Abstract
Background
Resveratrol, a natural polyphenol found in the skin of red grapes, is reported to have anti-inflammatory properties including protective effects against aging. Consequently, Resveratrol is a common nutritional supplement and additive in non-prescription lotions and creams marketed as anti-aging products. Studies in mice and with mouse bone marrow-derived mast cells (BMMCs) have indicated anti-allergic effects of Resveratrol. However, the effects of Resveratrol on human primary mast cells have not been reported.
Methods
Human mast cells were isolated and purified from normal skin tissue of different donors. The effect of Resveratrol on IgE-dependent release of allergic inflammatory mediators was determined using various immunoassays, Western blotting, and quantitative real-time PCR.
Results
Resveratrol at low concentrations (≤ 10 µM) inhibited PGD2 biosynthesis but not degranulation. Accordingly, COX-2 expression was inhibited but phosphorylation of Syk, Akt, p38, and p42/44 (ERKs) remained intact. Surprisingly, TNF production was significantly enhanced with Resveratrol. At high a concentration (100 µM), Resveratrol significantly inhibited all parameters analyzed except Syk phosphorylation.
Conclusions
Here, we show that Resveratrol at low concentrations exerts its anti-inflammatory properties by preferentially targeting the arachidonic acid pathway. We also demonstrate a previously unrecognized pro-inflammatory effect of Resveratrol – the enhancement of TNF production from human mature mast cells following IgE-dependent activation.
General significance
These findings suggest that Resveratrol as a therapeutic agent could inhibit PGD2-mediated inflammation but would be ineffective against histamine-mediated allergic reactions. However, Resveratrol could potentially exacerbate or promote allergic inflammation by enhancing IgE-dependent TNF production from mast cells in human skin.
Keywords: Resveratrol, Mast cells, Allergy, Prostaglandin D2, Tumor Necrosis Factor, Inflammation
1. Introduction
Resveratrol, a stilbenoid, is a natural polyphenol found predominantly in the skin of red grapes that has been studied extensively for its potential health benefits. Numerous studies have demonstrated protective properties of Resveratrol against inflammation, cardiovascular disease, cancer, and aging [1]. Consequently, Resveratrol has gained a reputation of being an elixir in red wine, and is a popular nutritional supplement and ingredient in over-the-counter skin care products. In human skin, Resveratrol was shown to protect against sun damage, enhance moisture and elasticity, reduce wrinkle depth and intensity of age spots, and protected keratinocytes from nitrous oxide-induced death [2–5].
Mast cells are the main effector cell type of IgE-mediated allergic reactions, and are important in innate immunity to parasitic and bacterial infections [6,7]. As such, mast cells are critical regulators of inflammation in skin [8], and are associated with dermatologic pathologies such as urticaria and atopic dermatitis [9]. The causative agents of allergic reactions are preformed mediators like histamine and serine neutral proteases stored in cytoplasmic granules that are released during degranulation, lipid mediators like Prostaglandin D2 (PGD2) that are biosynthesized from arachidonic acid [10], and de novo produced cytokines and chemokines [11]. Although mast cells can be activated by different methods, allergic reactions are mostly associated with crosslinking of the high affinity receptor for IgE, FcεRI, which induces a cascade of phosphorylation-dependent events initiated and regulated by receptor-proximal src kinases and spleen tyrosine kinase (Syk) [12–17]. In humans, two subsets of mast cells have been identified and characterized according to the expression of tryptase or chymase within cytoplasmic granules [18,19]. MCTC type cells express both tryptase and chymase and are the exclusive mast cell subset in skin, whereas MCT type cells express only tryptase and predominate in the lung. In addition, MCTC mast cells express complement factor 5a receptor (C5aR) whereas MCT cells do not [20], and skin mast cells express significantly less adenosine A3AR receptor compared to lung mast cells [21]. Thus, human mature mast cells are a heterogeneous group of tissue-resident cells that can express distinct functional phenotypes whose activation can be differentially regulated.
Various studies have demonstrated that Resveratrol inhibited the release of allergic mediators from murine bone marrow-derived mast cells (BMMCs) [22–24] and protected mice against induced atopic dermatitis or asthma [25–27]. However, the direct target of Resveratrol or the mechanism by which it inhibits mast cell responses has not been identified although phospholipase Cγ1 or ERK in the FcεRI pathway have been suggested [24]. Moreover, the effect of Resveratrol on the IgE-dependent response from human mature mast cells taken directly from human tissue has not been reported. Given that Resveratrol is present in many skin lotions and creams, we investigated the effect of Resveratrol on FcεRI-induced degranulation and production of PGD2 and cytokines from human mature mast cells that were isolated and purified from normal skin tissue. Here, we report that Resveratrol at relatively low concentrations blocked expression of COX-2 and inhibited PGD2 production, but did not affect degranulation. Our study also revealed the unexpected finding that Resveratrol enhanced pro-inflammatory TNF production. Further, we show that Syk kinase is not a target of Resveratrol even at high concentrations.
2. Materials and methods
2.1 Isolation and purification of human skin mast cells
Human skin mast cells were isolated and purified from fresh surgical specimens of human skin tissue obtained from the Cooperative Human Tissue Network of the National Cancer Institute, as approved by the human studies Internal Review Board at University of South Carolina. The tissues were mechanically disrupted with surgical scissors and then digested 3 × 1 h at 37°C with collagenase type II (Worthington Biochemical, Lakewood, NJ), hyaluronidase from bovine testes, and DNase I (Sigma-Aldrich, St. Louis, MO) in HBSS wash buffer (1× HBSS, 0.04% NaHCO3, 1% fetal bovine serum, 1% HEPES, 0.1% CaCl2) containing amphotericin B and Antibiotic/Antimycotic solution. After each digestion period, the samples were filtered through 40 µm nylon cell strainers. The filtered cells were collected by centrifugation, washed and re-suspended with wash buffer. After the final digestion, the collected cells were separated on Percoll by density centrifugation. The cells at the interface of buffer and Percoll layers were collected, washed and re-suspended at 5×105 cells/ml in serum-free X-VIVO 15™ media (Lonza, Walkersville, MD) containing recombinant human stem cell factor (SCF, 100 ng/ml) (PeproTech, Rocky Hill, NJ). The cells were transferred onto 24-well plates and maintained under standard culture conditions (37°C, 5% CO2) with weekly media changes. Purity was assessed cytochemically by metachromatic staining with acidic toluidine blue. Greater than 95% purity was achieved by 6 weeks of culture, and the mast cells were used in experiments after 8 weeks.
2.2 Viability Assay
Mast cell viability was determined by MTT reduction assay using the TACS® MTT Cell Proliferation Assay from Trevigen (Gaithersburg, MD) according to the manufacturer’s instructions. Briefly, human skin mast cells were plated in triplicate at 105 cells/well of a 96-well plate in 100 µl X-VIVO media + SCF. Resveratrol or DMSO was added, and the cells were incubated for 24, 48, or 72 h under normal culture conditions (37°C, 5% CO2). MTT Reagent was added to the cells and incubated for an additional 4 h. Detergent was added and the plate was incubated overnight at room temperature. Absorbance values at 570 nm were acquired with a BioTek Synergy HT microplate reader (BioTek, Winooski, VT).
2.3 Mast cell activation
Sensitized human skin mast cells (106/ml) were activated by crosslinking FcεRI with the hapten 4-hydroxy-3-nitrophenylacetyl conjugated to bovine serum albumin at a 16:1 molar ratio (NP-BSA; Biosearch Technologies, Novato, CA) at 37°C. For sensitization, 106 cells/ml were incubated in X-VIVO 15™ media or Tyrode’s buffer (135 mM NaCl, 1 mM MgCl2, 20 mM Hepes, 5 mM KCl, 1.8 mM CaCl2, 5.6 mM glucose; pH 7.4, 0.05% bovine serum albumin) containing 1 µg/ml chimeric human anti-NP IgE (human Fc + mouse Fab) (clone JW8/1; AbD Serotec, Raleigh, NC) for 3 h at 37°C. After washing to remove unbound IgE, the mast cells were re-suspended at 106 cells/ml in X-VIVO 15™ media or Tyrode’s buffer, pre-treated with Resveratrol (Sigma-Aldrich, St. Louis, MO) or DMSO (vehicle) for 1 h at 37°C, and then activated with 100 ng/ml NP-BSA for the indicated amount of time.
2.4 β-Hexosaminidase and PGD2 assays
Human skin mast cells were activated with 100 ng/ml NP-BSA for 30 min at 37°C in Tyrode’s buffer. After the incubation period, the mast cells and buffer were separated by centrifugation (2000 rpm × 5 min), and the pelleted cells were lysed with an equal volume of 1% Triton X-100. β-Hexosaminidase activity in supernatant and cell lysate was assayed by measuring the release of p-nitrophenol from substrate p-nitrophenyl N-acetyl-β-D-glucosaminide (pNAG; Sigma-Aldrich, St. Louis, MO) as described [28,29]. In a 96-well plate, 5 µl of supernatant or lysate were mixed with 45 µl of 4mM p-Nitrophenyl N-acetyl-β-D-Glucosaminide (pNAG) in citric acid buffer (pH 4.5) and incubated for 1 h at 37°C. The reaction was stopped by adding 150 µl of 0.2 M glycine, pH 10.7. Absorbance values at 405 nm were acquired with a BioTek Synergy HT microplate reader (BioTek, Winooski, VT). Percent degranulation was calculated as percent release of β-hexosaminidase using the formula: % β-hex release = ((supernatant)/(supernatant + lysate)) × 100. PGD2 in the supernatant was measured with a commercial enzyme immunoassay kit (Cayman Chemical, Ann Arbor, MI) according to the manufacturer’s instructions.
2.5 Cytokine ELISA
Sensitized human skin mast cells were activated with 100 ng/ml NP-BSA for 3 h at 37°C in X-VIVO 15™ media containing SCF and 100 µg/ml soybean trypsin inhibitor (SBTI; Sigma-Aldrich, St. Louis, MO). After the activation period, the mast cells and media were separated by centrifugation (2000 rpm × 5 min). IL-6 or TNF in supernatant was quantified by enzyme-linked immunosorbent assay (ELISA) in a 384-well format as described [11]. Capture (purified) and detection (biotinylated) rat antibodies (BD Biosciences) used were: IL-6 (MQ2-13A5 and MQ2-39C3), TNF (MAb1 and MAb11). Serially diluted recombinant cytokine standards (BD Biosciences, San Jose, CA) were used to generate standard curves. After developing with the substrate for peroxidase 2,2’-azino-bis-3-ethylbenzthiazoline-6-sulfonic acid (ABTS; Sigma-Aldrich, St. Louis, MO), absorbance values at 405 nm were obtained and cytokine concentrations in experimental samples determined with a BioTek Synergy HT microplate reader (BioTek, Winooski, VT) and Gen5 Data Analysis Software.
2.6 Quantitative RT-PCR
Cytokine and COX gene expression was determined from sensitized human skin mast cells activated with 100 ng/ml NP-BSA for 3 h or 30 min, respectively. RNA was isolated with the RNeasy Mini Kit (Qiagen, Valencia, CA). cDNA was synthesized from 350 ng total RNA with the SuperScript™ III First-Strand Synthesis System (Life Technologies, Grand Island, NY). The kits were used according to the manufacturer’s instructions. The PCR reaction mix was composed of 2 µl of cDNA, 1 µl each of sense and antisense primers (10 µM each) and 12.5 µl of iQ SYBR® Green Supermix (Bio-Rad, Hercules, CA) in a final volume of 25 µl. A hot-start PCR protocol (95°C × 5 min, (95°C × 30 sec, 55°C × 30 sec, 72°C × 30 sec) × 35 cycles, 95°C × 1 min, 55°C × 1 min) was performed on a CFX Connect Real Time PCR Detection System (Bio-Rad, Hercules, CA). Fold change in expression was determined by the 2ΔΔCt method. The oligonucleotide primers used were (5’-3’; forward and reverse): IL-6 (AGTGAGGAACAAGCCAGAGC and AAAGCTGCGCAGAATGAGAT), TNF (GACAAGCCTGTAGCCCATGT and TTATCTCTCAGCTCCACGCC), COX-1 (TCTTGCTGTTCCTGCTCCTG and GTTGGAGCGCACTGTGAGTA), COX-2 (ACTGCTCAACACCGGAATTT and CAAGGGAGTCGGGCAATCAT), and GAPDH (CAATGACCCCTTCATTGACC and TTGATTTTGGAGGGATCTCG).
2.7 Western blotting
Whole cell lysates were prepared from sensitized human skin mast cells (106/sample) that were activated with 100 ng/ml NP-BSA for 5 min at 37°C. Protein equivalents of 5×105 cells/lane were separated by reducing SDS-PAGE and transferred onto nitrocellulose membranes. Two-color staining for Akt, p38 and p42/44 was performed using Odyssey Blocking Buffer (LI-COR Biosciences, Lincoln, NE), and Syk immunoblotting was performed with 5% non-fat dry milk in 25 mM Tris, pH7.4, 0.15 M NaCl, 0.1% Tween-20 (TNT buffer) as previously described [30]. The primary antibodies used were: rabbit polyclonal anti-p38 MAPK, mouse monoclonal anti-phospho-p38 MAPK (Thr180/Tyr182)(28B10), rabbit polyclonal anti-p44/42 (Erk 1/2), mouse monoclonal anti-phospho-p42/44 (Erk1/2) (E10), rabbit polyclonal anti-Akt, mouse monoclonal anti-Akt (Thr308)(L32A4), rabbit polyclonal antibody against total Syk (Cell Signaling Technology, Danvers, MA), and mouse monoclonal antibody against human phospho-Syk (Tyr525) (R&D Systems, Minneapolis, MN). The secondary antibodies used were goat anti-rabbit IRDye 680RD and goat anti-mouse 800CW (LI-COR Biosciences, Lincoln, NE). The blots were scanned on an Odyssey® CLx Infrared Imaging System (LI-COR Biosciences, Lincoln, NE).
2.8 Statistical Analysis
Statistical analysis was performed using GraphPad Prism version 6.0c for Mac OS X, GraphPad Software, La Jolla California USA, www.graphpad.com
3. Results
3.1 Effect of Resveratrol on viability of human skin mast cells
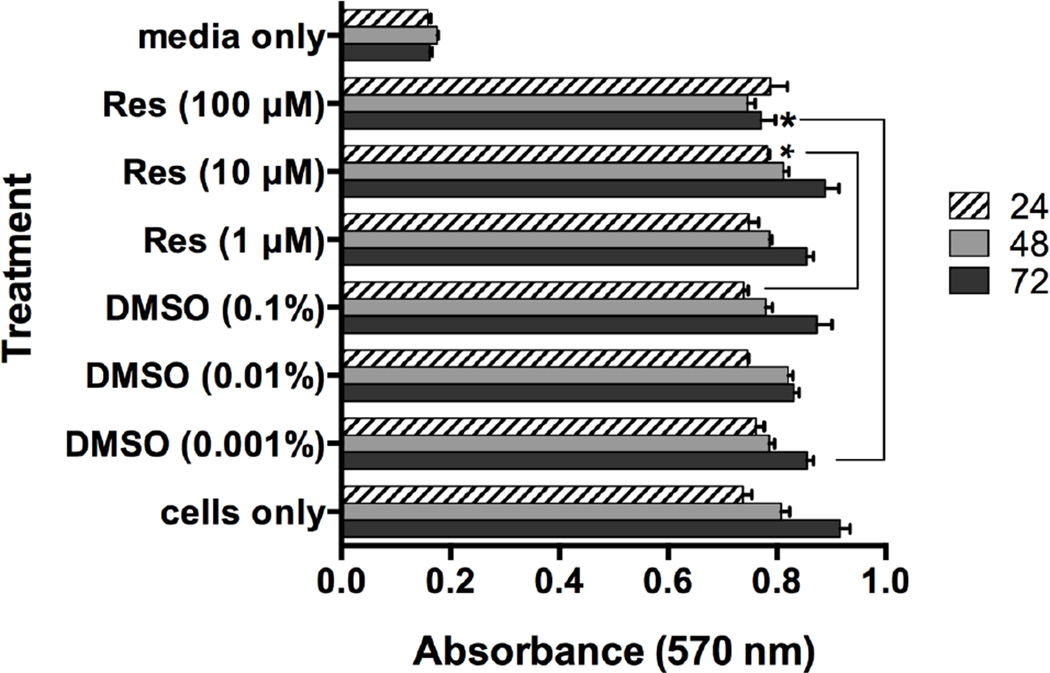
To begin our studies, we sought to determine the effect of Resveratrol on viability of human skin mast cells. Mast cells were cultured without or with 1, 10 or 100 µM Resveratrol or DMSO for up to 72 hours under normal culture conditions (37°C, 5% CO2). Cell viability was determined by MTT assay at each 24 h interval (Figure 1). No significant loss of viability was observed. In fact, a very slight but statistically significant increase in cell viability was observed with Resveratrol at 10 µM for 24 h or 100 µM for 72 h compared with vehicle control. Thus, Resveratrol did not induce cell death.
Figure 1. Effect of Resveratrol on viability of human skin mast cells.
Human skin mast cells were treated with 1, 10 or 100 µM Resveratrol or DMSO for 24, 48, or 72 h, and cell viability was determined by MTT reduction assay as described in Material and Methods. Absorbance values at 570 nm correspond to cell viability. Bars represent the mean ± SEM (n=3) of absorbance values obtained with mast cells isolated from normal skin tissue of different donors. *, p<0.05 by Student’s t-test comparing absorbance values from vehicle-treated cells to those treated with Resveratrol.
3.2 PGD2 biosynthesis is more sensitive than degranulation to inhibition by Resveratrol
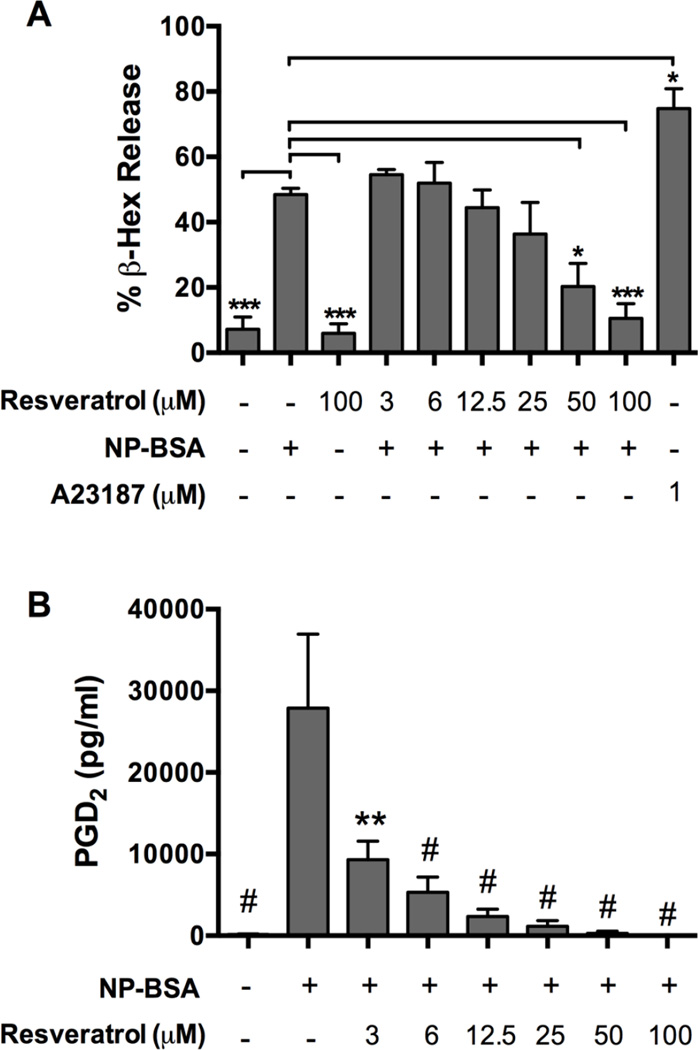
To determine the effect of Resveratrol on degranulation, human skin mast cells were sensitized with anti-NP IgE, pre-treated with a dose range of Resveratrol for 1 h then challenged for 30 min with 100 ng/ml NP-BSA. Degranulation was determined by β-hexosaminidase release assay. As shown in Figure 2A, Resveratrol dose-dependently inhibited FcεRI-induced degranulation. Mean percent degranulation ± S.E.M of control skin mast cells activated without Resveratrol was 48.5 ± 1.9% whereas that of mast cells pre-treated with Resveratrol at the indicated concentration was: 55 ± 3% (3 µM), 52 ± 6% (6 µM), 44 ± 9% (12.5 µM), 36 ± 17% (25 µM), 20 ± 12% (50 µM) and 10.5 ± 6% (100 µM). However, significant inhibition occurred only in mast cell pretreated with Resveratrol at 50 µM (p<0.05) and 100 µM (p<0.0001). Thus, FcεRI-induced degranulation of human skin mast cells is resistant to inhibition with Resveratrol at concentrations below 50 µM, but significantly inhibited with higher concentrations.
Figure 2. Effect of Resveratrol on FcεRI-induced degranulation and PGD2 production from human skin mast cells.
Dose-dependent effect of Resveratrol on Ag/IgE-induced degranulation (A) or PGD2 production (B) from human skin mast cells sensitized with chimeric human anti-NP IgE and challenged with 100 ng/ml NP-BSA. Percent degranulation was determined by β-hexosaminidase release assay. Calcium ionophore A23187 was used as a positive control for degranulation potential. Secreted PGD2 was measured by enzyme immunoassay. Data are expressed as the mean ± SEM of values obtained from independent experiments with mast cells from normal skin tissue of n=3 (A, degranulation) or n=5 (B, PGD2) different donors. Values were compared to those obtained from mast cells that were activated without Resveratrol (NP-BSA only). Statistical significance was determined by one-way ANOVA followed by Dunnett’s multiple comparisons test. *, p<0.05, **; p<0.01, ***; p<0.001; and #, p<0.0001.
Next, we determined the effect of Resveratrol on PGD2 production. IgE-sensitized skin mast cells were pre-treated with a dose range of Resveratrol for 1 h and then challenged for 30 min with 100 ng/ml NP-BSA. PGD2 in the supernatant was measured with commercial enzyme immunoassay kit. The data showed a strong inhibitory effect of Resveratrol on PGD2 biosynthesis with significant inhibition achieved at all concentrations tested (Figure 2B). Mast cells activated without Resveratrol pre-treatment produced 27,867 ± 9,099 pg/ml PGD2 whereas those pre-treated with the indicated amount of Resveratrol produced the following amount: 9,306 ± 2,276 pg/ml (3 µM), 5,325 ± 1,867 pg/ml (6 µM), 2,350 ± 914 pg/ml (12.5 µM), 1,165 ± 676 pg/ml (25 µM), 291 ± 259 pg/ml (50 µM), and 31 ± 14 pg/ml (100 µM). Thus, FcεRI-induced PGD2 biosynthesis is highly sensitive to inhibition by Resveratrol.
3.3 Resveratrol augments FcεRI-induced TNF production from human skin mast cells
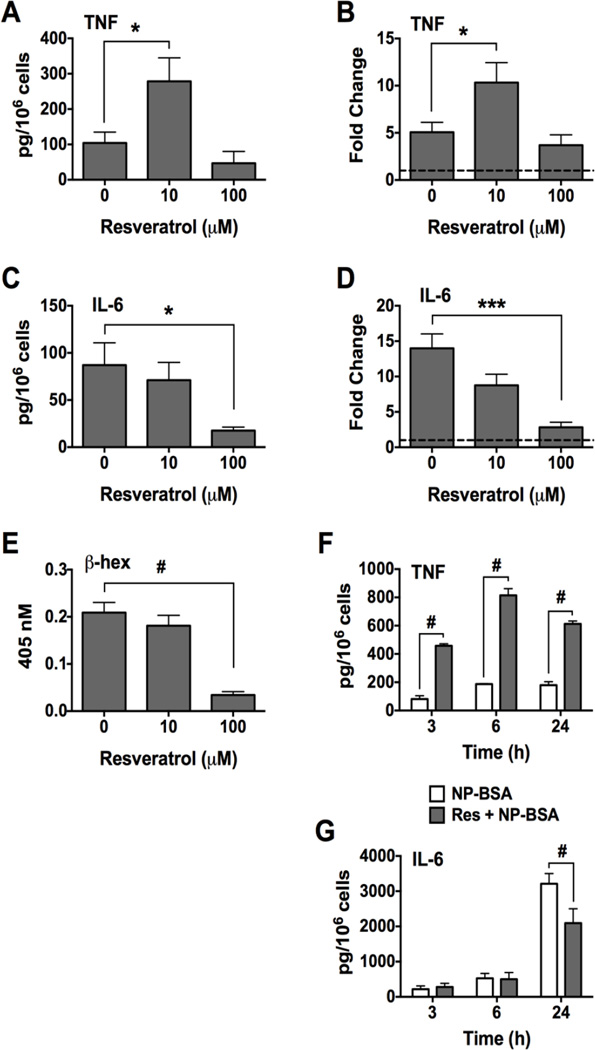
To evaluate the ability of Resveratrol to regulate pro-inflammatory cytokine production, IgE-sensitized human skin mast cells were pre-treated with 10 or 100 µM Resveratrol or DMSO for 1 h, and then challenged for 3 h with 100 ng/ml NP-BSA. Secreted IL-6 and TNF was measured using ELISA, and cytokine gene expression was determined by quantitative RT-PCR. Surprisingly, TNF production from mast cells pre-treated with 10 µM Resveratrol was significantly increased compared to that secreted from untreated mast cells (Figure 3A). Net TNF produced by control mast cells was 104 ± 31 pg/106 cells whereas mast cells pre-treated with 10 µM Resveratrol produced 279 ± 66 pg/106 cells. In accordance with increased TNF secretion, FcεRI-induced expression of TNF mRNA in Resveratrol-treated mast cells was increased 2-fold compared to non-treated activated mast cells: 5 ± 1 fold increase (non-treated) versus 10 ± 2 fold increase (Resveratrol treated) (Figure 3B). In contrast, IL-6 production was not significantly affected by 10 µM Resveratrol although some inhibition was apparent (Figure 3C and D). Thus, the enhancing effect of 10 µM Resveratrol on TNF did not extend to IL-6. At 100 µM, Resveratrol strongly inhibited IL-6 production and secretion (Figure 3C and D), but did not significantly affect TNF (Figure A and B). In time course experiments, 10 µM Resveratrol enhanced FcεRI-induced TNF production at 3, 6 and 24 h (Figure 3F), whereas IL-6 was inhibited at 24 h (Figure 3G). We also measured β-hexosaminidase activity in the sample supernatants to show that 10 µM Resveratrol had no affect on degranulation even with prolonged exposure, whereas 100 µM Resveratrol completely inhibited degranulation (Figure 3E). Thus, confirming our earlier finding (Figure 2A). These data reveal a previously unrecognized ability of Resveratrol at low concentration to enhance FcεRI-induced TNF production from human skin mast cells.
Figure 3. Effect of Resveratrol on FcεRI-induced production of TNF and IL-6 from human skin mast cells.
Human skin mast cells sensitized with anti-NP IgE were pre-treated with Resveratrol for 1 h and then challenged with antigen NP-BSA for 3 h (A – E), or 3, 6, or 24 h (F and G). Secreted cytokines in supernatant were measured by ELISA, and gene expression was assessed by quantitative RT-PCR. Fold change was determined by 2ΔΔCt method comparing to expression in non-activated mast cells (y=1, hatched line). Net secreted TNF (A, n=6) or IL-6 (C, n=5). Spontaneously released TNF was 8 ± 4 pg/106 cells, and IL-6 was 2 ± 2 pg/106 cells. Fold change in TNF (B, n=7) or IL-6 (D, n=7) gene expression. β-hexosaminidase in media (E, n=6). Time course evaluation for TNF (F, n=3) or IL-6 (G, n=3). Data shown is expressed as mean ± SEM of values from skin mast cells from different donor tissue in independent experiments. Statistical significance was determined by one-way ANOVA followed by Sidak’s multiple comparisons test. *, p<0.5; ***, p<0.001; and #, p<0.0001.
3.4 Resveratrol inhibits IgE-dependent COX-2 induction but not Syk phosphorylation
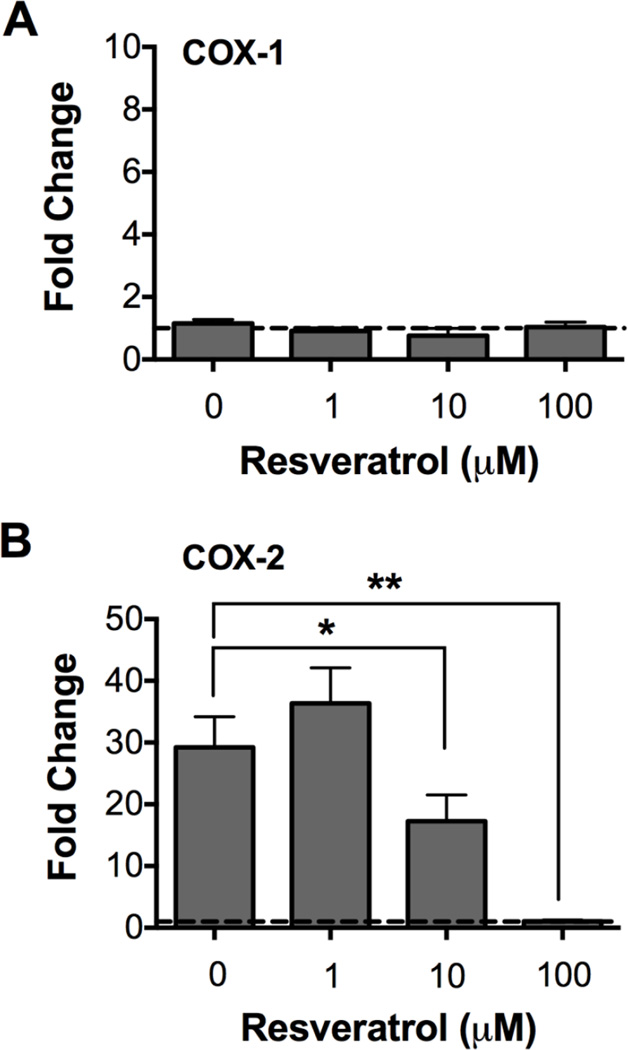
The finding that PGD2 biosynthesis was inhibited by Resveratrol at concentrations that did not affect degranulation (Figure 2) suggested that Resveratrol at relatively low concentrations preferentially inhibited the eicosanoid pathway downstream of FcεRI-proximal signaling events. To determine if this was the case, we first investigated the effect of Resveratrol on expression of the cyclooxygenases COX-1 and COX-2, key enzymes involved in the conversion of arachidonic acid to prostaglandins [31,32]. Both COX-1 and COX-2 are involved in PGD2 biosynthesis. However, whereas COX-1 is constitutively expressed in mast cells, COX-2 expression is an inducible event following FcεRI stimulation. In fact, COX-2 is the only major enzyme involved in eicosanoid production whose expression is induced in mast cells following FcεRI crosslinking; thus, making it an ideal candidate target for Resveratrol. To this end, IgE-sensitized skin mast cells were pre-treated with 1, 10 or 100 µM Resveratrol or DMSO for 1 h, and then challenged for 30 min with 100 ng/ml NP-BSA. Total RNA was isolated, and COX-1 and COX-2 expression was assessed by real time PCR. As expected, COX-1 expression was not affected whereas COX-2 expression was significantly increased after FcεRI crosslinking. Moreover, pre-treatment with Resveratrol had no effect on the constitutive expression of COX-1 (Figure 4A), whereas IgE-dependent COX-2 expression was significantly inhibited in skin mast cells pre-treated with 10 µM (p<0.05) or 100 µM Resveratrol (p<0.01) (Figure 4B). The fold increase ± SEM in COX-2 expression in FcεRI-activated control mast cells was 29 ± 5, and 36 ± 5, 17 ± 4, or 1 ± 0.2, respectively, in mast cells pre-treated with 1, 10 or 100 µM Resveratrol. Interestingly, COX-2 expression appeared to be slightly increased in skin mast cells pre-treated with 1 µM Resveratrol although the difference was not statistically significant (p=0.2). Thus, FcεRI induced COX-2 expression was significantly inhibited by Resveratrol.
Figure 4. Effect of Resveratrol on COX-1 and COX-2 expression in human skin mast cells.
Expression analysis of COX-1 (a, n=6) or COX-2 (b, n=5) in FcεRI-activated humans was performed by qRT-PCR in independent experiments with mast cells from different donor tissue. Fold change, expressed as mean ± SEM, was determined by 2ΔΔCt method comparing to expression in non-activated mast cells (y=1, hatched line). The data reflect the effect of Resveratrol on the constitutive expression of COX-1 and induction of COX-2. Statistical significance was determined by pairwise student’s t-test comparing to values from mast cells activated without Resveratrol pre-treatment. *, p<0.05; **, p<0.01.
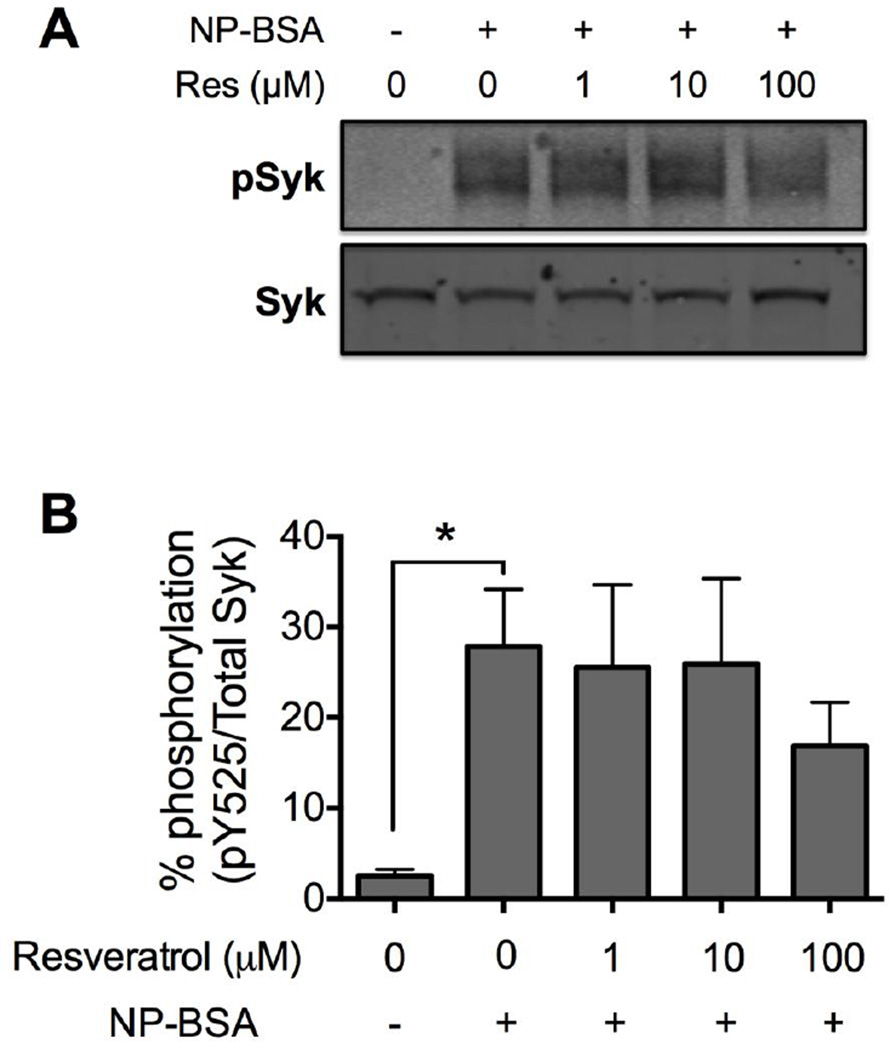
Our finding that high concentrations of Resveratrol were required to inhibit degranulation indicated that FcεRI-induced signals occurred normally with Resveratrol at low concentrations. To confirm this, we analyzed the effect of Resveratrol on Syk activation since degranulation is dependent on Syk activation [12,13,33]. Upon FcεRI crosslinking, Syk is rapidly recruited to ITAM residues on FcεRI γ chains where it is phosphorylated to propagate the cascade of signaling events [34]. Therefore, we analyzed the phosphorylation status of Syk at the activating tyrosine residue Y525 by SDS-PAGE and Western blotting. Whole cell lysates were prepared from IgE-sensitized human skin mast cells that had been pre-treated with 1, 10 or 100 µM Resveratrol or DMSO for 1 h and then activated for 5 min with 100 ng/ml NP-BSA. As expected, phosphorylated Syk was clearly detected in lysates from FcεRI-activated mast cells, but not in non-activated mast cells. Further, FcεRI-induced Syk phosphorylation was not affected in skin mast cells pre-treated with 1 or 10 µM Resveratrol (Figure 5) in accordance with the observation that Resveratrol at these concentrations did not affect degranulation (Figure 2A), which is dependent on Syk activation [13]. Interestingly, Resveratrol at 100 µM, a concentration that completely inhibited degranulation (Figure 2A), also did not significantly inhibit FcεRI-induced Syk phosphorylation (17 ± 5% versus 28 ± 6% in control cells; p=0.2) although an inhibitory trend was apparent. Thus, FcεRI-induced Syk phosphorylation was not inhibited with Resveratrol in human skin mast cells.
Figure 5. Effect of Resveratrol on FcεRI-induced phosphorylation of Syk in human skin mast cells.
Phosphorylation of Syk (Y525) was determined by quantitative infrared Western blotting of whole cell lysates of sensitized mast cells pre-treated with 1, 10 or 100 µM Resveratrol or DMSO (vehicle), and activated for 5 min with 100 ng/ml NP-BSA. The blot shown (a) is representative of 3 independent experiments with mast cells from skin tissue of different donors. Percent phosphorylation expressed as mean ± SEM (b, n=3) was determined as the ratio of phosphorylated Syk (Y525) to total Syk using fluorescent signal values obtained with an infrared imager. Statistical significance was determined by student’s t-test comparing to values from mast cells activated without Resveratrol pre-treatment. *, p<0.05.
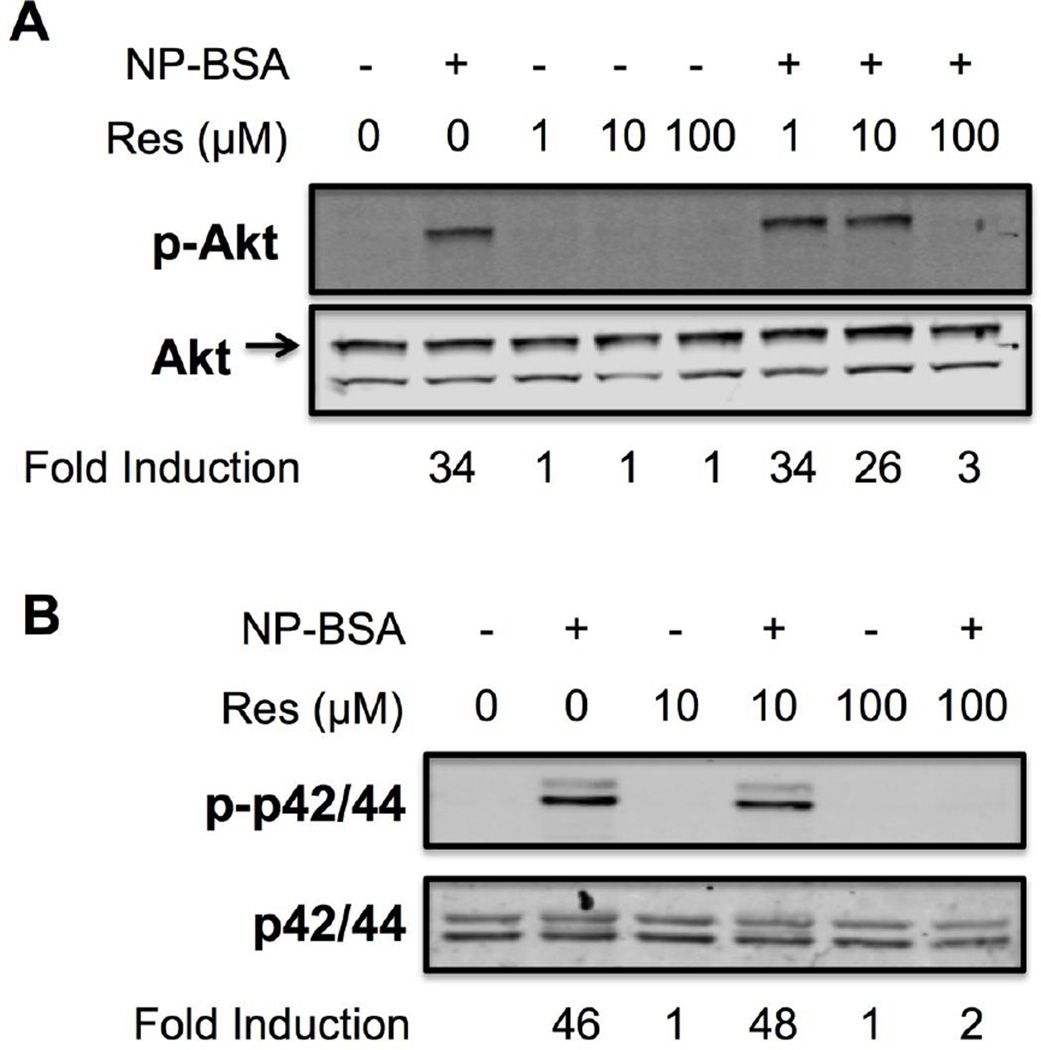
To determine the effect of Resveratrol on downstream signaling processes, we analyzed the phosphorylation status of Akt, p38 and p42/44 (ERK1/2) following FcεRI cross-linking since these intermediate signaling molecules have been implicated in activation of various transcription factors and cytokine production by mast cells [17,35,36]. To do so, SDS-PAGE and Western blotting were performed using whole cell lysates prepared from IgE-sensitized human skin mast cells that were pre-treated with Resveratrol at 1, 10 or 100 µM for 1 h, and challenged for 5 min with 100 ng/ml NP-BSA. As demonstrated in Figure 6, FcεRI-induced phosphorylation of Akt was minimally inhibited with 10 µM Resveratrol whereas p38 or p42/44 phosphorylation was not affected. In contrast, 100 µM Resveratrol completely inhibited the phosphorylation of these signaling molecules. These data indicate that the observed increase in TNF production from human skin mast cells pre-treated with 10 µM Resveratrol (Figure 3) was not due to an increase in FcεRI-induced activation of Akt, p38 or p42/44.
Figure 6. Effect of Resveratrol on FcεRI-induced phosphorylation of Akt, p42/44 and p38 in human skin mast cells.
Phosphorylation of Akt (a), p42/44 (b, upper panel) and p38 (b, lower panel) was determined by quantitative infrared Western blotting of whole cell lysates of sensitized mast cells pre-treated with 1, 10 or 100 µM Resveratrol or DMSO (vehicle), and activated for 5 min with 100 ng/ml NP-BSA. Fold induction of phosphorylation was determined from fluorescent signal values obtained with an infrared imager. The blots shown are representative of 3 independent experiments with mast cells from skin tissue of different donor.
4. Discussion
In this study, we investigated the effects of Resveratrol on IgE-dependent release of allergic and inflammatory mediators from mast cells that were isolated and purified from human skin tissue. Studies have shown that Resveratrol can inhibit mediator release from mouse mast cells [22–24]. However, there are currently no studies showing the direct effect of Resveratrol on human mature mast cells, and the mechanism or target of Resveratrol has not been identified. Here, we identified the COX-2 pathway leading to PGD2 biosynthesis as a target for inhibition by Resveratrol at low concentrations. In addition, we uncover the ability of Resveratrol to enhance the production of TNF from human skin mast cells, a previously unrecognized pro-inflammatory effect.
The finding that Resveratrol preferentially inhibited PGD2 production was corroborated by the observation that FcεRI-induced COX-2 expression but not Syk phosphorylation was inhibited by Resveratrol. In fact, Resveratrol failed to inhibit Syk phosphorylation even at an extremely high concentration (100 µM) that completely blocked downstream Akt, p38 or p42/44 phosphorylation. These findings provide the first evidence of preferential or selective inhibition of mediator release from human mature mast cells by Resveratrol at concentrations more likely to be achieved physiologically. In addition, these data demonstrate that FcεRI-proximal Syk is not a target of Resveratrol. Interestingly, Resveratrol is a non-hydroxylated analogue of naturally occurring piceatannol (3, 3’, 4, 5’-trans-trihydroxystilbene), a widely used inhibitor of Syk [37]. The finding that FcεRI-induced COX-2 expression was inhibited with a relatively low concentration of Resveratrol, while phosphorylation of receptor-proximal Syk or downstream signaling molecules Akt, p38 or p42/44 was not, indicates that Resveratrol selectively targeted the FcεRI-induced eicosanoid biosynthesis pathway to inhibit PGD2 production. These data suggest that Resveratrol as a therapeutic agent might protect against allergic effects mediated by PGD2 but not against pre-formed mediators like histamine, which are stored in cytoplasmic granules and released during degranulation.
Our data showing that Resveratrol at a relatively low concentration (10 µM) enhanced rather than inhibited TNF production was surprising given its reported anti-inflammatory properties. Resveratrol and other polyphenols have been shown to inhibit TNF and other cytokines from human mast cells. One study demonstrated that Resveratrol (10 or 50 µM) inhibited cytokine production from the human mast cell line HMC-1 following stimulation with phorbol ester (PMA) + ionomycin [38]. However, one critical difference from our study is that HMC-1 cells do not express FcεRI [39], and, thus, cannot be stimulated by IgE/Ag. Therefore, the signaling pathway(s) induced in HMC-1 transformed cells by PMA + iono are likely distinct from those initiated by FcεRI crosslinking in normal primary mast cells used in our study. The flavones, luteolin, quercetin, and baicalein (1 – 100 µM) have also been shown to inhibit TNF and other cytokines from human cultured mast cells, mast cell lines, or mouse BMMCs [40–43]. Recently, the novel flavone tetramethoxyluteolin (methlut; 1 – 100 µM), a structural analog of luteolin, was shown to potently inhibit TNF production from IgE/anti-IgE-activated human cord blood-derived mast cells or the LAD2 mast cell line [44]. Our finding is supported by a recent study demonstrating an increase in plasma TNF levels in healthy subjects given oral Resveratrol (5g), and in vitro (10 or 30 µM) from LPS-stimulated human peripheral blood mononuclear cells or monocytes pre-treated with Resveratrol [45]. Despite the numerous studies performed in mice or with mouse mast cells, there are no reported pro-inflammatory effects of Resveratrol or other polyphenols on allergic responses. Our finding and that of Gualdoni et al. [45] are the first pro-inflammatory effects of Resveratrol on human primary immune cells to be reported. Therefore, the possibility exists that Resveratrol has different effects on human and mouse mast cells that have yet to be fully elucidated with regard to IgE-dependent responses. It is not currently known if the potentiating effect is specific to MCTC type mast cells in skin or if TNF production from MCT mast cells in lung is also enhanced. Nevertheless, it appears that in addition to its well-documented anti-inflammatory properties, Resveratrol has pro-inflammatory effects in humans that could dampen its potentially healthful benefits for which it has garnered intense interest.
On the other hand, since Resveratrol is known to inhibit inflammation, it is possible that the increased amount of TNF induced by Resveratrol specifically from human skin mast cells is negated by Resveratrol’s overall anti-inflammatory properties. The observed increase in TNF reported here could also represent a localized response to Resveratrol in human skin particularly with regard to allergic inflammation. If so, this raises the issue of whether the overall physiological effect of Resveratrol is dependent on the route of delivery. This is particularly intriguing given that different methodologies to enhance the efficiency of topical administration of Resveratrol are currently being developed as a means to deliver the polyphenol [46–50]. Clearly, issues regarding route of delivery, solubility, and bioavailability will ultimately determine the efficacy of using Resveratrol or other polyphenols with limited solubility as therapeutic agents.
The mechanism by which Resveratrol enhances TNF production from human skin mast cells is not known. However, an effect on the NF-κB pathway is suspected since NF-κB is required for IgE-dependent TNF production from mast cells [51]. Indeed, enhanced TNF production by Resveratrol from LPS-stimulated human monocytes was associated with increased phosphorylation of p105, a component of the alternative pathway for NF-κB activation [45] suggesting that increased TNF production by Resveratrol could be due to increased NF-κB transcriptional activity. However, Resveratrol or other polyphenols were shown to inhibit NF-κB activation in human cultured mast cells, LAD2 or HMC-1 mast cell lines, or various transformed cell lines including U937, Jurkat, HeLa [38,44,52]. One critical question that needs to be addressed is: If NF-κB activation is involved in Resveratrol-induced enhancement of TNF production, how do we reconcile this with the observations that NF-κB is inhibited with Resveratrol? To answer this, additional studies are needed to determine the effect of Resveratrol on canonical and non-canonical NF-κB activation in human mature mast cells. Nevertheless, our finding that FcεRI-induced phosphorylation of p38 or p42/44, which are involved in cytokine production from FcεRI-activated mast cells, was not altered with Resveratrol at a concentration that enhanced TNF production suggests a target further downstream in the FcεRI pathway possibly NF-κB or other transcription factor(s).
Our study corroborates previous reports showing that high concentrations of Resveratrol inhibited mast cell degranulation and cytokine production. Here we show that Resveratrol at 100 µM strongly inhibited degranulation, PGD2 and cytokine production. However, whether the inhibitory effect with high concentrations of Resveratrol is physiologically relevant is questionable. It is unknown if human skin can absorb 50 – 100 µM Resveratrol, the concentration range required to significantly inhibit degranulation, via topical application, but such high concentrations are unlikely to be achieved in tissues at least via oral route [53]. Moreover, our data demonstrates that Resveratrol did not induce a loss in viability of human skin mast cells even at high concentrations indicating that the inhibitory effect was not due to cell toxicity.
Overall, we demonstrate that Resveratrol at relatively low concentrations: (1) Preferentially inhibits the COX-2 pathway leading to PGD2 production; and (2) Enhances TNF production from FcεRI-activated human skin mast cells. Together, these findings suggest that Resveratrol as a potential treatment for allergic reactions would be mostly effective against arachidonic acid-derived lipid mediators rather than histamine, but could also exacerbate or promote mast cell-mediated allergic inflammation in the skin.
Highlights.
Resveratrol targets the eicosanoid pathway for inhibition in human skin mast cells.
Resveratrol at low concentration inhibits PGD2 biosynthesis but not degranulation.
Resveratrol inhibits FcεRI-induced COX-2 expression but not Syk phosphorylation.
Resveratrol enhances FcεRI-induced TNF production from human skin mast cells.
Acknowledgments
This study was supported by National Institutes of Health grants P20 GM103641 and K01 HL092581-7.
Abbreviations
- IgE
Immunoglobulin E
- PGD2
Prostaglandin D2
- COX-1
cyclooxygenase-1
- COX-2
cyclooxygenase-2
- Syk
spleen tyrosine kinase
- TNF
tumor necrosis factor
- IL-6
interleukin-6
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Devon Shirley, Email: devon.shirley@uscmed.sc.edu.
Cody McHale, Email: mchalecc@email.sc.edu.
Gregorio Gomez, Email: gregorio.gomez@uscmed.sc.edu.
REFERENCES
- 1.Novelle MG, Wahl D, Diéguez C, Bernier M, de Cabo R. Resveratrol supplementation: Where are we now and where should we go? Ageing Res. Rev. 2015;21:1–15. doi: 10.1016/j.arr.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polonini HC, Lima LL, Gonçalves KM, do Carmo AMR, da Silva AD, Raposo NRB. Photoprotective activity of resveratrol analogues. Bioorg. Med. Chem. 2013;21:964–968. doi: 10.1016/j.bmc.2012.11.052. [DOI] [PubMed] [Google Scholar]
- 3.Wu Y, Jia L-L, Zheng Y-N, Xu X-G, Luo Y-J, Wang B, Chen JZS, Gao X-H, Chen H-D, Matsui M, Li Y-H. Resveratrate protects human skin from damage due to repetitive ultraviolet irradiation. J Eur Acad Dermatol Venereol. 2013;27:345–350. doi: 10.1111/j.1468-3083.2011.04414.x. [DOI] [PubMed] [Google Scholar]
- 4.Buonocore D, Lazzeretti A, Tocabens P, Nobile V, Cestone E, Santin G, Bottone MG, Marzatico F. Resveratrol-procyanidin blend: nutraceutical and antiaging efficacy evaluated in a placebocontrolled, double-blind study. Clin Cosmet Investig Dermatol. 2012;5:159–165. doi: 10.2147/CCID.S36102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bastianetto S, Dumont Y, Duranton A, Vercauteren F, Breton L, Quirion R. Protective action of resveratrol in human skin: possible involvement of specific receptor binding sites. PLoS ONE. 2010;5:e12935. doi: 10.1371/journal.pone.0012935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Theoharides TC, Valent P, Akin C. Mast Cells, Mastocytosis, and Related Disorders. N. Engl. J. Med. 2015;373:163–172. doi: 10.1056/NEJMra1409760. [DOI] [PubMed] [Google Scholar]
- 7.Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat. Med. 2012;18:693–704. doi: 10.1038/nm.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harvima IT, Nilsson G. Mast cells as regulators of skin inflammation and immunity. Acta Derm. Venereol. 2011;91:644–650. doi: 10.2340/00015555-1197. [DOI] [PubMed] [Google Scholar]
- 9.Ando T, Xiao W, Gao P, Namiranian S, Matsumoto K, Tomimori Y, Hong H, Yamashita H, Kimura M, Kashiwakura J-I, Hata TR, Izuhara K, Gurish MF, Roers A, Rafaels NM, Barnes KC, Jamora C, Kawakami Y, Kawakami T. Critical role for mast cell stat5 activity in skin inflammation. Cell Rep. 2014;6:366–376. doi: 10.1016/j.celrep.2013.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuoka T, Hirata M, Tanaka H, Takahashi Y, Murata T, Kabashima K, Sugimoto Y, Kobayashi T, Ushikubi F, Aze Y, Eguchi N, Urade Y, Yoshida N, Kimura K, Mizoguchi A, Honda Y, Nagai H, Narumiya S. Prostaglandin D2 as a mediator of allergic asthma. Science. 2000;287:2013–2017. doi: 10.1126/science.287.5460.2013. [DOI] [PubMed] [Google Scholar]
- 11.Zhao W, Oskeritzian CA, Pozez AL, Schwartz LB. Cytokine production by skin-derived mast cells: endogenous proteases are responsible for degradation of cytokines. J. Immunol. 2005;175:2635–2642. doi: 10.4049/jimmunol.175.4.2635. [DOI] [PubMed] [Google Scholar]
- 12.Siraganian RP, Zhang J, Suzuki K, Sada K. Protein tyrosine kinase Syk in mast cell signaling. Mol. Immunol. 2002;38:1229–1233. doi: 10.1016/s0161-5890(02)00068-8. [DOI] [PubMed] [Google Scholar]
- 13.Costello PS, Turner M, Walters AE, Cunningham CN, Bauer PH, Downward J, Tybulewicz VL. Critical role for the tyrosine kinase Syk in signalling through the high affinity IgE receptor of mast cells. Oncogene. 1996;13:2595–2605. [PubMed] [Google Scholar]
- 14.Gomez G, Schwartz L, Kepley C. Syk deficiency in human non-releaser lung mast cells. Clin. Immunol. 2007;125:112–115. doi: 10.1016/j.clim.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Odom S, Gomez G, Kovarova M, Furumoto Y, Ryan JJ, Wright HV, Gonzalez-Espinosa C, Hibbs ML, Harder KW, Rivera J. Negative regulation of immunoglobulin E-dependent allergic responses by Lyn kinase. J. Exp. Med. 2004;199:1491–1502. doi: 10.1084/jem.20040382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parravicini V, Gadina M, Kovarova M, Odom S, Gonzalez-Espinosa C, Furumoto Y, Saitoh S, Samelson LE, O'Shea JJ, Rivera J. Fyn kinase initiates complementary signals required for IgE-dependent mast cell degranulation. Nat. Immunol. 2002;3:741–748. doi: 10.1038/ni817. [DOI] [PubMed] [Google Scholar]
- 17.Gomez G, Gonzalez-Espinosa C, Odom S, Baez G, Cid ME, Ryan JJ, Rivera J. Impaired FcepsilonRI-dependent gene expression and defective eicosanoid and cytokine production as a consequence of Fyn deficiency in mast cells. J. Immunol. 2005;175:7602–7610. doi: 10.4049/jimmunol.175.11.7602. [DOI] [PubMed] [Google Scholar]
- 18.Irani AA, Schechter NM, Craig SS, DeBlois G, Schwartz LB. Two types of human mast cells that have distinct neutral protease compositions. Proc. Natl. Acad. Sci. U.S.a. 1986;83:4464–4468. doi: 10.1073/pnas.83.12.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irani AM, Goldstein SM, Wintroub BU, Bradford T, Schwartz LB. Human mast cell carboxypeptidase Selective localization to MCTC cells. J. Immunol. 1991;147:247–253. [PubMed] [Google Scholar]
- 20.Oskeritzian CA, Zhao W, Min H-K, Xia H-Z, Pozez A, Kiev J, Schwartz LB. Surface CD88 functionally distinguishes the MCTC from the MCT type of human lung mast cell. J. Allergy Clin. Immunol. 2005;115:1162–1168. doi: 10.1016/j.jaci.2005.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomez G, Zhao W, Schwartz LB. Disparity in FcεRI-induced degranulation of primary human lung and skin mast cells exposed to adenosine. J. Clin. Immunol. 2011;31:479–487. doi: 10.1007/s10875-011-9517-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baolin L, Inami Y, Tanaka H, Inagaki N, Iinuma M, Nagai H. Resveratrol inhibits the release of mediators from bone marrow-derived mouse mast cells in vitro. Planta Med. 2004;70:305–309. doi: 10.1055/s-2004-818940. [DOI] [PubMed] [Google Scholar]
- 23.Han S-Y, Bae J-Y, Park S-H, Kim Y-H, Park JHY, Kang Y-H. Resveratrol inhibits IgE-mediated basophilic mast cell degranulation and passive cutaneous anaphylaxis in mice. J. Nutr. 2013;143:632–639. doi: 10.3945/jn.112.173302. [DOI] [PubMed] [Google Scholar]
- 24.Koo N, Cho D, Kim Y, Choi HJ, Kim K-M. Effects of resveratrol on mast cell degranulation and tyrosine phosphorylation of the signaling components of the IgE receptor. Planta Med. 2006;72:659–661. doi: 10.1055/s-2006-931568. [DOI] [PubMed] [Google Scholar]
- 25.Karuppagounder V, Arumugam S, Thandavarayan RA, Pitchaimani V, Sreedhar R, Afrin R, Harima M, Suzuki H, Nomoto M, Miyashita S, Suzuki K, Watanabe K. Resveratrol attenuates HMGB1 signaling and inflammation in house dust mite-induced atopic dermatitis in mice. Int. Immunopharmacol. 2014;23:617–623. doi: 10.1016/j.intimp.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 26.Royce SG, Dang W, Yuan G, Tran J, El Osta A, Karagiannis TC, Tang MLK. Resveratrol has protective effects against airway remodeling and airway hyperreactivity in a murine model of allergic airways disease. Pathobiol Aging Age Relat Dis. 2011;1 doi: 10.3402/pba.v1i0.7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee M, Kim S, Kwon O-K, Oh S-R, Lee H-K, Ahn K. Anti-inflammatory and anti-asthmatic effects of resveratrol, a polyphenolic stilbene, in a mouse model of allergic asthma. Int. Immunopharmacol. 2009;9:418–424. doi: 10.1016/j.intimp.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz LB, Austen KF, Wasserman SI. Immunologic release of beta-hexosaminidase and beta-glucuronidase from purified rat serosal mast cells. J. Immunol. 1979;123:1445–1450. [PubMed] [Google Scholar]
- 29.Schwartz LB, Lewis RA, Seldin D, Austen KF. Acid hydrolases and tryptase from secretory granules of dispersed human lung mast cells. J. Immunol. 1981;126:1290–1294. [PubMed] [Google Scholar]
- 30.Gomez G, Nardone V, Lotfi-Emran S, Zhao W, Schwartz LB. Intracellular adenosine inhibits IgE-dependent degranulation of human skin mast cells. J. Clin. Immunol. 2013;33:1349–1359. doi: 10.1007/s10875-013-9950-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawata R, Reddy ST, Wolner B, Herschman HR. Prostaglandin synthase 1 and prostaglandin synthase 2 both participate in activation-induced prostaglandin D2 production in mast cells. J. Immunol. 1995;155:818–825. [PubMed] [Google Scholar]
- 32.Dichlberger A, Schlager S, Maaninka K, Schneider WJ, Kovanen PT. Adipose triglyceride lipase regulates eicosanoid production in activated human mast cells. J. Lipid Res. 2014;55:2471–2478. doi: 10.1194/jlr.M048553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang J, Berenstein EH, Evans RL, Siraganian RP. Transfection of Syk protein tyrosine kinase reconstitutes high affinity IgE receptor-mediated degranulation in a Syknegative variant of rat basophilic leukemia RBL-2H3 cells. J. Exp. Med. 1996;184:71–79. doi: 10.1084/jem.184.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shiue L, Green J, Green OM, Karas JL, Morgenstern JP, Ram MK, Taylor MK, Zoller MJ, Zydowsky LD, Bolen JB. Interaction of p72syk with the gamma and beta subunits of the high-affinity receptor for immunoglobulin E, Fc epsilon RI. Mol. Cell. Biol. 1995;15:272–281. doi: 10.1128/mcb.15.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakano N, Nishiyama C, Yagita H, Hara M, Motomura Y, Kubo M, Okumura K, Ogawa H. Notch Signaling Enhances FcεRI-Mediated Cytokine Production by Mast Cells through Direct and Indirect Mechanisms. J. Immunol. 2015;194:4535–4544. doi: 10.4049/jimmunol.1301850. [DOI] [PubMed] [Google Scholar]
- 36.Kitaura J, Asai K, Maeda-Yamamoto M, Kawakami Y, Kikkawa U, Kawakami T. Akt-dependent cytokine production in mast cells. J. Exp. Med. 2000;192:729–740. doi: 10.1084/jem.192.5.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piotrowska H, Kucinska M, Murias M. Biological activity of piceatannol: leaving the shadow of resveratrol. Mutat. Res. 2012;750:60–82. doi: 10.1016/j.mrrev.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 38.Kang O-H, Jang H-J, Chae H-S, Oh Y-C, Choi J-G, Lee Y-S, Kim J-H, Kim YC, Sohn DH, Park H, Kwon D-Y. Anti-inflammatory mechanisms of resveratrol in activated HMC-1 cells: pivotal roles of NF-kappaB and MAPK. Pharmacol. Res. 2009;59:330–337. doi: 10.1016/j.phrs.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 39.Nilsson G, Blom T, Kusche-Gullberg M, Kjellén L, Butterfield JH, Sundström C, Nilsson K, Hellman L. Phenotypic characterization of the human mast-cell line HMC-1, Scand. J. Immunol. 1994;39:489–498. doi: 10.1111/j.1365-3083.1994.tb03404.x. [DOI] [PubMed] [Google Scholar]
- 40.Kempuraj D, Tagen M, Iliopoulou BP, Clemons A, Vasiadi M, Boucher W, House M, Wolfberg A, Theoharides TC. Luteolin inhibits myelin basic protein-induced human mast cell activation and mast cell-dependent stimulation of Jurkat T cells. Br. J. Pharmacol. 2008;155:1076–1084. doi: 10.1038/bjp.2008.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kimata M, Inagaki N, Nagai H. Effects of luteolin and other flavonoids on IgEmediated allergic reactions. Planta Med. 2000;66:25–29. doi: 10.1055/s-2000-11107. [DOI] [PubMed] [Google Scholar]
- 42.Kimata M, Shichijo M, Miura T, Serizawa I, Inagaki N, Nagai H. Effects of luteolin, quercetin and baicalein on immunoglobulin E-mediated mediator release from human cultured mast cells. Clin. Exp. Allergy. 2000;30:501–508. doi: 10.1046/j.1365-2222.2000.00768.x. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka T, Takahashi R. Flavonoids and asthma. Nutrients. 2013;5:2128–2143. doi: 10.3390/nu5062128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weng Z, Patel AB, Panagiotidou S, Theoharides TC. The novel flavone tetramethoxyluteolin is a potent inhibitor of human mast cells. J. Allergy Clin. Immunol. 2015;135:1044. doi: 10.1016/j.jaci.2014.10.032. 52.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gualdoni GA, Kovarik JJ, Hofer J, Dose F, Pignitter M, Doberer D, Steinberger P, Somoza V, Wolzt M, Zlabinger GJ. Resveratrol enhances TNF-α production in human monocytes upon bacterial stimulation. Biochim. Biophys. Acta. 2014;1840:95–105. doi: 10.1016/j.bbagen.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 46.Scalia S, Trotta V, Iannuccelli V, Bianchi A. Enhancement of in vivo human skin penetration of resveratrol by chitosan-coated lipid microparticles. Colloids Surf B Biointerfaces. 2015;135:42–49. doi: 10.1016/j.colsurfb.2015.07.043. [DOI] [PubMed] [Google Scholar]
- 47.Pando D, Matos M, Gutiérrez G, Pazos C. Formulation of resveratrol entrapped niosomes for topical use. Colloids Surf B Biointerfaces. 2015;128:398–404. doi: 10.1016/j.colsurfb.2015.02.037. [DOI] [PubMed] [Google Scholar]
- 48.Yutani R, Teraoka R, Kitagawa S. Microemulsion Using Polyoxyethylene Sorbitan Trioleate and its Usage for Skin Delivery of Resveratrol to Protect Skin against UV-Induced Damage. Chem. Pharm. Bull. 2015;63:741–745. doi: 10.1248/cpb.c15-00378. [DOI] [PubMed] [Google Scholar]
- 49.Friedrich RB, Kann B, Coradini K, Offerhaus HL, Beck RCR, Windbergs M. Skin penetration behavior of lipid-core nanocapsules for simultaneous delivery of resveratrol and curcumin. Eur J Pharm Sci. 2015;78:204–213. doi: 10.1016/j.ejps.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 50.Cosco D, Paolino D, Maiuolo J, Marzio LD, Carafa M, Ventura CA, Fresta M. Ultradeformable liposomes as multidrug carrier of resveratrol and 5-fluorouracil for their topical delivery. Int J Pharm. 2015;489:1–10. doi: 10.1016/j.ijpharm.2015.04.056. [DOI] [PubMed] [Google Scholar]
- 51.Marquardt DL, Walker LL. Dependence of mast cell IgE-mediated cytokine production on nuclear factor-kappaB activity. J. Allergy Clin. Immunol. 2000;105:500–505. doi: 10.1067/mai.2000.104942. [DOI] [PubMed] [Google Scholar]
- 52.Manna SK, Mukhopadhyay A, Aggarwal BB. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation. J. Immunol. 2000;164:6509–6519. doi: 10.4049/jimmunol.164.12.6509. [DOI] [PubMed] [Google Scholar]
- 53.Walle T, Hsieh F, DeLegge MH, Oatis JE, Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004;32:1377–1382. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]