Abstract
Background
The impact of impaired vision on cognitive and psychosocial outcomes among long-term survivors of childhood low-grade gliomas has not been investigated previously, but could inform therapeutic decision-making.
Methods
Data from the Childhood Cancer Survivor Study was used to investigate psychological (measures of cognitive/emotional function) and socioeconomic (education, income, employment, marital status, independent living) outcomes among astroglial tumors survivors grouped by: (a) vision without impairment, (b) vision with impairment including unilateral blindness, visual field deficits or amblyopia, or (c) bilateral blindness. The effect of vision status on outcomes was examined using multivariable logistic regression, adjusting for age, gender, cranial radiation therapy and medical comorbidities.
Results
Among 1,233 survivors of childhood astroglial tumor ≥ 5 years post-diagnosis, 277 (22.5%) had visual impairment. In multivariable analysis, survivors with bilateral blindness were more likely to be unmarried (adjusted odds ratio [95% confidence interval]: 4.7 [1.5, 15.0]), live with a caregiver (3.1 [1.3, 7.5]), and be unemployed (2.2 [1.1, 4.5]) compared to those without visual impairment. Bilateral blindness had no measureable effect on cognitive or emotional outcomes, and vision with impairment was not significantly associated with any psychological or socio-economic outcomes.
Conclusions
Adult survivors of childhood astroglial tumors with bilateral blindness are more likely to live unmarried and dependently and be unemployed. Survivors with visual impairment but some remaining vision did not differ significantly with regard to psychological function and socioeconomic status from those without visual impairment.
Keywords: pediatric glioma, vision loss, late effects, childhood cancer survivors, optic pathway glioma
Introduction
Astroglial tumors are the most common brain tumor in children5 and can result in rapid vision loss by involvement of the visual pathways (as optic pathway gliomas, OPGs) or compression of visual circuits. In contrast to high grade malignancies, low grade astroglial tumors are associated with prolonged patient survival,6 even in cases where surgical resection is not practical.7 Therefore, preserving vision remains a high priority for both caregivers and families of individuals with astroglial tumors that threaten vision.8 Fortunately, only a portion of tumors that directly involve the optic pathway will progress and/or result in visual deficits.9, 10 However, identifying early vision loss can be challenging in children.11 Treatments such as chemotherapy and radiation therapy can expose children to risks of serious infection, life-threatening allergic reaction, as well as neurologic or endocrine dysfunction.12-14 Deciding whether and when to start therapy for low-grade gliomas affecting vision is a common clinical dilemma. We hypothesize that vision loss during childhood may affect academic and social development, and impair adult outcomes such as quality of life, emotional health, independence and financial security. Compared to adult-onset vision loss, childhood vision loss due to CNS tumors may cause disproportionate deficits in psychological and socio-economic outcomes due to a greater number of “blind years” as well as neurologic comorbidities, secondary to neurofibromatosis type 1 and the effect of treatment on a developing brain.15 Alternatively, early adaptation leading to neural reorganization and ready access to social supports may help ameliorate the impact of visual impairments.16
To better advise patients with progressive astroglial tumors that threaten their vision regarding the risks and benefits of therapy, we must first understand the long-term effects of vision loss in the pediatric population. The purpose of this study was to compare the psychological and socioeconomic late outcomes in long-term survivors of childhood astroglial tumors with and without vision loss.
Methods
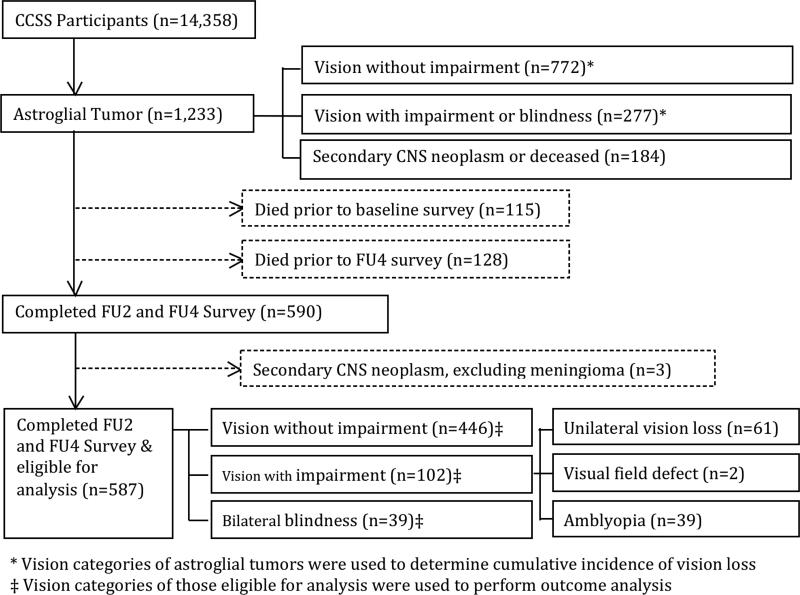
The CCSS is a multi-institutional retrospective study of individuals who survived at least 5 years after diagnosis of cancer.17-19 Participants in the CCSS cohort were younger than 21 years old when diagnosed between January 1, 1970 and December 31, 1986. No additional age limitations were placed for this analysis. The current analyses were limited to survivors of astroglial tumors. Cumulative incidence of vision loss was measured in all survivors (N= 1233), while analyses involving the impact of impaired vision on psychological or socioeconomic outcomes were limited to those who completed relevant questions from the Follow-up 2 (FU2; 2002-2005) and Follow-up 4 (FU4; 2007-2010) surveys (N= 587, Figure 1). Among 1233 astroglial survivors, 115 (9.3%) died prior to completing the baseline survey, and 128 (10.3%) died before the FU4 survey. Survivors who developed a second malignant neoplasm of the CNS were excluded from analyses (i.e., diagnosis of meningioma did not exclude a survivor). Institutional review board approval was obtained. Participants or legal guardians provided informed consent.
Figure 1.
Consort diagram of study participants with astroglial tumors.
Cumulative vision loss in all survivors of astroglial tumors was derived from answers to the baseline survey. Vision loss was defined as any positive response when respondents were asked if they have ever been told by a doctor or other health care professional that they are “legally blind in one or both eyes,” “have problems with double vision,” “any other problems with seeing with one or both eyes even when wearing glasses,” or if they described amblyopia or visual field deficits when asked about “other eye problems.” Vision impairment for multivariate analysis was defined categorically from the FU4 survey as (a) vision without impairment, (b) vision with impairment (including amblyopia, visual field deficits or unilateral blindness) or (c) bilateral blindness, as reported by survivors or their proxies. Vision with impairment was defined similar to above as “legally blind in only one eye”, “lazy eye (amblyopia)” or “any other trouble seeing with one or both eyes even when wearing glasses” or if respondents described visual field deficits. Bilateral blindness was defined as a positive response to the question of whether respondents had been told they are “legally blind in both eyes.”
Primary outcome variables included psychological and socioeconomic outcomes. Psychological outcomes utilized information from the FU2 survey and were derived from components of the Medical Outcomes Survey Short Form-36 (SF-36), the Brief Symptom Inventory-18 (BSI-18), the Cantril Ladder of Life and the CCSS neurocognitive questionnaire (NCQ). The SF-36 measured patient reported health outcomes and health-related quality of life over the last 4 weeks, including both physical and mental components.20 The BSI-18 was used to measure psychological distress and emotional health among survivors. This inventory includes a summary scale (global distress index) and three subscales (depression, anxiety, and somatization) and has been previously used in cancer survivor cohorts.21, 22 The Cantril Ladder of Life was used to measure life satisfaction among cancer survivors. Respondents rated their current lives on a 10-point scale ranging from “best possible life” to “worst possible life.”23 This global rating of life satisfaction has been used in previous studies of survivors of adult and childhood cancer.24, 25 Neurocognitive outcomes were assessed using the CCSS-NCQ, including subscales that measure task efficiency, emotional regulation, organization and memory. This scale was developed and validated in adult survivors of childhood cancer to assess neurocognitive impairment including executive dysfunction.26 Psychological impairment was determined in reference to standard norms and defined independently on each scale as follows: SF-36 T < 40; BSI-18 percentile > 10th; CCSS-NCQ percentile < 10th. The Cantril Ladder of Life (present) was considered impaired if subjects rated their current life satisfaction as less than 7 on a 10-point scale.
Socioeconomic outcomes included marital status, living independently, employment, income and education. Subjects were dichotomized as never married or married (including living as married, widowed or divorced). Living independently was defined as living alone or with a spouse/partner. Employment was defined as working full or part-time, and income was dichotomized as greater than or less than $20,000 per year. Education was dichotomized as no college attendance or some college attendance with or without a college degree. Socioeconomic and psychological measures were categorized consistent with previous reports to place outcomes of vision loss in the context of other late effects found in survivors of childhood brain tumors.
Potential covariates investigated for their effect on primary outcomes included age at diagnosis, age at survey, gender, history of cranial radiation therapy (CRT; none, ≤30Gy CRT, >30Gy CRT), presence of a meningioma, chronic conditions (coded according to Common Toxicity Criteria for Adverse Events27 and defined as any grade 3 or 4 medical condition, except vision impairment, occurring before 2004). Because proxy reporting may misclassify psychological and socioeconomic outcomes, a sensitivity analysis was performed to examine the effect of proxy reporting during the FU2 survey. Final models were reported without a variable for proxy reporting. Among survivors with vision loss, age at vision loss was dichotomized as <6 years and ≥6 years because this represented a meaningful division (age at school entry) and conveniently presented equal proportions of subjects in either group. In an additional analysis, age at interview was replaced by time from diagnosis. No significant changes were found in our multivariable model, and our final model includes age at interview.
Summary statistics were constructed using frequencies and proportions for categorical data elements and means and medians for continuous variables. Frequency distributions were examined to categorize relevant covariates according to reasonable groupings and consistent with previous CCSS manuscripts. Univariate logistic regression was used to evaluate associations between each outcome variable and visual function as well as for candidate covariates listed above. Covariates with a p-value of <0.20 for a univariate association were included in the multivariable models for the relevant outcome. Multivariable logistic regression analyses were used to examine associations between vision status and each outcome, controlling for covariates selected as indicated above. Results are presented as odds ratios (OR) with 95% confidence intervals (CI). SAS version 9.3 (Cary, N.C.) was used for analysis.
Results
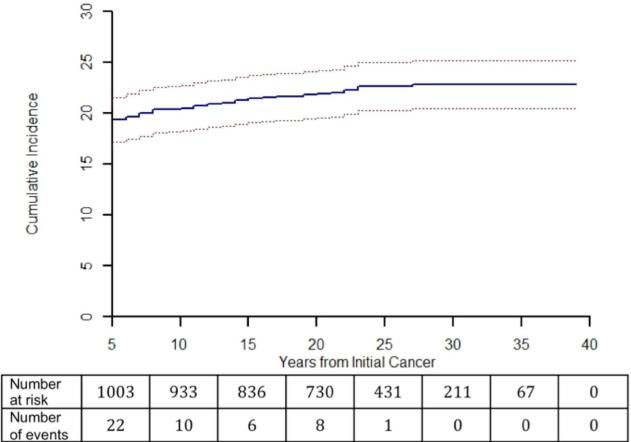
Among astroglial survivors, 277 (22.5%) had vision with impairment and 47 (3.8%) were bilaterally blind. The vast majority of vision impairment occurred within 5 years of diagnosis (Figure 2).
Figure 2.
Cumulative incidence of first reported vision loss vs. years from initial diagnosis in survivors of childhood astroglial tumors. Number of survivors at risk and number of events shown for each five year period.
Of the 1,233 survivors of astroglial tumors in the CCSS cohort identified at baseline survey, 646 were excluded from further analysis of psychological and socioeconomic outcomes due either to second CNS malignancies or lack of follow-up data. Evaluable survivors included in the analyses were more likely to be female, and less likely to have been exposed to chemotherapy or radiation (Table 1). Survivors with impaired vision were diagnosed at a younger age, were less likely to have undergone surgery and more likely to have used proxy reporting compared to survivors without vision impairment (Table 2).
Table 1.
Characteristics of survivors of childhood astroglial tumors who are and are not evaluable for study
| Characteristic | Evaluable for late outcomes (n=587) | Not evaluable for late outcomes (n=646) | P value |
|---|---|---|---|
| Age at baseline, years (sd) | 23.79 (7.30) | 23.26 (7.52) | 0.21 |
| Sex [n (%)] | |||
| Male | 285 (48.6) | 379 (58.7) | <0.001 |
| Female | 302 (51.4) | 267 (41.3) | |
| Age at diagnosis [n (%)] | |||
| ≤4 years | 210 (35.8) | 226 (35.0) | 0.34 |
| 5-9 years | 134 (22.8) | 129 (20.0) | |
| ≥10 years | 243 (41.4) | 291 (45.0) | |
| Treatment* | |||
| Surgery [n (%)] | |||
| Yes | 538 (98.0) | 527 (96.9) | 0.26 |
| No | 11 (2.0) | 17 (3.1) | |
| Chemotherapy [n (%)] | |||
| Yes | 87 (15.8) | 133 (24.4) | <0.001 |
| No | 463 (84.2) | 412 (75.6) | |
| Radiation [n (%)] | |||
| Yes | 324 (58.9) | 352 (64.8) | 0.046 |
| No | 226 (41.1) | 191 (35.2) | |
| Age at first vision problem [n (%)] | |||
| </=6 years | 73 (51.8) | 75 (57.6) | 0.39 |
| >6 years | 68 (48.2) | 56 (42.4) | |
| Vision status at baseline | |||
| Blind in one/both eye | 91 (15.8) | 126 (20.0) | 0.09 |
| No vision loss | 486 (84.2) | 503 (80.0) | |
38 evaluable survivors of astroglial tumors had missing treatment information (38 missing information on surgery, 37 missing information on radiation and chemotherapy), 103 inevaluable survivors had missing treatment information (102 missing information on surgery, 101 missing information on chemotherapy, 103 missing information on radiation).
Table 2.
Characteristics of survivors of childhood astroglial tumors eligible for study by vision status
| Characteristic | Vision without impairment (n=446) | Vision with impairment (n=102) | Bilateral blindness (n=39) | P-value |
|---|---|---|---|---|
| Age at Interview [n (%)] | ||||
| <30 years | 186 (41.7) | 58 (56.9) | 20 (51.3) | 0.08 |
| 30-39 years | 205 (46.0) | 34 (33.3) | 16 (41.0) | |
| ≥40 years | 55 (12.3) | 10 (9.8) | 3 (7.7) | |
| Sex [n (%)] | ||||
| Male | 224 (50.2) | 43 (42.2) | 18 (46.2) | 0.32 |
| Female | 222 (49.8) | 59 (57.8) | 21 (53.8) | |
| Age at diagnosis [n (%)] | ||||
| ≤4 years | 138 (30.9) | 54 (52.9) | 18 (46.2) | <0.001 |
| 5-9 years | 105 (23.5) | 18 (17.6) | 11 (28.2) | |
| ≥10 years | 203 (45.5) | 30 (29.4) | 10 (25.6) | |
| Treatment* | ||||
| Surgery [n (%)] | ||||
| Yes | 413 (98.8) | 91 (96.8) | 34 (91.9) | 0.03 |
| No | 5 (1.2) | 3 (3.2) | 3 (8.1) | |
| Chemotherapy [n (%)] | ||||
| Yes | 63 (15.0) | 14 (14.9) | 10 (27.0) | 0.17 |
| No | 356 (85.0) | 80 (85.1) | 27 (73.0) | |
| Radiation [n (%)] | ||||
| Yes | 246 (58.7) | 50 (53.2) | 28 (75.7) | 0.06 |
| No | 173 (41.3) | 44 (46.8) | 9 (24.3) | |
| Age at first vision problem | ||||
| ≤6 years | NA | 56 (54.9) | 17 (43.6) | 0.26 |
| >6 years | NA | 46 (45.1) | 22 (56.4) | |
| Proxy reporting* | ||||
| Yes | 88 (20.0) | 22 (21.8) | 16 (41.0) | 0.01 |
| No | 351 (80.0) | 79 (78.2) | 23 (59.0) | |
38 survivors of astroglial tumors have no information on surgery (28 with no vision loss, 8 with some vision loss, 2 with bilateral vision loss). 37 survivors of astroglial tumors have no information on chemotherapy or radiation (27 with no vision loss, 8 with some vision loss, 2 with bilateral vision loss). 8 survivors of astroglial tumor did not answer the question for the proxy reporting (7 with no vision loss, 1 with some vision loss).
In univariate analysis, survivors with bilateral blindness were more likely to never marry, live dependently, and/or not attend college and to demonstrate impairment on the Task Efficiency subscale of the NCQ (Table 3) when compared to those without vision impairment. They also demonstrated a trend (p=0.06) toward impairment in the physical component of the SF-36 and employment status compared to those without vision impairment. Impaired vision was not associated with measures of psychological distress (BSI-18), mental health-related quality of life (SF-36, mental component) or life satisfaction (Cantril Ladder of Life).
Table 3.
Univariate comparison of psychological and socioeconomic outcomes among survivors of childhood astroglial tumors by vision status
| Outcome | Vision Category | p value | ||||
|---|---|---|---|---|---|---|
| Vision without impairment | Vision with impairment | Bilateral blindness | ||||
| Psychological | Short Form-36 | |||||
| Physical | impaired | 57 | 11 | 10 | 0.06 | |
| not impaired | 301 | 70 | 21 | |||
| OR (95%CI) | reference | 0.83(0.41,1.66) | 2.52(1.13,5.62) | |||
| Mental | impaired | 70 | 18 | 4 | 0.55 | |
| not impaired | 288 | 63 | 27 | |||
| OR (95%CI) | reference | 1.18(0.66,2.11) | 0.61(0.21,1.80) | |||
| Brief Symptom Inventory-18 | ||||||
| GDI | impaired | 47 | 12 | 4 | 0.97 | |
| not impaired | 326 | 77 | 27 | |||
| OR (95%CI) | reference | 1.08(0.55,2.14) | 1.03(0.34,3.07) | |||
| Depression | impaired | 60 | 17 | 4 | 0.70 | |
| not impaired | 313 | 72 | 27 | |||
| OR (95%CI) | reference | 1.23(0.68,2.24) | 0.77(0.26,2.29) | |||
| Anxiety | impaired | 36 | 7 | 3 | 0.85 | |
| not impaired | 337 | 82 | 28 | |||
| OR (95%CI) | reference | 0.80(0.34,1.86) | 1.00(0.29,3.46) | |||
| Somatization | impaired | 48 | 14 | 6 | 0.43 | |
| not impaired | 325 | 75 | 25 | |||
| OR (95%CI) | reference | 1.26(0.66,2.41) | 1.63(0.63,4.17) | |||
| Cantril Ladder of Life | ||||||
| Life Satisfaction (present) | impaired | 129 | 25 | 8 | 0.46 | |
| not impaired | 225 | 55 | 22 | |||
| OR (95%CI) | reference | 0.79(0.47,1.33) | 0.63(0.28,1.47) | |||
| NeuroCognitive Questionnaire | ||||||
| Task Efficiency | impaired | 133 | 40 | 16 | 0.049 | |
| not impaired | 209 | 40 | 12 | |||
| OR (95%CI) | reference | 1.57(0.96,2.56) | 2.10(0.96,4.57) | |||
| Emotional Regulation | impaired | 48 | 12 | 6 | 0.56 | |
| not impaired | 307 | 68 | 24 | |||
| OR (95%CI) | reference | 1.13(0.57,2.24) | 1.60(0.62,4.11) | |||
| Organization | impaired | 71 | 17 | 7 | 0.86 | |
| not impaired | 283 | 65 | 23 | |||
| OR (95%CI) | reference | 1.04(0.58,1.89) | 1.21(0.50,2.94) | |||
| Memory | impaired | 91 | 21 | 10 | 0.67 | |
| not impaired | 259 | 60 | 20 | |||
| OR (95%CI) | reference | 1.00(0.57,1.73) | 1.42(0.64,3.15) | |||
| Socioeconomic | Marital Status | |||||
| Never Married | 275 | 70 | 34 | <0.001 | ||
| Married | 170 | 31 | 4 | |||
| OR (95% CI) | reference | 1.40(0.88,2.22) | 5.26(1.83,15.07) | |||
| Living Arrangement | ||||||
| Living Dependently | 212 | 57 | 31 | <0.001 | ||
| Living Independently | 234 | 45 | 8 | |||
| OR (95% CI) | reference | 1.40(0.91,2.16) | 4.28(1.92,9.51) | |||
| Employment Status | ||||||
| Unemployed | 166 | 43 | 22 | 0.05 | ||
| Employed | 280 | 59 | 17 | |||
| OR (95% CI) | reference | 1.23(0.79,1.90) | 2.18(1.13,4.23) | |||
| Income | ||||||
| Income <=$20,000 | 269 | 68 | 27 | 0.11 | ||
| Income >$20,000 | 158 | 28 | 8 | |||
| OR (95% CI) | reference | 1.43(0.88,2.31) | 1.98(0.88,4.47) | |||
| Education | ||||||
| Less than College | 149 | 35 | 22 | 0.02 | ||
| College | 290 | 64 | 16 | |||
| OR (95% CI) | reference | 1.06(0.67,1.68) | 2.68(1.36,5.25) | |||
OR = odds ratio, 95%CI = 95% confidence interval, GDI = Global Disability Index. Bold text indicates statistical significance (p<0.05)
Table 4 displays the associations between impaired vision and each psychological and socioeconomic outcome, adjusting for age, gender, prior CRT, and chronic health conditions. Survivors with bilateral blindness were more likely to be unmarried (OR 4.74 [1.49, 15.00]), live dependently (OR 3.12 [1.30, 7.48]) and be unemployed (OR 2.17 [1.06, 4.46]) compared to those without vision impairment. Survivors with bilateral blindness may be less likely to attend college (OR 2.05 [0.99, 4.23]), but this did not achieve statistical significance. The multivariable analysis was repeated to include proxy reporting as a potential covariate. This model demonstrated that bilateral blindness was associated with similar late effects as those shown above, including being unmarried (OR 3.94 [1.22, 12.74]) and living dependently (OR 2.98 [1.23, 7.21]) (full model not shown). Impaired vision other than bilateral blindness was not associated with any of the psychological or socioeconomic outcomes. In survivors with visual impairment, there was no effect of age at first onset of visual impairment on psychological or socioeconomic outcomes (data not shown).
Table 4.
Multivariate analysis reporting odds ratio (95%CI) for associations between psychological and socioeconomic outcomes and vision status among survivors of childhood astroglial tumor.
| Psychological Status | Socioeconomic Status | ||||||
|---|---|---|---|---|---|---|---|
| SF-36 Physical | Task Efficiency | Not married | Live dependently | Not employed | Income ≤$20,000 | Education < College | |
| Number of Analyzed Subjects (Total N=587) | 436 | 419 | 530 | 533 | 533 | 510 | 525 |
| Vision Status | |||||||
| Vision without impairment | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Vision with impairment | 0.81(0.38,1.74) | 1.65(0.97,2.81) | 1.19(0.68,2.10) | 1.15(0.70,1.90) | 1.29(0.79,2.09) | 1.17(0.67,2.02) | 0.93(0.56,1.55) |
| Bilateral Vision Loss | 2.02(0.85,4.84) | 1.71(0.74,3.93) | 4.74(1.49,15.00) | 3.12(1.30,7.48) | 2.17(1.06,4.46) | 1.58(0.61,4.08) | 2.05(0.99,4.23) |
| Age at Diagnosis | |||||||
| >/=10 | * | * | * | Reference | * | Reference | Reference |
| 5-9 | 1.23(0.75,2.03) | 0.57(0.34,0.98) | 1.41(0.86,2.30) | ||||
| </=4 | 1.97(1.19,3.27) | 1.32(0.75,2.29) | 2.01(1.29,3.12) | ||||
| Age at Interview, years | |||||||
| >=40 | * | * | Reference | Reference | * | Reference | * |
| 30-39 | 2.49(1.31,4.72) | 1.62(0.83,3.16) | 1.81(0.96,3.42) | ||||
| <30 | 21.49(10.43,44.26) | 4.40(2.12,9.16) | 5.10(2.45,10.63) | ||||
| Sex | |||||||
| Male | * | * | Reference | * | Reference | * | * |
| Female | 0.47(0.30,0.72) | 1.68(1.16,2.44) | |||||
| Cranial Radiation | |||||||
| None | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| ≤30Gy | 13.18(3.29,52.73) | 0.29(0.06,1.43) | 0.90(0.27,3.04) | 0.98(0.31,3.06) | 2.41(0.78,7.46) | 1.17(0.35,3.88) | 0.53(0.14,1.98) |
| >30Gy | 1.89(1.01,3.55) | 2.06(1.34,3.18) | 2.19(1.39,3.45) | 1.83(1.23,2.73) | 1.74(1.17,2.59) | 1.87(1.23,2.85) | 2.05(1.37,3.06) |
| Medical Comorbidity (Grade 3-5) | |||||||
| No | Reference | Reference | Reference | Ref. | Ref. | Reference | Reference |
| Yes | 5.36(2.92,9.87) | 2.50(1.63,3.85) | 1.85(1.17,2.95) | 1.83(1.22,2.73) | 2.83(1.92,4.15) | 2.67(1.71,4.17) | 1.84(1.25,2.72) |
Variable does not contribute to the overall multivariable model
Discussion
This analysis suggests that bilateral blindness may be an important determinant of marital status, independent living and employment among adult survivors of childhood astroglial tumors. Our results also suggest that survivors with some visual impairment were not significantly different from those without visual impairment. Given this treatment era, when few patients diagnosed with high-grade astroglial tumors (such as anaplastic astrocytoma and glioblastoma multiforme) survived five years from diagnosis, these findings are most directly relevant to aging survivors of low grade gliomas such as optic pathway gliomas (OPGs).28, 29
OPGs are the most common astroglial tumors to threaten vision. These tumors have a very high overall survival rate,29 but can cause some vision impairment in up to half of affected patients.30 Up to 70% of OPGs are associated with NF1,31 and NF1-associated tumors are often diagnosed at an early age and rarely cause new vision loss after the age of 10 years.32 In our cohort, 72% of astroglial survivors with impaired vision or bilateral blindness were diagnosed before 10 years of age, compared to 54% of those with unimpaired vision. Only 10% of the cohort died before FU4 survey, compared to 18% of all CNS tumor survivors in the CCSS,33 suggesting a low overall late mortality consistent with low grade tumors. In addition, the median age at first visual deficit was 6 years, and few late visual deficits occurred (Figure 2), suggesting that the cohort with impaired vision is likely enriched for NF1-associated optic pathway tumors.
Few studies have examined the impact of tumor-associated vision loss. In 36 children with OPG, vision loss was associated with decreased vision-specific quality of life, and bilateral vision impairment was associated with greater difficulty with social interactions and pleasurable activities by parent report.1 A small series of children with visual impairment not associated with CNS tumor (N=24, mean age 10.13+/−2.89) reported diminished vision-specific quality of life and that extent of visual impairment correlated with decreased quality of life measures.2 In British birth cohort studies, all-cause visual impairment among adults has been associated with higher rates of unemployment (OR [95% CI]: 4.6 [2.7 – 8.0]) and lower socioeconomic status (1.9 [1.3 – 2.7]).3, 4 These results are consistent with our findings that demonstrate an association between marital status, independent living and employment with bilateral blindness but not with vision with some impairment.
Although our study demonstrates an association between bilateral blindness in adult survivors of childhood astroglial tumors and certain socio-economic outcomes, it is important to note that many outcomes were unaffected by childhood vision loss. Adult survivors with childhood blindness failed to show any significant psychological distress, neurocognitive impairment or income deficit. Survivors with some remaining vision showed no significant impairment in any measured outcome. This lack of effect is seen, despite our sample of those with impaired vision or bilateral blindness being likely enriched for subjects with neurofibromatosis type 1, who frequently have learning differences and attention disorders that may hinder educational and employment opportunities.15, 34 It is impossible to directly compare vision-specific quality of life from other studies of children with tumor-related vision loss to measures in this study; however, adult survivors with childhood vision loss showed no mental or physical impairment on the SF-36 or decreased life satisfaction on the Cantril Ladder of Life compared to survivors of similar tumors without vision loss.
The modest long-term effects of vision loss seen in this study are consistent with recent evidence in retinoblastoma that shows few cognitive or social attainment deficits in adult survivors with vision loss.35 Studies have suggested that neural reorganization after early vision loss can ameliorate sensory deficits and may be associated with superior cognitive outcomes in subjects with early vision loss (occurring before 1 year of age),16, 35 although age at first onset of impaired vision was not associated with any adult outcomes in our study (data not shown).
Determining an optimal time to treat gliomas (such as OPGs) that threaten vision can be challenging. Traditional endpoints used to determine the need for treatment, such as radiographic progression, have not been associated with the important functional endpoint of vision loss.36-38 Recent expert opinion suggests that visual outcomes may be the most important endpoint for future clinical trials.39 However, before exposing children to the risks associated with chemotherapy or radiation, it is important to understand the impact that childhood vision loss may have on adult life. Our data suggest that tumors threatening vision in a single eye, such as solitary optic nerve gliomas, may have limited impact on adult psychological and socioeconomic outcomes.
This study represents one of the largest samples of adult survivors of astroglial tumors in the literature, suggesting that the effect size of any unrecognized association is likely small. Taken as a whole, this suggests that adult survivors of astroglial tumors adapt well to early impairment of vision, although survivors with bilateral vision loss may experience worse socioeconomic outcomes. The challenges of limited vision (including limitations in driving and difficulties with activities of daily living) should not be minimized; however, this remains promising news for children with OPG who may have permanent vision loss despite our best current therapies.
This study is subject to certain important limitations. Ten percent of astroglial survivors died before the FU4 survey, excluding them from analysis of psychological or socioeconomic outcomes. Subjects included in analysis also differed significantly from those excluded in terms of gender and exposure to chemotherapy and radiation, which may have resulted in an underestimate of negative outcomes.40, 41 Self-report may overestimate or underestimate the severity of vision loss, and initial visual symptoms may be present earlier than realized by the survivor. However, many of our findings are comparable to those reported with direct assessment.35 A significant portion of respondents used proxies to complete their surveys (41% with bilateral vision loss, 22% with some vision loss and 20% with no vision loss). Proxy reporting was not included in the main multivariable model because its strong association with vision loss may reduce the ability to find a significant association between vision loss and late outcomes. Proxy reports of observed behaviors (such as the socio-economic outcomes in this study) are generally closely correlated with self-report. Proxy reporting would have been included as a potential covariate in affective outcomes (such as depression and anxiety), which were excluded in univariate analysis. A multivariable analysis including proxy reporting demonstrates no difference in the outcomes associated with vision loss.
While our analysis investigates late effects of vision in survivors of low-grade glioma in the largest population yet published, precise tumor location and glioma subtype may predict adverse outcomes but were not available for the current study. In addition, Vision-specific quality of life was not assessed in the CCSS questionnaires and should be assessed in future studies of long-term survivors with vision loss to determine whether vision-specific quality of life deficits found in other studies persist into adulthood.1 This is especially true since more general measures of health-related quality of life (SF-36) failed to show a significant difference between survivors with and without vision loss in multivariable analysis.
Conclusions
Understanding the impact of childhood vision loss in adult survivors of childhood astroglial tumors is important as it may help guide clinical decision-making about potential therapies for astroglial tumors that threaten vision. Our study demonstrates that adult survivors of childhood astroglial tumors who are blind in both eyes are more likely to be unmarried, live dependently and be unemployed compared to survivors with unimpaired vision. Trends toward significance were also found between bilateral blindness and a lower level of attained education. However, there was no difference in psychological measures between adult survivors who were blind and those that had unimpaired vision, and survivors with vision but some impairment were not significantly different from those with normal vision in either psychological or socio-economic outcomes.
Acknowledgement
The authors would like to thank Grant T. Liu for his expertise and assistance in defining age categories for first vision loss.
Funding: National Cancer Institute (CA055727-21, GT Armstrong, PI). Additional support for PdB provided by National Institutes of Health (CA076917-15), and from the Johns Hopkins University School of Medicine and the Neurofibromatosis Therapeutic Acceleration Program (NTAP). The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of The Johns Hopkins University School of Medicine. Investigators at St. Jude Children's Research Hospital also funded by ALSAC.
Footnotes
Conflict of Interest: None
References
- 1.Avery RA, Hardy KK. Vision specific quality of life in children with optic pathway gliomas. J Neurooncol. 2014;116:341–347. doi: 10.1007/s11060-013-1300-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chadha RK, Subramanian A. The effect of visual impairment on quality of life of children aged 3-16 years. Br J Ophthalmol. 2011;95:642–645. doi: 10.1136/bjo.2010.182386. [DOI] [PubMed] [Google Scholar]
- 3.Rahi JS, Cumberland PM, Peckham CS. Visual function in working-age adults: early life influences and associations with health and social outcomes. Ophthalmology. 2009;116:1866–1871. doi: 10.1016/j.ophtha.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Rahi JS, Cumberland PM, Peckham CS. Visual impairment and vision-related quality of life in working-age adults: findings in the 1958 British birth cohort. Ophthalmology. 2009;116:270–274. doi: 10.1016/j.ophtha.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 5.Qaddoumi I, Sultan I, Gajjar A. Outcome and prognostic features in pediatric gliomas: a review of 6212 cases from the Surveillance, Epidemiology, and End Results database. Cancer. 2009;115:5761–5770. doi: 10.1002/cncr.24663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sievert AJ, Fisher MJ. Pediatric low-grade gliomas. J Child Neurol. 2009;24:1397–1408. doi: 10.1177/0883073809342005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alvord EC, Lofton S. Gliomas of the optic nerve or chiasm: outcome by patients' age, tumor site, and treatment. J Neurosurg. 1988;68:85–98. doi: 10.3171/jns.1988.68.1.0085. [DOI] [PubMed] [Google Scholar]
- 8.Avery RA, Ferner RE, Listernick R, Fisher MJ, Gutmann DH, Liu GT. Visual acuity in children with low grade gliomas of the visual pathway: implications for patient care and clinical research. J Neurooncol. 2012;110:1–7. doi: 10.1007/s11060-012-0944-y. [DOI] [PubMed] [Google Scholar]
- 9.Czyzyk E, Jozwiak S, Roszkowski M, Schwartz RA. Optic pathway gliomas in children with and without neurofibromatosis 1. J Child Neurol. 2003;18:471–478. doi: 10.1177/08830738030180070401. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong GT, Conklin HM, Huang S, et al. Survival and long-term health and cognitive outcomes after low-grade glioma. Neuro Oncol. 2011;13:223–234. doi: 10.1093/neuonc/noq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pilling RF, Lloyd IC, Huson S. Utility of optic pathway glioma screening in young children with neurofibromatosis type I: questions generated by a clinical audit. Eye (Lond) 2010;24:1603–1605. doi: 10.1038/eye.2010.99. [DOI] [PubMed] [Google Scholar]
- 12.Armstrong GT, Liu Q, Yasui Y, et al. Long-term outcomes among adult survivors of childhood central nervous system malignancies in the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2009;101:946–958. doi: 10.1093/jnci/djp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowers DC, Liu Y, Leisenring W, et al. Late-occurring stroke among long-term survivors of childhood leukemia and brain tumors: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2006;24:5277–5282. doi: 10.1200/JCO.2006.07.2884. [DOI] [PubMed] [Google Scholar]
- 14.Ater JL, Zhou T, Holmes E, et al. Randomized study of two chemotherapy regimens for treatment of low-grade glioma in young children: a report from the Children's Oncology Group. J Clin Oncol. 2012;30:2641–2647. doi: 10.1200/JCO.2011.36.6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hyman SL, Shores A, North KN. The nature and frequency of cognitive deficits in children with neurofibromatosis type 1. Neurology. 2005;65:1037–1044. doi: 10.1212/01.wnl.0000179303.72345.ce. [DOI] [PubMed] [Google Scholar]
- 16.Merabet LB, Pascual-Leone A. Neural reorganization following sensory loss: the opportunity of change. Nat Rev Neurosci. 2010;11:44–52. doi: 10.1038/nrn2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27:2308–2318. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robison LL, Mertens AC, Boice JD, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: a multi-institutional collaborative project. Med Pediatr Oncol. 2002;38:229–239. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 19.Leisenring WM, Mertens AC, Armstrong GT, et al. Pediatric cancer survivorship research: experience of the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2319–2327. doi: 10.1200/JCO.2008.21.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ware JE, Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 21.Recklitis CJ, Parsons SK, Shih MC, Mertens A, Robison LL, Zeltzer L. Factor structure of the brief symptom inventory--18 in adult survivors of childhood cancer: results from the childhood cancer survivor study. Psychol Assess. 2006;18:22–32. doi: 10.1037/1040-3590.18.1.22. [DOI] [PubMed] [Google Scholar]
- 22.Zeltzer LK, Lu Q, Leisenring W, et al. Psychosocial outcomes and health-related quality of life in adult childhood cancer survivors: a report from the childhood cancer survivor study. Cancer Epidemiol Biomarkers Prev. 2008;17:435–446. doi: 10.1158/1055-9965.EPI-07-2541. [DOI] [PubMed] [Google Scholar]
- 23.Cantril H. The pattern of human concerns. Rutgers University Press; New Brunswick (NJ): 1965. [Google Scholar]
- 24.Ganz PA, Desmond KA, Leedham B, Rowland JH, Meyerowitz BE, Belin TR. Quality of life in long-term, disease-free survivors of breast cancer: a follow-up study. J Natl Cancer Inst. 2002;94:39–49. doi: 10.1093/jnci/94.1.39. [DOI] [PubMed] [Google Scholar]
- 25.Zeltzer LK, Recklitis C, Buchbinder D, et al. Psychological status in childhood cancer survivors: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2396–2404. doi: 10.1200/JCO.2008.21.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krull KR, Gioia G, Ness KK, et al. Reliability and validity of the Childhood Cancer Survivor Study Neurocognitive Questionnaire. Cancer. 2008;113:2188–2197. doi: 10.1002/cncr.23809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrell FE. Regression Modeling Strategies With Applications to Linear Models, Logistic Regression and Survival Analysis. Springer; New York: 2001. [Google Scholar]
- 28.Broniscer A. Past, present, and future strategies in the treatment of high-grade glioma in children. Cancer Invest. 2006;24:77–81. doi: 10.1080/07357900500449702. [DOI] [PubMed] [Google Scholar]
- 29.Ostrom QT, Gittleman H, Farah P, et al. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro Oncol. 2013;15(Suppl 2):ii1–56. doi: 10.1093/neuonc/not151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Listernick R, Ferner RE, Liu GT, Gutmann DH. Optic pathway gliomas in neurofibromatosis-1: controversies and recommendations. Ann Neurol. 2007;61:189–198. doi: 10.1002/ana.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Listernick R, Louis DN, Packer RJ, Gutmann DH. Optic pathway gliomas in children with neurofibromatosis 1: consensus statement from the NF1 optic pathway glioma task force. Annals of Neurology. 1997;41:143–149. doi: 10.1002/ana.410410204. [DOI] [PubMed] [Google Scholar]
- 32.King A, Listernick R, Charrow J, Piersall L, Gutmann DH. Optic pathway gliomas in neurofibromatosis type 1: the effect of presenting symptoms on outcome. Am J Med Genet A. 2003;122A:95–99. doi: 10.1002/ajmg.a.20211. [DOI] [PubMed] [Google Scholar]
- 33.Armstrong GT, Kawashima T, Leisenring W, et al. Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study. J Clin Oncol. 2014;32:1218–1227. doi: 10.1200/JCO.2013.51.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hersh JH. Health supervision for children with neurofibromatosis. Pediatrics. 2008;121:633–642. doi: 10.1542/peds.2007-3364. [DOI] [PubMed] [Google Scholar]
- 35.Brinkman TM, Merchant TE, Li Z, et al. Cognitive function and social attainment in adult survivors of retinoblastoma: A report from the St. Jude Lifetime Cohort Study. Cancer. 2014 doi: 10.1002/cncr.28924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Campagna M, Opocher E, Viscardi E, et al. Optic pathway glioma: long-term visual outcome in children without neurofibromatosis type-1. Pediatr Blood Cancer. 2010;55:1083–1088. doi: 10.1002/pbc.22748. [DOI] [PubMed] [Google Scholar]
- 37.Shofty B, Ben-Sira L, Freedman S, et al. Visual outcome following chemotherapy for progressive optic pathway gliomas. Pediatr Blood Cancer. 2011;57:481–485. doi: 10.1002/pbc.22967. [DOI] [PubMed] [Google Scholar]
- 38.Fisher M, Loguidice M, Gutmann D, et al. Visual outcomes in children with neurofibromatosis type 1 associated optic pathway glioma following chemotherapy: a multi-center retrospective analysis. NANOS. 2010 doi: 10.1093/neuonc/nos076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fisher MJ, Avery RA, Allen JC, et al. Functional outcome measures for NF1-associated optic pathway glioma clinical trials. Neurology. 2013;81:S15–24. doi: 10.1212/01.wnl.0000435745.95155.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whelan KF, Stratton K, Kawashima T, et al. Ocular late effects in childhood and adolescent cancer survivors: a report from the childhood cancer survivor study. Pediatr Blood Cancer. 2010;54:103–109. doi: 10.1002/pbc.22277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Packer RJ, Gurney JG, Punyko JA, et al. Long-term neurologic and neurosensory sequelae in adult survivors of a childhood brain tumor: childhood cancer survivor study. J Clin Oncol. 2003;21:3255–3261. doi: 10.1200/JCO.2003.01.202. [DOI] [PubMed] [Google Scholar]