Abstract
In January 2015, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) invited an expert panel to a workshop to address numerous knowledge gaps, and to provide evidence-based guidelines for the diagnosis and management of pregnant women with what had been commonly called chorioamnionitis and the infants born to these women. The panel noted that the term chorioamnionitis has been used to label a heterogeneous array of conditions characterized by infection and inflammation or both, with a consequent great variation in clinical practice for mothers and their newborns. Therefore, the panel proposed to replace the term chorioamnionitis with a more general, descriptive term, “intrauterine inflammation or infection or both,” abbreviated as “Triple I.” The panel proposed a classification for Triple I and recommended approaches to evaluation and management of pregnant women and their newborns with a diagnosis of Triple I. It is particularly important to recognize that an isolated maternal fever is not synonymous with chorioamnionitis. A research agenda was proposed to further refine the definition and management of this complex group of conditions. This article provides a summary of the workshop presentations and discussions.
Introduction
The term chorioamnionitis has been in existence for several decades.1 In the strictest sense, the term implies that a pregnant woman has an “inflammatory or an infectious” disorder of the chorion, amnion, or both. This diagnosis often implies that the mother and her fetus may be at an increased risk for developing serious infectious consequences. Because of its connotation, the mere entry of chorioamnionitis in the patient’s record triggers a series of investigations and management decisions in the mother and in the infant, irrespective of probable cause or clinical findings. Due to the imprecise nature of the definitional elements and to the heterogeneity of clinical manifestations, there is no unanimity in the approaches for diagnostic work-up, or for obstetric and neonatal management. To address these wide-ranging issues, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), Society for Maternal Fetal Medicine, American College of Obstetricians and Gynecologists, and American Academy of Pediatrics invited a group of maternal and neonatal experts to a workshop on January 26–27, 2015. In this article, we provide a brief summary of the workshop discussions and the expert opinion concerning management and evaluation of what has heretofore been labeled chorioamnionitis. This is not a formal Consensus Development Conference recommendation by the National Institutes of Health.
Review of the Current Understanding of Chorioamnionitis
The term chorioamnionitis has transitioned from its original autological scope (to express what it describes) to a more heterological term (not corresponding), essentially becoming an out-of-date misnomer. Although the term literally points to "inflammation limited to the chorion and amnion layers of the fetal membranes," it is often used when other intrauterine components are involved, such as amniotic fluid or the decidua. Adding to the confusion, the term is commonly used to denote clinical suspicion of intra-uterine inflammation or infection even before any laboratory or pathologic evidence of infection or inflammation is uncovered. The findings on such an examination are often not conclusive, are not available until after the infant is delivered, and are not always aligned with clinical features. The term chorioamnionitis does not consistently convey the degree and severity of maternal or fetal illness, which makes it difficult to assess the consequences of this diagnosis for the mother or baby.
In its current usage, the term chorioamnionitis refers to a heterogeneous group of conditions that include inflammation as well as infections of varying degrees of severity and duration. Inflammation includes a reaction that results in tissue edema, swelling and irritation. Infection includes inflammation with concurrent invasion of bacteria, virus, fungus or other infectious agent. Often a designation of chorioamnionitis is made when any combination (or even one) of the following elements are noted: maternal fever, maternal or fetal tachycardia or both, elevated maternal white blood cell (WBC) count, uterine tenderness, and purulent fluid or purulent discharge from the cervical os. However, the presence of one (or even more than one) of these signs and symptoms does not necessarily indicate intrauterine infection – or actual chorioamnionitis, is present.
Intrauterine infection may lead to serious maternal complications, such as sepsis, prolonged labor, wound infection, need for hysterectomy, post-partum endometritis, post-partum hemorrhage, adult respiratory distress syndrome (ARDS), intensive care unit (ICU) admission, and in rare instances, maternal mortality. However, by erring on the side of treatment for any suspected chorioamnionitis, health care providers may not be fully considering the adverse effects of unnecessary treatment. Treatment with antimicrobial agents for fever during labor is generally safe for the mother, with relatively few side effects. However, rare instances of anaphylaxis2,3 have been reported, with serious implications for the fetus when utero-placental blood flow and oxygenation are adversely affected. The prevalence of anaphylaxis was found to be 2.7 cases per 100,000 deliveries.2 In addition, a diagnosis of maternal chorioamnionitis has significant implications for the evaluation and management of the newborn infant. It often leads to additional laboratory evaluation, unnecessary treatment, and hospitalization in higher acuity units.4–6 For all these reasons, the workshop participants agreed that there is a need to change the prevailing and unsubstantiated perceptions associated with the term chorioamnionitis.
Maternal fever can occur due to intrauterine or extrauterine causes. Infectious causes can include pyelonephritis, upper and lower respiratory tract infections such as influenza, as well as infections in other organ systems. Non-infectious causes of fever include use of epidural analgesia during labor,7,8 hyperthyroidism, dehydration, elevated ambient temperature, and the use of pyrogens such as prostaglandin E2 (PGE2) for the induction of labor. It may not always be possible to differentiate between intra- and extra-uterine causes of fever, or to categorically exclude chorioamnionitis, particularly early in its presentation. For these reasons, a plan to “rule out chorioamnionitis” or to treat “presumptive chorioamnionitis” is sometimes made and entered into the medical records, which often triggers an unnecessary work-up for “sepsis” and antimicrobial treatment for the newborn. Since not every intrapartum fever is of infectious origin, treating all fevers with antimicrobial agents will result in over treatment of mothers.
The neonatal team might interpret maternal antimicrobial treatment itself as evidence of potential maternal and fetal infection, leading to additional neonatal laboratory testing and treatment of the infant with antimicrobial agents for varying duration. Thus, a diagnosis of “chorioamnionitis” has serious implications for the management of the newborn infant. The guidelines developed by the Centers for Disease Control (CDC),9 American Academy of Pediatrics (AAP),10 and the National Institute of Child Health and Clinical Excellence (NICE)11 differ in some of their specifics, but all three guidelines recommend treatment of well-appearing infants born to women with suspected or proven chorioamnionitis. For example, for well appearing infants born to women with suspected chorioamnionitis, both the CDC9 and the Committee on Fetus and Newborn of the American Academy of Pediatrics10 recommend a blood culture at birth followed by treatment and subsequent laboratory tests (e.g., white blood cell and differential count, C-reactive protein, or platelet count). The National Institute for Health and Clinical Excellence guideline from the United Kingdom11 recommends blood culture and CRP determination followed by initiation of antimicrobial agents for any neonate whose mother received antimicrobial agents for confirmed or suspected bacterial infection including chorioamnionitis.
The consequences of the three sets of guidelines outlined above include a significant increase in the number of infants exposed to antimicrobial agents in an attempt to treat rare cases of early onset sepsis (EOS), as well as an increase in the workload for health care providers and cost.4–6 In addition, many newborns are treated with antimicrobial agents for prolonged periods despite negative blood culture results.5,6 Since administration of antimicrobial agents oftentimes is accompanied by admission to a neonatal intensive care unit (NICU), a large number of newborns are additionally exposed to the NICU environment where there is increased risk of acquiring infections with multi-drug resistant bacteria. Infants in NICUs are also separated from their families, which may have consequences for mother-infant attachment and successful breastfeeding. Antimicrobial agents also alter the gut microbiota.12,13 The overall implications are even more concerning considering the likelihood of an infectious etiology is small. Since the early 1970s, neonatal care providers have been rightly concerned about early onset sepsis (EOS), especially group B streptococcal (GBS) disease because of its high morbidity and mortality. Much of this concern began in an era before routine maternal screening for GBS, and intrapartum antimicrobial prophylaxis. However, following publication of GBS management guidelines by several professional societies and organizations, the incidence of early-onset GBS sepsis has dropped significantly.14 The authors found no concomitant increase in E. Coli sepsis during the study period from 2006–2009.14
Although confirmed maternal infection needs to be treated with appropriate antimicrobial agents (which also treat the fetus), they are frequently given for febrile episodes with a low likelihood of intrauterine infection.. Therefore, giving antimicrobial agents to a newborn infant simply based on an isolated maternal fever will likely treat many infants with a very low likelihood of infection. Because such circumstances are relatively common, some consensus around the management of well-appearing infants exposed to antimicrobial agents in utero, and how to target investigation and treatment of infants at highest risk for EOS is needed.
Ideally, antimicrobial treatment of the newborn at high risk of early onset sepsis should be initiated immediately after birth, but restricted only to newborns that might benefit from treatment (i.e., those likely to be infected). Unfortunately at this time, diagnostic tests with the ability to identify newborn infants likely to be infected are not clinically available. One approach to limiting the unnecessary use of antimicrobials is to use the “sepsis calculator” developed by Puopolo et al15 to estimate the probability of EOS using maternal risk factors in infants ≥ 34 weeks gestation. The model uses three categorical variables: GBS status (positive, negative, uncertain), maternal intrapartum antimicrobial treatment (GBS specific or broad spectrum), and intrapartum prophylaxis (IAP) or treatment given ≥ 4 hours prior to delivery (yes, no) in addition to the following continuous variables: highest maternal intrapartum temperature (centigrade or Fahrenheit), gestational age (weeks and days), and duration of ROM (hours). A predicted probability per 1,000 live births can be estimated using the calculator (http://www.dor.kaiser.org/external/DORExternal/research/InfectionProbabilityCalculator.aspx). In a retrospective study, Shakib et.al16 demonstrated that the use of the sepsis calculator in a population of well appearing infants (≥ 34 weeks) with a clinical diagnosis ofchorioamnionitis would have reduced the proportion of infants having laboratory tests and antimicrobial agents to 12% of the total and would not have missed any cases of culture positive EOS.16
Escobar et al17 recently refined the sepsis calculator developed by Puopolo15 by combining the same risk factors for sepsis described above (pretest probability) and the infant’s clinical presentation (clinically ill, equivocal presentation or well appearing) during the first 6–12 hours of life (post-test probability) to estimate the probability of sepsis in infants ≥ 34 weeks gestation. Escobar demonstrated that in well-appearing infants with risk factors for sepsis, the incidence of EOS is extremely low [sepsis rate of 0.11/1000 (0.08–0.13)], but not quite zero. Both algorithms will need further modification as new data are generated.
There is general consensus that infants who have persistent signs associated with sepsis, whether or not born to mothers with a diagnosis of “chorioamnionitis” (suspected or proven) ought to receive broad-spectrum antimicrobials after appropriate cultures are taken. However, some newborns will initially be symptomatic immediately after birth and will become asymptomatic over the ensuing 4–6 hours. Those infants should be managed as if they were healthy appearing. The management of the well-appearing asymptomatic infant born to a mother with a “chorioamnionitis” diagnosis remains controversial. As noted above, CDC,9 AAP,10 and NICE11 recommend a diagnostic evaluation and antimicrobial coverage. Given that clinicians may have a low threshold for labeling the patient as having “chorioamnionitis,” and this decision does not take into consideration the resulting neonatal interventions, it is important to re-evaluate the approach to this group of women and neonates.
Intrauterine Inflammation,Infection or both (Triple I)
The workshop participants noted that use of the term chorioamnionitis convey a definitive infectious etiology when this may not always be the case. Providers often use this term even when the only sign is a maternal fever. The panel of experts agreed that maternal fever alone should not automatically lead to a diagnosis of infection (or chorioamnionitis) and to antimicrobial therapy. They also sought to develop new terminology to better describe various scenarios associated with fever or infection during the intrapartum period.
In order to clarify this issue, the panel recommended new terminology that differentiates the mere presence of fever from infection or inflammation or both, and clarifies that inflammation can occur without infection. Therefore given the historical inconsistency in use, the panel proposed to altogether discontinue the intrapartum use of the term chorioamnionitis and instead use “Intrauterine Inflammation or Infection or both” or Triple I as shown in Table 1. Under the new proposal, Triple I is diagnosed when fever is present with one or more of the following:
Fetal Tachycardia (> 160 bpm for 10 minutes or longer)18
Maternal WBC > 15,000 in absence of corticosteroids
Purulent fluid from the cervical os (cloudy or yellowish thick discharge confirmed visually on speculum exam to be coming from the cervical canal)
Biochemical or microbiologic amniotic fluid results consistent with microbial invasion of the amniotic cavity (see below)
Table: 1.
Features of Isolated Maternal Fever, and Triple I with Classification*
| Terminology | Features and Comments |
|---|---|
| Isolated Maternal Fever (“Documented” Fever) | Maternal oral temperature ≥ 39.0°C (102.2° F) on any one occasion is “documented fever.” If the oral temperature ≥ 38.0°C (100.4°F) but ≤ 39.0° C (102.2° F), repeat the measurement in 30 minutes; if the repeat value, too remains ≥ 38.0°C (100.4 °F) it is “documented fever. |
| Suspected Triple I | Fever without a clear source plus any of the following:
|
| Confirmed Triple I | All of the above plus
|
Fever in the absence of any of the above criteria, should be categorized as “isolated maternal fever.” Isolated maternal fever can include but is not limited to fever secondary to epidural anesthesia, prostaglandin use, dehydration, hyperthyroidism, and excess ambient heat. In the clinical situation of labor with fever and unknown GBS status at ≥ 37 weeks gestation, intrapartum prophylaxis should be initiated as per CDC guidelines.9
The panel also recommended that the diagnosis of fever be standardized as follows: maternal temperature ≥ 39.0 °C or 102.2 °F on one reading constitutes a fever. If the temperature is ≥ 38.0 °C or 100.4 ° F but less than 39.0 °C or 102.2 °F, the temperature should be re-taken in 30 minutes for confirmation. A repeat temperature ≥ 38.0 °C or 100.4 °F constitutes a documented fever.19,20 For the diagnosis of fever, temperature should be measured orally.21
The panel suggests that Triple I be categorized as suspected or confirmed. Without confirmation, Triple I should be qualified with the term “suspected.” In order to be confirmed, Triple I should be accompanied by objective laboratory findings of infection in amniotic fluid (AF) (e.g. positive gram stain for bacteria, low AF glucose, high WBC count in the absence of a bloody tap, or positive AF culture results) or histopathological evidence of infection or inflammation or both in the placenta, fetal membranes or the umbilical cord vessels (funisitis).19,22,23 Obviously, the histopathological evidence would be applied in retrospect.
Cases can thus be categorized as follows (Table 1):
Isolated maternal fever (not Triple I)
Suspected Triple I
Confirmed Triple I
Biomarkers
Members of the panel agreed on the critical need for discovery, validation and implementation in clinical workflow of biomarkers that could objectively assess the level of risk for EOS. Biomarkers with potential to guide neonatal management can either be antenatal or postnatal.
Antenatal markers should be aimed at diagnosing Triple I and assessing its severity. In combination with gestational age and clinical manifestations, such biomarkers have potential to play an active role in the management as noted below:
Consideration for admission or transfer to a health care facility with maternal fetal medicine service and level 3 or 4 NICU if warranted by the clinical assessment;
Decision for expectant management versus delivery;
Decision to perform a cervical cerclage or to withhold such procedure;
Timing for steroid administration;
Decision whether to initiate tocolytic treatment;
Decision whether antimicrobial treatment of the mother is needed;
Since most intrauterine infections have a subclinical stage,24 one should recognize the challenges of interpreting results of antenatal markers of Triple I. The first challenge results from the compartmentalization of the gestational sac from the maternal systemic circulation.25 As a result, studies focusing on markers traditionally associated with inflammatory or infectious processes have failed to show clinical utility when these markers are assessed in the maternal circulation. Although some authors have proposed using amniotic fluid analysis to rule out Triple I in women with PPROM managed expectantly, a recent Cochrane review found that the quality of evidence is poor.26 While a meta-analysis was not possible due to the small number of studies, it is clear that high quality evidence is needed to guide clinical practice related to the role of amniocentesis and amniotic fluid analysis in management of PPROM. There is a similar paucity of data regarding the need for amniocentesis in women presenting with preterm labor and intact membranes or advanced cervical effacement. Recent studies recommend ruling out Triple I using amniotic fluid analysis before surgical placement of a foreign body such as cervical cerclage.27–29 For example, subclinical microbial invasion of amniotic fluid was found in 9% of women with a sonographically short cervix (< 25 mm in the mid trimester).30
Even when analyzed in amniotic fluid, there is controversy as to which biomarkers are most informative and whether they are markers of intra-amniotic infection, intra-amniotic inflammation or both. In the few institutions where amniocentesis is performed to confirm Triple I, the laboratory tests that are used for clinical management are glucose concentration, lactate dehydrogenase (LDH) activity, WBC and RBC counts, Gram stain and bacterial cultures. Culture results are usually not available in time for decision-making. Therefore, clinicians must rely on the remaining analyses, which have turnaround times in hours. Unfortunately, the tests noted above (glucose, LDH, WBC count and Gram stain) do not always concur in ruling out or confirming Triple I; therefore, the interpretation of the test results may not be straightforward.31 Studies of biomarkers of Triple I are confounded by the lack of a gold standard for diagnosis. Bacterial cultures depend on the choice of media and do not routinely identify all species, some of which are known etiologic agents of Triple I32 and of EOS.33 Moreover, amniotic fluid inflammation has been linked to poor pregnancy and neonatal outcomes27 even in the absence of infection. Biomarkers also have different diagnostic accuracy in various subgroups of women (PPROM versus preterm labor intact membranes versus short cervix). This makes them less practical in the clinical setting as the patient’s condition may evolve from one to the other. Despite a plethora of hypothesis driven and “omics” discovery studies (primarily proteomics and metabolomics) only a few biomarkers have been validated or tested clinically.
To overcome the need for amniocentesis, many investigators have searched for markers informative of Triple I in biological fluids that can be sampled non-invasively (urine) or through minimally invasive approaches (maternal blood, cervico-vaginal secretions, vaginal amniotic or vaginal washings fluid in PPROM cases). Maternal blood has been the compartment most extensively explored, but so far none of the markers are sensitive enough to diagnose Triple I or to estimate its severity. The issue of specificity is more difficult to evaluate as the majority of published studies fail to include cases with other types of systemic inflammatory conditions with overlapping symptomatology (pyelonephritis, appendicitis, and other conditions).
Postnatal markers have the potential to impact the postnatal care of the newborn. Indeed, postnatal markers could be particularly useful because they:
Remove some of the subjectivity from the interpretation of symptoms of sepsis which are nonspecific in newborns or may not be apparent to an untrained provider;
Help with the decision to admit a newborn to an intensive care unit and to promptly initiate broad spectrum antimicrobial therapy;
Guide the duration of antimicrobial therapy;
Facilitate counseling of the mother and family with respect to probable cause of preterm birth and future pregnancies
Cord blood and neonatal blood, sampled within 72 hours of birth are the biological fluids most often explored for markers indicative of EOS. The chief advantage of cord blood is that it is available in relatively large quantities immediately upon delivery; its sampling is technically easy to perform and does not pose a risk of infection or hemorrhage for the baby. Its disadvantage is that some analytes of placental origin might be present in increased concentration in cord blood compared to neonatal blood (although this has not been systematically addressed). Cord blood levels of C-reactive protein (CRP), procalcitonin (PCT), interleukin-6 (IL-6), interleukin 8 and more recently of haptoglobin (Hp) and Hp-related protein (HpRP) have been studied alone, in combination and as add-ons to hematologic indices.34–38
Blood obtained from the neonate following birth is the biological fluid most often used to test for sepsis biomarkers (including hematological indices) which are used in some centers to guide initiation and duration of antimicrobial therapy. Postnatal blood samples are also used for bacterial cultures. The problem with blood sampling after birth is that it poses a small risk for the baby and the amount of blood that can be safely obtained is severely limited, especially in VLBW infants. Most importantly, the interpretation of some biomarkers such as CRP and IL-6 is confounded by physiological changes that occur in the immediate postnatal period, which affect their specificity.39,40 Other soluble or cell-adhesion molecules have been suggested as markers for identifying newborns with EOS, but none are accurate and or widely available enough for current clinical use.41
Commercial development of a diagnostic test for sepsis generally requires reporting of sensitivity and specificity, which is not possible for EOS since an accurate gold standard does not exist and there is no established consensus on the definition for neonatal sepsis.42 Despite claims that the neonatal blood cultures are "the gold standard for EOS" their use is severely limited by both false negative and false positive results.42 Therefore any new biomarker that is technically superior at identification of true disease will appear inferior when compared to blood culture results. Accordingly, novel biomarkers should be assessed against clinically important neonatal outcomes.
Maternal Management
Isolated fever, and suspected or confirmed Triple I are not, by themselves, indications for cesarean delivery. The approach to antimicrobial treatment in the mother is similar to the one for the neonate. In the presence of isolated fever, particularly in the late preterm and term patient following epidural analgesia, it may be appropriate to avoid antimicrobial agents and monitor the patient for additional signs or symptoms of infection.
The choice of antimicrobial agents in the case of suspected Triple I should be guided by the prevalent microorganisms causing intrauterine infection. In general, a combination of ampicillin and gentamicin should cover most relevant pathogens. If a cesarean section is performed, the addition of anaerobic coverage after delivery may be considered (clindamycin or metronidazole)to decrease the risk of endometritis.
In women treated intrapartum with antimicrobial agents for suspected or confirmed Triple I, continuation of antimicrobial agents postpartum should not be automatic, but rather based on risk factors for postpartum endometritis. Women who have a vaginal delivery are less likely to have postpartum endometritis, and therefore are candidates for discontinuing antimicrobial agents after delivery. Even in women undergoing cesarean delivery, one more dose of antimicrobial agents after delivery has the same efficacy as continuing for longer duration.43–45 The presence of other maternal factors in the postpartum period, such as bacteremia, sepsis and persistent fever may be used to guide duration of antimicrobial therapy.
Controlling the maternal temperature with antipyretics and judicious hydration may be required. Since antipyretics may prevent or mask further fever, a decision regarding the likelihood of infection should be made before they are given.
Neonatal Management
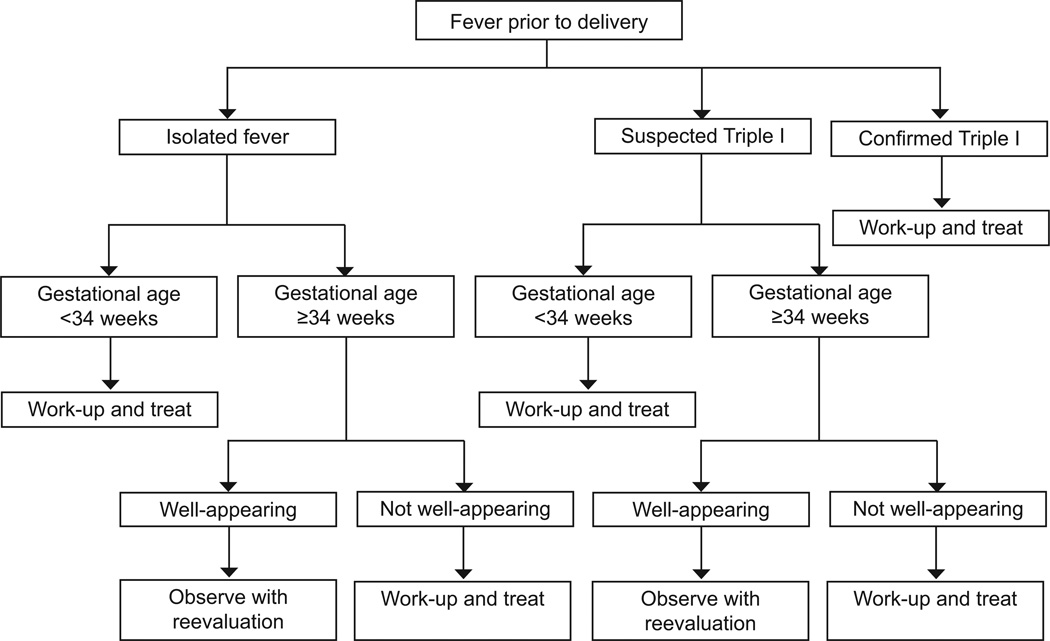
We recommend that neonatal management be guided by the maternal category of isolated fever, suspected Triple I or confirmed Triple I, gestational age at delivery, and clinical evaluation of the neonate. Clearly, for the appropriate neonatal treatment to be applied, communication of the diagnosis between obstetric and neonatal teams is essential. A proposed algorithm for neonatal management is provided in Figure 1. Typically, management is different for late preterm and term infants compared to infants born < 34 weeks gestation.
Figure 1.
Proposed algorithm for neonatal management.
Late preterm and term neonates
In cases of isolated maternal fever not attributable to Triple I, current evidence suggests that treatment is not beneficial for well-appearing late preterm and term infants, regardless of whether or not the mother was given antimicrobial agents. Conversely, when there is confirmed Triple I, these infants should be assessed and treated per current guidelines.9–11 When Triple I is suspected, but not confirmed, care should be individualized, but the majority of well-appearing late preterm and term infants can be observed without receiving antimicrobial agents provided they remain asymptomatic. The sepsis calculator of Puopolo et al15 may help with decision to treat or not to treat in cases with suspected Triple I. Using the original “sepsis calculator”, if the hypothetical risk of sepsis ranged from 0.65 to 1.54 per 1,000 live births (based solely on historical risk factors) 823 well-appearing infants born to women with suspected Triple I would need treatment to capture the one truly -infected infant (the number needed to treat). Such newborns account for 11% of all live births. If the risk of sepsis at birth was less than 0.65 per 1,000, 9,370 newborns would need treatment to identify the one truly infected infant. All infants born to women with suspected or proven chorioamnionitis who are not treated need frequent close observations.
Neonates < 34 0/7 weeks of gestation
There is currently no sepsis calculator for newborns < 34 weeks gestation. However, premature infants born to women with risk factors for sepsis (including suspected or confirmed Triple I) are at a much higher risk for EOS.46,47 Therefore, the threshold for evaluation and treatment of these neonates should be significantly lower when compared to the late preterm and term infants. There is a strong inverse relationship between gestational age at birth and the likelihood of an infectious etiology, especially when Triple I is suspected or confirmed. Therefore, infants born at < 34 0/7 weeks gestation to women with suspected or proven Triple I should be started on antimicrobial agents as soon as cultures are obtained. Healthy appearing premature infants < 34 weeks gestation born to women with isolated maternal fever might be observed if laboratory testing is not suggestive of sepsis, but this recommendation is not evidence based. Furthermore as the degree of prematurity increases, most of these infants will be symptomatic and not meet the designation of “healthy appearing”.
Duration of Infant Antimicrobial Therapy
Once antimicrobial therapy is started, evidence to guide the duration of treatment is limited. The NICE guidelines11 suggest 36 hours of antimicrobial agents for term newborns while awaiting negative blood culture results. Studies are warranted to guide clinical practice for duration of antimicrobial treatment when cultures are negative. In most well-appearing infants there is no compelling evidence that antimicrobial agents need to be continued beyond 48 hours, especially when blood cultures are negative, and irrespective of how “abnormal” laboratory data are found in these newborns. Information regarding duration of antimicrobial therapy for “rule out sepsis” predates routine GBS screening and prophylaxis. Duration of antimicrobial agents was based on information from the 1970’s assessing how long cultures generally needed to be evaluated to determine if bacteria were present.48 There is ongoing concern about the validity of blood cultures in infants born to women who received broad-spectrum antimicrobial agents prior to delivery. More research is needed to address this concern, because it is a common reason for treating newborn with antimicrobial agents for five or more days.
Cost Implications
Depending on individual hospital practice, some well-appearing newborns that undergo evaluation or treatment for possible infection may be admitted to transitional care units, special care units, or newborn intensive care units (NICU). Costs vary widely depending where the evaluation and care are rendered in the hospital. The workshop participants agreed that evaluation of the well-appearing neonate can be performed in the regular nursery or in the mother-baby unit.
Systems Issues
The management of isolated maternal fever and Triple I require important practical and logistical issues to be addressed. Communication regarding the maternal diagnosis among providers for optimal maternal and neonatal management is necessary. Improved communication also should occur during the postpartum period, as the maternal course, laboratory results, and histopathology results in the hours and days after delivery may be relevant to the management and treatment of the newborn infant. Communication at the time of patient handoff (shift change) also is key to insuring continuity of care. Institution of a checklist that would convey information needed to assess and manage the infant may be helpful. Box 1 provides items that could be potentially included on such a list. Further, systems to communicate this postnatal information to the neonatal team should be established as well as neonatal information (i.e. positive culture) to the obstetric team.
Education of obstetric and pediatric or neonatal staff is important for communication, identification, and appropriate treatment of mothers and infants at risk for Triple I. Programs for recognition, evaluation, and intervention for triple I should be introduced in labor and delivery, postpartum, and neonatal wards. Audit and feedback mechanisms can be utilized to determine whether guidelines are being followed and to identify opportunities for quality improvement.
Research Opportunities and Gaps
Multiple areas in need of further investigation were identified during the workshop (Table 2). Key areas for study include accurate identification of infection during labor and appropriate treatment of mothers to avoid poor maternal and neonatal outcomes. Much work is needed in the neonatal arena, particularly evidence-based studies for the management of the well-appearing late preterm and term infant. Trials evaluating the effects of withholding antimicrobial agents, as well as discontinuation of treatment after short periods of time (24–48 hours), could greatly reduce antimicrobial exposure of newborns and shorten hospital length of stay. Biomarkers and prediction models are likely to facilitate management of mothers and their newborns.
Table 2.
Research gaps and opportunities
| Area | Maternal Topics | Neonatal Topics |
|---|---|---|
| Prevention of infection | Yes | Yes |
| Prediction of infection | Yes Colonization versus infection |
Yes |
| Scoring system for probability of sepsis; infection prediction models to guide clinical management | Yes, placental histology, microbiome | Yes – need to define by GA, microbiome |
| Isolated fever in labor | Management – antipyretics, NSAIDs, antimicrobial agents | Management evaluation and antimicrobial agents |
| Biomarkers | Prediction, Consensus for design of biomarker validation studies and/or reporting of accuracy | Prediction, guidance for management, consensus for design of biomarker validation studies or reporting of accuracy |
| Outcomes | In hospital; subsequent reproductive outcomes | In-hospital, morbidities; longer term outcomes including neurodevelopment |
| Antimicrobial agents | Timing, duration, selection of antimicrobial agents used | Timing, duration, selection of antimicrobial agents used |
| Post-partum events | Fever, clinical course and its relationship to newborn’s care and management | |
| “Epidural fever” investigation | Management and treatment | Management and treatment |
| Maternal Fever | Timing, duration, height and impact on clinical care and course | Timing, duration, height and impact on clinical care and course |
| Duration of antimicrobial therapy | Timing and selection of antimicrobial agents | Term infant- well appearing-Term infant –symptomatic Term infant – resolved minor symptoms Preterm infant |
| Link studies – mother and baby cohorts | Impact of infection on neurodevelopment impairment or CP | |
| Observation versus treatment | Low risk cohorts | |
| Corticosteroids | Effects –short and long term | Effects prenatal and post-natal |
| Microbiome – maternal-fetal microbiome ecosystem | Perturbations, influence of GI flora on GU flora | Symbiosis versus pathology |
Discussion
Clinical use of the term chorioamnionitis is outdated and overused, and implies the presence of infection. Use of the phrase maternal chorioamnionitis has significant implications for both mother and baby. The expert panel recommended the use of new terminology, specifically Triple I, with the term chorioamnionitis restricted to pathologic diagnosis. The participants identified many gaps in research and opportunities to advance knowledge to affect care for the health of mothers and babies. Better evidence to guide appropriate provision of care is desperately needed.
Supplementary Material
Box 1. Checklist of items to include in communication between the obstetric and neonatal team.
Gestational age
Maternal tachycardia
Fetal tachycardia
Maternal white blood cell count > 15,000
Maternal GBS status
Duration of rupture of membranes
Duration of labor
Purulent fluid
Amniotic fluid evaluation
Highest maternal temperature
Epidural anesthesia use
Prostaglandin use
Antimicrobial agent(s)used
Antipyretic used
Spontaneous preterm birth
Prior spontaneous preterm birth
Acknowledgments
The National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD staff had input into conference and manuscript. The content of the summary is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The workshop was co-funded by the American College of Obstetricians and Gynecologists, American Academy of Pediatrics, and the Society of Maternal-Fetal Medicine.
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
References
- 1.Blanc WA. Pathways of fetal and early neonatal infection. Viral placentitis, bacterial and fungal chorioamnionitis. J Pediatr. 1961;59:473–496. doi: 10.1016/s0022-3476(61)80232-1. [DOI] [PubMed] [Google Scholar]
- 2.Mulla ZD, Ebrahim MS, Gonzalez JL. Anaphylaxis in the obstetric patient: analysis of a statewide hospital discharge database. Ann Allergy Asthma Immunol. 2010;104(1):55–59. doi: 10.1016/j.anai.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Berenguer A, Couto A, Brites V, Fernandes R. Anaphylaxis in pregnancy: a rare cause of neonatal mortality. BMJ Case Rep. 2013 Jan 11; doi: 10.1136/bcr-2012-007055. 2013. pii: bcr2012007055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mukhopadhyay S, Dukhovny D, Mao W, Eichenwald EC, Puopolo KM. 2010 Perinatal GBS Prevention Guideline and Resource Utilization. Pediatrics. 2014;133(2):196–203. doi: 10.1542/peds.2013-1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiser C, Nawab U, McKenna K, Aghai ZH. Role of Guidelines on Length of Therapy in Chorioamnionitis and Neonatal Sepsis. Pediatrics. 2014;133(6):992–998. doi: 10.1542/peds.2013-2927. [DOI] [PubMed] [Google Scholar]
- 6.Mukherjee A, Davidson L, Anguvaa L, Duffy DA, Kennea N. NICE neonatal early onset sepsis guidance: greater consistency, but more investigations, and greater length of stay. Arch Dis Child Fetal Neonatal Ed. 2014;0:F1–F2. doi: 10.1136/archdischild-2014-306349. [DOI] [PubMed] [Google Scholar]
- 7.Riley LE, Celi AC, Onderdonk AB, Roberts DJ, Johnson LC, Tsen LC, Leffert L, Pian-Smith MC, Heffner LJ, Haas ST, Lieberman ES. Association of epidural-related fever and noninfectious inflammation in term labor. Obstet Gynecol. 2011 Mar;117(3):588–595. doi: 10.1097/AOG.0b013e31820b0503. [DOI] [PubMed] [Google Scholar]
- 8.Sharma SK, Rogers BB, Alexander JM, McIntire DD, Leveno KJ. A randomized trial of the effects of antibiotic prophylaxis on epidural-related fever in labor. Anesth Analg. 2014;118(3):604–610. doi: 10.1213/ANE.0b013e3182a5d539. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Prevention of perinatal group B streptococcal disease—revised guidelines from CDC, 2010. MMWR Recomm Rep. 2010;59(RR-10):1–36. [PubMed] [Google Scholar]
- 10.Polin R The Committee on Fetus and Newborn. Management of Neonates with Suspected or Proven Early-Onset Bacterial Sepsis. Pediatrics. 2012;129(5):1006–1015. doi: 10.1542/peds.2012-0541. [DOI] [PubMed] [Google Scholar]
- 11.NICE. Intrapartum care: care of healthy women and their babies during childbirth. NICE clinical guideline 190. 2014:1–108. [Google Scholar]
- 12.Madan JC, Salari RC, Saxena D, Davidson L, O’Toole GA, Moore JH, et al. Gu.t microbial colonization in premature neonates predicts neonatal sepsis. Ach Dis Child Fetal Neonatal Ed. 2012;97(6):F456–F462. doi: 10.1136/fetalneonatal-2011-301373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azad MB, Konya T, Persaud RR, Guttman DS, Chari RS, Field CJ, et al. Impact of maternal intrapartum antibiotics, method of birth and breastfeeding on gut microbiota during the first year of life: a prospective cohort study. BJOG. 2015 doi: 10.1111/1471-0528.13601. [DOI] [PubMed] [Google Scholar]
- 14.Stoll BJ, Hansen NI, Sánchez PJ, Faix RG, Poindexter BB, Van Meurs KP, et al. Early Onset Neonatal Sepsis: The Burden of Group B Streptococcal and E. coli Disease Continues. Pediatrics. 2011;127(5):817–826. doi: 10.1542/peds.2010-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puopolo KM, Draper D, Wi S, Newman TB, Zupancic J, Lieberman E, et al. Estimating the probability of neonatal early-onset infection on the basis of maternal risk factors. Pediatrics. 2011 Nov;128(5):e1155–e1163. doi: 10.1542/peds.2010-3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shakib J, Buchi K, Smith E, Young PC. With chorioamnionitis: is it a time for a kinder, gentler approach? Academic Pediatrics. 2015;15:340–344. doi: 10.1016/j.acap.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Escobar GJ, Puopolo KM, Wi S, Turk BJ, Kuzniewicz MW, Walsh EM, et al. Stratification of risk of early-onset sepsis in newborns ≥ 34 weeks' gestation. Pediatrics. 2014;133(1):30–36. doi: 10.1542/peds.2013-1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macones GA, Hankins GD, Spong CY, Hauth J, Moore T. The 2008 National Institute of Child Health and Human Development workshop report on electronic fetal monitoring: update on definitions, interpretation, and research guidelines. J Obstet Gynecol Neonatal Nurs. 2008;37(5):510–515. doi: 10.1111/j.1552-6909.2008.00284.x. [DOI] [PubMed] [Google Scholar]
- 19.Tita AT, Andrews WW. Diagnosis and management of clinical chorioamnionitis. Clin Perinatol. 2010;37(2):339–354. doi: 10.1016/j.clp.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avila C, Willins JL, Jackson M, Mathai J, Jabsky M, Kong A, et al. Usefulness of two clinical chorioamnionitis definitions in predicting neonatal infectious outcomes: a systematic review. Am J Perinatol. 2015;32:1001–1009. doi: 10.1055/s-0035-1547325. [DOI] [PubMed] [Google Scholar]
- 21.Banerjee S, Cashman P, Yentis SM, Steer PJ. Maternal temperature monitoring during labor: concordance and variability among monitoring sites. Obstet Gynecol. 2004;103(2):287–293. doi: 10.1097/01.AOG.0000100155.85379.88. [DOI] [PubMed] [Google Scholar]
- 22.Buhimschi CS, Bhandari V, Hamar BD, Bahtiyar MO, Zhao G, Sfakianaki AK, et al. Proteomic profiling of the amniotic fluid to detect inflammation, infection, and neonatal sepsis. PLoS Med. 2007;4(1):e18. doi: 10.1371/journal.pmed.0040018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdel-Razeq SS, Buhimschi IA, Bahtiyar MO, Rosenberg VA, Dulay AT, Han CS, et al. Interpretation of amniotic fluid white blood cell count in "bloody tap" amniocenteses in women with symptoms of preterm labor. Obstet Gynecol. 2010;116(2 Pt 1):344–354. doi: 10.1097/AOG.0b013e3181e8fec6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342(20):1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 25.Dulay AT, Buhimschi IA, Zhao G, Bahtiyar MO, Thung SF, Cackovic M, et al. Compartmentalization of acute phase reactants Interleukin-6, C-Reactive Protein and Procalcitonin as biomarkers of intra-amniotic infection and chorioamnionitis. Cytokine. 2015 May 6; doi: 10.1016/j.cyto.2015.04.014. pii: S1043-4666(15)00165-9. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharp GC, Stock SJ, Norman JE. Fetal assessment methods for improving neonatal and maternal outcomes in preterm prelabour rupture of membranes. Cochrane Database Syst Rev. 2014 Oct 3;10:CD010209. doi: 10.1002/14651858.CD010209.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aguin E, Aguin T, Cordoba M, Aguin V, Roberts R, Albayrak S, et al. Amniotic fluid inflammation with negative culture and outcome after cervical cerclage. J Matern Fetal Neonatal Med. 2012;25(10):1990–1994. doi: 10.3109/14767058.2012.667177. [DOI] [PubMed] [Google Scholar]
- 28.Vaisbuch E, Hassan SS, Mazaki-Tovi S, Nhan-Chang CL, Kusanovic JP, Chaiworapongsa T, et al. Patients with an asymptomatic short cervix (<or=15 mm) have a high rate of subclinical intraamniotic inflammation: implications for patient counseling. Am J Obstet Gynecol. 2010;202(5):433.e1–433.e8. doi: 10.1016/j.ajog.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lisonkova S, Sabr Y, Joseph KS. Diagnosis of subclinical amniotic fluid infection prior to rescue cerclage using gram stain and glucose tests: an individual patient meta-analysis. J Obstet Gynaecol Can. 2014;36(2):116–122. doi: 10.1016/S1701-2163(15)30656-3. [DOI] [PubMed] [Google Scholar]
- 30.Hassan S, Romero R, Hendler I, Gomez R, Khalek N, Espinoza J, et al. A sonographic short cervix as the only clinical manifestation of intra-amniotic infection. J Perinat Med. 2006;34(1):13–19. doi: 10.1515/JPM.2006.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buhimschi CS, Bhandari V, Hamar BD, Bahtiyar MO, Zhao G, Sfakianaki AK, et al. Proteomic profiling of the amniotic fluid to detect inflammation, infection, and neonatal sepsis. PLoS Med. 2007;4(1):e18. doi: 10.1371/journal.pmed.0040018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han YW, Shen T, Chung P, Buhimschi IA, Buhimschi CS. Uncultivated bacteria as etiologic agents of intra-amniotic inflammation leading to preterm birth. J Clin Microbiol. 2009;47(1):38–47. doi: 10.1128/JCM.01206-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X, Buhimschi CS, Temoin S, Bhandari V, Han YW, Buhimschi IA. Comparative microbial analysis of paired amniotic fluid and cord blood from pregnancies complicated by preterm birth and early-onset neonatal sepsis. PLoS One. 2013;8(2):e56131. doi: 10.1371/journal.pone.0056131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steinberger E, Hofer N, Resch B. Cord blood procalcitonin and Interleukin-6 are highly sensitive and specific in the prediction of early-onset sepsis in preterm infants. Scand J Clin Lab Invest. 2014;74(5):432–436. doi: 10.3109/00365513.2014.900696. [DOI] [PubMed] [Google Scholar]
- 35.Cobo T, Kacerovsky M, Andrys C, Drahosova M, Musilova I, Hornychova H, Jacobsson B. Umbilical cord blood IL-6 as predictor of early-onset neonatal sepsis in women with preterm prelabour rupture of membranes. PLoS One. 2013;8(7):e69341. doi: 10.1371/journal.pone.0069341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howman RA, Charles AK, Jacques A, Doherty DA, Simmer K, Strunk T, Richmond PC, Cole CH, Burgner DP. Inflammatory and haematological markers in the maternal, umbilical cord and infant circulation in histological chorioamnionitis. PLoS One. 2012;7(12):e51836. doi: 10.1371/journal.pone.0051836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fan Y, Yu JL. Umbilical blood biomarkers for predicting early-onset neonatal sepsis. World J Pediatr. 2012;8(2):101–108. doi: 10.1007/s12519-012-0347-3. [DOI] [PubMed] [Google Scholar]
- 38.Buhimschi CS, Bhandari V, Dulay AT, Nayeri UA, Abdel-Razeq SS, Pettker CM, et al. Proteomics mapping of cord blood identifies haptoglobin "switch-on" pattern as biomarker of early-onset neonatal sepsis in preterm newborns. PLoS One. 2011;6(10):e26111. doi: 10.1371/journal.pone.0026111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiesa C, Signore F, Assumma M, Buffone E, Tramontozzi P, Osborn JF, et al. Serial measurements of C-reactive protein and interleukin-6 in the immediate postnatal period: reference intervals and analysis of maternal and perinatal confounders. Clin Chem. 2001;47(6):1016–1222. [PubMed] [Google Scholar]
- 40.Chiesa C, Pellegrini G, Panero A, Osborn JF, Signore F, Assumma M, et al. C-reactive protein, interleukin-6, and procalcitonin in the immediate postnatal period: influence of illness severity, risk status, antenatal and perinatal complications, and infection. Clin Chem. 2003;49(1):60–68. doi: 10.1373/49.1.60. [DOI] [PubMed] [Google Scholar]
- 41.Delanghe JR, Speeckaert MM. Translational research and biomarkers in neonatal sepsis. Clin Chim Acta. 2015 Feb 4; doi: 10.1016/j.cca.2015.01.031. pii: S0009-8981(15)00053-4. [DOI] [PubMed] [Google Scholar]
- 42.Wynn JL, Wong HR, Shanley TP, Bizzarro MJ, Saiman L, Polin RA. Time for a neonatal-specific consensus definition for sepsis. Pediatr Crit Care Med. 2014;15(6):523–528. doi: 10.1097/PCC.0000000000000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turnquest MA, How HY, Cook CR, O'Rourke TP, Cureton AC, Spinnato JA, Brown HL. Chorioamnionitis: is continuation of antibiotic therapy necessary after cesarean section? Am J Obstet Gynecol. 1998;179(5):1261–1266. doi: 10.1016/s0002-9378(98)70143-7. [DOI] [PubMed] [Google Scholar]
- 44.Edwards RK, Duff P. Single additional dose postpartum therapy for women with chorioamnionitis. Obstet Gynecol. 2003;102:957–961. doi: 10.1016/s0029-7844(03)00863-9. [DOI] [PubMed] [Google Scholar]
- 45.Black LP, Hinson L, Duff P. Limited course of antibiotic treatment for choriomanionitis. Obstet Gynecol. 2012;119:1102–1105. doi: 10.1097/AOG.0b013e31824b2e29. [DOI] [PubMed] [Google Scholar]
- 46.Mukhopadhyay S, Puopolo KM. Neonatal Early-Onset Sepsis: Epidemiology and Risk Assessment. NeoReviews. 2015;16(4):e221–e230. [Google Scholar]
- 47.Thomas W, Speer CP. Chorioamnionitis: important risk factor or innocent bystander for neonatal outcome? Neonatology. 2011;99(3):177–187. doi: 10.1159/000320170. [DOI] [PubMed] [Google Scholar]
- 48.Wientzen RL, McCracken GH. Pathogenesis and management of neonatal sepsis and meningitis. Curr Probl Pediatr. 1977 Dec 8;(2):1–61. doi: 10.1016/s0045-9380(77)80005-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.