Abstract
While persons with dementia are frequently hospitalized, relatively little is known about the health profile, patterns of health care use, and mortality rates for patients with dementia who access care in the emergency department (ED). We linked data from our hospital system with Medicare and Medicaid claims, Minimum Data Set, and Outcome and Assessment Information Set data to evaluate 175,652 ED visits made by 10,354 individuals with dementia and 15,020 individuals without dementia over 11 years. Survival rates after ED visits and associated charges were examined. Patients with dementia visited the ED more frequently, were hospitalized more often than patients without dementia, and had an increased odds of returning to the ED within 30 days of an index ED visit compared to persons who never had a dementia diagnosis (odds ratio 2.29, P<0.001). Survival rates differed significantly between patients by dementia status (P<0.001). Mean Medicare payments for ED services were significantly higher among patients with dementia. These results show that older adults with dementia are frequent ED visitors who have greater comorbidity, incur higher charges, are admitted to hospitals at higher rates, return to EDs at higher rates, and have higher mortality after an ED visit than patients without dementia.
Keywords: survival rate, emergency department, dementia diagnosis
INTRODUCTION
In 2009, older adults made approximately 19.8 million visits to American emergency departments (EDs).(1) Older adults’ ED visits have been characterized as among the longest for all patients, with this group receiving more testing and incurring higher bills than younger visitors.(2, 3) To compound matters, when older adults visit the ED, a large proportion of them will be admitted to the hospital.(4) An increasing number of researchers believe however that many hospitalizations of vulnerable older adults could be avoided and that hospital-based services are over-utilized for care that could have been provided in lower cost settings.(5-9)
Dementia represents a prototype condition that both induces vulnerability in older adults and places them at increased risk of accessing hospital and acute care services.(10) Care of persons with dementia is expensive, resulting in $157 billion to $215 billion annually in costs.(11) Given projections of a large increase in the number of adults with dementia by 2050,(12) the development of dementia care management programs that avoid “use of costly and aggressive treatments that may be of limited clinical benefit” has been identified as a priority in the field.(13)
The extant literature contains comparatively little data though about the care experiences and health care trajectories of persons with dementia who seek care in the ED. While studies have shown that patients with dementia are admitted to the hospital at higher rates and have increased health costs over patients without dementia,(8, 14, 15) we lack a comprehensive understanding of the patient profile, health characteristics, medical charges, and long-term health outcomes of patients with dementia who seek care in the ED. Such longitudinal data are needed in order to develop programs that appropriately address the acute care needs of older adults with dementia. The objective of this study was to describe the clinical characteristics, care patterns, associated medical costs, and health outcomes for older adults with and without dementia who seek care in the ED using a comprehensive database of more than 32,000 older adults followed for an 11-year period.
METHODS
This study was approved by the Indiana University Institutional Review Board and the Centers for Medicare and Medicaid Services Privacy Board. In these analyses, we report data from a merged database of local electronic medical record data, Medicare claims, Indiana Medicaid claims, resident-level Minimum Data Set (MDS), and Outcome and Assessment Information Set (OASIS) data from 1999 through 2009. MDS and OASIS data were used to identify the patterns of transitions in care but we did not access clinical and functional status data available in these databases because such data are only available on the subset of subjects who utilized these sites of care. For the larger group of subjects without nursing home or home health care use, we did not have access to functional status data.
The study was conducted at Eskenazi Health Services (formerly known as Wishard Health Services), an urban, public hospital system in Indianapolis. Eskenazi Health Services consists of a 293-bed teaching hospital and 10 federally qualified health centers. Though patients were enrolled in this study through Eskenazi Health Services, data utilization for patients was captured at other hospitals or sites of care through the Medicare, Medicaid, MDS, and OASIS datasets.
Our database consisted of observations for 32,697 patients aged 65 and older who sought care at Eskenazi Health over an 11 year period. We identified prevalent cases of dementia using International Classification of Disease codes 290.0 to 290.43, 291.2, 294.0 to 294.9, 331.0 to 331.9, 333.0, and 797, as we have described previously.(10) Prevalent cases of dementia were based on these codes existing in any of the linked data sets on or before the date of individuals’ first ED visit; incident cases were defined by the appearance of these codes for the first time in any of the data sets after their first ED visit. We have used these methods to identify patients with dementia in previous work.(10, 16, 17)
Comorbid conditions were identified in a similar manner according to ICD codes applied by providers. These conditions and their corresponding ICD codes were: arthritis (714. and 715.), cancer (140.-172. and 174.-239.), coronary artery disease (410., 411., 412., 413. and 414.), congestive heart failure (428. and 398.91), chronic obstructive pulmonary disease (491., 492., and 496.), diabetes (250.), hypertension (401.), liver disease (570.-573.), renal disease (585.), and stroke (433.1 and 434.1).
The study sample was divided into two strata, those patients with dementia and those without dementia. First, we compiled demographic and medical characteristics of these two groups stratified by ED use; we subsequently compared these using chi-square and t tests. Next, we examined annual rates of ED use by persons with dementia at the time of their ED visit as compared to persons who did not have dementia identified at their ED visit. We then examined patient disposition after ED use as well as diagnosis codes stratified by patient's dementia status at the time of their ED visit. Next, we evaluated differences between these groups in time to return to the ED and in mortality following a first ED visit stratified by dementia status. Finally, total Medicare and Medicaid costs were calculated for patients who ever developed dementia and who never developed dementia; these results were then compared using a Wald test to account for zero costs and skewness in cost distributions.(18) SAS software was used for all analyses. P ≤ .05 was considered statistically significant.
RESULTS
Characteristics of Study Population
Among the 32,697 individuals in the study sample, 11,069 individuals (33.9%) were identified as having dementia, while 21,628 individuals (66.1%) were not identified as having dementia during the 11 year study period. The mean follow-up time for patients was 8.5 years (SD 2.8). During the study, 175,652 ED visits were made by 10,354 individuals with dementia and 15,020 individuals without dementia. Clinical characteristics of study subjects including demographic and comorbidities, stratified by presence of dementia at any time and use of the ED, are compared in Table 1. Patients with a diagnosis of dementia were more likely to be older (P<0.001) and more likely to be female (P<0.001). Patients with dementia who visited the ED had the highest number of mean comorbidities, 8.0 (SD 2.5), as well the highest percentage of individuals affected by each of the reported comorbidities.
Table 1.
Descriptive Characteristics of Older Adults Without and With Dementia
| All Subjects | Never Diagnosed with Dementia | Ever Diagnosed with Dementia | p-value* | |||
|---|---|---|---|---|---|---|
| n=32,697 | No ED n=6,608 | ED n=15,020 | No ED n=715 | ED n=10,354 | ||
| Demographics | ||||||
| Age, mean (SD)** | 68.3 (8.5) | 65.0 (8.1) | 66.5 (7.2) | 73.5 (9.9) | 72.8 (8.5) | <.001 |
| Female, n (%) | 19406 (59.4) | 3546 (53.7) | 8925 (59.4) | 423 (59.2) | 6512 (62.9) | <.001 |
| Black, n (%) | 11596 (35.5) | 2007 (30.4) | 5265 (35.1) | 263 (36.8) | 4061 (39.2) | <.001 |
| No. of months of observation, mean (SD)*** | 102.4 (33.1) | 84.6 (45.9) | 108.7 (28.0) | 82.9 (38.1) | 106.0 (24.0) | <.001 |
| Comorbid conditions, n (%)**** | ||||||
| Arthritis | 18480 (56.5) | 1808 (27.4) | 8971 (59.7) | 265 (37.1) | 7436 (71.8) | <.001 |
| Cancer | 13169 (40.3) | 1485 (22.5) | 6755 (45.0) | 201 (28.1) | 4728 (45.7) | <.001 |
| Coronary artery disease | 15316 (46.8) | 1048 (15.9) | 7344 (48.9) | 197 (27.6) | 6727 (65.0) | <.001 |
| Congestive heart failure | 12568 (38.4) | 598 (9.1) | 5613 (37.4) | 195 (27.3) | 6162 (59.5) | <.001 |
| Chronic obstructive pulmonary disease | 13511 (41.3) | 863 (13.1) | 6650 (44.3) | 167 (23.4) | 5831 (56.3) | <.001 |
| Diabetes | 14851 (45.4) | 1673 (25.3) | 6934 (46.2) | 231 (32.3) | 3013 (58.1) | <.001 |
| Hypertension | 27303 (83.5) | 3829 (57.9) | 13294 (88.5) | 467 (65.3) | 9713 (93.8) | <.001 |
| Liver disease | 4586 (14.0) | 276 (4.2) | 2369 (15.8) | 42 (5.9) | 1899 (18.3) | <.001 |
| Renal disease | 1540 (4.7) | 118 (1.8) | 778 (5.2) | 13 (1.8) | 631 (6.1) | <.001 |
| Stroke | 5561 (17.0) | 233 (3.5) | 1934 (12.9) | 75 (10.5) | 3319 (32.1) | <.001 |
| Number of comorbid conditions, mean (SD) | 5.4 (3.1) | 2.1 (1.9) | 5.0 (2.4) | 4.6 (2.4) | 8.0 (2.5) | <.001 |
p-value based upon chi-square test for categorical variables and general linear model for continuous variables.
age as of January 1, 1999; potential range was 55-105.
Time period measured was January 1, 1999 to December 31, 2009; potential range was 1 day to 132 months.
comorbid conditions were measured at any time up until the subject's last observation date.
Annual Rates of ED Use
The annual rate of ED use among individuals varied by dementia status. Over the entire period of observation, 77% of all subjects visited the ED. Among individuals with a dementia diagnosis at or prior to the time of an ED visit, between 37% and 54% of individuals visited the ED in a given year, while 20% to 31% of individuals without a dementia diagnosis made an ED visit in a given year.
Patient Disposition and ED Return Rates
Patient disposition after ED use is shown in table 2. Patients with a current dementia diagnosis were admitted to the hospital at higher rates (39.7%) than patients without a current dementia diagnosis (29.6%) (P<0.001). Patients with dementia were admitted to the hospital at higher rates after adjustment for age, race, gender, and number of comorbidities. The majority of patients (52.9%) with dementia diagnoses were discharged. The ten most common primary diagnosis codes recorded for an individual with and without a current dementia diagnosis and sorted by disposition are displayed in table 3. Diagnosis codes for patients with dementia who were discharged and for patients without dementia who were discharged were similar. Fifty-eight percent of individuals with a dementia diagnosis had at least one ED visit within 30 days after an index ED visit, as compared to 38% of individuals who never had a dementia diagnosis (odds ratio 2.29, P<0.001). This pattern of increased 30 day ED return visits among patients with a dementia diagnosis persisted after adjusting for age, race, gender, and number of comorbidities (odds ratio 1.37, P<0.001).
Table 2.
Patient Disposition Following Emergency Department (ED) Use
| All ED visits | ED Visits When Not Demented | ED Visits When Demented | |
|---|---|---|---|
| Home | 107770 (61.4) | 72576 (66.5) | 35194 (52.9) |
| Nursing Home | 2070 (1.2) | 314 (0.3) | 1756 (2.6) |
| Observation | 5043 (2.9) | 2962 (2.7) | 2081 (3.1) |
| Hospital | 58783 (33.5) | 32328 (29.6) | 26455 (39.7) |
| Death | 294 (0.2) | 156 (0.1) | 138 (0.2) |
| Other | 1692 (0.1) | 252 (0.2) | 944 (1.4) |
| Total | 175652 (100.0) | 109084 (100.0) | 66568 (100.0) |
Notes: N (%) shown in columns.
Table 3.
Ten Most Frequent ED Primary Diagnosis Codes by Dementia Status and Discharge Status
| When Not Demented | When Demented | |||
|---|---|---|---|---|
| Not Discharged | Discharged | Not Discharged | Discharged | |
| 1 | 428.0 Congestive Heart Failure | 786.50 Chest pain NOS | 486 Pneumonia, Organism NOS | 599.0 Urinary Tract Infection NOS |
| 2 | 491.21 Obstructive Chronic Bronchitis with Acute Exacerbation | 599.0 Urinary Tract Infection NOS | 428.0 Congestive Heart Failure | 786.50 Chest pain NOS |
| 3 | 486 Pneumonia, Organism NOS | 789.00 Abdominal Pain Unspecified Site | 599.0 Urinary Tract Infection NOS | 789.00 Abdominal Pain Unspecified Site |
| 4 | 414.01 Coronary Atherosclerosis Native Vessel | 729.5 Pain in Limb | 491.21 Obstructive Chronic Bronchitis with Acute Exacerbation | 780.4 Dizziness and Giddiness |
| 5 | 786.50 Chest pain NOS | 491.21 Obstructive Chronic Bronchitis with Acute Exacerbation | 584.9 Acute renal failure NOS | 729.5 Pain in Limb |
| 6 | 786.59 Chest pain NEC | 401.9 Hypertension NOS | 507.0 Food/Vomit Pneumonitis | 780.39 Convulsions NEC |
| 7 | 410.71 Subendocardial infarct, initial | 780.4 Dizziness and Giddiness | 434.91 Cerebral Artery Occlusion NOS with Infarct | 780.79 Malaise and Fatigue NEC |
| 8 | 427.31 Atrial fibrillation | 786.59 Chest pain NEC | 038.9 Septicemia NOS | 401.9 Hypertension NOS |
| 9 | 584.9 Acute renal failure NOS | 490 Bronchitis NOS | 780.2 Syncope and Collapse | 784.0 Headache |
| 10 | 434.91 Cerebral Artery Occlusion NOS with Infarct | 784.0 Headache | 786.50 Chest pain NOS | 491.21 Obstructive Chronic Bronchitis with Acute Exacerbation |
Patient Mortality Rates after an Initial ED Visit
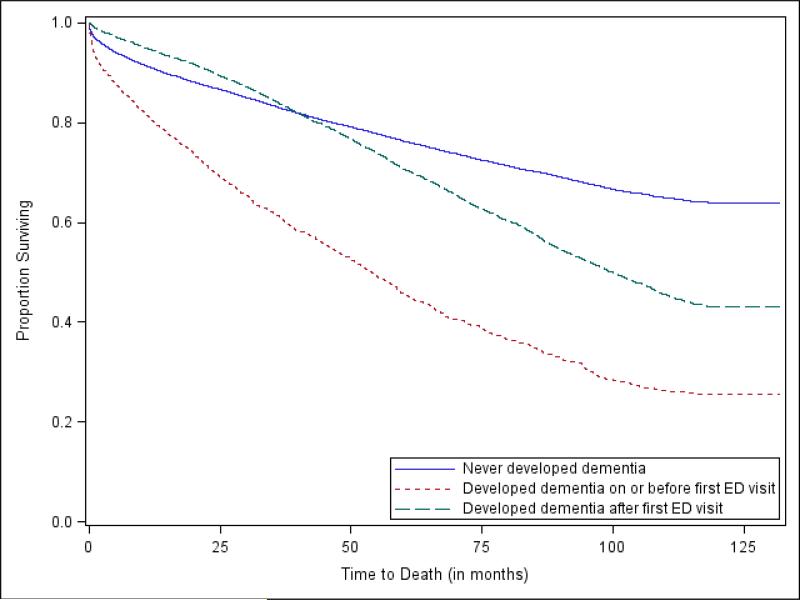
Mortality rates differed significantly among patients with dementia at the time of their first ED visit, patients who subsequently developed dementia, and patients who never developed dementia. Kaplan-Meier estimates of survival function as calculated from the time of patients’ first ED visit are demonstrated in Figure 1. At 12 months, 80.5% of individuals with dementia were alive as compared to 91% of individuals without dementia and 94.6% of individuals who subsequently developed dementia. At 60 months, 45.7% of individuals with dementia were still alive as compared to 76.3% of individuals without dementia and 70.8% of individuals who later developed dementia. Compared to patients who never developed dementia as the reference group, patients with dementia at the time of their first ED visit had a hazard ratio of 2.99 (P<0.001) for death while patients who were diagnosed after their first ED visit with dementia had a hazard ratio of 1.53 for death (P<0.001). These findings persisted after adjustment for age, race, gender, and number of comorbidities.
Figure 1.
Kaplan-Meier estimates of survival by dementia status.
Patient Mortality Rates Following an ED Visit with Discharge
Over a shorter time period, mortality rates in the 6 months following an ED visit differed significantly among patients who were discharged based on their dementia status at the time of their ED visit (P<0.001). Among those patients discharged, 92.9% of individuals with dementia at the time of their ED visit were alive at 6 months after their ED visit as compared to 97.7% of individuals without dementia.
Medicare and Medicaid Payments for ED Services
Table 4 displays ED-related Medicare and Medicaid payments for our cohort based on dementia diagnosis. The mean Medicaid payment was increased ($199 versus $134, P<0.001) for patients with dementia as compared to those without dementia. Mean Medicare ED payments for patients with dementia at any time during the study period were 75% higher than for patients without dementia ($6028 vs. $3454, P<0.001). These Medicaid and Medicare cost findings persisted after adjustment for age, race, gender, and number of comorbidities.
Table 4.
ED payment amounts for Medicaid and Medicare between 1999 and 2009, N=25,374 (number of subjects with an ED visit).*
| Total (N=25,374) | Never Had Dementia (N=15,020) | Ever Had Dementia (N=10,354) | P-value | |
|---|---|---|---|---|
| ED Medicaid payments**, mean (SD); median; range | 161 (1680); 0; 0-106,497 | 134 (1813); 0; 0-106,497 | 199 (1466); 0; 0-46,263 | <.001 |
| ED Medicare payments, mean (SD); median; range | 4504 (7659); 2069; 0-199,968 | 3454 (6349); 1466; 0-135,276 | 6028 (9020); 3247; 0-199,968 | <.001 |
A Wald test to account for zero costs and skewness in cost distributions was used to make comparisons(18)
Subjects having no Medicaid costs were assumed to have zero Medicaid costs.
DISCUSSION
To our knowledge, this is the first observational cohort study of a population of older adults with dementia to describe their health profile, patterns of ED use, ED-related health care costs, and health outcomes. Additional strengths of the study include a large population of older adults followed for 11 years and access to utilization of nursing facilities and home health care. Our findings show that the ED is a site where a significant amount of care is accessed by older adults with dementia, with 37% to 54% of these patients making a visit to the ED in any given year. Further, these data underscore that individuals with dementia who seek care in the ED have more medical comorbidity, accrue higher ED charges, and die at an accelerated rate in the time following an initial ED visit compared with individuals without dementia. From our analyses adjusted for the available covariates, we conclude that these patients’ health outcomes and their increased costs for ED care cannot be explained fully by their increased comorbidity and appear to be driven, at least in part, by the dementing illness.
Our findings are consistent with earlier studies that have documented the increased use of hospital and acute care services and greater health care costs to funding agencies among older adults with dementia.(8, 19) Indeed, the higher rate of Medicare expenditures among individuals in these studies is driven in large part by their increased rates of hospitalization.(19) Our study demonstrates though that ED-associated costs for such individuals are higher also as compared to older adults without dementia. Given that many patients’ hospitalizations begin with an ED visit and that a large percentage of older adults with dementia who seek care in the ED will be admitted to the hospital, further study of the care that is provided to older adults with dementia in this important location is warranted. To date, patients with dementia and other cognitive impairments have been recognized as more difficult for ED providers to assess and treat.(20, 21) We, however, know comparatively little about the decision making of emergency providers when treating this vulnerable population, the care preferences of patients with dementia and their caregivers who seek care in this environment, or alternatives that might be readily offered to patients with dementia who wish to avoid admission to the hospital from the ED.
In our study, we were intrigued by the finding that a large number of patients with dementia who sought care in the ED were subsequently discharged. Using our methods, we do not have insight into the “appropriateness” of these disposition decisions, but we can examine this observed phenomenon from two contrasting perspectives. Assuming that the medical care (including the disposition decision) provided to these patients was “appropriate,” we might question how medically necessary these visits were (even if perceived as needed by the patient or caregiver) and by extension, whether some proportion of these patients may have received care in a different, potentially lower cost venue. Alternatively, if the decisions to discharge the patient were flawed, either due to missed medical complications or due to incomplete assessments of the safety of the patient's home environment, this might explain the increased odds of death that we observed among those patients with a dementia diagnosis who were discharged. In other veins of literature among cognitively impaired adults in the ED, unrecognized delirium has been associated with a 20% higher risk of death in patients discharged home at 6 months.(22) Given that only 16% to 35% of delirium cases are recognized in the ED(21) and that one of the greatest risk factors for delirium in the ED is pre-existing dementia,(23) it is intriguing to speculate whether unrecognized delirium could be driving part of this higher mortality rate among older patients with dementia. Further work will be needed to explore these findings.
Our study demonstrates that dementia is a marker both of an increased risk of return to the ED in the next 30 days and of death in the following months and years when compared to the outcomes of older adults without dementia who visit the ED. These findings persist after adjustment for relevant covariates and remind us that an ED visit is a sentinel event in the life of an older adult with dementia.(24) After an ED visit, follow-up with the patient's primary care physician to ensure resolution of the patient's acute care needs is certainly indicated. Beyond this, it may also be useful for the patient's primary care doctor to consider whether the patient with dementia and his or her family are eligible for referral to community based support services or dementia-care programs. In our own community, dementia-focused collaborative care programs, like the Healthy Aging Brain Center and Aging Brain Care Medical Home, are available that provide support and care to older adults with dementia and their caregivers, while other programs like Geriatric Resources for Assessment and Care of Elders (GRACE) team services provide more broad-based geriatric care.(25-28) While these programs have not been shown to affect mortality, they have been successful at improving quality of care, improving selected health outcomes, and/or producing cost savings.(25, 28-30) Federally-sponsored initiatives, funded through the Centers for Medicare and Medicaid Innovation, have been more recently developed that seek to improve transitions for older adults with dementia and reduce unnecessary health care utilization.(31)
From this work, we can identify several opportunities for future research. To this point, we know little about emergency providers’ decision making when encountering persons with dementia; patients’ or caregivers’ preferences for care in the ED; and acceptable alternatives to ED care for persons with dementia. These are all areas of inquiry that deserve future exploration. Beyond these, future work will be needed to explore the influence of dementia severity and functional impairment on patients’ use of the ED as well as their impact on patients’ care trajectories after ED visits. Given the critical role that caregivers play in supporting cognitively impaired adults, it will be also be important to explore the effect of formal and informal caregiver support on ED use and patients’ post-ED trajectories. Finally, it may be useful to explore patterns of ED use among community-dwelling and assisted living-dwelling older adults as well as short and long-stay nursing residents, as different care settings may lend themselves towards different approaches to providing excellent, goal-concordant acute care to persons with dementia.
Our study has several limitations which are worth noting. First, our ability to draw conclusions on the causal relationship between our observations and health care outcomes is limited by our methodology, a secondary dataset analysis of administrative data. While administrative data provide information on large numbers of individuals and are relatively easy to obtain, they are also subject to several factors that may limit their utility, including the accuracy of the encoded data, the completeness of coding, reimbursement policies, and provider responses to those policies.(32, 33) Though we do not have precise estimates of the sensitivity and specificity of the ICD-9 codes that were used to diagnose dementia in this population, a very similar grouping of ICD-9 codes had a specificity of 84% and a sensitivity of 69% for detecting dementia among a Medicaid population in our state.(33) In this work, we have analyzed a large cohort of older adults, both with and without dementia, over an extended period of time in an entire health system and their associated Medicare and Medicaid charges. Next, we relied upon physician diagnoses of dementia to identify patients with dementia. Though dementia is known to be under recognized in older patients or recognized later in the course of illness, this misclassification would tend to decrease the observed differences between groups. Additionally, we have limited information on the functional status of the patients enrolled in our study; we do not have data on the severity of patients’ dementia; and we lack information to gauge the urgency of patients’ ED visits or resulting hospitalizations. We were therefore unable to account for the effect of these factors on the described study outcomes. We were also unable to evaluate the effect that our local Senior Care Program, including the Healthy Aging Brain Center, Aging Brain Care Medical Home, and GRACE services, may have had upon patient outcomes. Finally, our study was performed among a cohort of older adults in a single, urban public hospital system. As a consequence, these results may not be generalizable to other health care settings. As a federally qualified health center (FQHC) though, our hospital system cares for many vulnerable patients with low incomes. Therefore our findings may provide insights into the health trajectories of similar patient populations across the nation enrolled in FQHCs.
In conclusion, we found that older adults with dementia were frequent visitors to EDs who had higher numbers of comorbidities, received more costly care, were admitted to hospitals at higher rates, and had higher return rates than patients without dementia. Survival and ED return rates differed also according to the patients’ dementia status.
ACKNOWLEDGEMENTS
This work was supported by grants from the National Institute on Aging (5P30AG024967, 1K23AG043498, 5K24AG024078). We would like to acknowledge gratefully the contributions of Roberta Ambuehl for her assistance in data management for this study.
Source of Funding:
Dr. LaMantia presented an abstract of this work at the 2015 American Geriatrics Society Annual Scientific Meeting. Dr. LaMantia also received an award at that meeting with accompanying travel stipend sponsored by Merck Pharmaceuticals, but administered by the American Geriatrics Society. Merck Pharmaceuticals has not sponsored this work or had any input into its design or conduct. The authors alone made decisions regarding the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest
The authors declare no relevant financial interests related to this manuscript.
REFERENCES
- 1.Pines JM, Mullins PM, Cooper JK, Feng LB, Roth KE. National trends in emergency department use, care patterns, and quality of care of older adults in the United States. J Am Geriatr Soc. 2013;61(1):12–7. doi: 10.1111/jgs.12072. Epub 2013/01/15. doi: 10.1111/jgs.12072. PubMed PMID: 23311549. [DOI] [PubMed] [Google Scholar]
- 2.Baum SA, Rubenstein LZ. Old people in the emergency room: age-related differences in emergency department use and care. J Am Geriatr Soc. 1987;35(5):398–404. doi: 10.1111/j.1532-5415.1987.tb04660.x. Epub 1987/05/01. PubMed PMID: 3571788. [DOI] [PubMed] [Google Scholar]
- 3.Lowenstein SR, Crescenzi CA, Kern DC, Steel K. Care of the elderly in the emergency department. Ann Emerg Med. 1986;15(5):528–35. doi: 10.1016/s0196-0644(86)80987-8. Epub 1986/05/01. PubMed PMID: 3963531. [DOI] [PubMed] [Google Scholar]
- 4.LaMantia MA, Platts-Mills TF, Biese K, Khandelwal C, Forbach C, Cairns CB, et al. Predicting hospital admission and returns to the emergency department for elderly patients. Acad Emerg Med. 2010;17(3):252–9. doi: 10.1111/j.1553-2712.2009.00675.x. Epub 2010/04/08. doi: 10.1111/j.1553-2712.2009.00675.x. PubMed PMID: 20370757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walsh EG, Wiener JM, Haber S, Bragg A, Freiman M, Ouslander JG. Potentially avoidable hospitalizations of dually eligible Medicare and Medicaid beneficiaries from nursing facility and Home- and Community-Based Services waiver programs. J Am Geriatr Soc. 2012;60(5):821–9. doi: 10.1111/j.1532-5415.2012.03920.x. Epub 2012/03/31. doi: 10.1111/j.1532-5415.2012.03920.x. PubMed PMID: 22458363. [DOI] [PubMed] [Google Scholar]
- 6.Lin PJ, Fillit HM, Cohen JT, Neumann PJ. Potentially avoidable hospitalizations among Medicare beneficiaries with Alzheimer's disease and related disorders. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2013;9(1):30–8. doi: 10.1016/j.jalz.2012.11.002. Epub 2013/01/12. doi: 10.1016/j.jalz.2012.11.002. PubMed PMID: 23305822. [DOI] [PubMed] [Google Scholar]
- 7.Lyketsos CG. Prevention of Unnecessary Hospitalization for Patients With Dementia. JAMA: The Journal of the American Medical Association. 2012;307(2):197–8. doi: 10.1001/jama.2011.2005. [DOI] [PubMed] [Google Scholar]
- 8.Phelan EA, Borson S, Grothaus L, Balch S, Larson EB. Association of incident dementia with hospitalizations. JAMA: The Journal of the American Medical Association. 2012;307(2):165–72. doi: 10.1001/jama.2011.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carpenter CR, Platts-Mills TF. Evolving prehospital, emergency department, and “inpatient” management models for geriatric emergencies. Clin Geriatr Med. 2013;29(1):31–47. doi: 10.1016/j.cger.2012.09.003. Epub 2012/11/28. doi: 10.1016/j.cger.2012.09.003. PubMed PMID: 23177599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Callahan CM, Arling G, Tu W, Rosenman MB, Counsell SR, Stump TE, et al. Transitions in care for older adults with and without dementia. J Am Geriatr Soc. 2012;60(5):813–20. doi: 10.1111/j.1532-5415.2012.03905.x. Epub 2012/05/17. doi: 10.1111/j.1532-5415.2012.03905.x. PubMed PMID: 22587849; PubMed Central PMCID: PMC3354737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. N Engl J Med. 2013;368(14):1326–34. doi: 10.1056/NEJMsa1204629. Epub 2013/04/05. doi: 10.1056/NEJMsa1204629. PubMed PMID: 23550670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology. 2013;80(19):1778–83. doi: 10.1212/WNL.0b013e31828726f5. Epub 2013/02/08. doi: 10.1212/WNL.0b013e31828726f5. PubMed PMID: 23390181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell SL, Black BS, Ersek M, Hanson LC, Miller SC, Sachs GA, et al. Advanced dementia: state of the art and priorities for the next decade. Ann Intern Med. 2012;156(1 Pt 1):45–51. doi: 10.1059/0003-4819-156-1-201201030-00008. Epub 2012/01/04. doi: 10.1059/0003-4819-156-1-201201030-00008. PubMed PMID: 22213494; PubMed Central PMCID: PMC3261500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luengo-Fernandez R, Leal J, Gray AM. Cost of dementia in the pre-enlargement countries of the European Union. J Alzheimers Dis. 2011;27(1):187–96. doi: 10.3233/JAD-2011-102019. doi: 10.3233/JAD-2011-102019. PubMed PMID: 21860095. [DOI] [PubMed] [Google Scholar]
- 15.Gutterman EM, Markowitz JS, Lewis B, Fillit H. Cost of Alzheimer's disease and related dementia in managed-medicare. J Am Geriatr Soc. 1999;47(9):1065–71. doi: 10.1111/j.1532-5415.1999.tb05228.x. PubMed PMID: 10484247. [DOI] [PubMed] [Google Scholar]
- 16.Arling G, Tu W, Stump TE, Rosenman MB, Counsell SR, Callahan CM. Impact of dementia on payments for long-term and acute care in an elderly cohort. Med Care. 2013;51(7):575–81. doi: 10.1097/MLR.0b013e31828d4d4a. doi: 10.1097/MLR.0b013e31828d4d4a. PubMed PMID: 23756644; PubMed Central PMCID: PMC3680786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Unroe KT, Sachs GA, Hickman SE, Stump TE, Tu WZ, Callahan CM. Hospice Use Among Nursing Home Patients. Journal of the American Medical Directors Association. 2013;14(4):254–9. doi: 10.1016/j.jamda.2012.10.006. doi: DOI 10.1016/j.jamda.2012.10.006. PubMed PMID: WOS:000317281200007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tu W, Zhou XH. A Wald test comparing medical costs based on log-normal distributions with zero valued costs. Stat Med. 1999;18(20):2749–61. doi: 10.1002/(sici)1097-0258(19991030)18:20<2749::aid-sim195>3.0.co;2-c. PubMed PMID: 10521864. [DOI] [PubMed] [Google Scholar]
- 19.Bynum JP, Rabins PV, Weller W, Niefeld M, Anderson GF, Wu AW. The relationship between a dementia diagnosis, chronic illness, medicare expenditures, and hospital use. J Am Geriatr Soc. 2004;52(2):187–94. doi: 10.1111/j.1532-5415.2004.52054.x. PubMed PMID: 14728626. [DOI] [PubMed] [Google Scholar]
- 20.Terrell KM, Hustey FM, Hwang U, Gerson LW, Wenger NS, Miller DK, et al. Quality Indicators for Geriatric Emergency Care. Acad Emerg Med. 2009;16(5):441–9. doi: 10.1111/j.1553-2712.2009.00382.x. doi: 10.1111/j.1553-2712.2009.00382.x. PubMed PMID: ISI:000265548300012. [DOI] [PubMed] [Google Scholar]
- 21.Lamantia MA, Messina FC, Hobgood CD, Miller DK. Screening for Delirium in the Emergency Department: A Systematic Review. Ann Emerg Med. 2014;63(5):551–60. e2. doi: 10.1016/j.annemergmed.2013.11.010. doi: 10.1016/j.annemergmed.2013.11.010. PubMed PMID: 24355431. [DOI] [PubMed] [Google Scholar]
- 22.Kakuma R, du Fort GG, Arsenault L, Perrault A, Platt RW, Monette J, et al. Delirium in older emergency department patients discharged home: Effect on survival. J Am Geriatr Soc. 2003;51(4):443–50. doi: 10.1046/j.1532-5415.2003.51151.x. PubMed PMID: ISI:000181803000001. [DOI] [PubMed] [Google Scholar]
- 23.Han JH, Zimmerman EE, Cutler N, Schnelle J, Morandi A, Dittus RS, et al. Delirium in older emergency department patients: recognition, risk factors, and psychomotor subtypes. Acad Emerg Med. 2009;16(3):193–200. doi: 10.1111/j.1553-2712.2008.00339.x. Epub 2009/01/22. doi: 10.1111/j.1553-2712.2008.00339.x. PubMed PMID: 19154565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernstein E. Repeat visits by elder emergency department patients: sentinel events. Acad Emerg Med. 1997;4(6):538–9. doi: 10.1111/j.1553-2712.1997.tb03573.x. PubMed PMID: 9189183. [DOI] [PubMed] [Google Scholar]
- 25.Boustani MA, Sachs GA, Alder CA, Munger S, Schubert CC, Austrom MG, et al. Implementing innovative models of dementia care: The Healthy Aging Brain Center. Aging Ment Health. 2011;15(1):13–22. doi: 10.1080/13607863.2010.496445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Callahan CM, Boustani MA, Weiner M, Beck RA, Livin LR, Kellams JJ, et al. Implementing dementia care models in primary care settings: The Aging Brain Care Medical Home. Aging Ment Health. 2011;15(1):5–12. doi: 10.1080/13607861003801052. Epub 2010/10/15. doi: 10.1080/13607861003801052. PubMed PMID: 20945236; PubMed Central PMCID: PMC3030631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Counsell SR, Callahan CM, Buttar AB, Clark DO, Frank KI. Geriatric Resources for Assessment and Care of Elders (GRACE): a new model of primary care for low-income seniors. J Am Geriatr Soc. 2006;54(7):1136–41. doi: 10.1111/j.1532-5415.2006.00791.x. doi: 10.1111/j.1532-5415.2006.00791.x. PubMed PMID: 16866688. [DOI] [PubMed] [Google Scholar]
- 28.LaMantia MA, Alder CA, Callahan CM, Gao S, French DD, Austrom MG, et al. The Aging Brain Care Medical Home: Preliminary Data. J Am Geriatr Soc. 2015;63(6):1209–13. doi: 10.1111/jgs.13447. doi: 10.1111/jgs.13447. PubMed PMID: 26096394. [DOI] [PubMed] [Google Scholar]
- 29.Counsell SR, Callahan CM, Clark DO, Tu W, Buttar AB, Stump TE, et al. Geriatric care management for low-income seniors: a randomized controlled trial. JAMA. 2007;298(22):2623–33. doi: 10.1001/jama.298.22.2623. Epub 2007/12/13. doi: 10.1001/jama.298.22.2623. PubMed PMID: 18073358. [DOI] [PubMed] [Google Scholar]
- 30.French DD, Lamantia MA, Livin LR, Herceg D, Alder CA, Boustani MA. Healthy aging brain center improved care coordination and produced net savings. Health Aff (Millwood) 2014;33(4):613–8. doi: 10.1377/hlthaff.2013.1221. doi: 10.1377/hlthaff.2013.1221. PubMed PMID: 24711322. [DOI] [PubMed] [Google Scholar]
- 31.Callahan CM, Sachs GA, Lamantia MA, Unroe KT, Arling G, Boustani MA. Redesigning systems of care for older adults with Alzheimer's disease. Health Aff (Millwood) 2014;33(4):626–32. doi: 10.1377/hlthaff.2013.1260. doi: 10.1377/hlthaff.2013.1260. PubMed PMID: 24711324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iezzoni LI. Assessing quality using administrative data. Ann Intern Med. 1997;127(8 Pt 2):666–74. doi: 10.7326/0003-4819-127-8_part_2-199710151-00048. PubMed PMID: 9382378. [DOI] [PubMed] [Google Scholar]
- 33.Bharmal MF, Weiner M, Sands LP, Xu H, Craig BA, Thomas J., 3rd. Impact of patient selection criteria on prevalence estimates and prevalence of diagnosed dementia in a Medicaid population. Alzheimer Dis Assoc Disord. 2007;21(2):92–100. doi: 10.1097/WAD.0b013e31805c0835. doi: 10.1097/WAD.0b013e31805c0835. PubMed PMID: 17545733. [DOI] [PubMed] [Google Scholar]