Introduction
Febuxostat is a unique xanthine oxidase inhibitor that was approved for the prevention of gout in the United States in 2009.1 Unlike allopurinol, febuxostat is not a nucleoside analogue and has a higher specificity for xanthine oxidase. Although this drug has not been on the market for a long time, febuxostat may be safer than allopurinol.2,3 Importantly, febuxostat appears to be safer than allopurinol, which has been linked to many instances of severe hypersensitivity reactions, including skin rash with eosinophilia and systemic manifestations (DRESS syndrome), Stevens Johnson syndrome, acute nephritis, acute icteric hepatitis and acute liver failure.3,4 While febuxostat is not infrequently associated with serum enzyme elevations during treatment, it has yet to be linked to cases of clinically apparent acute liver injury.3, 4 We report a case of acute liver injury with jaundice due to febuxostat and which was identified in a prospective study of drug-induced liver injury in the United States.5
Case
A 34 year old man with obesity and severe gout developed fatigue, nausea, and abdominal pain followed by dark urine, jaundice and itching 2 months after adding febuxostat (40 mg daily) to his regular regimen of colchicine (1.2 mg) and allopurinol (300 mg) per day. He had no history of liver disease, alcohol use or risk factors for viral hepatitis. His other medical problems included obesity, hypertension, dyslipidemia, sleep apnea, depression and inactive sarcoidosis. Other medications included simvastatin, lisinopril, hydrochlorothiazide, ranitidine, amitriptyline and acetaminophen with codeine (1-2 tablets daily). He denied use of herbals or over-the-counter products.
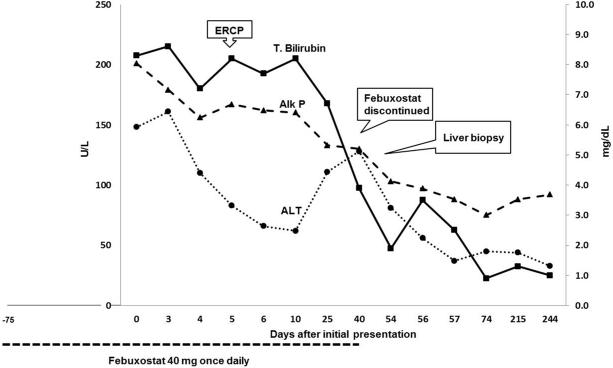
At presentation, physical examination showed severe obesity (BMI 63.3 kg/m2) and jaundice, but no fever, rash or signs of chronic liver disease. Serum bilirubin was 8.3 mg/dL, alkaline phosphatase (Alk P) 201 U/L, alanine aminotransferase (ALT) 148 U/L and amylase 779 U/L. Complete blood count was normal with 5% eosinophils (normal 1-4%); INR was 1.09. Tests for hepatitis A, B, C and E were negative as were antinuclear and smooth muscle antibodies. Abdominal ultrasound and computerized tomography showed gallstones but no evidence of biliary obstruction. An ERCP confirmed these findings. Due to elevated bilirubin and acute pancreatitis in the context of extreme obesity, a decision to perform ERCP rather than a MRCP was made. At that time, allopurinol, hydrochlorothiazide and lisinopril were stopped, while febuxostat was continued. Laboratory results improved minimally (Figure 1). A liver biopsy showed changes suggestive of intrahepatic cholestasis with mild inflammation and necrosis but without bile duct injury or fibrosis (Figure 2). Forty days after presentation, febuxostat was finally discontinued and was promptly associated with symptom resolution and normalization of liver tests. Allopurinol, lisinopril and hydrochlorothiazide were restarted and liver tests remained normal at the 6 month follow up visit per protocol.
Figure 1.
Schematic representation of exposure, dechallenge, clinical events with trends of serum liver biochemistries during the clinical course and follow up.
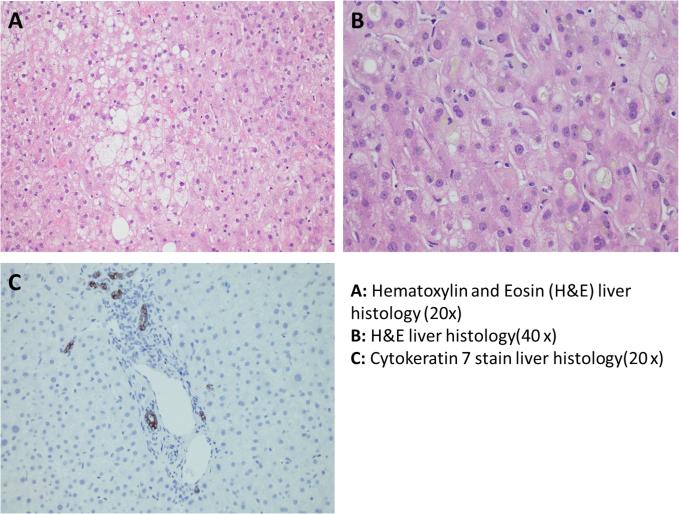
Figure 2.
Light microscopy with magnification of liver histology with stains. Panel A. Hematoxylin and Eosin (H&E) – 20×: At low power, the biopsy shows hepatocytes with significant ballooning injury. Panel B. H&E – 40×: At high power, the biopsy shows marked cellular and canalicular cholestasis, as well as ballooned hepatocytes. Panel C. CK7 – 20×: The cytokeratin 7 immunohistochemical stain shows mild ductular reaction.
Discussion
The clinical course, serum enzyme elevations, liver histology and response to stopping treatment were most consistent with the diagnosis of febuxostat-induced acute liver injury. Other common causes of acute liver injury were excluded and other potentially hepatotoxic drugs were restarted without recurrence of injury. Adjudication by 3 reviewers in the Drug-Induced Liver Network scored the case as “highly likely” due to febuxostat.5 The latency of 2 months, cholestatic pattern of serum enzyme elevations and liver histology of cholestatic hepatitis were typical of drug-induced liver injury, but somewhat atypical of allopurinol hepatotoxicity, which usually causes hepatocellular injury features of hypersensitivity (fever, rash, eosinophilia) and acute hepatitis with eosinophils and granulomas on liver biopsy. While febuxostat was not linked to instances of clinically apparent liver injury in pre-licensure studies, it was associated with serum enzyme elevations.3 In this case, febuxostat was associated with jaundice probably because of its continuation after onset of liver injury. Thus, febuxostat is a rare but convincing cause of clinically apparent liver injury presenting with a cholestatic pattern of liver test abnormalities without evidence of hypersensitivity or autoimmune features and with rapid improvement once therapy is discontinued.
Acknowledgements
The authors thank the members of the Drug-Induced Liver Injury Network (names given in reference 5) for support and Drs. Jay H. Hoofnagle and Romil Saxena for help in preparing the manuscript.
Abbreviations
- ALT
alanine aminotransferase
- Alk P
alkaline phosphatase
Footnotes
Financial Conflicts of Interest: Dr. Chalasani serves as a consultant for Merck, Sanofi, Salix, Abbott, Lilly, and Mochida for drug hepatotoxicity. He has research funding from Intercept, Lilly, Gilead, and GenFit. Dr. Vuppalanchi served on the Speaker's Bureau for Gilead and as a consultant to Pfizer and Bristol Myers Squibb for drug hepatotoxicity. Dr. Bohm declares no conflict of interest.
References
- 1.Bruce SP. Febuxostat: a selective xanthine oxidase inhibitor for the treatment of hyperuricemia and gout. Ann Pharmacother. 2006;40:2187–94. doi: 10.1345/aph.1H121. [DOI] [PubMed] [Google Scholar]
- 2.Becker MA, Schumacher HR, MacDonald PA, Lloyd E, Lademacher C. Clinical efficacy and safety of successful longterm urate lowering with febuxostat or allopurinol in patients with gout. J Rheumatol. 2009;36:1273–82. doi: 10.3899/jrheum.080814. [DOI] [PubMed] [Google Scholar]
- 3.Becker MA, Schumacher HR, Jr., Wortmann RL, MacDonald PA, Palo WA, Eustace D, Vernillet L, Joseph-Ridge N. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005;353:2450–61. doi: 10.1056/NEJMoa050373. [DOI] [PubMed] [Google Scholar]
- 4.Febuxostat. http://livertox.nlm.nih.gov [last accessed August 10, 2014]
- 5.Fontana RJ, Watkins PB, Bonkovsky HL, Chalasani N, Davern T, Serrano J, Rochon J. DILIN Study Group. Drug-Induced Liver Injury Network (DILIN) prospective study: rationale, design and conduct. Drug Safety. 2009;32:55–68. doi: 10.2165/00002018-200932010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]