Abstract
Objectives
To assess whether risk of severe maternal morbidity at delivery differed for women who conceived using assisted reproductive technology (ART), those with indicators of subfertility but no ART (“subfertile”), and those who had neither ART nor subfertility (“fertile”).
Methods
This retrospective cohort study was part of the larger Massachusetts Outcomes Study of Assisted Reproductive Technology (MOSART). To construct the MOSART database and identify ART deliveries, we linked ART treatment records to birth certificates and maternal and infant hospitalization records occurring in Massachusetts between 2004 and 2010. An algorithm of ICD-9-CM diagnosis and procedure codes identified severe maternal morbidity. We used Logistic Generalized Estimating Equations to estimate odds of severe maternal morbidity associated with fertility status, adjusting for maternal demographic and health factors and gestational age, stratifying on plurality and method of delivery.
Results
The prevalence of severe maternal morbidity among this population (n = 458,918) was 1.16%. The overall, crude prevalences of severe maternal morbidity among fertile, subfertile and ART deliveries were 1.09%, 1.44% and 3.14%, respectively. The most common indicator of severe maternal morbidity was blood transfusion. In multivariable analyses, among singletons, ART was associated with increased odds of severe maternal morbidity compared to both fertile (Vaginal: aOR 2.27, 95% CI: 1.78 – 2.88; cesarean: aOR 1.67, 95% CI: 1.40 – 1.98, respectively) and subfertile (vaginal: aOR 1.97, 95% CI: 1.30 – 3.00; cesarean: aOR 1.75, 95% CI: 1.30 – 2.35, respectively) deliveries. Among twins, only cesarean ART deliveries had significantly greater severe maternal morbidity compared to cesarean fertile deliveries (aOR 1.48, 95% CI: 1.14, 1.93).
Conclusions
Women who conceive through ART may have elevated risk severe maternal morbidity at delivery, largely indicated by blood transfusion, even when compared with a subfertile population. Further research should elucidate mechanisms underlying this risk.
INTRODUCTION
The rate of pregnancy-related death in the United States has been on the rise since the 1980’s, increasing from 7.2 per 100,000 in 1987 to 17.8 in 2011.1 However, because mortality is so rare, surveillance efforts have also targeted severe maternal morbidity (SMM), defined as having any of twenty-five ICD-9 hospitalization codes for procedures and conditions indicating a potentially life threatening situation.2 The SMM rate more than doubled in the United States between 2001 and 2011, increasing from 78.6, to 162.8 per 10,000 delivery hospitalizations.2 During the same period, the proportion of infants born as a result of assisted reproductive technology (ART), defined as “fertility treatments in which both eggs and embryos are handled in the laboratory,”3 increased from 0.9% to 1.5%.3;4
While the profile of the ART patient population tends to reflect greater social advantage and factors protective against SMM than the general maternity population,5 ART has been associated with known risk factors for SMM including placenta accreta,6 plural births7 and cesarean delivery.8 Furthermore, women who use ART to conceive are more likely to have underlying health and fertility problems which necessitated the use of ART.9;10 As a result, previous research has encountered difficulty in distinguishing the direct effects of ART on maternal outcomes from other contributing health issues.11 The objective of this analysis was to examine whether ART use contributed to the frequency and type of severe maternal morbidities at the time of delivery apart from maternal fertility status and other health factors.
MATERIALS AND METHODS
This is one in a series of retrospective cohort analyses evaluating the effect of maternal fertility status (subfertile women treated with ART, subfertile women not treated with ART, and fertile women) on the course and outcome of pregnancy. This analysis is part of a larger population-based study of ART in Massachusetts.9;10;12-16 The project known as the Massachusetts Outcome Study of Assisted Reproductive Technology (MOSART) took place under a Memorandum of Understanding for use of the SART CORS data and was approved by Institutional Review Boards of Boston University, the Massachusetts Department of Public Health (MDPH), all investigator institutions, and by the SART Research Committee.
Data for these analyses were obtained from two sources 1) the Society for Assisted Reproductive Technology Clinic Outcome Reporting System (SART CORS) online database and 2) the birth certificates, fetal death records, and hospital utilization data in the Massachusetts-based Pregnancy to Early Life Longitudinal (PELL) Data System.17
SART CORS is a clinic and treatment-based data system that contains cycle-based ART data from more than 90% of United States ART clinics and over 97% of ART cycles.7 SART CORS data contain patient demographics (age, race, diagnosis), ART treatment data (fresh versus frozen state, oocyte type, micromanipulation procedures and embryos transferred) and outcome data (cancellation, pregnancy, live birth, birth outcomes) on all cycles of ART at participating clinics and data from this system are available for research from 2004 onward. All Massachusetts clinics report their data to SART CORS.
PELL is a relational data system composed of individual databases linked together by randomly-generated unique IDs for mother and infant. The linked database contains information on more than 99% of all births and fetal deaths in Massachusetts from 1998-2010 and links these to corresponding hospital utilization data (hospital admissions, observational stays, and emergency room visits) for individual women and their children. MDPH and the Massachusetts Center for Health Information and Analysis are the custodians of the PELL data.
The construction of the MOSART database involved a linkage of the SART CORS and PELL data systems for all deliveries to Massachusetts residents in Massachusetts hospitals between July 1, 2004 and December 31, 2010. In order to capture births plausibly attributable to ART treatment cycles beginning on January 1, 2004, we included births occurring from July 1, 2004, through December 31, 2010, the last date that birth records were available to us at the time. A detailed description of the PELL and SART CORS data, their linkage and validation have been published previously.14 PELL records were matched against eligible ART treatment cycle records – those occurring to Massachusetts resident women or to women who obtained treatment in a Massachusetts clinic during the same timeframe (43,214 eligible cycles for 17,547 women). Among our total sample of PELL deliveries, 13,981 (2.70%) linked to ART treatment cycles in SART CORS, indicating that these infants were conceived using ART. We limited our analysis to singleton or twin deliveries and excluded deliveries with incomplete data on the key predictors or outcomes of interest and those to women under age eighteen.
An algorithm was developed to identify deliveries to women with indicators of subfertility who did not use ART to become pregnant.10 Briefly, subfertile deliveries were identified using three sources: (1) birth certificate items indicating the use of fertility treatment for the current, or a prior pregnancy within the past five years; (2) hospital contact within five years prior to the current pregnancy for a condition specifically related to infertility (ICD-9 codes 628.0, 628.2, 628.3, 628.8, 628.9, V230); or (3) an ART treatment cycle for a prior pregnancy attempt occurring after January 1, 2004 but no ART cycle linked to the index delivery, as reported to SART CORS. From the pool of deliveries that met at least one of these criteria, we eliminated ART-assisted deliveries identified in the SART CORS database in the study period and duplicate cases which resulted in 8,984 non-ART singleton or twin deliveries between July 1, 2004 and December 31, 2010 to women with at least one indicator of subfertility.
The Centers for Disease Control and Prevention defines severe maternal morbidity using delivery hospitalization data and International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis and procedure codes that indicate a potentially life-threatening maternal condition or complication.18 Delivery hospitalizations with severe maternal morbidities were identified using a published algorithm that includes 25 specific ICD-9-CM diagnosis and procedure codes (Table 1) with indicators of organ-system failure that likely represent specific well-defined severe events.2 Irrespective of the pregnancy outcome, women who had any ICD-9-CM codes that indicate such a potentially severe event were designated as having severe maternal morbidity.2 We applied this algorithm to all deliveries occurring at 20 weeks or greater gestational age in our study population, and evaluated the differences by maternal fertility group and plurality. The primary outcome measure was severe maternal morbidity (SMM), defined as any of the 25 factors listed in Table 1.2
Table 1.
| Severe Maternal Morbidity Indicator |
ICD-9-CM Codes | ICD-9-CM Diagnosis Code |
ICD-9-CM Procedure Code |
|---|---|---|---|
| 1. Acute myocardial infarction | 410.xx | x | |
| 2. Acute renal failure | 584.x, 669.3x | x | |
| 3. Adult respiratory distress syndrome |
518.5, 518.81, 518.82, 518.84,799.1 | x | |
| 4. Amniotic fluid embolism | 673.1x | x | |
| 5. Aneurysm | 441.xx | x | |
| 6. Cardiac arrest/ventricular fibrillation |
427.41, 427.42, 427.5 | x | |
| 7. Disseminated intravascular coagulation |
286.6, 286.9, 666.3x | x | |
| 8. Eclampsia | 642.6x | x | |
| 9. Heart failure during procedure or surgery |
669.4x, 997.1 | x | |
| 10. Internal injuries of thorax, abdomen, and pelvis |
860.xx—869.xx | x | |
| 11. Intracranial injuries | 800.xx, 801.xx, 803.xx, 804.xx, 851.xx- 854.xx |
x | |
| 12. Puerperal cerebrovascular disorders |
430, 431, 432.x, 433.xx, 434.xx, 436, 437.x, 671.5x, 674.0x, 997.2, 999.2 |
x | |
| 13. Pulmonary edema | 428.1, 518.4 | x | |
| 14. Severe anesthesia complications |
668.0x, 668.1x, 668.2x | x | |
| 15. Sepsis | 038.xx, 995.91, 995.92 | x | |
| 16. Shock | 669.1x, 785.5x, 995.0, 995.4, 998.0 | x | |
| 17. Sickle cell anemia with crisis | 282.62, 282.64, 282.69 | x | |
| 18. Thrombotic embolism | 415.1x, 673.0x, 673.2x, 673.3x, 673.8x | x | |
| 19. Blood transfusion | 99.0x | x | |
| 20. Cardio monitoring | 89.6x | x | |
| 21. Conversion of cardiac rhythm | 99.6x | x | |
| 22. Hysterectomy | 68.3x-68.9 | x | |
| 23. Operations on heart and pericardium |
35.xx, 36.xx, 37.xx, 39.xx | x | |
| 24. Temporary tracheostomy | 31.1 | x | |
| 25. Ventilation | 93.90, 96.01-96.05, 96.7x | x |
Note: ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification
To estimate the association of ART with risk of any severe maternal morbidity during the delivery hospitalization, we analyzed the data using Logistic Generalized Estimating Equations (GEE). The GEE model accounted for correlation between multiple infants born to the same woman during the time period studied, as there were women who had more than one delivery in the MOSART data system. Because of the known maternal health risks associated with both plurality and cesarean delivery,17;19 and the association of these with fertility treatment, models were run separately for singleton and twin pregnancies, and for vaginal and cesarean deliveries.
We tested potential confounders of the association between fertility status of the delivery and subsequent SMM. The final model included maternal age, education, race and ethnicity, marital status, payer for delivery, smoking during pregnancy, parity, and gestational or chronic hypertension or diabetes. In order to further adjust for the potentially confounding effects of certain maternal health conditions, our models also included selected obstetric or gynecologic health conditions found in the hospital discharge records and known to be associated with infertility including endometriosis, pelvic inflammatory disease, polycystic ovarian syndrome, intrauterine synchiae, peritoneal adhesions, abdominal/ovarian/tubal pregnancy, and absence of menstruation and combined these into a single dichotomous measure as selected OB GYN conditions.20 We tested for interaction effects between the main independent variable (fertility and treatment status of the delivery) and select demographic characteristics, (e.g. maternal age and race and ethnicity), associated with each of the perinatal outcomes. We examined the Type 3 analysis of effects for significance, finding no set of interaction terms meeting the criterion of p < 0.05.
We performed a count of the most common severe morbidities reported on maternal hospital discharge records and calculated the proportion of all deliveries affected by a given morbidity, reporting those which were most prevalent, by fertility status, plurality, and method of delivery. Finally, because blood transfusion was found to be the most common indicator of severe morbidity, we performed a post-hoc analysis examining the prevalence of transfusion within the ART group, across diagnoses recorded in SART CORS. Diagnoses tested included uterine factor, diminished ovarian reserve, endometriosis, male factor, ovulatory disorder, other factors, tubal factor, and unknown factors. The data were analyzed using SAS software version 9.2 (SAS Institute Inc., Cary, NC, USA.)
RESULTS
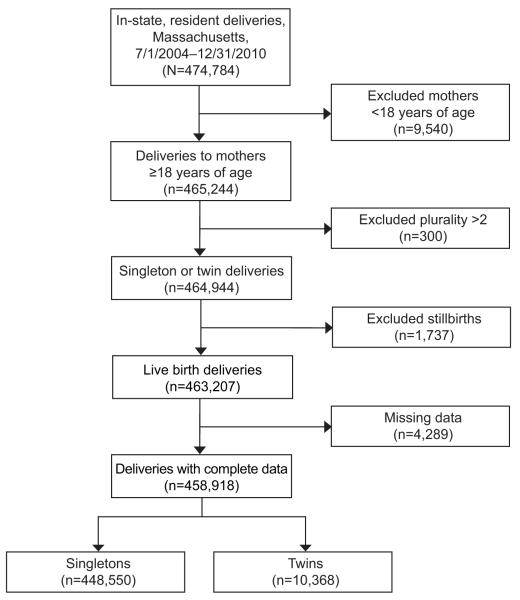
Out of a total of 474,482 deliveries occurring between July 1, 2004 and December 31, 2010 in Massachusetts and included in the MOSART database 458,918 (96.7%) were born to mothers 18 or older, were live-born singletons or twins, and had complete data on the variables of interest (Figure 1). A description of the study population by fertility status group and plurality is shown in Table 2. Women who underwent ART, those with subfertility who did not undergo ART, and those in the fertile group differed significantly on multiple socio-demographic and health characteristics. Compared to the fertile group, the ART and subfertile groups were more likely to be over 40 (20.6% and 14.8% vs 3.7%) white, (85.2% and 84.6% vs 67.1%) and to have private health insurance at the time of delivery (95.0% and 90.4% vs 57.9%. Women in the ART and subfertile groups were also more likely to have delivered twins (26.0% and 8.3% vs 1.4%), have a preterm birth (21.7% and 11.7% vs 6.9%), and a Cesarean delivery 54.2% and 45.3% vs 31.3%). All demographic comparisons across the three groups were significant at a level of p < 0.001.
Figure 1.
Flow chart of study population selection.
Table 2.
Description of the Study Population* (Massachusetts births, July 1, 2004 – December 31, 2010)
| Characteristics | ART | Subfertile | Fertile |
|---|---|---|---|
| Total (deliveries) | 13,677 | 8,754 | 436,487 |
| Mother’s age (%) | |||
| <30 | 8.3 | 11.9 | 47.5 |
| 30-39 | 71.1 | 73.3 | 48.8 |
| 40+ | 20.6 | 14.8 | 3.7 |
| Mother’s race and ethnicity (%) | |||
| Non-Hispanic Black | 3.1 | 3.1 | 8.9 |
| Non-Hispanic White | 85.2 | 84.6 | 67.1 |
| Hispanic | 3.5 | 4.4 | 14.2 |
| Asian/Pacific Islander | 7.2 | 6.8 | 7.7 |
| American Indian/Alaskan Native | 1.0 | 1.1 | 2.1 |
| Mother’s education (%) | |||
| <=High School | 9.2 | 11.9 | 36.5 |
| Some post-High School | 15.6 | 17.6 | 21.9 |
| Bachelor’s degree or greater | 75.2 | 70.6 | 41.6 |
| Unmarried (%) | 4.1 | 5.6 | 33.6 |
| Private health insurance (%) | 95.0 | 90.4 | 57.9 |
| Primiparous (%) | 54.6 | 37.2 | 44.6 |
| Smoking during pregnancy (%) | 0.7 | 1.7 | 7.5 |
| Selected OB/GYN conditions† (%) | 8.6 | 7.4 | 3.1 |
| Plurality at birth (%) | |||
| Singletons | 74.0 | 91.7 | 98.6 |
| Twins | 26.0 | 8.3 | 1.4 |
| Length of Gestation (%) | |||
| <32 Weeks | 3.7 | 1.8 | 1.0 |
| 32-36 Weeks | 18.0 | 9.9 | 5.9 |
| ≥37 Weeks | 78.3 | 88.3 | 93.1 |
| Method of delivery (%) | |||
| Vaginal | 44.6 | 51.8 | 66.7 |
| VBAC | 1.2 | 3.0 | 2.0 |
| Primary Cesarean | 40.7 | 23.8 | 18.5 |
| Repeat Cesarean | 13.5 | 21.5 | 12.8 |
All differences across the three fertility groups, between ART and SUBFERTILE and between ART and FERTILE were significant at a level of at least p < 0.01.
Selected conditions include: endometriosis, polycystic ovarian syndrome, tubal/abdominal/ovarian pregnancy, pelvic inflammatory disease, and peritoneal adhesions.
Among our total study population, we identified 5,318 deliveries (1.16%) which involved an SMM. The overall prevalences of SMM among fertile, subfertile and ART deliveries were 1.09%, 1.44% and 3.14%, (data not shown). Among women with singleton pregnancies, those with cesarean and ART combined had a significantly higher rate of SMM compared to women with cesarean and subfertile (OR 1.84, 95% CI 1.37-2.46) or cesarean and fertile deliveries (OR 1.65, 95% CI 1.40-1.94) with crude prevalences being 3.37% versus 1.86% and 2.07% respectively, (Table 3). In the adjusted analysis, cesarean ART deliveries had 1.75 times higher odds (95%CI 1.30-2.35) compared to cesarean subfertile deliveries and 1.67 times higher odds of SMM (95%CI 1.40-1.98) compared to cesarean fertile deliveries. Among women with singletons who delivered vaginally, ART pregnancies had the highest proportion of any severe morbidity compared with subfertile (OR 2.21 95%CI 1.46-3.36) and fertile deliveries (OR 2.46 95%CI 1.96-3.09) with crude prevalences being 1.44%, versus 0.65% and 0.59%, respectively. In the adjusted analysis, women with ART who delivered vaginally had 1.97 times higher odds (95%CI 1.30-3.00) than women with subfertile deliveries and 2.27 times higher odds of SMM (95%CI 1.78-2.88) than those with fertile deliveries.
Table 3.
Risk of Severe Maternal Morbidity by Fertility Group Status, Plurality, and Mode of Delivery
| Plurality |
Reference
group |
Fertility
status |
Vaginal Deliveries | Cesarean Deliveries | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total in subgroup |
% with SMM* |
OR (95% CI) | aOR† (95% CI) | Total in subgroup |
% with SMM* |
OR (95% CI) | aOR† (95% CI) | |||
| Singletons | Fertile as ref. |
Fertile | 297,800 | 0.59 | 1.00 (ref) | 1.00 (ref) | 132,600 | 2.07 | 1.00 (ref) | 1.00 (ref) |
| Subfertile | 4,585 | 0.65 | 1.11 (0.77, 1.60) | 1.16 (0.80, 1.68) | 3,442 | 1.86 | 0.90 (0.70, 1.15) | 0.95 (0.73, 1.23) | ||
| ART | 5,492 | 1.44 | 2.46 (1.96, 3.09) | 2.27 (1.78, 2.88) | 4,631 | 3.37 | 1.65 (1.40, 1.94) | 1.67 (1.40, 1.98) | ||
| Subfertile as ref. |
Subfertile | 4,585 | 0.65 | 1.00 (ref) | 1.00 (ref) | 3,442 | 1.86 | 1.00 (ref) | 1.00 (ref) | |
| ART | 5,492 | 1.44 | 2.21 (1.46, 3.36) | 1.97 (1.30, 3.00) | 4,631 | 3.37 | 1.84 (1.37, 2.46) | 1.75 (1.30, 2.35) | ||
| Twins | Fertile as ref. |
Fertile | 1,928 | 3.94 | 1.00 (ref) | 1.00 (ref) | 4,159 | 4.26 | 1.00 (ref) | 1.00 (ref) |
| Subfertile | 209 | 4.31 | 1.10 (0.54, 2.22) | 1.19 (0.56, 2.55) | 518 | 4.44 | 1.05 (0.67, 1.63) | 1.15 (0.72, 1.84) | ||
| ART | 778 | 4.50 | 1.15 (0.76, 1.73) | 1.24 (0.76, 2.01) | 2,776 | 5.76 | 1.38 (1.11, 1.71) | 1.48 (1.14, 1.93) | ||
| Subfertile as ref. |
Subfertile | 209 | 4.31 | 1.00 (ref) | 1.00 (ref) | 518 | 4.44 | 1.00 (ref) | 1.00 (ref) | |
| ART | 778 | 4.50 | 1.05 (0.49, 2.21) | 1.03 (0.48, 2.18) | 2,776 | 5.76 | 1.32 (0.84, 2.06) | 1.27 (0.81,1.99) | ||
Unadjusted %.
Models adjusted for maternal age, education, race/ethnicity, marital status, insurance, parity, smoking during pregnancy, pre-pregnancy hypertension, pre-pregnancy diabetes mellitus, the selected obstetric and gynecologic conditions (endometriosis, polycystic ovarian syndrome, pelvic inflammatory disease, and peritoneal adhesions), and length of gestation.
Twin deliveries were associated rates of SMM ranging from 3.94% among fertile, vaginal deliveries, to 5.76% among ART, cesarean deliveries. Among twin deliveries, the only difference in SMM was found between ART and fertile cesarean groups (aOR 1.48, 95% CI: 1.14 – 1.93). The subfertile group did not differ significantly from a fertile group in any model.
The most common indicator of SMM across all deliveries, and for each subgroup, was blood transfusion, (n = 3,466, 0.76%), involved in nearly two thirds of all SMM cases. Prevalence of transfusion varied across the groups, with ART cesarean twin deliveries having the highest (4.03%) and vaginal singleton deliveries to fertile women the lowest (0.42%) transfusion rates. (Data not shown)
We examined hospital discharge records post-hoc to see what other conditions accompanied transfusions and found that almost 90% of deliveries that involved a transfusion also had an indication of a hemorrhagic or anemic condition (data not shown). We also sub-categorized ART deliveries by those involving male infertility, female infertility, both male and female infertility, and unexplained infertility to examine whether maternal and/or paternal factors were associated with increased risk of SMM and found no significant differences (data not shown). When we calculated the prevalence of transfusion by specific infertility diagnoses recorded in SART CORS, only women with “uterine factor” had a significantly higher prevalence of transfusion than those without, however, only 16 (5.3%) of the 300 transfusion cases were affected by uterine-factors, (data not shown).
DISCUSSION
Women who conceived using ART and delivered singleton infants were significantly more likely than women with subfertile and fertile deliveries to suffer a severe morbidity regardless of method of delivery and adjusting for demographic and health factors. The risk of SMM in women with singleton subfertile births did not differ significantly from that of the fertile group. Among twin deliveries, there were fewer significant differences, perhaps owing to smaller numbers and the higher occurrence of maternal morbidities associated with plural births in general.19 It should be noted, however, that the absolute increase in risk associated with ART was very small, under 2 percentage points, for all sub-group comparisons.
The overall prevalence of SMM among Massachusetts mothers (1.16%) in this study, was consistent with previously reported national estimates for similar time periods. Using a national sample, Kuklina et al. found a SMM prevalence of 0.81% in 2004–0521 and Callaghan et al. found 1.29% in 2008-2009.2 The most common indicator of a SMM event in our sample, as in these previous studies, was blood transfusion, which largely accounted for greater SMM observed among the ART groups. However, no distinct co-morbidity patterns were observed that would explain the higher transfusion rates. Rates of blood transfusion are dependent at least in part on guidelines around acceptable maternal hemoglobin levels, and these guidelines have changed over time.27 Therefore, studies of SMM, particularly time trend analyses should be mindful of changes in practice standards for management of hemorrhage and anemia in obstetric patients.
This study of the association of ART with SMM is important for its inclusion of a non-ART but subfertile comparison group10. A Dutch study found 2.5 times greater risk of SMM among ART compared to fertile births, though the researchers did not include a subfertile comparison group.22 A study by Silberstein, et al. compared the risks of certain severe adverse outcomes among groups with ART, non-ART ovulation induction and spontaneous conceptions, observing a significant trend of highest to lowest risk across the three groups, respectively.23 We observed comparable trends across our three comparison groups.
The MOSART database allowed us to identify infants conceived with ART, and distinguish them from non-ART, subfertile pregnancies. Additionally, this population-level database included nearly all births to women 18 and older in Massachusetts over more than a 6 year period. Our stratification by plurality and method of delivery, and comparison of ART to subfertile deliveries allowed us to account for the higher prevalence of cesarean and plural births among ART pregnancies,17;19 and estimate the contribution of ART to severe morbidity apart from underlying health issues and complications more common to ART pregnancies. This approach addressed a common limitation of prior ART studies.10
One study limitation was the lack of information on non-ART fertility treatments in the subfertile group. Therefore, we could not distinguish all sub-types of subfertile pregnancies. In addition, some subfertile deliveries may have been misclassified as “fertile,” though this is unlikely to have affected the much larger fertile group. Similarly, some women who delivered in Massachusetts may have received ART out of state and thus would be misclassified as fertile or subfertile. Similarly, we could not determine whether events possibly accompanying ART such as gonadotropin medications and fetal losses contributed to the greater observed SMM. Lastly, our analysis of only Massachusetts births may raise concerns around generalizability, however our findings largely corresponded to previous national data on the nature and prevalence of SMM by fertility groups, as noted above, suggesting that they may well translate to other settings.
In conclusion, women who conceived via assisted reproductive technology and delivered singleton infants were found to be at elevated risk of severe morbidities during their delivery hospitalizations compared to women who had indicators of subfertility but did not use ART to conceive, to women who had neither indicators of subfertility nor ART. Blood transfusion was the most common indicator of a severe morbid event for all deliveries, however, further research is needed toward understanding mechanisms and accompanying conditions which may underlie the elevated risk of severe morbidity among women who undergo ART.
Acknowledgments
Supported by Grants R01HD064595 and R01HD067270 from the National Institute of Child Health and Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Child Health and Human Development or the National Institutes of Health.
The authors thank additional members of the MOSART team: Lan Hoang, Howard Cabral, Mark D. Hornstein, Bruce Cohen, and Donna Richard, as well as the SART Research Committee for their comments. SART wishes to thank all of its members for providing clinical information to the SART CORS database for use by patients and researchers. Without the efforts of our members, this research would not have been possible.
Footnotes
Presented at the 2014 City MatCH Leadership and MCH Epidemiology Conference, Phoenix, AZ, September, 17-19, 2014.
Financial Disclosure
Barbara Luke is a consultant to the Society for Assisted Reproductive Technology. The other authors did not report any potential conflicts of interest.
References
- (1).Centers for Disease Control and Prevention. Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion [Accessed July 14, 2015];Pregnancy Mortality Surveillance System. 2014 http://www.cdc gov/reproductivehealth/maternalinfanthealth/pmss html [serial online]
- (2).Callaghan WM, Creanga AA, Kuklina EV. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstet Gynecol. 2012;120:1029–1036. doi: 10.1097/aog.0b013e31826d60c5. [DOI] [PubMed] [Google Scholar]
- (3).Sunderam S, Kissin DM, Crawford SB, Folger SG, Jamieson DJ, Barfield WD. Assisted reproductive technology surveillance--United States, 2011. MMWR Surveill Summ. 2014;63:1–28. [PubMed] [Google Scholar]
- (4).Wright VC, Schieve LA, Reynolds MA, Jeng G, Kissin D. Assisted reproductive technology surveillance--United States, 2001. MMWR Surveill Summ. 2004;53:1–20. [PubMed] [Google Scholar]
- (5).Chandra A, Copen CE, Stephen EH. Infertility service use in the United States: Data from the National Survey of Family Growth, 1982–2010. National Center for Health Statistics; Hyattsville, MD: 2014. National health statistics reports; no 73. 2014. [PubMed] [Google Scholar]
- (6).Esh-Broder E, Ariel I, Abas-Bashir N, Bdolah Y, Celnikier DH. Placenta accreta is associated with IVF pregnancies: a retrospective chart review. BJOG. 2011;118:1084–1089. doi: 10.1111/j.1471-0528.2011.02976.x. [DOI] [PubMed] [Google Scholar]
- (7).Centers for Disease Control and Prevention. American Society for Reproductive Medicine. Society for Reproductive Technology . 2012 Assisted Reproductive Technology National Summary Report. US Dept of Health and Human Services; Atlanta, GA: 2014. [Google Scholar]
- (8).Sheiner E, Shoham-Vardi I, Hershkovitz R, Katz M, Mazor M. Infertility treatment is an independent risk factor for cesarean section among nulliparous women aged 40 and above. Am J Obstet Gynecol. 2001;185:888–892. doi: 10.1067/mob.2001.117308. [DOI] [PubMed] [Google Scholar]
- (9).Declercq E, Luke B, Belanoff C, et al. Perinatal outcomes associated with assisted reproductive technology: the Massachusetts Outcomes Study of Assisted Reproductive Technologies (MOSART) Fertil Steril. 2015;103:888–895. doi: 10.1016/j.fertnstert.2014.12.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Declercq ER, Belanoff C, Diop H, et al. Identifying women with indicators of subfertility in a statewide population database: operationalizing the missing link in assisted reproductive technology research. Fertil Steril. 2014;101:463–471. doi: 10.1016/j.fertnstert.2013.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Thomopoulos C, Tsioufis C, Michalopoulou H, Makris T, Papademetriou V, Stefanadis C. Assisted reproductive technology and pregnancy-related hypertensive complications: a systematic review. J Hum Hypertens. 2013;27:148–157. doi: 10.1038/jhh.2012.13. [DOI] [PubMed] [Google Scholar]
- (12).Luke B, Stern JE, Kotelchuck M, et al. Adverse pregnancy outcomes after in vitro fertilization: effect of number of embryos transferred and plurality at conception. Fertil Steril. 2015;104:79–86. doi: 10.1016/j.fertnstert.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Stern JE, Luke B, Tobias M, Gopal D, Hornstein MD, Diop H. Adverse pregnancy and birth outcomes associated with underlying diagnosis with and without assisted reproductive technology treatment. Fertil Steril. 2015;103:1438–1445. doi: 10.1016/j.fertnstert.2015.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Kotelchuck M, Hoang L, Stern JE, Diop H, Belanoff C, Declercq E. The MOSART database: linking the SART CORS clinical database to the population-based Massachusetts PELL reproductive public health data system. Matern Child Health J. 2014;18:2167–2178. doi: 10.1007/s10995-014-1465-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Stern JE, Kotelchuck M, Luke B, Declercq E, Cabral H, Diop H. Calculating length of gestation from the Society for Assisted Reproductive Technology Clinic Outcome Reporting System (SART CORS) database versus vital records may alter reported rates of prematurity. Fertil Steril. 2014;101:1315–1320. doi: 10.1016/j.fertnstert.2014.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Stern JE, Luke B, Hornstein MD, et al. The effect of father's age in fertile, subfertile, and assisted reproductive technology pregnancies: a population based cohort study. J Assist Reprod Genet. 2014;31:1437–1444. doi: 10.1007/s10815-014-0327-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Declercq E, Barger M, Cabral HJ, et al. Maternal outcomes associated with planned primary cesarean births compared with planned vaginal births. Obstet Gynecol. 2007;109:669–677. doi: 10.1097/01.AOG.0000255668.20639.40. [DOI] [PubMed] [Google Scholar]
- (18).Centers for Disease Control and Prevention [Accessed July 11, 2015];Severe Maternal Morbidity in the United States. 2014 http://www.cdc.gov/reproductivehealth/MaternalInfantHealth/SevereMaternalMorbidityhtml#r1 [serial online]
- (19).Luke B, Brown MB. Contemporary risks of maternal morbidity and adverse outcomes with increasing maternal age and plurality. Fertil Steril. 2007;88:283–293. doi: 10.1016/j.fertnstert.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Adamson GD, Baker VL. Subfertility: causes, treatment and outcome. Best Pract Res Clin Obstet Gynaecol. 2003;17:169–185. doi: 10.1016/s1521-6934(02)00146-3. [DOI] [PubMed] [Google Scholar]
- (21).Kuklina EV, Meikle SF, Jamieson DJ, et al. Severe obstetric morbidity in the United States: 1998-2005. Obstet Gynecol. 2009;113:293–299. doi: 10.1097/AOG.0b013e3181954e5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).van Roosmalen J, Zwart J. Severe acute maternal morbidity in high-income countries. Best Pract Res Clin Obstet Gynaecol. 2009;23:297–304. doi: 10.1016/j.bpobgyn.2009.01.004. [DOI] [PubMed] [Google Scholar]
- (23).Silberstein T, Levy A, Harlev A, Saphier O, Sheiner E. Perinatal outcome of pregnancies following in vitro fertilization and ovulation induction. J Matern Fetal Neonatal Med. 2014;27:1316–1319. doi: 10.3109/14767058.2013.856415. [DOI] [PubMed] [Google Scholar]
- (24).Zhang Z, Macaluso M, Cohen B, et al. Accuracy of assisted reproductive technology information on the Massachusetts birth certificate, 1997-2000. Fertil Steril. 2010;94:1657–1661. doi: 10.1016/j.fertnstert.2009.10.059. [DOI] [PubMed] [Google Scholar]
- (25).Cohen B, Bernson D, Sappenfield W, et al. Accuracy of assisted reproductive technology information on birth certificates: Florida and Massachusetts, 2004-06. Paediatr Perinat Epidemiol. 2014;28:181–190. doi: 10.1111/ppe.12110. [DOI] [PubMed] [Google Scholar]
- (26).Small MJ, James AH, Kershaw T, Thames B, Gunatilake R, Brown H. Near-miss maternal mortality: cardiac dysfunction as the principal cause of obstetric intensive care unit admissions. Obstet Gynecol. 2012;119:250–255. doi: 10.1097/AOG.0b013e31824265c7. [DOI] [PubMed] [Google Scholar]
- (27).Jansen AJG, van Rhenen DJ, Steegers EAP, Duvekot JJ. Postpartum Hemorrhage and Transfusion of Blood and Blood Components. Obstet Gynecol Surv. 2005;60:663–671. doi: 10.1097/01.ogx.0000180909.31293.cf. [DOI] [PubMed] [Google Scholar]