D+/R- | 125 (29) | ||
| D-/R+ | 59 (14) | ||
| D-/R- | 110 (26) | ||
| Missing | 15 (4) | ||
| Graft type | N/A | ||
| Bone marrow | 86 (21) | ||
| Peripheral blood | 336 (79) | ||
| GVHD prophylaxis | N/A | ||
| Ex-vivo TCD or CD34 selection | 12 (2) | ||
| CsA + MTX | 91 (21) | ||
| Tacrolimus + MTX | 195 (46) | ||
| CsA/Tac + MMF | 58 (14) | ||
| Tac + Sirolimus | 29 (7) | ||
| Othersb | 37 (9) | ||
| ATG/Alemtuzumab | N/A | ||
| ATG alone | 53 (13) | ||
| Alemtuzumab alone | 9 (2) | ||
| No ATG or alemtuzumab | 360 (85) | ||
| Year | |||
| 2002-2007 | 318 (75) | 72 (65) | |
| 2008-2011 | 104 (25) | 36 (35) | |
| Median follow-up of survivors (range), monthsc | 65 (6-130) | 48 (<1-94) |
other cytogenetic abnormalities for HCT cohort include: hypodiploidy, n=7; hyperdiploidy, n=11; more than 3 complexity, n=7; normal karyotype, n=54; other specified, n=189.
other GVHD prophylaxis include: cyclophosphamide alone, n=3; FK506 + others, n=9; FK506 alone, n=11; CsA based, n=7; unknown, n=7
Median follow-up of survivors counted from date of 1st complete remission HCT: Hematopoietic Cell Transplantation; Chemo: chemotherapy cohort; CR: Complete Remission; MTX: Methotrexate; MMF: Mycophenolate Mofetil; Tac: CsA: cyclosporine A; Tacrolimus; CMV: cytomegalovirus; ATG: Anti-thymocyte globulin; TCD: T cell depletion.
108 cases in chemo cohort but 1 lost to follow-up on day of CR1, so 107 were included in all analyses.
Median time to achieve CR1 was longer in the HCT group (8 weeks, interquartile range 4-14) vs. (chemo 4 weeks, interquartile range 4-5, p<0.001). Within the HCT cohort, conditioning regimen intensity was myeloablative for 94%, and 86% of all HCT recipients received TBI. RDs comprised 42% of HCTs, with the remainder consisting of HLA 8/8 matched URD (40%) or 7/8 matched (18%). Hematopoietic cell source was peripheral blood in 79%. For GVHD prophylaxis 15% of HCT recipients received in vivo T cell depletion with ATG or alemtuzumab and an additional 2% underwent ex vivo T cell depletion.
Ten of the chemotherapy patients were taken off protocol and censored at the time they underwent HCT in CR1 at investigator discretion; two of these patients relapsed 102 and 305 days post-HCT. Eight remaining patients were alive in CR1 with median follow-up time of 33 months (range 11-68) post-HCT.
Causes of death for both cohorts are listed in the supplementary online document. Within the chemotherapy cohort, 5 patients experienced lethal toxicities during post-remission chemotherapy. Relapse accounted for 69% of the deaths in the chemotherapy cohort, with 23% from non-relapse causes. In contrast, within the HCT cohort, 28% of the deaths were related to leukemic relapse and 70% were attributable to HCT-related toxicities.
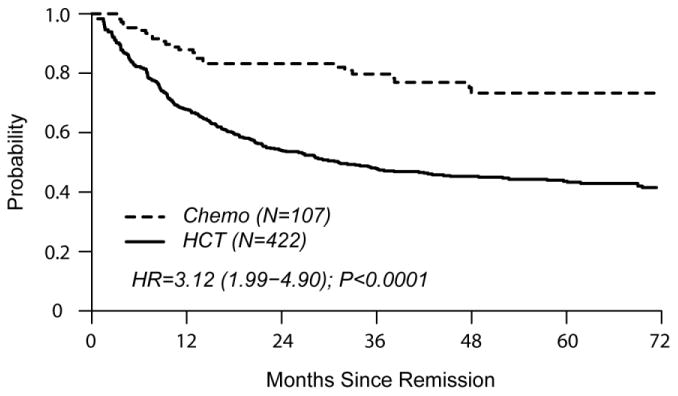
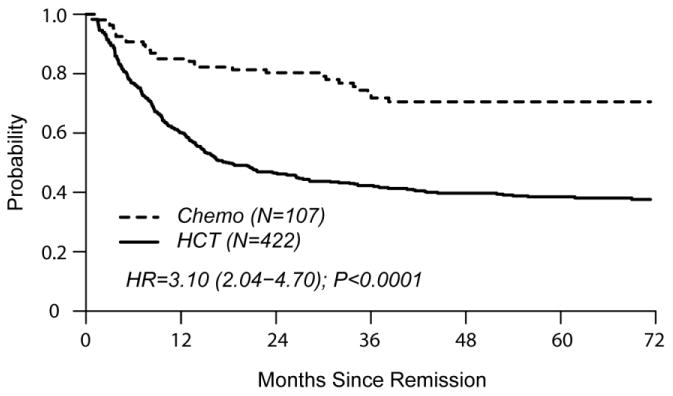
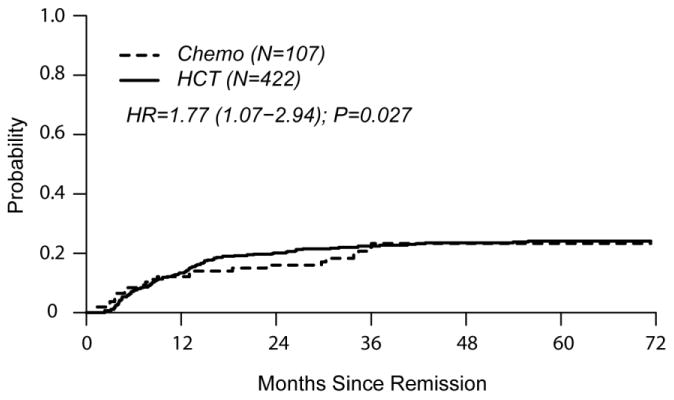
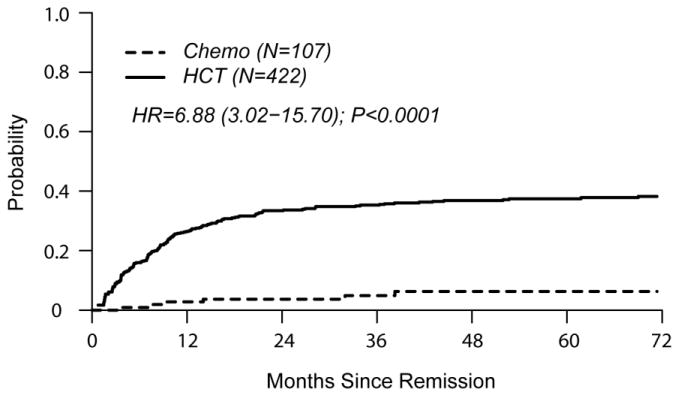
In univariate comparisons at 4 years of follow-up, cumulative incidence of relapse was similar in both groups (HCT 24% [19-28] vs. chemo 23% [15-32] p=0.97). TRM was higher in the HCT cohort (HCT 37% [31-42] vs. chemo 6% [3 – 12], p<0.0001). DFS favored the chemo cohort (HCT 40% [35-45] vs. chemo 71% [60-79], p<0.0001). OS was also in favor of chemo (HCT 45% [40-50] vs. chemo 73% [63-81], p<0.0001).
Multivariable Analysis
The single independent factor predictive of treatment failure (relapse or death) was HCT (HR 3.10 [2.04 – 4.70], p<0.001) (Table 2). HCT was also independently associated with poorer OS (HR 3.12 [1.99 – 4.90], p<0.0001). Factors significantly associated with relapse were HCT (HR 1.77 [1.07 – 2.94]) and CNS involvement at diagnosis (HR 1.90 [1.07 – 3.40]). TRM was associated with HCT (HR 6.88 [3.02 – 15.70]), older age (HR 1.69 [1.16 – 2.44], and B cell phenotype (HR 2.26 [1.30 – 3.93]).
Table 2. Multivariable Analyses.
| Outcome | Number | Hazard Ratio (95% CI) | p-value | |
|---|---|---|---|---|
|
| ||||
| TRM | ||||
|
| ||||
| Main: Chemo | 107 | 1.00 | ||
| HCT | 422 | 6.88 (3.02 – 15.70) | <0.01 | |
|
| ||||
| Age: 19 - 29 | 214 | 1.00 | ||
| 30 - 50 | 315 | 1.69 (1.16 – 2.44) | <0.01 | |
|
| ||||
| FAB: T-cell/Unspecified | 102 | 1.00 | ||
| B-cell | 427 | 2.26 (1.30 – 3.93) | <0.01 | |
|
| ||||
| Relapse | ||||
|
| ||||
| Main: Chemo | 107 | 1.00 | ||
| HCT | 422 | 1.77 (1.07 – 2.94) | 0.03 | |
|
| ||||
| CNS Involvement: No | 481 | 1.00 | ||
| Yes | 39 | 1.90 (1.07 – 3.40) | 0.03 | |
| Missing | 9 | 3.51 (1.35 – 9.16) | 0.01 | |
|
| ||||
| Treatment Failure (inverse of DFS) | ||||
|
| ||||
| Main: Chemo | 107 | 1.00 | ||
| HCT | 422 | 3.10 (2.04 – 4.70) | <0.01 | |
|
| ||||
| CNS Involvement: No | 481 | 1.00 | ||
| Yes | 39 | 1.53 (0.99 – 2.35) | 0.05 | |
| Missing | 9 | 2.21 (0.88 – 5.57) | 0.09 | |
|
| ||||
| Overall Mortality | ||||
|
| ||||
| Main: Chemo | 107 | 1.00 | ||
| HCT | 422 | 3.12 (1.99 – 4.90) | <0.01 | |
TRM: treatment related mortality; N: Number; Chemo; Chemotherapy cohort; HCT: Hematopoietic Cell Transplant; DFS: disease-free survival; OS: overall survival; CI: Confidence Interval; CNS: Central Nervous System.
As a sensitivity analysis, we excluded the 10 patients in the chemo cohort who underwent HCT in CR1. This did not change the key outcomes. Similar to the primary analysis, treatment failure (relapse or death) was higher in the HCT cohort (HR=3.05 [2.00 – 4.63], P<0.0001. Relapse and TRM were greater in the HCT cohort (HR 1.75 [1.05 – 2.90], p=0.0308, and HR=6.74 [2.96 – 15.37], respectively). Overall Survival favored the chemotherapy cohort (HR=3.08[1.96 –4.83], P<0.0001.
Figures 2a and 2b show left-truncated Kaplan-Meier DFS and OS curves comparing outcomes of chemotherapy and HCT. Hazard Ratios for these outcomes favor the chemo cohort (3.10 [2.04-4.70] and 3.12 [1.99-4.90], respectively). Figures 2c and 2d demonstrate left-truncated cumulative incidence of relapse and TRM. In these analyses, both relapse and TRM were inferior in the HCT cohort (HR=1.77 [1.07-2.94] and HR=6.88 [3.02-15.70], respectively.
Figure 2.
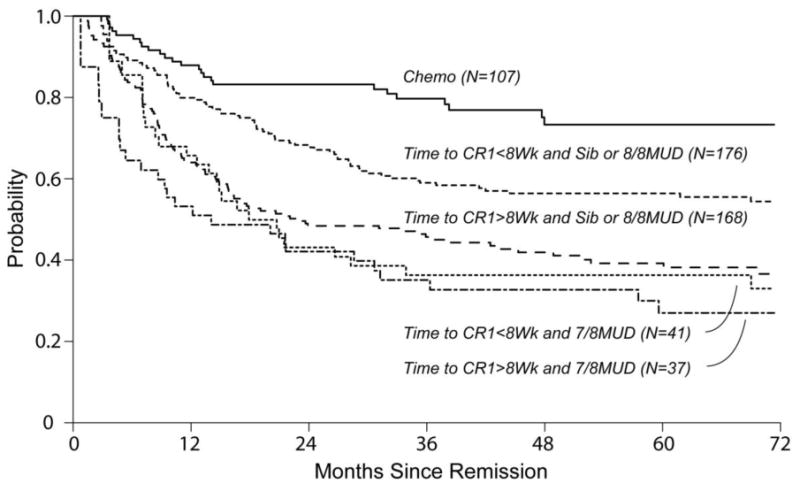
Comparison of outcomes of Chemotherapy only regimen (chemo) and allogeneic hematopoietic cell transplantation (HCT)
(a) Overall Survival
(b) Disease Free Survival
(c) Cumulative Incidence of Relapse
(d) Cumulative Incidence of Treatment Related Mortality
(e) Kaplan Meier Estimate of Overall Survival Comparing Chemotherapy Patients To Sub-Groups Of HCT Recipients
Exploratory multivariable regression analysis was performed within the HCT cohort in order to establish predictors of outcome for these patients. Time to CR of >8 weeks and the use of HLA mismatched URDs (7/8) were significantly associated with inferior OS after HCT. These factors were then adjusted for in the analysis of HCT vs. chemo outcomes (supplementary online material). This demonstrated that the better OS associated with chemotherapy was maintained (HCT HR 2.14 [1.36-3.35]) even when restricting the HCT cohort to only those recipients using RDs or 8/8 URDs and those with time from diagnosis to CR1 <8 weeks (Figure 2e).
Discussion
In younger adults with Ph- ALL in CR1, we show that the administration of a non-HCT, pediatric–inspired chemotherapy regimen resulted in clinical outcomes that were superior to allogeneic HCT. A striking finding was that HCT did not appear to reduce relapse rates as compared to the non-HCT approach. The HCT also patients experienced higher TRM. Overall, this resulted in poorer DFS and OS compared to patients who received the pediatric-inspired regimen.
Our results differ from the largest donor-versus-no donor clinical trial conducted in adults with ALL in CR1 that demonstrated better overall survival for HCT recipients.3 The value of HCT has been further supported by meta-analyses, suggesting that for adults with Ph- ALL in CR1, HCT offers an OS advantage of approximately 10% compared to non-HCT therapy. 6,8 The contradictory findings in our current study are likely to be a consequence of the characteristics of the non-HCT chemotherapy arm, which is adopted from the intensive approach offered to children and adolescents with high-risk ALL.4,5 The DFCI regimen, similar to other pediatric regimens for ALL, offers a prolonged (> 2 years), intensive protocol, with particular emphasis on the post-remission administration of high cumulative doses of non-myelosuppressive agents including corticosteroids, vincristine, and L-Asparaginase. In addition, this regimen includes extensive CNS prophylaxis with intrathecal chemotherapy, cranial irradiation, and high dose intravenous methotrexate.4,5,7 When comparing outcomes of adolescents and younger adults who have received pediatric regimens to those of similar age who received less intensive regimens at adult ALL centers, the intensive approach has consistently resulted in better prevention of relapse and more favorable clinical outcomes.19-21 Pediatric-inspired regimens have recently been evaluated for adults as old as 60 years, with promising outcomes in both clinical trial and regular hospital settings.7,11-14
This study was not a randomized controlled trial, and thus we actively addressed potential biases that may result from measurable differences in baseline clinical characteristics and in time to definitive post-remission treatment. We selected patients who were treated over the same time period (2002-2011) and geographic regions (Canada and US) with identical eligibility criteria for age (18-50). We then deployed multivariable Cox regression analysis to account for imbalances between the two cohorts including age, baseline white blood cell count, and cytogenetic risk category. We also used left-truncation to adjust for a time bias which might favor HCT patients, as these patients would need to survive in CR1 long enough to receive the HCT.
Within the HCT cohort, a longer time from diagnosis to CR1 may have been an indication of patients who required several cycles of chemotherapy to achieve CR1; this may adversely influence outcomes for the HCT cohort. However, when we limited the HCT cohort only to those whose time from diagnosis to CR1 was less than 8 weeks, the key clinical outcomes still favored the chemotherapy cohort.
The advantage of pediatric-inspired chemotherapy over HCT was noted regardless of donor type in the HCT arm. Because the use of a partially HLA matched (7/8) URD may confer poorer outcomes after HCT, we performed a subgroup analysis restricted to RD or only 8/8 URD. Using this adjustment, our results still showed a survival advantage for the chemotherapy cohort.
Although follow-up in the chemo group was shorter than in the HCT cohort (48 vs. 65 months), the majority of events related to relapse or to TRM occurred within the first 48 months in both cohorts. Thus, the shorter median follow-up time in the chemo cohort is unlikely to change the key results in this study. Overall, adjusting for all of potential biases that we could recognize, we continued to find a significant advantage in favor of chemotherapy compared to HCT, the latter offering no reduction in relapse risk.
HCT outcomes were limited by excess TRM. Lethal toxicities following HCT, primarily as a result of GVHD or infection, remain major limiting factors to HCT for ALL. Thankfully, improvements in survival following HCT have been noted,22 although outcomes after HCT for adults with ALL do not appear to have improved as much as those seen in children and adolescents.23 The BFM group have demonstrated that for children with ALL who received an allogeneic HCT, TRM as low as 4% after related donor transplants and 10% after unrelated donor transplants can be achieved24. This suggests that young adults tolerate allogeneic HCT much more poorly than in children, further emphasizing our concerns about the risks of HCT in younger adults with ALL. However, regardless of any foreseeable improvements in TRM after HCT, this procedure did not appear to reduce the relapse rate, and hence the added risk of TRM was not counterbalanced by a reduction in ALL relapse.
Older age (>30 years) was independently associated with excess TRM. This is not surprising, and highlights the need for greater attention to the management of co-morbidities, and general supportive care regardless of whether patients are assigned to a HCT or a non-HCT post-remission strategy. For the HCT cohort, selection of the optimal conditioning regimens is important. The majority of the HCT patients in the current analysis underwent myeloablative conditioning, which has been shown in some25 but not all26 studies to pose a higher risk of TRM compared to reduced intensity conditioning (RIC) approaches in adult ALL. With increasing use of RIC approaches that lack TBI, especially those who are older than 40 to 50 years or with comorbidities, the outcomes of ALL patients undergoing HCT may improve, necessitating further analyses of post-HCT outcomes in the RIC era.27,28 Although the incidence of post-remission TRM was significantly lower in the chemo cohort, it was still at an appreciable level (6%). Pediatric-inspired regimens require vigilance with respect to infectious, venous thromboembolic, and other complications.7 Centers that choose to use such regimens need to build rigorous supportive care strategies into their protocols.
Too few data were available regarding the use of alternative graft sources (umbilical cord blood or haploidentical donors) to include in this analysis. Recently, registry studies and clinical trial outcomes have suggested that HCTs using such cell sources are promising modalities, albeit in need of further study.29-31
Unfortunately, we lacked data for either cohort on novel molecular markers in ALL, such as IKZF132; however, in North American leukemia centers, these tests were unlikely to have been performed frequently over the time period of study and thus would not have influenced assignment to either chemotherapy or HCT.
In ALL, MRD is an important predictor of subsequent relapse.32-34 Data on post-treatment MRD (as a measure of the depth of remission) were unfortunately not available for the majority of patients in either the HCT or chemotherapy arms. It is possible that the 10 patients in the chemo cohort who proceeded to HCT while in CR1 represented a subgroup at particularly high risk for relapse (e.g. MRD positivity or other adverse molecular markers). As a sensitivity analysis, we removed these patients from the chemo cohort. Outcomes continued to show no advantage to receiving HCT in CR1 in terms of relapse risk, TRM, DFS, or OS. MRD guided therapy, although popular in pediatric ALL, was not widely applied for adults during the period of study and thus could not further inform the reported comparisons.35 Future studies will need to incorporate MRD reporting into their results.
In summary, we show that a pediatric-inspired chemotherapy regimen for adults with Ph-ALL results in durable leukemia-free survival, and is associated with superior outcomes compared to allogeneic transplantation. These findings should help to inform health care providers and patients about the relative merits of HCT versus intensive, non-HCT approaches in adult Ph-ALL, and will also provide rationale for the development of prospective clinical trials in this area.
Supplementary Material
Figure 1.
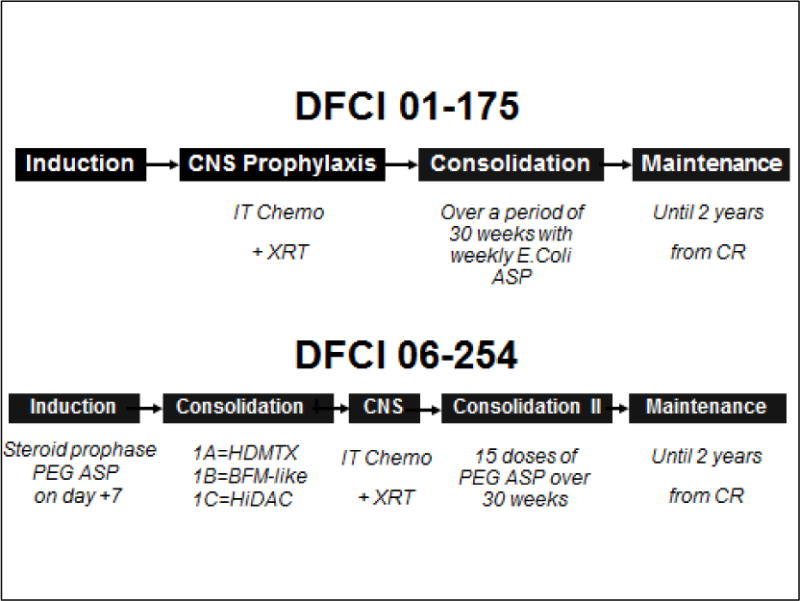
Overall Schema of the Dana Farber Cancer Institute (DFCI) adult ALL Consortium regimens in trials 01-175 and 06-254 trials11,16
CNS: Central Nervous System; IT: Intrathecal; XRT: Cranial Irradiation; ASP: L-Asparaginase; CR: Complete Remission; PEG: Pegylated; HDMTX: High dose Methotrexate; BFML Berlin-Frankfurt-Münster; HiDAC: High dose cytarabine;
Acknowledgments
The DFCI ALL Consortium trials in younger adults were supported by grant P01CA068484 from the National Cancer Institute (NCI) to SES.
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-13-1-0039 and N00014-14-1-0028 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals; Allos Therapeutics, Inc.; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; *Blue Cross and Blue Shield Association; *Celgene Corporation; Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Fresenius-Biotech North America, Inc.; *Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.;*Gentium SpA; Genzyme Corporation; GlaxoSmithKline; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; Jeff Gordon Children's Foundation; Kiadis Pharma; The Leukemia & Lymphoma Society; Medac GmbH; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; *Milliman USA, Inc.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Perkin Elmer, Inc.; *Remedy Informatics; *Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; St. Baldrick's Foundation; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; *Tarix Pharmaceuticals; *TerumoBCT; *Teva Neuroscience, Inc.; *THERAKOS, Inc.; University of Minnesota; University of Utah; and *Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
*Corporate Members
Footnotes
Previous presentations: Presented in part as an oral abstract at the 2014 American Society of Hematology Meeting, San Francisco, CA; December, 2014.
Conflict-of-interest disclosure: The authors do not have conflicts of interest to declare.
References
- 1.Stock W, Johnson JL, Stone RM, et al. Dose intensification of daunorubicin and cytarabine during treatment of adult acute lymphoblastic leukemia: results of Cancer and Leukemia Group B Study 19802. Cancer. 2013;119(1):90. doi: 10.1002/cncr.27617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas D, O'Brien S, Faderl S, et al. Anthracycline dose intensification in adult acute lymphoblastic leukemia: lack of benefit in the context of the fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone regimen. Cancer. 2010;116(19):4580–458. doi: 10.1002/cncr.25319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldstone AH, Richards SM, Lazarus HM, et al. In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the International ALL Trial (MRC UKALL XII/ECOG E2993) Blood. 2008;111(4):1827–1833. doi: 10.1182/blood-2007-10-116582. [DOI] [PubMed] [Google Scholar]
- 4.Silverman LB, Stevenson KE, O'Brien JE, et al. Long-term results of Dana-Farber Cancer Institute ALL Consortium protocols for children with newly diagnosed acute lymphoblastic leukemia (1985-2000) Leukemia. 2010;24(2):320–334. doi: 10.1038/leu.2009.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barry E, DeAngelo DJ, Neuberg D, et al. Favorable outcome for adolescents with acute lymphoblastic leukemia treated on Dana-Farber Cancer Institute Acute Lymphoblastic Leukemia Consortium Protocols. J Clin Oncol. 2007;25(7):813–819. doi: 10.1200/JCO.2006.08.6397. [DOI] [PubMed] [Google Scholar]
- 6.Pidala J, Djulbegovic B, Anasetti C, Kharfan-Dabaja M, Kumar A. Allogeneic hematopoietic cell transplantation for adult acute lymphoblastic leukemia (ALL) in first complete remission. Cochrane Database Syst Rev. 2011;(10):CD008818. doi: 10.1002/14651858.CD008818.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oliansky DM, Larson RA, Weisdorf D, et al. The Role of Cytotoxic Therapy with Hematopoietic Stem Cell Transplantation in the Treatment of Adult Acute Lymphoblastic Leukemia: Update of the 2006 Evidence-Based Review. Biol Blood Marrow Transplant. 2012;18(1):16–17. doi: 10.1016/j.bbmt.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Gupta V, Richards S, Rowe J Acute Leukemia Stem Cell Transplantation Trialists' Collaborative Group. Allogeneic, but not autologous, hematopoietic cell transplantation improves survival only among younger adults with acute lymphoblastic leukemia in first remission: an individual patient data meta-analysis. Blood. 2013;121(2):339–350. doi: 10.1182/blood-2012-07-445098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marks DI, Perez WS, He W, et al. Unrelated donor transplants in adults with Philadelphia-negative acute lymphoblastic leukemia in first complete remission. Blood. 2008;112(2):426–434. doi: 10.1182/blood-2007-12-128918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishiwaki S, Inamoto Y, Sakamaki H, et al. Allogeneic stem cell transplantation for adult Philadelphia chromosome-negative acute lymphocytic leukemia: comparable survival rates but different risk factors between related and unrelated transplantation in first complete remission. Blood. 2010;116(20):4368–4375. doi: 10.1182/blood-2010-02-269571. [DOI] [PubMed] [Google Scholar]
- 11.DeAngelo DJ, Dahlberg S, Silverman LB, et al. A Multicenter Phase II Study Using a Dose Intensified Pediatric Regimen in Adults with Untreated Acute Lymphoblastic Leukemia. Leukemia. 2013 doi: 10.1038/leu.2014.229. e-pub ahead of print 31 July 2013. [DOI] [Google Scholar]
- 12.Huguet F, Leguay T, Raffoux E, et al. Pediatric-inspired therapy in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: the GRAALL-2003 study. J Clin Oncol. 2009;27(6):911–918. doi: 10.1200/JCO.2008.18.6916. [DOI] [PubMed] [Google Scholar]
- 13.Hayakawa F, Sakura T, Yujiri T, et al. Markedly improved outcomes and acceptable toxicity in adolescents and young adults with acute lymphoblastic leukemia following treatment with a pediatric protocol: A phase II study by the Japan Adult Leukemia Study Group. Blood Cancer. 2014;J4:e252. doi: 10.1038/bcj.2014.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brandwein JM, Atenafu EG, Schuh AC, et al. Predictors of outcome in adults with BCR-ABL negative acute lymphoblastic leukemia treated with a pediatric-based regimen. Leuk Res. 2014;38(5):532–536. doi: 10.1016/j.leukres.2013.10.025. [DOI] [PubMed] [Google Scholar]
- 15.Stock W, Luger S, Advani AS, et al. ASH meeting. San Francisco: Dec, 2014. Favorable Outcomes for Older Adolescents and Young Adults (AYA) with Acute Lymphoblastic Leukemia (ALL): Early Results of U.S. Intergroup Trial C10403. Abstract #796, 56th. [Google Scholar]
- 16.DeAngelo DJ, Stevenson K, Neuberg DS, et al. A Multicenter Phase II Study Using a Dose Intensified Pegylated-Asparaginase Pediatric Regimen in Adults with Untreated Acute Lymphoblastic Leukemia: A DFCI ALL Consortium Trial. ASH Annual Meeting. 2015 Dec 5; Abstract#80. [Google Scholar]
- 17.Cain KC, Harlow SD, Little RJ, et al. Bias due to left truncation and left censoring in longitudinal studies of developmental and disease processes. Am J Epidemiol. 2011;173(9):1078–1084. doi: 10.1093/aje/kwq481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 19.Boissel N, Auclerc MF, Lheritier V, et al. Should adolescents with acute lymphoblastic leukemia be treated as old children or young adults? Comparison of the French FRALLE-93 and LALA-94 trials. J Clin Oncol. 2003;21:774–780. doi: 10.1200/JCO.2003.02.053. [DOI] [PubMed] [Google Scholar]
- 20.Stock W, La M, Sanford B, et al. What determines the outcomes for adolescents and young adults with acute lymphoblastic leukemia treated on cooperative group protocols? A comparison of Children's Cancer Group and Cancer and Leukemia Group B studies. Blood. 2008;112(5):1646–1654. doi: 10.1182/blood-2008-01-130237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramanujachar R, Richards S, Hann I, et al. Adolescents with acute lymphoblastic leukaemia: outcome on UK national paediatric (ALL97) and adult (UKALLXII/E2993) trials. Pediatric Blood Cancer. 2007;48(3):254–261. doi: 10.1002/pbc.20749. [DOI] [PubMed] [Google Scholar]
- 22.Hahn T, McCarthy PL, Jr, Hassebroek A, et al. Significant improvement in survival after allogeneic hematopoietic cell transplantation during a period of significantly increased use, older recipient age, and use of unrelated donors. J Clin Oncol. 2013;31(19):2437–49. doi: 10.1200/JCO.2012.46.6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burke MJ, Gossai N, Wagner JE, et al. Survival differences between adolescents/young adults and children with B precursor acute lymphoblastic leukemia after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2013;19(1):138–142. doi: 10.1016/j.bbmt.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peters C, Schrappe M, von Stackelberg A, et al. Stem-cell transplantation in children with acute lymphoblastic leukemia: A prospective international multicenter trial comparing sibling donors with matched unrelated donors-The ALL-SCT-BFM-2003 trial. J Clin Oncol . 2015 Apr 10;33(11):1265–74. doi: 10.1200/JCO.2014.58.9747. [DOI] [PubMed] [Google Scholar]
- 25.Mohty M, Labopin M, Volin L, et al. Acute Leukemia Working Party of EBMT. Reduced-intensity versus conventional myeloablative conditioning allogeneic stem cell transplantation for patients with acute lymphoblastic leukemia: a retrospective study from the European Group for Blood and Marrow Transplantation. Blood. 2010;116(22):4439–4443. doi: 10.1182/blood-2010-02-266551. [DOI] [PubMed] [Google Scholar]
- 26.Marks DI, Wang T, Pérez WS, et al. The outcome of full-intensity and reduced-intensity conditioning matched sibling or unrelated donor transplantation in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia in first and second complete remission. Blood. 2010;116(3):366–374. doi: 10.1182/blood-2010-01-264077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kunter G, Perkins JB, Pidala J, et al. Pharmacokinetically-targeted BU and fludarabine as conditioning before allogeneic hematopoietic cell transplantation for adults with ALL in first remission. Bone Marrow Transplant. 2014;49(1):11–16. doi: 10.1038/bmt.2013.121. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka J, Kanamori H, Nishiwaki S, et al. Reduced-intensity vs myeloablative conditioning allogeneic hematopoietic SCT for patients aged over 45 years with ALL in remission: a study from the Adult ALL Working Group of the Japan Society for Hematopoietic Cell Transplantation (JSHCT) Bone Marrow Transplant. 2013;48(11):1389–1394. doi: 10.1038/bmt.2013.68. [DOI] [PubMed] [Google Scholar]
- 29.Brunstein CG, Fuchs EJ, Carter SL, et al. Blood and Marrow Transplant Clinical Trials Network. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118(2):282–288. doi: 10.1182/blood-2011-03-344853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marks DI, Woo KA, Zhong X, et al. Unrelated umbilical cord blood transplant for adult acute lymphoblastic leukemia in first and second complete remission: a comparison with allografts from adult unrelated donors. Haematologica. 2014;99(2):322–328. doi: 10.3324/haematol.2013.094193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun YQ, Wang J, Jiang Q, et al. Haploidentical hematopoietic SCT may be superior to conventional consolidation/maintenance chemotherapy as post-remission therapy for high-risk adult ALL. Bone Marrow Transplant. 2015;50:20–25. doi: 10.1038/bmt.2014.195. [DOI] [PubMed] [Google Scholar]
- 32.Dhédin N, Huynh A, Maury S, et al. Role of allogeneic stem cell transplantation in adult patients with Ph-negative acute lymphoblastic leukemia. Blood. 2015;125(16):2486–96. doi: 10.1182/blood-2014-09-599894. [DOI] [PubMed] [Google Scholar]
- 33.Bruggemann M, Raff T, Flohr T, et al. Clinical significance of minimal residual disease quantification in adult patients with standard-risk acute lymphoblastic leukemia. Blood. 2006;107:1116–1123. doi: 10.1182/blood-2005-07-2708. [DOI] [PubMed] [Google Scholar]
- 34.Ribera JM, Oriol A, Morgades M, et al. Treatment of high-risk Philadelphia chromosome-negative acute lymphoblastic leukemia in adolescents and adults according to early cytologic response and minimal residual disease after consolidation assessed by flow cytometry: final results of the PETHEMA ALL-AR-03 trial. J Clin Oncol. 2014;32(15):1595–1604. doi: 10.1200/JCO.2013.52.2425. [DOI] [PubMed] [Google Scholar]
- 35.Paulson K, Szwajcer D, Raymond CB, Seftel MD. The role of hematopoietic cell transplantation in adult ALL: clinical equipoise persists. Leuk Res. 2014;38(2):176–179. doi: 10.1016/j.leukres.2013.10.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


