Abstract
The last 25 years have been characterized by dramatic improvements in short-term patient and allograft survival after kidney transplantation. Long-term patient and allograft survival remains limited by cardiovascular disease and chronic allograft injury, among other factors. Cardiovascular disease remains a significant contributor to mortality in native chronic kidney disease as well, as cardiovascular mortality in chronic kidney disease more than doubles that of the general population. The chronic kidney disease-mineral bone disorder (CKD-MBD) is a syndrome recently coined to embody the biochemical, skeletal, and cardiovascular pathophysiology that results from disrupting the complex systems biology between the kidney, skeleton, and cardiovascular system in native and transplant kidney disease. The CKD-MBD is a unique kidney disease-specific syndrome containing novel cardiovascular risk factors, with an impact reaching far beyond traditional notions of renal osteodystrophy and hyperparathyroidism. This Overview reviews current knowledge of the pathophysiology of the CKD-MBD, including emerging concepts surrounding the importance of circulating pathogenic factors released from the injured kidney that directly cause cardiovascular disease in native and transplant chronic kidney disease, with potential application to mechanisms of chronic allograft injury and vasculopathy.
INTRODUCTION
In kidney transplantation, the last 25 years have been characterized by dramatic improvements in short-term patient and allograft survival, credited in large part to advances in surgical techniques, organ preservation, immunosuppression and medical care of the recipient. However, during this same period improvements in long-term patient and allograft survival have been less dramatic (1). The leading causes of death and allograft loss in kidney transplant recipients are cardiovascular disease and chronic allograft injury, respectively (1–3). Cardiovascular disease is also the leading cause of mortality in native chronic kidney disease (CKD) and end-stage renal disease (ESRD) (4). Importantly, the mortality rate from CKD-associated cardiovascular disease eclipses the mortality rate from progressive CKD by two-fold (4). Although cardiovascular risk is inversely related to glomerular filtration rate (GFR), it is already substantial in early stages of CKD with mildly depressed GFR (5). Kidney transplantation improves but does not neutralize the enormous cardiovascular risk of CKD, reflecting either residual cardiovascular risk of ESRD and/or de novo cardiovascular risk factors introduced by the kidney transplant milieu (3, 6). Kidney transplant recipients are uniquely exposed to recurrent CKD pathophysiology, first in the setting of progressive native CKD and then again with progressive transplant CKD due to chronic allograft injury. Therefore, the immense burden of cardiovascular risk is relevant across the entire continuum of CKD, ESRD, and kidney transplantation.
CKD-associated cardiovascular risk cannot be fully attributed to traditional risk factors such as smoking, hypertension, hypercholesterolemia and diabetes mellitus (7, 8). Non-traditional cardiovascular risk factors in CKD include hyperphosphatemia, vascular stiffness, vascular calcification, and fibroblast growth factor-23 (FGF23), all of which are components of a progressive disturbance in systems biology between the renal, skeletal, and cardiovascular systems known as the chronic kidney disease-mineral bone disorder (CKD-MBD) (9). In recent years, much has been discovered about the pathophysiology of the CKD-MBD, including its potential origins in early CKD or at the time of acute kidney injury (10, 11). In addition, there is an emerging role for novel circulating pathogenic factors in the CKD-MBD such as FGF23, klotho, and Wingless/integrated-1 (Wnt)-inhibitors, including their contribution to cardiovascular dysfunction in native and transplant CKD (11–15). The purpose of this Overview is to briefly review the pathophysiology of the CKD-MBD and highlight emerging pathogenic concepts that underscore its importance in kidney transplantation.
Beyond Renal Osteodystrophy: Redefining the CKD-MBD
Disordered bone and mineral homeostasis is a hallmark consequence of progressive CKD, a condition known as renal osteodystrophy (9). Traditional definitions of renal osteodystrophy have included descriptions of altered bone morphology and remodeling derived from bone biopsies in patients with CKD, which are now performed infrequently in clinical practice. Furthermore, these definitions did not capture the full clinical spectrum of disordered mineral metabolism in CKD. In 2006, the Kidney Disease: Improving Global Outcomes (KDIGO) group enshrined the CKD-MBD as a systemic disorder of mineral and bone metabolism due to CKD, manifested by abnormalities in circulating biomarkers, bone histomorphometry, and/or extraskeletal calcification (Table 1). This new syndrome better illustrated the association between mineral bone disorders and the significant cardiovascular morbidity and mortality in CKD. The traditional term renal osteodystrophy was reserved only for describing disturbances in bone morphology in CKD. In 2009, KDIGO reaffirmed this recommendation in their clinical practice guideline for management of the CKD-MBD in adults and children with native CKD, but recognized the need for management of the syndrome in all kidney transplant recipients (CKD stages 1T-5T), since an amalgamation of pre- and post-transplant CKD-MBD physiology is likely to be present even in those with excellent allograft function (16).
Table 1.
Classification framework for the CKD-MBD including standard and alternative metrics for assessment, adapted from KDIGO 2006 and 2009 guidelines. Italicized metrics are not discussed specifically in this review.
| CKD-MBD Classificationa | Clinical Features | Standard Metrics | Investigational or Alternative Metrics | ||
|---|---|---|---|---|---|
| Laboratory Abnormalities (L) | FGF23 | ||||
| Hyperphosphatemia | Phosphorus | DKK1 | |||
| Secondary Hyperparathyroidism | Intact PTH | Sclerostin | |||
| Hypocalcemia | Calcium (total and ionized) | Soluble klotho | |||
| Vitamin D Deficiency | 25- and 1,25-hydroxyvitamin D | Activin-A | |||
| Acidemia | Bicarbonate | Tartrate-resistant acid phosphatase 5b (TRAP-5b) | |||
| Alkaline Phosphatase | Procollagen type 1 N-terminal peptide (P1NP) | ||||
| Osteocalcin | |||||
| Bone Disease (B) | Renal osteodystrophy (based on bone biopsy) | Bone biopsy | Quantitative Ultrasonography | ||
| Osteitis Fibrosa | Turnover (low, normal, high) | High-performance liquid chromatography (HPLC) | |||
| Osteomalacia | Mineralization (normal, abnormal) | Electron microscopy | |||
| Adynamic Done Disease | Volume (low, normal, high) | Micro-CT | |||
| Mild Hyperparathyroid-related Disease | Bone mineral density (DXA) scan | MRI | |||
| Mixed Uremic Osteodystrophy | Bone mineral density (quantitative CT) scan | Radioisotope studies | |||
| Osteoporosis (low bone mineral density) | Plain bone radiographs | ||||
| Fractures | Linear growth | ||||
| Bone pain | |||||
| Bone deformities | |||||
| Impaired growth velocity | |||||
| Abnormal height | |||||
| Extraskeletal Calcification (C) | Coronary Artery Calcification | Echocardiography | Multislice or electron beam CT | ||
| Generalized Vascular Calcification | Pulse wave velocity | Cardiac MRI | |||
| Valvular Calcification | Abdominal radiograph | ||||
| Vascular Stiffness | Vascular ultrasound | ||||
| Systolic/Diastolic Dysfunction | |||||
| Joint and Soft Tissue Calcification | |||||
CKD-MBD categories rarely occur in isolation. The majority of patients with CKD-MBD will be classified as either L, LB, LC, or LBC. For example, a patient with hyperphosphatemia and vascular calcification would be classified as CKD-MBD-LC.
Key Pathogenic Components of the CKD-MBD
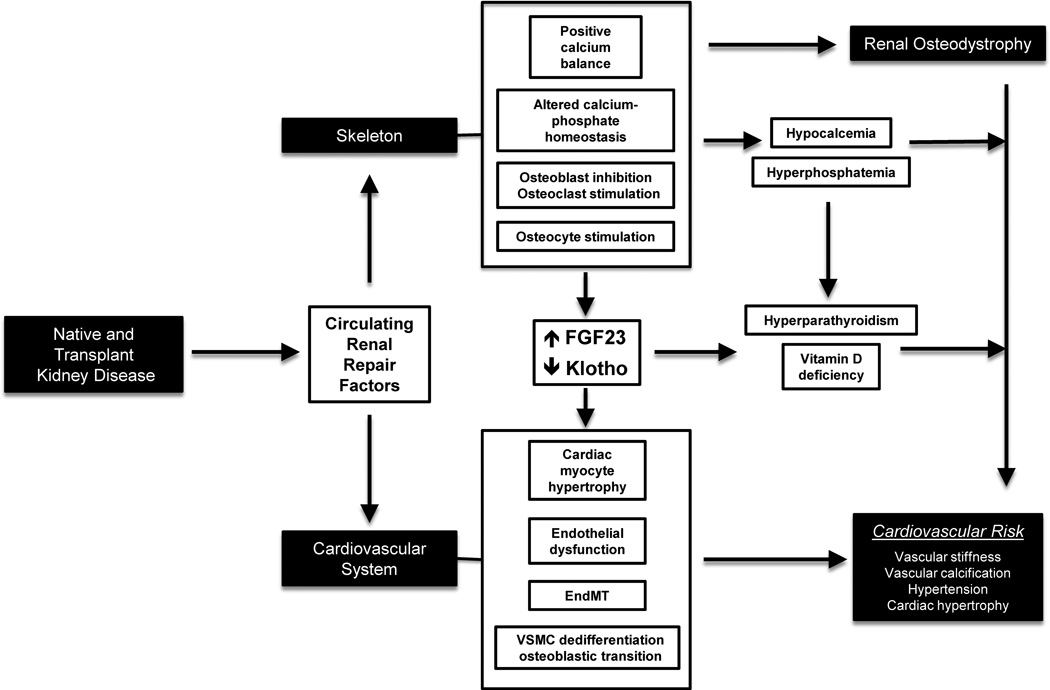
In early CKD, the inception of the CKD-MBD is characterized by kidney, skeletal, and vascular manifestations mediated through circulating pathogenic factors and altered phosphate homeostasis. In advanced CKD, there are additional manifestations that augment biochemical, skeletal, and vascular pathophysiology of the CKD-MBD (Figure 1).
Figure 1.
Diagram illustrating the complex systems biology between the kidney, skeleton, and cardiovascular system that is perturbed in native and transplant kidney disease, producing the components of the CKD-MBD. Recent mechanistic studies suggest that native and transplant kidney diseases produce circulating renal repair factors (e.g., DKK1, sclerostin, and activin-A) that may have systemic effects on the skeleton and cardiovascular system. (Top) In the skeleton, CKD alters calcium-phosphate homeostasis that contributes to varying degrees of hypocalcemia and hyperphosphatemia. Also, there is stimulation of osteocyte function with increased FGF23 secretion, along with decreased renal, parathyroid, and circulating klotho expression. Hypocalcemia, hyperphosphatemia, increased FGF23 levels, and decreased klotho expression all contribute to secondary hyperparathyroidism and vitamin D deficiency in CKD, and collectively have been associated with cardiovascular risk. In addition, there is inhibition of osteoblast function and stimulation of osteoclast function in this setting that produces abnormal skeletal remodeling and high-turnover renal osteodystrophy. (Bottom) In the cardiovascular system, studies in humans and animal models suggest that circulating renal repair factors and mineral-bone disturbances all contribute to cardiovascular risk in the CKD-MBD. Specifically, circulating renal repair factors have been implicated in key cellular events such as cardiac myocyte hypertrophy, endothelial dysfunction, endothelial-to-mesenchymal transition (EndMT), and osteoblastic transition of dedifferentiated vascular smooth muscle cells (VSMC). Paired with mineral-bone disturbances derived from the skeletal component of the CKD-MBD, this may contribute to vascular calcification, vascular stiffness, hypertension, and cardiac hypertrophy, all of which are associated with cardiovascular risk.
Inception of the CKD-MBD in Early CKD
FGF23
FGF23 is the original phosphatonin (hormone regulating phosphate excretion) discovered in studies of autosomal dominant hypophosphatemic rickets and oncogenic osteomalacia (17). FGF23 is produced by osteocytes and osteoblasts, and it represents direct bone-kidney and bone–parathyroid connections in the multiorgan systems biology involved in the CKD-MBD. Circulating FGF23 levels rise after mild renal injury and progressively increase several fold during the course of CKD due to increased osteocyte secretion as well as decreased catabolism by the injured kidney. Several studies in animal models and humans with early native CKD have demonstrated that FGF23 levels rise prior to changes in calcium, phosphorus, or PTH levels and could serve as an early biomarker associated with the CKD-MBD (18–24). However, more studies are needed to confirm the pathophysiologic link between FGF23 and other mineral metabolites in CKD beyond these temporal relationships. Additional studies of FGF23 and mineral metabolism are especially needed in kidney transplantation before there is broad clinical application of FGF23 as a biomarker associated with the early CKD-MBD (25–27).
Since its identification of its pivotal role in the CKD-MBD, the pathophysiologic importance of FGF23 in native and transplant CKD has greatly expanded. FGF23 levels have been associated with cardiovascular risk and progression of native CKD, and have been associated with kidney transplant loss and mortality, all effects that persist after adjusting for GFR and traditional cardiovascular risk factors as potential confounding factors (12, 28–31). In humans and animal models, Faul et al demonstrated that FGF23 is not only a biomarker associated with cardiovascular risk in CKD, but is also a direct pathogenic factor causing left ventricular hypertrophy (LVH) through activation of the calcineurin-NFAT pathway in cardiac myocytes (32). However, despite its emerging role as a predictive biomarker, the role of FGF23 as a therapeutic target to improve patient and allograft outcomes in native and transplant CKD remains largely speculative at present (33, 34).
Recently, the pathogenic nature of circulating FGF23 has become more intriguing, as Andrukhova et al showed that FGF23 directly regulates the abundance of the thiazide-sensitive sodium-chloride transporter (NCC) in the distal convoluted tubule, leading to increased distal sodium reabsorption, effective circulating volume expansion, hypertension, and cardiac hypertrophy, effects that were abrogated by a thiazide diuretic (35). Interestingly, these FGF23-mediated effects on cardiovascular pathophysiology were augmented in animal models ingesting a low sodium diet (which stimulates aldosterone secretion), raising the possibility of an interaction between FGF23 and the renin-angiotensin-aldosterone axis in CKD-stimulated cardiovascular disease. Furthermore, Humalda et al demonstrated that humans with higher baseline FGF23 levels had a reduced antiproteinuric response to dietary sodium restriction and ACE inhibitor therapy, which has been associated with heighted cardiovascular and end-stage renal disease (ESRD) risk in CKD (36).
There are important effects on FGF23 introduced by the kidney transplant milieu with unknown long-term consequences. In the early post-transplant period, restoration of GFR leads to a rapid decline in FGF23 levels approaching those of patients with normal kidney function within six months (26, 27, 37). However, despite this initial improvement FGF23 levels rise again with declining allograft function and are predictive of poor patient and allograft outcomes (12, 29). Steroids are commonly used in maintenance immunosuppression regimens after transplant, with some studies reporting an association with higher FGF23 levels but others showing no association (26, 38, 39). Studies in mice have shown that calcineurin inhibitors may decrease FGF23 expression but this trend has not been demonstrated in humans (40).
Klotho
FGF23 signaling through FGF receptors typically requires the co-receptor function of membrane-bound klotho. Klotho is highly expressed in very few tissues and defines the targets of FGF23 as the proximal and distal renal tubules, the parathyroid glands and the brain (41). Klotho also circulates as a physiologically active hormone after either being cleaved at the cell surface by ADAM-10 and -17 in the distal renal tubule or through alternative splicing of the klotho gene transcript (42). Soluble klotho directly regulates calcium and phosphorus excretion in the kidney and participates in systemic mineral homeostasis by regulating 1-alpha hydroxylase activity, PTH and FGF23 secretion (43). Klotho expression is significantly reduced by native and transplant kidney injuries such as acute kidney injury, glomerulonephritis, calcineurin inhibitor use and chronic allograft injury (41, 44). The resulting klotho deficiency limits its regulation of FGF23 production and leaves hyperphosphatemia as the principal regulator of FGF23 secretion in CKD. Furthermore, the loss of membrane-bound klotho expression in native and transplant CKD limits FGF23-stimulated signal transduction through FGF receptor/klotho complexes. One result is the loss of negative feedback to FGF23 secretion and the continual production of FGF23 and secretion by the osteocyte. In late CKD, the very high levels of FGF23 may permit anomalous FGF receptor activation independent of Klotho and result in unique FGF23-stimulated pathologies such as cardiac myocyte hypertrophy (32). In addition, recent mechanistic studies have directly linked klotho deficiency with cardiovascular disease including vascular calcification, vascular stiffness, and uremic vasculopathy (15).
Additional Manifestations of the CKD-MBD in Advanced CKD
Hyperphosphatemia
As renal injury decreases the number of functioning nephrons, phosphate excretion is maintained by reducing the tubular reabsorption of filtered phosphate in the remaining nephrons under the influence of FGF23 and PTH (45). The effects of FGF23 on phosphate excretion are limited by proximal tubular klotho deficiency in CKD, and PTH becomes the major adaptive mechanism maintaining phosphate homeostasis. In stage 4–5 CKD (GFR < 30% of normal), this adaptation is no longer adequate and hyperphosphatemia develops despite high PTH and FGF23 levels (45).
CKD contributes to hyperphosphatemia and vascular calcification through inhibition of skeletal function. Skeletal inhibition increases phosphate reabsorption and decreases phosphate deposition resulting in increased serum phosphorus levels (46). Hyperphosphatemia stimulates osteoblastic transition in the vasculature and directly contributes to extraskeletal mineralization (47).
Hyperphosphatemia exerts other important effects in the CKD-MBD axis. In the kidney, hyperphosphatemia suppresses 1-alpha-hydroxylase activity that further contributes to calcitriol deficiency (48). In the parathyroid gland, hyperphosphatemia directly stimulates parathyroid cells independent of calcium and calcitriol levels, producing nodular hyperplasia and increasing PTH secretion (49). In the skeleton, phosphorus stimulates FGF23 secretion from osteocytes (50).
An important consideration in many kidney transplant recipients is the induction of profound hypophosphatemia in the immediate post-transplant period. This immediate phenomenon has been linked to proximal tubular dysfunction, persistent hyperparathyroidism, and persistently elevated FGF23 levels in the setting of sudden correction of GFR (26, 51), but it can also persist beyond the early post-transplant period (52). Chronic hypophosphatemia is a risk factor for musculoskeletal weakness, osteomalacia and rickets and may contribute to the elevated fracture risk that persists in kidney transplant recipients (53). Steroid-based immunosuppressive regimens may reduce activity of renal and intestinal phosphate cotransporters and increase the risk for hypophosphatemia after kidney transplantation. Patients that switch from a calcineurin inhibitor to sirolimus may have a tendency toward hypophosphatemia due to its effect on renal klotho expression and sodium-phosphate cotransport (54).
Hypocalcemia
Hypocalcemia is rare in early native and transplant CKD but is more common in late CKD, where there are substantial alterations in hormones that regulate calcium homeostasis, including 25- and 1,25-hydroxyvitamin D, PTH, FGF23, and phosphorus (55–59). There is an important distinction between calcium balance and calcium homeostasis, with the former referring to total body calcium stores and the latter referring to regulation of circulating ionized calcium levels through hormonal influences (56). Therefore, patients with hypocalcemia can have increased calcium balance and vice-versa. Hypocalcemia exerts hormonal actions by stimulating PTH secretion via inactivation of a calcium-sensing receptor (CASR) on chief cells of the parathyroid gland (56), an effect inhibited by calcimimetic drugs such as cinacalcet. Hypercalcemia has the reverse effect on these influences under physiologic conditions, but can also be associated with secondary hyperparathyroidism or excessive vitamin D supplementation (56). Recently, elegant calcium balance studies in CKD patients have demonstrated the presence of neutral calcium balance regardless of serum calcium level, and intake of calcium above the dietary reference intake (from sources including calcium-based phosphorus binders) for as little as 3 weeks can produce positive calcium balance, at least temporarily, in the setting of skeletal inhibition from the CKD-MBD, which further increases the risk of extraskeletal calcification (60).
Vitamin D deficiency
In early CKD, the physiologic actions of FGF23 secretion from the osteocyte include inhibition of 1-alpha-hydroxylase and stimulation of 24-hydroxylase in proximal renal tubules, thereby decreasing calcitriol production and producing 25-hydroxyvitamin D deficiency (61). As CKD advances, the decrease in functioning nephron mass combined with hyperphosphatemia and increased FGF23 levels results in calcitriol (1,25-hydroxyvitamin D) deficiency as well (16). Calcitriol deficiency decreases intestinal calcium absorption leading to hypocalcemia and diminishes tissue levels of vitamin D receptors, which in the parathyroid gland results in resistance to calcitriol-mediated regulation and stimulation of PTH secretion leading to secondary hyperparathyroidism (62). 25-hydroxyvitamin D and calcitriol deficiency have been linked to cardiovascular disease and altered immune function in patients with native and transplant kidney disease. Repletion of 25- and 1,25-hydroxyvitamin D has been associated with reduced inflammation and dampened immune responses that could be beneficial toward long-term patient and graft survival (63, 64).
Hyperparathyroidism
In native CKD, there is somewhat of a paradox in that PTH regulates secretion of FGF23 and is required for the early stimulation of FGF23 secretion (65), whereas many studies have shown FGF23 as the earliest detectable abnormality of the CKD-MBD (18). Thus, there is a regulation of PTH secretion early in native CKD that remains to be clarified. As reviewed above, as native CKD progresses there is increased production of PTH and nodular hyperplasia of the parathyroid glands that becomes insensitive to regulation from calcium homeostasis, phosphorus, and calcitriol. Sustained elevations in PTH levels, while adaptive to maintain osteoblast surfaces, are associated with an abnormal phenotype of osteoblast function and osteocyte stimulation with relatively less type 1 collagen and more RANKL ligand production than anabolic osteoblasts (66). New studies discussed below indicate that other factors such as FGF23 and activin may impact osteoblast function besides PTH, and produce the mineralization disorder of CKD changing the material properties of bone. The outcome is a high turnover renal osteodystrophy, excess bone resorption, skeletal frailty and elevated fracture risk. After kidney transplantation, some patients have initial normalization of PTH levels until declining transplant function recapitulates the CKD-MBD with late recurrence of secondary hyperparathyroidism. However, in others there is sufficient parathyroid gland hyperplasia prior to transplant to cause persistent hyperparathyroidism despite correction of GFR and mineral abnormalities post-transplant. In the early post-transplant period this scenario can be associated with hypercalcemia and hypophosphatemia, requiring treatment with bisphosphonates, calcimimetics, or subtotal parathyroidectomy. These mineral abnormalities collectively increase the risk for nephrocalcinosis, fractures, and cardiovascular disease in kidney transplant recipients (67).
Renal osteodystrophy
With progressive loss of renal function, cancellous bone volume may be increased along with a loss of cortical bone, but this is in part due to deposition of woven immature collagen fibrils instead of lamellar mature fibrils. Thus, bone strength suffers despite an apparent increase in mass detected by dual energy x-ray absorptiometry (DXA) (68). Patients with advanced CKD could have a loss or gain in bone volume depending on overall bone balance. When the bone balance is positive, osteosclerosis may be observed when osteoblasts are active in depositing new bone composed primarily of immature woven collagen. However, this scenario is rare in the 21st century due to improved therapy of secondary hyperparathyroidism. When the bone balance is negative both cortical and cancellous bone loss occurs, resulting in osteopenia or osteoporosis detected by DXA. The prevalence of osteoporosis in CKD patients exceeds that of the general population and is a major public health concern in CKD (69). With high-turnover renal osteodystrophy, as in secondary hyperparathyroidism with osteitis fibrosa, bone resorption rates are in excess of bone formation and osteopenia progressing to osteoporosis may result (70). With low-turnover renal osteodystrophy, as occurs with over-treatment of secondary hyperparathyroidism that inappropriately suppresses PTH-stimulated skeletal remodeling, both bone formation and resorption rates may be reduced although resorption is still in relative excess and loss of bone mass occurs (71). Therefore, osteoporosis may be observed with either high-turnover or low-turnover renal osteodystrophy. The impact of this phenomenon extends far beyond bone health in CKD, as excessive bone resorption rates contribute to hyperphosphatemia with stimulation of heterotopic mineralization including vascular calcification (47). This disrupted systems biology linkskidney, skeletal, and parathyroid dysfunction to cardiovascular risk and mortality through the CKD-MBD.
These abnormalities in bone architecture can persist or recur after kidney transplantation despite initial correction of many mineral bone disturbances. As reviewed above, persistent secondary hyperparathyroidism, vitamin D deficiency and hypophosphatemia can prevent correction of renal osteodystrophy and contribute to increased fracture risk (53). Even if transplantation successfully corrects these mineral bone disturbances, the eventual decline in kidney function due to chronic allograft injury can result in recapitulation of the CKD-MBD and recurrent renal osteodystrophy.
Cardiovascular disease
The CKD-MBD is a contributing factor to vascular stiffness and calcification that increases the systolic blood pressure, pulse pressure, pulse wave velocity, and left ventricular mass, all of which are surrogates for cardiovascular risk in the general population and in those with CKD (7, 72). Structural and functional abnormalities of the vasculature are seen in early CKD, including vascular stiffness and endothelial dysfunction that progress to vascular calcification, a common phenomenon in the aging general population that is accelerated in CKD to the highest level seen in clinical medicine. Vascular calcification further intensifies vascular stiffness and promotes the development of LVH, all processes that contribute to cardiovascular risk and excess cardiac mortality in native and transplant CKD.
In animal models with mild renal insufficiency (equivalent to human stage 2 CKD with type 2 diabetes), we have demonstrated that expression of proteins involved in the contractile apparatus of aortic smooth muscle cells are decreased, reflecting a dedifferentiated state of the vasculature in early CKD (11). Within the developmental program of mesenchymal stem cells and early vascular progenitors, dedifferentiated vascular smooth muscle cells are susceptible to osteoblastic transition, which contributes to vascular calcification in CKD. Osteoblastic transition of vascular smooth muscle cells produces CKD-stimulated calcification of atherosclerotic plaques as well as the tunica media, resulting in either neointimal or medial vascular calcification (73). This is akin to the significant burden of vascular calcification seen in human CKD that is augmented further by the presence of diabetes mellitus (7, 74), a common comorbidity in patients with native and transplant CKD.
Emerging Concepts in the Systems Biology of the CKD-MBD
The Wnt pathway and reactivation of renal repair mechanisms in the CKD-MBD
Kidney injuries produce circulating signals that directly affect the vasculature, the myocardium and the skeleton. These signals are derived from reactivation of developmental programs of nephrogenesis in an attempt at kidney repair, which are typically silent in the normal adult kidney (11). The classic example is the reactivation of the Wnt pathway that controls tubular epithelial proliferation and polarity during nephrogenesis and is a driving force in renal fibrosis (75). Activation of the canonical Wnt pathway increases expression of downstream transcriptional targets, including Wnt inhibitors that function in a negative feedback loop to autoregulate Wnt activation. These Wnt inhibitors include Dickkopf-1 (Dkk1), soluble frizzled related proteins, Wnt-modulator in surface ectoderm (Wise), and sclerostin among others. While Wnts are strictly autocrine/paracrine factors, the Wnt inhibitors also function as circulating systemic factors (76). The role of Wnt in renal development largely precedes the invasion of the microcirculation forming the glomerulus and the peritubular capillaries. Therefore, while the Wnt inhibitors did not evolve as circulating factors produced by the normal kidney, during kidney injury and repair they are released into the systemic circulation and may inhibit the physiologic roles of Wnt in extrarenal tissues (11).
We and others have recently shown this “unintended” systemic inhibition of Wnt activity stimulated by kidney disease to have major consequences in the skeleton and vasculature. In animal models of early CKD, incomplete recovery from acute kidney injury led to increased expression of Wnt inhibitors (e.g., Dkk1, sclerostin) in the injured kidney and increased levels in the systemic circulation (11). The skeleton was affected through both changes in remodeling (decreased bone formation rates) and in secretory properties of the osteocytes (increased FGF23 secretion). The cardiovascular system was affected through loss of vascular contractile machinery and dedifferentiation of vascular smooth muscle cells, stimulation of osteoblastic transition and vascular calcification, and promotion of cardiac hypertrophy (11, 77). Neutralization of circulating Dkk1 using a monoclonal antibody resulted in increased bone formation rates and bone volume, improved vascular function, and decreased osteoblastic transition and vascular calcification (11). It is important to note that these experiments were conducted using an ldlr−/−, high fat-fed, type 2 diabetic mouse with incomplete recovery from acute kidney injury as the cause of CKD. While this model experiences reductions in GFR that mimic human stage 2 CKD, there are important distinctions from the human condition that may prevent broad translation of these findings. There were concurrent increases in circulating FGF23 and intact PTH that may be distinct from patterns in early human CKD (18, 23). Whereas this animal model of early CKD had increases in circulating DKK1 and sclerostin, patterns of these factors in studies of human CKD are more variable (78, 79). Likewise, we detected increased circulating soluble klotho levels, which is inconsistent with the direct correlation observed between GFR and soluble klotho in most human and animal studies (15, 43, 80). There was also a decrease in bone formation rates in this mouse model of stage 2 CKD, which may not be consistently present in early human CKD (81).
Circulating renal repair factors induce vascular endothelial-to-mesenchymal transition in the CKD-MBD
Recent studies demonstrate that Wnt inhibitors, namely Dkk1, are capable of stimulating endothelial-to-mesenchymal transition (EndMT) in the vasculature through downregulation of Wnt7b and signaling through activin-like kinase/Smad 1/5 pathways, leading to vascular calcification and fibrosis in both endothelial cell cultures and atherogenic (non-CKD) animal models (82). Similar to the Wnt pathway, EndMT is a physiologic process involved in normal cardiac development that is reactivated in various adult disease states, and has been implicated in cardiac and vascular fibrosis, renal fibrosis, and progression of native and transplant CKD (83).
We recently expanded and confirmed these findings in our animal models of early native CKD, including the discovery of another renal repair factor that is released from the injured kidney into the systemic circulation, activin-A (77). As with our previous studies involving Dkk1, we demonstrated that incomplete recovery from acute kidney injury increases activin-A expression in the kidney and increases circulating activin-A levels, reproducing the vascular phenotype of the CKD-MBD. Furthermore, using lineage studies we showed that kidney injury stimulated EndMT in the cardiovascular system that contributed to vascular dedifferentiation, calcification, and cardiac hypertrophy, and an activin ligand trap abrogated these deleterious systemic effects, similar to our findings using a Dkk1 monoclonal antibody. Interestingly, we found that systemic inhibition of activin-A signaling decreased Wnt activation and in the kidney and cardiovascular system and reduced circulating levels of the Wnt inhibitor Dkk1, suggesting that activin-A acts upstream of Wnt activation and systemic Wnt inhibition in the CKD-MBD. Inhibition of activin-A signaling also reduced the amount of collagen deposition and renal fibrosis while increasing renal klotho expression in our animal model of early CKD, suggesting its potential as a therapeutic target in the early CKD-MBD (77). While the pathophysiologic role of circulating Wnt-inhibitors and activin-A in the early CKD-MBD is promising, whether these factors can play a meaningful diagnostic or therapeutic role in human CKD remains to be seen.
Application of Emerging Concepts in the CKD-MBD to Kidney Transplantation
While these concepts have been primarily demonstrated in translational models of early native CKD, there is emerging support for their potential translation into humans and application to the kidney transplant paradigm. We and others have demonstrated increased circulating levels of pathogenic CKD-MBD factors (e.g., FGF23, Dkk1, sclerostin, activin-A) in humans with stage 2–3 native CKD versus no CKD (11, 14) and in kidney transplant recipients with chronic allograft injury versus normal transplant biopsies (84), suggesting they may play an important role as functional biomarkers of the early CKD-MBD in both native and transplant CKD. However, other studies have reported significant variability in circulating levels of these factors across the spectrum of native and transplant CKD, cautioning against their use as reliable biomarkers in humans at present (22, 78, 85, 86).
Kidney transplantation is an interesting clinical correlate to these translational models of incomplete recovery from kidney injury, as it involves ischemia-reperfusion injury to a previously healthy donor kidney followed by cumulative chronic allograft injury. This could reactivate renal repair mechanisms resulting in increased activin and Wnt expression with systemic exposure to activin-A and Wnt inhibitors implicated in the early CKD-MBD and cardiovascular disease. In a rodent model of renal chronic allograft injury, von Toerne et al recently demonstrated this concept using immunohistochemistry and transcriptional profiling of renal allograft tissue (87). Chronic allograft injury was accompanied by early and sustained altered expression of components of the transforming growth factor-beta and canonical Wnt pathways, which contain both activin-A and Wnt inhibitors. Expression of nuclear beta-catenin, a marker of canonical Wnt activation, was increased in chronic allograft injury and was highly localized to the allograft endothelium (glomerular and peritubular capillaries). Renal allograft expression of select Wnt inhibitors (soluble frizzled protein -1, -4, and -5; Wnt inhibitory factor-1, and Dkk3) were measured by microarray and qPCR and were not differentially expressed in chronic allograft injury; circulating levels of these or other Wnt-inhibitors were not measured. Of interest, renal expression of Wnt7b was downregulated in the setting of chronic allograft injury, which was the aforementioned driver of EndMT and the early CKD-MBD in the cardiovascular system in models of native CKD. Induction of EndMT in the allograft or cardiovascular system was not directly assessed in this study, but overall there was strong evidence of early and sustained reactivation of renal repair mechanisms involved the CKD-MBD and association of these pathways with renal allograft vasculopathy (87).
Qian et al recently addressed these emerging concepts therapeutically in a similar rat model of renal chronic allograft injury (88). The Wnt inhibitor Wise was expressed in the kidney allograft by day 3 post-transplant and showed increased tissue expression by month 6 post-transplant. Treatment with a neutralizing anti-Wise antibody throughout the post-transplant period improved renal allograft function and survival, reduced chronic allograft injury scores, reduced inflammatory cell infiltrates, and reduced expression of inflammatory and fibrotic transcripts (88).
Summary and Future Directions
The CKD-MBD defines a disruption in the systems biology between the injured kidney, skeleton, and cardiovascular system that has a profoundly negative impact on survival in native and transplant CKD. Despite correcting many metabolic disturbances associated with progressive CKD, kidney transplantation does not fully mitigate CKD-associated cardiovascular risk, whether due to residual CKD-MBD physiology and/or recapitulation of the full CKD-MBD syndrome with declining allograft function. Recent translational discoveries have introduced a new paradigm where kidney injury directly leads to skeletal and cardiovascular injury through the production of pathogenic circulating factors during attempted renal repair, including molecules that inhibit the canonical Wnt pathway and stimulate endothelial-to-mesenchymal transition, both processes that have been implicated in chronic allograft injury as well as cardiovascular disease. Future studies must clarify whether incomplete recovery from acute kidney injury, such as in kidney transplantation, is sufficient to stimulate these disturbances in the kidney-skeletal-cardiovascular axis that contribute to decreased patient and allograft survival. This would identify the early CKD-MBD as an important therapeutic target for improving long-term outcomes in transplantation as well as native CKD.
Acknowledgments
Funding: M.E.S. is supported by NIH grants UL1 TR000448, KL2 TR000450, and L40 DK099748-01. K.A.H. is supported by NIH grants R01 DK070790 and R01 DK089137.
K.A.H. receives research grant support from Celgene and is a consultant for Celgene and Vifor.
ABBREVIATIONS
- ACTRIIA
Activin Receptor Type IIA
- CKD
chronic kidney disease
- GFR
glomerular filtration rate
- ESRD
end-stage renal disease
- CKD-MBD
chronic kidney disease-mineral bone disorder
- FGF23
fibroblast growth factor-23
- DKK1
Dickkopf-related protein-1
- PTH
parathyroid hormone
- KDIGO
Kidney Disease Improving Global Outcomes
- Wnt
portmanteau of wingless and integrated-1
- Wise
Wnt-modulator in surface ectoderm
- LVH
left ventricular hypertrophy
- DXA
dual energy x-ray absorptiometry
- EndMT
endothelial-to-mesenchymal transition
- VSMC
vascular smooth muscle cell
Footnotes
Disclosures: M.E.S. has no financial disclosures.
M.E.S. conceived, drafted and helped to revise the manuscript, and approved the final version for publication.
K.A.H. edited the first and subsequent drafts of the manuscript and approved the final version for publication.
Contributor Information
Michael E. Seifert, Email: Seifert_m@kids.wustl.edu.
Keith A. Hruska, Email: Hruska_k@kids.wustl.edu.
REFERENCES
- 1.USRDS. USRDS 2012 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. 2012 [Google Scholar]
- 2.NAPRTCS. 2010 Annual Transplant Report. 2010 Available from: https://web.emmes.com/study/ped/annlrept/2010_Report.pdf. [Google Scholar]
- 3.Ortiz A, Covic A, Fliser D, et al. Epidemiology, contributors to, clinical trials of mortality risk in chronic kidney failure. The Lancet. 2014;383(9931):1831. doi: 10.1016/S0140-6736(14)60384-6. [DOI] [PubMed] [Google Scholar]
- 4.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 5.Culleton BF, Larson MG, Wilson PW, Evans JC, Parfrey PS, Levy D. Cardiovascular disease and mortality in a community-based cohort with mild renal insufficiency. Kidney Int. 1999;56(6):2214. doi: 10.1046/j.1523-1755.1999.00773.x. [DOI] [PubMed] [Google Scholar]
- 6.Bullington N, Kartel J, Khoury P, Mitsnefes M. Left ventricular hypertrophy in pediatric kidney transplant recipients: long-term follow-up study. Pediatr Transplant. 2006;10(7):811. doi: 10.1111/j.1399-3046.2006.00565.x. [DOI] [PubMed] [Google Scholar]
- 7.Foley RN, Murray AM, Li S, et al. Chronic Kidney Disease and the Risk for Cardiovascular Disease, Renal Replacement, and Death in the United States Medicare Population, 1998 to 1999. Journal of the American Society of Nephrology. 2005;16(2):489. doi: 10.1681/ASN.2004030203. [DOI] [PubMed] [Google Scholar]
- 8.Baber U, Gutierrez OM, Levitan EB, et al. Risk for recurrent coronary heart disease and all-cause mortality among individuals with chronic kidney disease compared with diabetes mellitus, metabolic syndrome, and cigarette smokers. Am Heart J. 2013;166(2):373. doi: 10.1016/j.ahj.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moe S, Drueke T, Cunningham J, et al. Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2006;69(11):1945. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- 10.Christov M, Waikar SS, Pereira RC, et al. Plasma FGF23 levels increase rapidly after acute kidney injury. Kidney Int. 2013;84(4):776. doi: 10.1038/ki.2013.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang Y, Ginsberg C, Seifert M, et al. CKD-Induced Wingless/Integration1 Inhibitors and Phosphorus Cause the CKD–Mineral and Bone Disorder. Journal of the American Society of Nephrology. 2014;25(8):1760. doi: 10.1681/ASN.2013080818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolf M, Molnar MZ, Amaral AP, et al. Elevated Fibroblast Growth Factor 23 is a Risk Factor for Kidney Transplant Loss and Mortality. J Am Soc Nephrol. 2011;22(5):956. doi: 10.1681/ASN.2010080894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seifert ME, de las Fuentes L, Ginsberg C, et al. Left Ventricular Mass Progression despite Stable Blood Pressure and Kidney Function in Stage 3 Chronic Kidney Disease. American Journal of Nephrology. 2014;39(5):392. doi: 10.1159/000362251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seifert ME, de Las Fuentes L, Rothstein M, et al. Effects of Phosphate Binder Therapy on Vascular Stiffness in Early-Stage Chronic Kidney Disease. American journal of nephrology. 2013;38(2):158. doi: 10.1159/000353569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu MC, Shi M, Zhang J, et al. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2011;22(1):124. doi: 10.1681/ASN.2009121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Kidney Int Suppl. 2009;(113):S1. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 17.Consortium A. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nature Genetics. 2000;26(3):345. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- 18.Isakova T, Wahl P, Vargas GS, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79(12):1370. doi: 10.1038/ki.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Portale AA, Wolf M, Jüppner H, et al. Disordered FGF23 and Mineral Metabolism in Children with CKD. Clinical Journal of the American Society of Nephrology. 2014;9(2):344. doi: 10.2215/CJN.05840513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scialla JJ, Astor BC, Isakova T, Xie H, Appel LJ, Wolf M. Mineral Metabolites and CKD Progression in African Americans. Journal of the American Society of Nephrology. 2013;24(1):125. doi: 10.1681/ASN.2012070713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hasegawa H, Nagano N, Urakawa I, et al. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int. 78(10):975. doi: 10.1038/ki.2010.313. [DOI] [PubMed] [Google Scholar]
- 22.Wan M, Smith C, Shah V, et al. Fibroblast growth factor 23 and soluble klotho in children with chronic kidney disease. Nephrology Dialysis Transplantation. 2013;28(1):153. doi: 10.1093/ndt/gfs411. [DOI] [PubMed] [Google Scholar]
- 23.Pereira RC, Juppner H, Azucena-Serrano CE, Yadin O, Salusky IB, Wesseling-Perry K. Patterns of FGF-23, DMP1, and MEPE expression in patients with chronic kidney disease. Bone. 2009;45(6):1161. doi: 10.1016/j.bone.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stubbs JR, He N, Idiculla A, et al. Longitudinal evaluation of FGF23 changes and mineral metabolism abnormalities in a mouse model of chronic kidney disease. Journal of Bone and Mineral Research. 2012;27(1):38. doi: 10.1002/jbmr.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith ER. The Use of Fibroblast Growth Factor 23 Testing in Patients with Kidney Disease. Clinical Journal of the American Society of Nephrology. 2014;9(7):1283. doi: 10.2215/CJN.10941013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wesseling-Perry K, Pereira R, Tsai E, Ettenger R, Jüppner H, Salusky I. FGF23 and mineral metabolism in the early post-renal transplantation period. Pediatric Nephrology. 2013;28(11):2207. doi: 10.1007/s00467-013-2547-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yilmaz MI, Sonmez A, Saglam M, et al. A Longitudinal Study of Inflammation, CKD-Mineral Bone Disorder, and Carotid Atherosclerosis after Renal Transplantation. Clinical Journal of the American Society of Nephrology. 2015;10(3):471. doi: 10.2215/CJN.07860814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fliser D, Kollerits B, Fau - Neyer U, Neyer U, Fau - Ankerst DP, et al. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol. 2007;18(9):2600. doi: 10.1681/ASN.2006080936. [DOI] [PubMed] [Google Scholar]
- 29.Baia LC, Humalda JK, Vervloet MG, Navis G, Bakker SJL, de Borst MH. Fibroblast Growth Factor 23 and Cardiovascular Mortality after Kidney Transplantation. Clinical Journal of the American Society of Nephrology. 2013;8(11):1968. doi: 10.2215/CJN.01880213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scialla JJ, Xie H, Rahman M, et al. Fibroblast Growth Factor-23 and Cardiovascular Events in CKD. Journal of the American Society of Nephrology. 2014;25(2):349. doi: 10.1681/ASN.2013050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isakova T, Xie H, Yang W, et al. FIbroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305(23):2432. doi: 10.1001/jama.2011.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faul C, Amaral AP, Oskouei B, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121(11):4393. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chue CD, Townend JN, Moody WE, et al. Cardiovascular effects of sevelamer in stage 3 CKD. J Am Soc Nephrol. 2013;24(5):842. doi: 10.1681/ASN.2012070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Isakova T, Gutiérrez OM, Smith K, et al. Pilot study of dietary phosphorus restriction and phosphorus binders to target fibroblast growth factor 23 in patients with chronic kidney disease. Nephrol Dial Transplant. 2011;26(2):584. doi: 10.1093/ndt/gfq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andrukhova O, Slavic S, Smorodchenko A, et al. FGF23 regulates renal sodium handling and blood pressure. EMBO Mol Med. 2014;6(6):744. doi: 10.1002/emmm.201303716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Humalda JK, Lambers Heerspink HJ, Kwakernaak AJ, et al. Fibroblast Growth Factor 23 and the Antiproteinuric Response to Dietary Sodium Restriction During Renin-Angiotensin-Aldosterone System Blockade. American Journal of Kidney Diseases. 2014 doi: 10.1053/j.ajkd.2014.07.022. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 37.Bhan I, Shah A, Holmes J, et al. Post-transplant hypophosphatemia: Tertiary /`Hyper-Phosphatoninism/'? Kidney Int. 2006;70(8):1486. doi: 10.1038/sj.ki.5001788. [DOI] [PubMed] [Google Scholar]
- 38.Sánchez Fructuoso AI, Maestro ML, Pérez-Flores I, et al. Serum level of fibroblast growth factor 23 in maintenance renal transplant patients. Nephrology Dialysis Transplantation. 2012;27(11):4227. doi: 10.1093/ndt/gfs409. [DOI] [PubMed] [Google Scholar]
- 39.Bacchetta J, Dubourg L, Harambat J, et al. The Influence of Glomerular Filtration Rate and Age on Fibroblast Growth Factor 23 Serum Levels in Pediatric Chronic Kidney Disease. The Journal of Clinical Endocrinology & Metabolism. 2010;95(4):1741. doi: 10.1210/jc.2009-1576. [DOI] [PubMed] [Google Scholar]
- 40.Han DH, Piao SG, Song J-H, et al. Effect of sirolimus on calcineurin inhibitor-induced nephrotoxicity using renal expression of KLOTHO, an antiaging gene. Transplantation. 2010;90(2):135. doi: 10.1097/TP.0b013e3181e117b4. [DOI] [PubMed] [Google Scholar]
- 41.Hu MC, Kuro-o M, Moe OW. Klotho and kidney disease. J Nephrol. 23(Suppl 16):S136. [PMC free article] [PubMed] [Google Scholar]
- 42.Chen CD, Podvin S, Gillespie E, Leeman SE, Abraham CR. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci U. S. A. 2007;104(50):19796. doi: 10.1073/pnas.0709805104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sakan H, Nakatani K, Asai O, et al. Reduced Renal α-Klotho Expression in CKD Patients and Its Effect on Renal Phosphate Handling and Vitamin D Metabolism. PLoS One. 2014;9(1):e86301. doi: 10.1371/journal.pone.0086301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu MC, Shi M, Zhang J, Quinones H, Kuro-o M, Moe OW. Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int. 78(12):1240. doi: 10.1038/ki.2010.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silver J, Rodriguez M, Slatopolsky E. FGF23 and PTH--double agents at the heart of CKD. Nephrol Dial Transplant. 2012;27(5):1715. doi: 10.1093/ndt/gfs050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davies MR, Lund RJ, Mathew S, Hruska KA. Low turnover osteodystrophy and vascular calcification are amenable to skeletal anabolism in an animal model of chronic kidney disease and the metabolic syndrome. J Am Soc Nephrol. 2005;16(4):917. doi: 10.1681/ASN.2004100835. [DOI] [PubMed] [Google Scholar]
- 47.Mathew S, Tustison KS, Sugatani T, Chaudhary LR, Rifas L, Hruska KA. The mechanism of phosphorus as a cardiovascular risk factor in chronic kidney disease. J Am Soc Nephrol. 2008;19(6):1092. doi: 10.1681/ASN.2007070760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hruska KA, Mathew S, Lund R, Qiu P, Pratt R. Hyperphosphatemia of chronic kidney disease. Kidney Int. 2008;74(2):148. doi: 10.1038/ki.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moallem E, Kilav R, Fau - Silver J, Silver J, Fau - Naveh-Many T, Naveh-Many T. RNA-Protein binding and post-transcriptional regulation of parathyroid hormone gene expression by calcium and phosphate. J Biol Chem. 1998;273(9):5253. doi: 10.1074/jbc.273.9.5253. [DOI] [PubMed] [Google Scholar]
- 50.Larsson T, Nisbeth U, Ljunggren O, Juppner H, Jonsson KB. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 2003;64(6):2272. doi: 10.1046/j.1523-1755.2003.00328.x. [DOI] [PubMed] [Google Scholar]
- 51.Seeherunvong W, Wolf M. Tertiary excess of fibroblast growth factor 23 and hypophosphatemia following kidney transplantation. Pediatr Transplant. 2011;15(1):37. doi: 10.1111/j.1399-3046.2010.01405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bonthuis M, Busutti M, van Stralen KJ, et al. Mineral Metabolism in European Children Living with a Renal Transplant: A European Society for Paediatric Nephrology/European Renal Association–European Dialysis and Transplant Association Registry Study. Clinical Journal of the American Society of Nephrology. 2015;10(5):767. doi: 10.2215/CJN.06200614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sukumaran Nair S, Lenihan CR, Montez-Rath ME, Lowenberg DW, Chertow GM, Winkelmayer WC. Temporal Trends in the Incidence, Treatment and Outcomes of Hip Fracture After First Kidney Transplantation in the United States. American Journal of Transplantation. 2014;14(4):943. doi: 10.1111/ajt.12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tataranni T, Biondi G, Cariello M, et al. Rapamycin-Induced Hypophosphatemia and Insulin Resistance Are Associated With mTORC2 Activation and Klotho Expression. American Journal of Transplantation. 2011;11(8):1656. doi: 10.1111/j.1600-6143.2011.03590.x. [DOI] [PubMed] [Google Scholar]
- 55.Staples AO, Greenbaum LA, Smith JM, et al. Association between clinical risk factors and progression of chronic kidney disease in children. Clin J Am Soc Nephrol. 2010;5(12):2172. doi: 10.2215/CJN.07851109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peacock M. Calcium Metabolism in Health and Disease. Clinical Journal of the American Society of Nephrology. 2010;5(Supplement 1):S23. doi: 10.2215/CJN.05910809. [DOI] [PubMed] [Google Scholar]
- 57.van Husen M, Lehnhardt A, Fischer A-K, et al. Fibroblast growth factor 23 and calcium phosphate homeostasis after pediatric renal transplantation. Pediatric Transplantation. 2012;16(5):443. doi: 10.1111/j.1399-3046.2012.01702.x. [DOI] [PubMed] [Google Scholar]
- 58.van Husen M, Fischer AK, Lehnhardt A, et al. Fibroblast growth factor 23 and bone metabolism in children with chronic kidney disease. Kidney Int. 78(2):200. doi: 10.1038/ki.2010.107. [DOI] [PubMed] [Google Scholar]
- 59.Bonthuis M, Busutti M, van Stralen KJ, et al. Mineral Metabolism in European Children Living with a Renal Transplant: A European Society for Paediatric Nephrology/European Renal Association–European Dialysis and Transplant Association Registry Study. Clinical Journal of the American Society of Nephrology. 2015 doi: 10.2215/CJN.06200614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hill KM, Martin BR, Wastney ME, et al. Oral calcium carbonate affects calcium but not phosphorus balance in stage 3–4 chronic kidney disease. Kidney Int. 2013;83(5):959. doi: 10.1038/ki.2012.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prie D, Friedlander G. Reciprocal control of 1,25-dihydroxyvitamin D and FGF23 formation involving the FGF23/Klotho system. Clin J Am Soc Nephrol. 5(9):1717. doi: 10.2215/CJN.02680310. [DOI] [PubMed] [Google Scholar]
- 62.Naveh-Many T, Marx R, Keshet E, Pike JW, Silver J. Regulation of 1,25-dihydroxyvitamin D3 receptor gene expression by 1,25-dihydroxyvitamin D3 in the parathyroid in vivo. J Clin Invest. 1990;86(6):1968. doi: 10.1172/JCI114931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baeke F, Gysemans C, Korf H, Mathieu C. Vitamin D insufficiency: implications for the immune system. Pediatric Nephrology. 2010;25(9):1597. doi: 10.1007/s00467-010-1452-y. [DOI] [PubMed] [Google Scholar]
- 64.Hullett DA, Laeseke PF, Malin G, Nessel R, Sollinger HW, Becker BN. Prevention of chronic allograft nephropathy with vitamin D*. Transplant International. 2005;18(10):1175. doi: 10.1111/j.1432-2277.2005.00187.x. [DOI] [PubMed] [Google Scholar]
- 65.Meir T, Durlacher K, Pan Z, et al. Parathyroid hormone activates the orphan nuclear receptor Nurr1 to induce FGF23 transcription. Kidney Int. 2014;86(6):1106. doi: 10.1038/ki.2014.215. [DOI] [PubMed] [Google Scholar]
- 66.Wesseling-Perry K, Salusky IB. Chronic kidney disease: mineral and bone disorder in children. Semin Nephrol. 2013;33(2):169. doi: 10.1016/j.semnephrol.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Evenepoel P, Cooper K, Holdaas H, et al. A Randomized Study Evaluating Cinacalcet to Treat Hypercalcemia in Renal Transplant Recipients With Persistent Hyperparathyroidism. American Journal of Transplantation. 2014;14(11):2545. doi: 10.1111/ajt.12911. [DOI] [PubMed] [Google Scholar]
- 68.Malluche HH, Porter DS, Pienkowski D. Evaluating bone quality in patients with chronic kidney disease. Nat Rev Nephrol. 2013;9(11):671. doi: 10.1038/nrneph.2013.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cunningham J, Sprague SM, Cannata-Andia J, et al. Osteoporosis in chronic kidney disease. Am J Kidney Dis. 2004;43(3):566. doi: 10.1053/j.ajkd.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 70.Rix M, Andreassen H, Eskildsen P, Langdahl B, Olgaard K. Bone mineral density and biochemical markers of bone turnover in patients with predialysis chronic renal failure. Kidney Int. 1999;56(3):1084. doi: 10.1046/j.1523-1755.1999.00617.x. [DOI] [PubMed] [Google Scholar]
- 71.Coco M, Rush H. Increased incidence of hip fractures in dialysis patients with low serum parathyroid hormone. Am J Kidney Dis. 2000;36(6):1115. doi: 10.1053/ajkd.2000.19812. [DOI] [PubMed] [Google Scholar]
- 72.Ix JH, Katz R, De Boer IH, et al. Association of chronic kidney disease with the spectrum of ankle brachial index the CHS (Cardiovascular Health Study) J Am Coll Cardiol. 2009;54(13):1176. doi: 10.1016/j.jacc.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moe SM, Chen NX. Pathophysiology of Vascular Calcification in Chronic Kidney Disease. Circ Res. 2004;95(6):560. doi: 10.1161/01.RES.0000141775.67189.98. [DOI] [PubMed] [Google Scholar]
- 74.Kronmal RA, McClelland RL, Detrano R, et al. Risk Factors for the Progression of Coronary Artery Calcification in Asymptomatic Subjects: Results From the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2007;115(21):2722. doi: 10.1161/CIRCULATIONAHA.106.674143. [DOI] [PubMed] [Google Scholar]
- 75.Kawakami T, Ren S, Duffield JS. Wnt signalling in kidney diseases: dual roles in renal injury and repair. J Pathol. 2013;229(2):221. doi: 10.1002/path.4121. [DOI] [PubMed] [Google Scholar]
- 76.Diarra D, Stolina M, Polzer K, et al. Dickkopf-1 is a master regulator of joint remodeling. Nature Med. 2007;13(2):156. doi: 10.1038/nm1538. [DOI] [PubMed] [Google Scholar]
- 77.Hruska KA, Agapova OA, Fang Y, Sugatani T, Seifert ME, Sung V. Chronic Kidney Disease (CKD) Stimulates Activin and Endothelial to Mesenchymal Transition (EnMT), which Causes Vascular Calcification and is Inhibited by an Activin Ligand Trap [Abstract] Journal of the American Society of Nephrology. 2014;25:95A. [Google Scholar]
- 78.Cejka D, Herberth J, Branscum AJ, et al. Sclerostin and Dickkopf-1 in renal osteodystrophy. Clin J Am Soc Nephrol. 2011;6(4):877. doi: 10.2215/CJN.06550810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Morena M, Jaussent I, Dupuy A-M, et al. Osteoprotegerin and sclerostin in chronic kidney disease prior to dialysis: potential partners in vascular calcifications. Nephrology Dialysis Transplantation. 2015 doi: 10.1093/ndt/gfv081. [DOI] [PubMed] [Google Scholar]
- 80.Kitagawa M, Sugiyama H, Morinaga H, et al. A decreased level of serum soluble Klotho is an independent biomarker associated with arterial stiffness in patients with chronic kidney disease. PLoS One. 2013;8(2):e56695. doi: 10.1371/journal.pone.0056695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wesseling-Perry K, Pereira RC, Tseng C-H, et al. Early Skeletal and Biochemical Alterations in Pediatric Chronic Kidney Disease. Clinical Journal of the American Society of Nephrology. 2012;7(1):146. doi: 10.2215/CJN.05940611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cheng SL, Shao JS, Behrmann A, Krchma K, Towler DA. Dkk1 and MSX2-Wnt7b signaling reciprocally regulate the endothelial-mesenchymal transition in aortic endothelial cells. Arterioscler Thromb Vasc Biol. 2013;33(7):1679. doi: 10.1161/ATVBAHA.113.300647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hertig A, Gangadhar T, Kalluri R. Renal studies provide an insight into cardiac extracellular matrix remodeling during health and disease. J Mol Cell Cardiol. 2010;48(3):497. doi: 10.1016/j.yjmcc.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Seifert ME, Chishti AS, Chiang ML, Selewski DT, Gipson DS, Hruska KA. Elevated Fibroblast Growth Factor 23 Levels Are Associated with Chronic Rejection and Declining Kidney Transplant Function. Journal of the American Society of Nephrology. 2013;24:865A. [Google Scholar]
- 85.Pelletier S, Dubourg L, Carlier M-C, Hadj-Aissa A, Fouque D. The Relation between Renal Function and Serum Sclerostin in Adult Patients with CKD. Clinical Journal of the American Society of Nephrology. 2013;8(5):819. doi: 10.2215/CJN.07670712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Seiler S, Rogacev KS, Roth HJ, et al. Associations of FGF-23 and sKlotho with Cardiovascular Outcomes among Patients with CKD Stages 2–4. Clinical Journal of the American Society of Nephrology. 2014;9(6):1049. doi: 10.2215/CJN.07870713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.von Toerne C, Schmidt C, Adams J, et al. Wnt pathway regulation in chronic renal allograft damage. Am J Transplant. 2009;9(10):2223. doi: 10.1111/j.1600-6143.2009.02762.x. [DOI] [PubMed] [Google Scholar]
- 88.Qian X, Yuan X, Vonderfecht S, et al. Inhibition of WISE preserves renal allograft function. J Am Soc Nephrol. 2013;24(1):66. doi: 10.1681/ASN.2012010012. [DOI] [PMC free article] [PubMed] [Google Scholar]