Abstract
Lifestyle factors have been well-studied in relation to breast cancer prognosis overall, however, associations of lifestyle and late outcomes (>5 after diagnosis) have been much less studied, and no studies have focused on ER+ breast cancer survivors, who may have high risk of late recurrence and mortality. We utilized a large prospective pooling study to evaluate the associations of lifestyle factors with late recurrence and all-cause mortality among 6,295 5-year ER+ stage I–III breast cancer survivors. Pooled and harmonized data were available on clinical factors and lifestyle factors (pre-to-post-diagnosis weight change, BMI (kg/m2), recreational physical activity (PA), alcohol intake, and smoking history), measured on average 2.1 years after diagnosis. Updated information for weight only was available. Study heterogeneity was evaluated by the Q statistic. Multivariable Cox regression models were stratified by study. Adjusting for clinical factors and potential confounders, ≥10% weight gain and obesity (BMI 30–34.99 and ≥35) were associated with increased risk of late recurrence (HRs (95% CIs): 1.24 (1.00–1.53), 1.40 (1.05–1.86) and 1.41 (1.02–1.93), respectively). Daily alcohol intake was associated with late recurrence, 1.28 (1.01–1.62). PA was inversely associated with late all-cause mortality (0.81 (0.71–0.93) and 0.71 (0.61–0.82) for 4.9–<17.4 and ≥17.4 MET-h/wk). A U-shaped association was observed for late all-cause mortality and BMI using updated weight (1.42 (1.15–1.74) and 1.40 (1.09–1.81), <21.5 and ≥ 35, respectively). Smoking was associated with increased risk of late outcomes. In this large prospective pooling project, modifiable lifestyle factors were associated with late outcomes among long-term ER+ breast cancer survivors.
Keywords: lifestyle factors, recurrence, mortality, breast cancer, prospective, cohort
INTRODUCTION
In 2011, a meta-analysis of 20 clinical trials reported that even among women treated with tamoxifen for 5 years, there was considerable risk of recurrence in later years for women with estrogen receptor positive (ER+) breast cancer.1 Specifically, the probability of breast cancer recurrence was 25.9% at 10 years and 33.0% at 15 years. Studies have also shown that, compared to women with ER- breast cancer, women with ER+ breast cancer have a better prognosis in the first several years after diagnosis, but may have higher risk of recurrence in later years after diagnosis.2–8 Despite this, risk factors for late outcomes are not yet established.
Modifiable lifestyle factors, such as body mass index (BMI), weight change, and physical activity, have been well-studied in relation to overall breast cancer prognosis.9–13 Evidence is most consistent for an association of obesity at or around the time of diagnosis with poorer prognosis, and an association of physical activity with reduced risk of mortality in breast cancer survivors. While the importance of lifestyle factors in overall breast cancer prognosis has been demonstrated in many studies, associations of lifestyle factors with late outcomes (>5 after diagnosis) have been much less studied, especially in ER+ breast cancer survivors. Some studies have examined associations for tumor characteristics and molecular markers with late recurrence specifically in ER+ breast cancer;14–16 however, no studies to date have investigated modifiable lifestyle factors.
Late breast cancer outcomes are a major concern in ER+ breast cancer, which accounts for close to two-thirds of all breast cancer diagnosed.1, 6, 16 Therefore, it is of critical importance to understand potentially modifiable factors that may be uniquely associated with these late breast cancer outcomes among women with ER+ breast cancer. The After Breast Cancer Pooling Project (ABCPP) includes data from several long-term (>10 years), prospective cohorts of breast cancer survivors, providing the opportunity to evaluate the role of lifestyle factors after diagnosis in long-term breast cancer outcomes among a large sample of ER+ survivors. The purpose of the present study was to evaluate the associations of post-diagnosis lifestyle factors that have been well-studied in association with breast cancer prognosis overall with late breast cancer outcomes among ER+ breast cancer survivors.
MATERIALS AND METHODS
After Breast Cancer Pooling Project
The ABCPP includes pooled data on 18,363 breast cancer survivors aged 20 to 83 years from four prospective cohorts recruited from U.S. sites and Shanghai, China diagnosed with invasive breast cancer between 1976 and 2004.17 Three cohorts recruited only breast cancer patients: the Shanghai Breast Cancer Survival Study (SBCSS),18 the Life After Cancer Epidemiology (LACE) Study,19 and the Women’s Healthy Eating & Living (WHEL) Study.20 The WHEL study was an intervention trial (1995–2006) designed to test adoption of a diet high in vegetables, fruit, and fiber and low in fat among breast cancer survivors. The findings were null, and therefore WHEL was treated as a cohort study.21 The fourth cohort consists of breast cancer patients participating in the Nurses’ Health Study (NHS).22 WHEL and LACE only enrolled participants who had completed primary treatment. All participants provided informed consent. Institutional review board approval was obtained for each study and for the ABCPP. Pooled and harmonized data were available for post-diagnosis lifestyle factors, cancer treatment, tumor characteristics, socio-demographics, and select major comorbidities.17
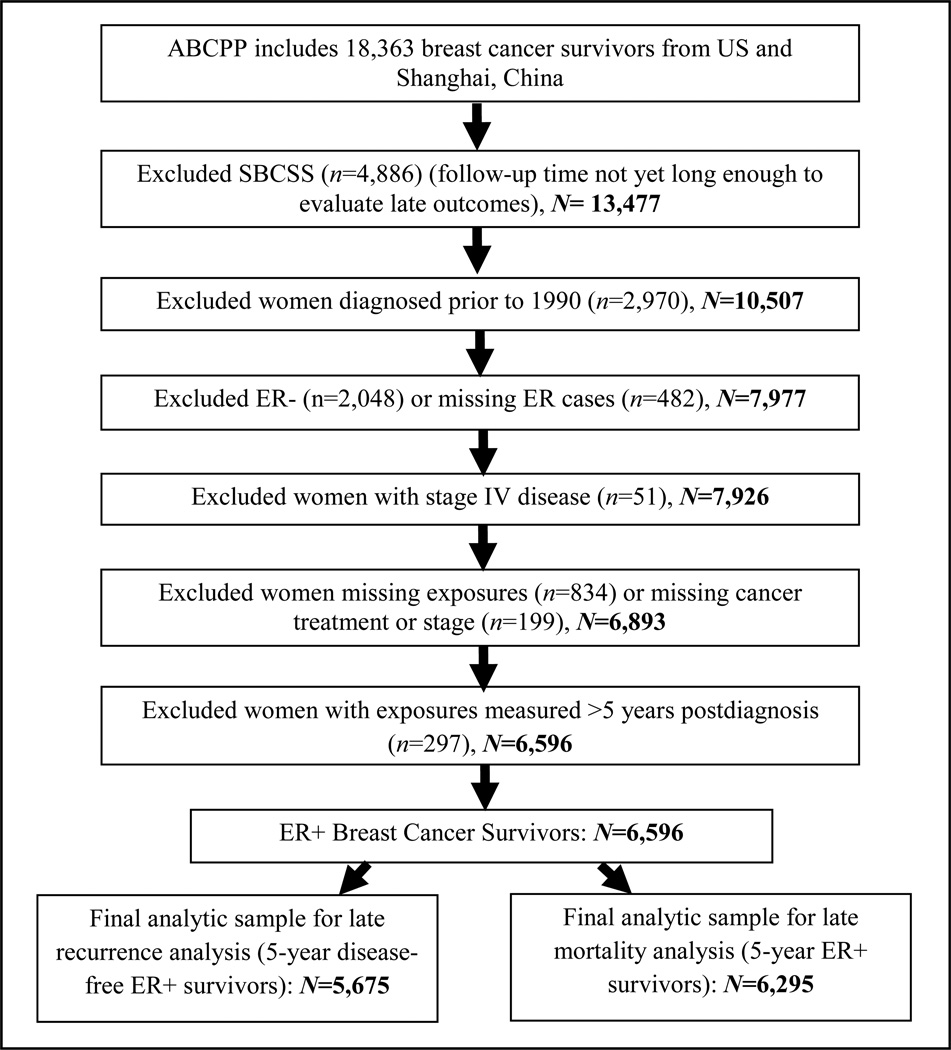
The present study included breast cancer survivors from the U.S. cohorts only, as the SBCSS cohort is the most recent cohort, and does not yet have enough long-term follow-up time for the evaluation of late outcomes (≥5 years after diagnosis). A detailed description of the study exclusions is shown in Figure 1. A total of 921 women were excluded from the recurrence analysis due to event/loss to follow-up prior to 5-years after diagnosis, resulting in 5,675 5-year disease-free survivors. A total of 599 women were excluded from the mortality analysis due to death/loss to follow-up prior to 5-years after diagnosis, resulting in 6,295 5-year ER+ survivors.
Figure 1.
Post-diagnosis Lifestyle Factors
Lifestyle factors were initially assessed at a mean of 2 years post-diagnosis. If the first post-diagnosis survey was <1 year after diagnosis, the second post-diagnosis survey was used for measurement of lifestyle factors. Height and weight after diagnosis were measured in-person by study staff for WHEL and were self-reported in the NHS and LACE. Pre-diagnosis weight was self-reported after diagnosis for LACE and WHEL participants at cohort enrollment and on a pre-diagnosis mailed questionnaire for the NHS. Absolute weight change was calculated as weight at first post-diagnosis assessment minus pre-diagnosis weight (at about 1 year prior to diagnosis of breast cancer). We classified percent weight change pre- to post-diagnosis with the following categories based on our previous work: stable (within 5%), moderate loss (5–10%), large loss (≥10%), moderate gain (5–10%), large gain (≥10%).23,24
Post-diagnosis BMI was calculated as weight in kg divided by height in meters squared and initially categorized using the World Health Organization classifications: underweight (<18.5 kg/m2), normal weight (18.5–24.99 kg/m2), overweight (25–29.99 kg/m2), and obese (≥30 kg/m2). The sample size for <18.5kg/m2 was too small for stable estimates, and therefore we re-classified women in the lowest two BMI categories as follows: <21.5 kg/m2 and 21.5–24.99 kg/m2. We further classified obese women as obese (BMI (30–<34.99 kg/m2) and severely obese (BMI ≥35 kg/m2), sample sizes were too small to examine the morbidly obese (>40 kg/m2) group.
Self-reported information on recreational physical activity was available for all cohorts, and was converted into metabolic equivalents (METs)25 in MET-hours/week for all activities combined. The physical activity assessments used in each cohort were previously evaluated for reproducibility and validity.26–28 Physical activity was classified based on tertiles (0–<4.9, 4.9–17.4, ≥17.4) and as meeting (yes or no) the U.S. 2008 recommendations (≥10-MET-h/w, equivalent to about 2.5 hours of moderate intensity activity per week),29 as results were similar regardless of classification only those for the tertile categorization are shown for multivariable models.
Post-diagnosis alcohol intake was assessed in each cohort via food frequency questionnaires.30 Alcohol intake was classified using cutpoints: <0.36 g/day (non-drinkers), 0.36–6 g/day, >6–<12 g/day, ≥12 g/day (6 g is equivalent to about one-half of an alcoholic beverage), and these cut points were used previously in our research.30 Smoking status was assessed at the first post-diagnosis survey, including information on current smoking and past smoking habits. Pack-years were calculated using the number of years smoked and number of cigarettes smoked. Smoking status at about two years post-diagnosis was categorized as never, former (<20 pack-years, ≥20 pack-years),31 and current (sample size was not large enough to examine pack-years of exposure among current smokers). Updated weight information was available for all cohorts at a second post-diagnosis time point (weight was the only lifestyle factor with updated information available). The updated weight was used to create updated post-diagnosis BMI and weight change (pre-diagnosis to the second post-diagnosis weight) variables, using the same classifications as above.
Clinical Characteristics and Additional Covariates
Data on treatment included chemotherapy (yes, no), radiotherapy (yes, no), mastectomy (yes, no), and hormonal therapy (yes, no). Most women received tamoxifen, as the majority of cases were diagnosed before aromatase inhibitors were widely available. Tumor characteristics included estrogen receptor (ER) status, progesterone receptor (PR) status, and AJCC 6th edition stage (I, II, III, IV). Age at diagnosis, race/ethnicity, education, and family history of breast cancer were available for all cohorts. Menopausal status at diagnosis (or pre-diagnosis measurement closest to diagnosis for NHS) was classified as premenopausal, postmenopausal, and unclear/unknown.
Outcome Ascertainment
Detailed methods on outcome and follow-up have been previously published for the ABCPP,17 and each cohort (WHEL,20 LACE,19 NHS32). Briefly, during active follow-up each cohort followed participants to ascertain breast cancer outcomes (recurrence, metastasis, new primary breast cancer (except NHS), overall mortality, and cause-specific mortality). For the WHEL study, outcomes were obtained via semi-annual telephone contact and clinic visits through the end of the trial (June 2006) with all reported events confirmed by medical records review.21 Active follow-up for over half the cohort continued until June 2010 with subsequent follow-up for mortality outcomes only via linkage to death registries. For the LACE study, outcomes were ascertained on a semi-annual basis via mailed surveys until 5 years post-diagnosis and yearly thereafter, and medical records were obtained to verify any reported breast cancer outcomes.19 For the NHS, recurrences were collected via questionnaires to breast cancer patients (if a woman died of breast cancer without self-report of a recurrence, the date of recurrence was assigned as 1 year prior to death). For all cohorts, mortality information was obtained via periodic linkages to the Social Security Index and the National Death Index, and for LACE, periodic linkages were also made to Kaiser Permanente Northern California electronic data sources, while for NHS deaths were also reported through next of kin and the post office. Cause of death information was obtained from the National Death Index, state death certificates, and/or medical records.
Statistical Analysis
Outcomes for the present analysis included late (≥5 years) disease-free survival (hereafter referred to as recurrence for brevity) with an event defined as recurrence, metastasis, new breast primary or breast cancer death, whichever occurred first; and late (≥5 years) all-cause mortality. Follow-up time started at 5 years post-diagnosis,33 and the recurrence analysis included 5 year disease-free survivors and the mortality analysis included 5 year survivors, regardless of whether they had a recurrence.34 The exit date was date of death (or recurrence for the recurrence analysis) or date of last contact (i.e., date of last follow-up survey or last registry linkage, whichever was most recent).
Initially, study-specific adjusted HRs and their corresponding 95% CIs were derived from Cox regression models. The Q statistic was used to test for heterogeneity in risk estimates across studies.35 If heterogeneity was observed, we conducted a random-effects meta-analysis, with study-specific hazards ratios using inverse-variance weights in random-effects models.36 If heterogeneity was not observed, we conducted a pooled analysis using combined data with HRs and 95% CIs from Cox regression models stratified by study (i.e., study was as a variable in the STRATA statement).36 The Q statistic was statistically significant for 4 models for only a specific category of the exposure, including (1) late recurrence and post-diagnosis BMI 25–29.99 kg/m2 (P =0.026), (2) late mortality and weight loss ≥10% (P =0.036), (3) late mortality and post-diagnosis BMI 30–34.99 kg/m2 (P =0.016), and (4) late mortality and alcohol intake of 6–<12 g/day (P =0.0095). To be consistent, all results for these associations were from a random effects meta-analysis,36 all other results shown are from the individually pooled analysis, and we provide a footnote to indicate if the results displayed in the Tables are from the random effects meta-analysis (see17, 36 for additional details on the analytic approach).
Covariates selected a priori included clinical characteristics and known breast cancer prognostic factors (age at diagnosis, stage, PR status, race/ethnicity, mastectomy, chemotherapy, radiotherapy, hormonal therapy, and menopausal status), and select major comorbidities available for all cohorts (diabetes, hypertension). Weight change models were adjusted for pre-diagnosis BMI. Multivariable models were also adjusted for the lifestyle factors of interest (when these variables were not the main exposures being modeled). Time between exposure measurement and start of follow-up was included as a covariate.
For comparison, we also evaluated associations for each lifestyle factor and early recurrence and all-cause mortality (event within 5 years after diagnosis) (Supplemental Information, Table S1). It is important to note that (1) women survived on average 2 years before they were enrolled in the cohorts and (2) lifestyle factors were measured on average 2 years after diagnosis and up to four years after diagnosis, therefore, investigations of post-diagnosis lifestyle in association with early events are limited in the present analysis, in particular as survivors are ER+ breast cancer survivors, who have better survival in the first five years after diagnosis, which further reduces number of early events.
Tests for linear trend were calculated using the Wald test. The proportional hazards assumption was evaluated by testing the statistical significance of interaction terms for each covariate and survival time for all models. All analyses were performed using SAS (version 9.4; SAS Institute, Cary, NC). Tests of statistical significance were two-sided and P<0.05 were considered statistically significant.
RESULTS
Table 1 displays the number of events, follow-up time, clinical characteristics and post-diagnosis lifestyle data by cohort and combined for women diagnosed with ER+ breast cancer. About 49% of deaths were due to breast cancer, 17% were due to other cancers, 13% were due to CVD, and 21% were due to other causes. Disease-free survival was 92.7% at 5-years and 84.9% at 10-years. Overall survival was 96.7% at 5-years and 86.6% at 10-years.
Table 1.
Follow-up time, events, clinical characteristics, and lifestyle factors for ER+ breast cancer survivors by cohort and combined (N=6,596)
| WHEL (N=2,118) |
LACE (N =1,543) |
NHS (N =2,935) |
ALL (N =6,596) |
|||||
|---|---|---|---|---|---|---|---|---|
| Median follow-up time for mortality (SD), years since diagnosis | 13.6 (3.0) | 12.6 (2.9) | 10.5 (4.1) | 12.0 (3.8) | ||||
| Median follow-up time for recurrence (SD), years since diagnosis | 10.9 (3.5) | 11.9 (3.6) | 9.6 (4.4) | 10.6 (4.0) | ||||
| Total deaths, n | 374 | 387 | 666 | 1427 | ||||
| Recurrencea, n | 377 | 319 | 613 | 1309 | ||||
| Year of diagnosis, range | 1991–2000 | 1996–2000 | 1990–2004 | 1990–2004 | ||||
| Age at diagnosis, mean SD | 52.2 | 8.7 | 59.4 | 10.5 | 64.6 | 7.5 | 59.4 | 10.2 |
| Chemotherapy, n % | 1332 | 62.9 | 763 | 49.5 | 951 | 32.4 | 3046 | 46.2 |
| Radiotherapy, n % | 1320 | 62.3 | 958 | 62.1 | 1785 | 60.8 | 4063 | 61.6 |
| Mastectomy, n % | 1080 | 51.0 | 776 | 50.3 | 1347 | 45.9 | 3203 | 48.6 |
| Hormonal therapy, n % | 1766 | 83.4 | 1433 | 92.9 | 2490 | 84.8 | 5689 | 86.3 |
| TNM stage, n % | ||||||||
| I | 871 | 41.1 | 761 | 49.3 | 1876 | 63.9 | 3508 | 53.2 |
| II | 922 | 43.5 | 619 | 40.1 | 813 | 27.7 | 2354 | 35.7 |
| III | 325 | 15.3 | 163 | 10.6 | 246 | 8.4 | 734 | 11.1 |
| PR+, n % | 1768 | 84.2 | 1268 | 82.2 | 2296 | 80.0 | 5332 | 81.9 |
| Postmenopausal, n % | 1052 | 49.7 | 1047 | 67.9 | 2709 | 92.3 | 4808 | 72.9 |
| Years between diagnosis and measurement of post-diagnosis lifestyle factors, mean (range) | 2.2 (1.0–4.0) | 2.1 (1.0–3.7) | 2.1 (1.0–4.9)b | 2.1 (1.0–4.9) | ||||
| Years between diagnosis and 1st post-diagnosis weight measurement, mean, (SD) | 2.2 (0.83) | 2.1 (0.60) | 2.1 (0.70) | 2.1 (0.72) | ||||
| Years between diagnosis and 2nd post-diagnosis weight measurement, mean, (SD) | 3.7(0.99) | 7.0 (9.4) | 4.1 (0.87) | 4.6 (1.6) | ||||
| Pre- to post-diagnosis weight change, n % | ||||||||
| Stable (±5%) | 910 | 43.5 | 730 | 47.9 | 1760 | 60.8 | 3400 | 52.2 |
| Weight loss of 5–10% | 172 | 8.2 | 153 | 10.0 | 317 | 10.9 | 642 | 9.9 |
| Weight loss of ≥10% | 94 | 4.5 | 106 | 7.0 | 174 | 6.0 | 374 | 5.7 |
| Weight gain of 5–10% | 370 | 17.7 | 254 | 16.7 | 435 | 15.0 | 1059 | 16.3 |
| Weight gain of ≥10% | 546 | 26.1 | 282 | 18.5 | 211 | 7.3 | 1039 | 16.0 |
| BMI at 2 years post-diagnosis (kg/m2), n % | ||||||||
| <21.5 | 264 | 12.5 | 187 | 12.1 | 376 | 12.8 | 827 | 12.5 |
| 21.5–24.99 | 637 | 30.1 | 420 | 27.2 | 901 | 30.7 | 1958 | 29.7 |
| 25–29.99 | 672 | 31.7 | 531 | 34.4 | 1002 | 34.1 | 2205 | 33.4 |
| 30–34.99 | 331 | 15.6 | 249 | 16.1 | 456 | 15.5 | 1036 | 15.7 |
| ≥35 | 214 | 10.1 | 156 | 10.1 | 200 | 6.8 | 570 | 8.6 |
| Post-diagnosis recreational physical activity, n % | ||||||||
| MET-h/wk | ||||||||
| <4.9 | 634 | 29.9 | 573 | 37.1 | 960 | 32.7 | 2167 | 32.9 |
| 4.9–<17.4 | 743 | 35.1 | 475 | 30.8 | 968 | 33.0 | 2186 | 33.1 |
| ≥17.4 | 741 | 35.0 | 495 | 32.1 | 1007 | 34.3 | 2243 | 34.0 |
| Alcohol consumption (g/day), n % | ||||||||
| Non-drinker | 751 | 35.5 | 714 | 47.7 | 1140 | 41.8 | 2605 | 41.1 |
| 0.36–<6 | 717 | 33.9 | 387 | 25.9 | 838 | 30.7 | 1942 | 30.6 |
| 6–<12 | 263 | 12.4 | 144 | 9.6 | 296 | 10.8 | 703 | 11.1 |
| ≥12 | 386 | 18.2 | 252 | 16.8 | 456 | 16.7 | 1094 | 17.2 |
| Smoking status, n % | ||||||||
| Never | 1115 | 52.9 | 817 | 53.7 | 1222 | 42.1 | 3154 | 48.3 |
| Former <20 pack-years | 653 | 31.0 | 381 | 25.1 | 803 | 27.7 | 1837 | 28.1 |
| Former ≥20 pack-years | 245 | 11.6 | 212 | 13.9 | 633 | 21.8 | 1090 | 16.7 |
| Current | 95 | 4.5 | 111 | 7.3 | 244 | 8.4 | 450 | 6.9 |
Table excludes missing, where applicable.
Includes first breast cancer event (recurrence, metastasis, new breast primary, or death due to breast cancer).
For NHS, this date is for BMI measurement, as the dates vary by lifestyle factor (exercise, mean: 2.4 (range: 1.0–4.99); alcohol, mean: 3.0(range: 1.0–4.99), smoking, mean:2.0 (range: 1.0–3.7)).
Table 2 displays results for the associations of lifestyle factors and late recurrence. Table 3 displays results for the associations of lifestyle factors and all-cause mortality. A non-significant inverse association between ≥10% pre-to-post diagnosis weight loss and late recurrence was observed(HR: 0.67; 95% CI: 0.42–1.05). Pre-to-post diagnosis weight gain ≥10% was associated with increased risk of late breast cancer recurrence (HR: 1.24, 95%: 1.00–1.53). Weight loss and weight gain were not significantly associated with late all-cause mortality.
Table 2.
Hazard ratiosa for post-diagnosis lifestyle factors in association with late recurrence (≥5 years) among ER+ breast cancer survivors (N=5,675)b
| Events | Cohort | HR | (95% CI) | |
|---|---|---|---|---|
| Pre- to post-diagnosis weight change | ||||
| Loss of 5–10% | 44 | 547 | 0.77 | (0.56–1.07) |
| Loss of ≥10% | 20 | 313 | 0.67 | (0.42–1.05) |
| Stable | 282 | 2898 | 1.00 | (reference) |
| Gain of 5–10% | 109 | 927 | 1.05 | (0.84–1.31) |
| Gain of ≥10% | 138 | 919 | 1.24 | (1.00–1.53) |
| BMI at 2 years post-diagnosis (kg/m2)c | ||||
| <21.5 | 68 | 704 | 1.17 | (0.87–1.57) |
| 21.5–24.99 | 138 | 1712 | 1.00 | (reference) |
| 25–29.99 | 230 | 1892 | 1.49 | (0.98–2.25) |
| 30–34.99 | 107 | 876 | 1.40 | (1.05–1.86) |
| ≥35 | 61 | 491 | 1.41 | (1.02–1.93) |
| Ptrend | 0.007 | |||
| Post-diagnosis BMI using second available weight measurement (kg/m2)d | ||||
| <21.5 | 61 | 653 | 1.36 | (0.99–1.86) |
| 21.5–24.99 | 110 | 1558 | 1.00 | (reference |
| 25–29.99 | 194 | 1750 | 1.59 | (1.25–2.01) |
| 30–34.99 | 94 | 821 | 1.62 | (1.22–2.15) |
| ≥35 | 51 | 421 | 1.65 | (1.16–2.32) |
| Ptrend | 0.0003 | |||
| Post-diagnosis recreational physical activity (MET-h/wk) | ||||
| 0–<4.9 | 218 | 1856 | 1.00 | (reference) |
| 4.9–<17.4 | 200 | 1876 | 0.93 | (0.76–1.13) |
| ≥17.4 | 186 | 1943 | 0.89 | (0.73–1.09) |
| Ptrend | 0.27 | |||
| Post-diagnosis alcohol consumption (g/day) | ||||
| Non-drinker (0–<0.36) | 233 | 2267 | 1.00 | (reference) |
| 0.36–6 | 186 | 1668 | 1.09 | (0.89–1.32) |
| <6–<12 | 61 | 608 | 1.06 | (0.79–1.42) |
| ≥12 (≥1 drink/day) | 113 | 973 | 1.28 | (1.01–1.62) |
| Ptrend | 0.06 | |||
| Smoking status at first post-diagnosis survey | ||||
| Never | 284 | 2773 | 1.00 | (reference) |
| Former <20 pack-years | 164 | 1603 | 1.04 | (0.86–1.27) |
| Former ≥20 pack-years | 106 | 894 | 1.32 | (1.05–1.66) |
| Current | 43 | 353 | 1.30 | (0.94–1.81) |
Adjusted for age at diagnosis, TNM stage, PR status, chemotherapy, radiotherapy, surgery, hormonal therapy, race/ethnicity, menopausal status, comorbidity (diabetes, hypertension), other studied lifestyle factors (as appropriate), and time between exposure measurement and 5-year post diagnosis date, stratified by study. Models for weight change also adjusted for pre-diagnosis BMI.
Table is limited to women who were 5-year disease-free survivors and not missing date of recurrence. In addition, specific models excluded the following: 80 women missing pre-diagnosis BMI (for weight change models), 245 women missing alcohol intake (alcohol models), and 64 women missing pack-years information(smoking models).
Q statistic was statistically significant for one exposure category for one model (post-diagnosis BMI 25–29.99 kg/m2 (P =0.026)); all results for this model were from random effects models.1
Using second post-diagnosis weight instead of first post-diagnosis weight, assessed at on average 4.6 years after diagnosis.
Model excludes women with second weight measured after recurrence (n=31). Excludes an additional 441 women missing second measurement of BMI.
Table 3.
Hazard ratiosa for post-diagnosis lifestyle factors in association with late all-cause mortality (≥5 years)among ER+ breast cancer survivors (N=6,259)b
| Events | Cohort | HR | (95% CI) | |
|---|---|---|---|---|
| Pre- to post-diagnosis weight changec | ||||
| Loss of 5–10% | 129 | 595 | 1.16 | (0.95–1.41) |
| Loss of ≥10% | 69 | 348 | 1.17 | (0.53–2.59) |
| Stable | 599 | 3217 | 1.00 | (reference) |
| Gain of 5–10% | 199 | 1021 | 1.08 | (0.85–1.36) |
| Gain of ≥10% | 187 | 1001 | 1.06 | (0.82–1.38) |
| BMI at 2 years post-diagnosis (kg/m2)c | ||||
| <21.5 | 151 | 784 | 1.19 | (0.98–1.45) |
| 21.5–24.99 | 314 | 1877 | 1.00 | (reference) |
| 25–29.9 | 400 | 2093 | 1.05 | (0.81–1.37) |
| 30–34.99 | 211 | 970 | 1.12 | (0.78–1.63) |
| ≥35 | 133 | 535 | 1.37 | (0.93–2.01) |
| Ptrend | 0.19 | |||
| Post-diagnosis BMI using second available weight measurement (kg/m2)d | ||||
| <21.5 | 144 | 716 | 1.42 | (1.15–1.74) |
| 21.5–24.99 | 244 | 1702 | 1.00 | (reference) |
| 25–29.9 | 320 | 1927 | 1.06 | (0.90–1.26) |
| 30–34.99 | 162 | 891 | 1.11 | (0.91–1.36) |
| ≥35 | 92 | 445 | 1.40 | (1.09–1.81) |
| Ptrend | 0.013 | |||
| Post-diagnosis recreational physical activity (MET-h/wk) | ||||
| 0–<4.9 | 503 | 2027 | 1.00 | (reference) |
| 4.9–<17.4 | 382 | 2076 | 0.81 | (0.71–0.93) |
| ≥17.4 | 324 | 2156 | 0.71 | (0.61–0.82) |
| Ptrend | <0.0001 | |||
| Post-diagnosis alcohol consumption (g/day)c | ||||
| Non-drinker | 529 | 2491 | 1.00 | (reference) |
| 0.36–6 | 328 | 1864 | 0.94 | (0.81–1.08) |
| <6–<12 | 121 | 676 | 1.00 | (0.64–1.57) |
| ≥12 | 185 | 1055 | 0.93 | (0.75–1.17) |
| Ptrend | 0.29 | |||
| Smoking status at first post-diagnosis survey | ||||
| Never | 513 | 3045 | 1.00 | (reference) |
| Former <20 pack-years | 268 | 1751 | 0.94 | (0.81–1.09) |
| Former ≥20 pack-years | 266 | 996 | 1.46 | (1.25–1.70) |
| Current | 144 | 408 | 2.20 | (1.82–2.66) |
Adjusted for age at diagnosis, TNM stage, PR status, chemotherapy, radiotherapy, surgery, hormonal therapy, race/ethnicity, menopausal status, comorbidity (diabetes, hypertension), studied lifestyle factors (as appropriate), and time between exposure measurement and 5-year post diagnosis date, stratified by study. Models for weight change also adjusted for pre-diagnosis BMI.
Table limited to 5-year survivors. In addition, specific models excluded the following: 82 missing pre-diagnosis BMI (for weight change models), 252 missing alcohol intake (for alcohol models), 65 missing pack-year information (smoking models).
The Q statistic was statistically significant for one exposure category for three models (weight loss ≥10%, P =0.036, post-diagnosis BMI 30–34.99 kg/m2, P =0.016, alcohol intake of 6–<12 g/day, P =0.0095); therefore, the results were from a random effects meta-analysis for these models.1
Using second post-diagnosis weight instead of first post-diagnosis weight, assessed at on average 4.6 years after diagnosis.
Model excludes women with second weight measured after recurrence (n=31). Excludes and additional 547 women missing second measurement of BMI.
High BMI at about 2 years after diagnosis was associated with increased risk of late recurrence (HR: 1.40, 95% CI: 1.05–1.86) and (HR: 1.41, 95% CI:1.02–1.93) for BMI 30–34.99 and ≥35 kg/m2, respectively). While there was an overall pattern of a U-shaped association for higher BMI and late all-cause mortality, results were not statistically significant. Higher BMI was associated with increased risk of breast cancer-specific mortality, HRs (95% CIs)): 1.33 (1.07–1.66), 1.18 (0.90–1.54), and 1.43 (1.04–1.97) for 25–29.9 kg/m2, 30–34.99 kg/m2, and ≥35 kg/m2, respectively (reference = 21.5–24.99 kg/m2). Updated information on weight only was available for all cohorts (mean of 4.6 years after diagnosis, with some measurements up to 9.9 years after diagnosis). The association for high post-diagnosis BMI and increased risk of late recurrence was again observed, with evidence for a stronger association using the updated weight. For mortality, we observed a significant U-shaped association, with increased risk for both low BMI (<21.5 kg/m2) and high BMI (≥35 kg/m2).
Post-diagnosis recreational physical activity was not associated with late recurrence. Higher levels of post-diagnosis recreational physical activity were strongly inversely associated with late all-cause mortality (HR: 0.81, 95% CI: 0.71–0.93 and HR: 0.71, 95% CI: 0.61–0.82 for 4.9–<17.4 and ≥17.4 MET-h/wk, respectively, Ptrend<0.0001). Post-diagnosis alcohol intake ≥1 drink/day was associated with increased risk of late recurrence (HR: 1.28, 95% CI: 1.01–1.62), however, a consistent trend for increasing intake was not observed. Post-diagnosis alcohol intake was not significantly associated with late all-cause mortality. Compared to never smokers, positive associations were observed for former smokers of ≥20 pack-years and current smokers and risk of late recurrence (HR: 1.32, 95% CI: 1.05–1.66 and HR: 1.30, 95% CI: 0.94–1.81, respectively). Strong positive associations were also observed for former smokers of ≥20 pack-years and current smokers with late all-cause mortality. Formers smokers of ≥20 pack-years and current smokers also had increased risk of breast cancer-specific mortality, HRs (95% CIs): 1.27 (1.01–1.61) and 1.75 (1.30–2.35), respectively.
DISCUSSION
In this prospective, pooled analysis of over 6,500 ER+ breast cancer survivors who had survived on average two years at study entry, we found that large post-diagnosis weight gain, obesity, and daily alcohol consumption (≥ 1 drink/day) were associated with increased risk of late recurrence (≥5 years after diagnosis). Physical activity was inversely associated with late all-cause mortality, but not late recurrence. Current and heavy former smoking was associated with increased risk of late recurrence and all-cause mortality. To our knowledge, our study is the first to specifically focus on the evaluation of post-diagnosis lifestyle factors and late outcomes in long-term ER+ breast cancer survivors, a group that is continuing to increase and has been shown to have a higher risk of late outcomes. Our findings demonstrate that lifestyle factors after diagnosis may have a long-term impact on breast cancer outcomes among 5-year survivors. These results support the critical need for the incorporation of lifestyle recommendations and modifications into long-term survivorship care plans,23, 37 in particular promotion of regular exercise participation, avoidance of large weight gain, careful consideration of the risks and benefits of moderate alcohol consumption, and smoking cessation.
While some studies have evaluated tumor/molecular markers in association with late outcomes in ER+ breast cancer survivors14–16 or among all 5-year breast cancer survivors,34, 38 none of these studies have evaluated lifestyle factors. We did identify one study of pre-diagnosis BMI and breast cancer survival that investigated associations by time since diagnosis among all breast cancer subtypes using registry-linked data from Denmark.39 That study reported that the association of pre-diagnosis obesity and risk of distant metastasis varied by time since diagnosis, with stronger associations observed in the later time period (5–10 years after diagnosis). Although our study differs from the Denmark study in that we evaluated post-diagnosis BMI, have follow-up beyond 10 years, and focused on ER+ breast cancer, our findings of increased risk of late recurrence for high post-diagnosis BMI are supported by this earlier study.
We also found that BMI at both 2.1 and 4.6 years after diagnosis (on average) were associated with increased risk of recurrence. However, for all-cause mortality, results were inconsistent by time point of post-diagnosis weight. Specifically, BMI at 2 years post-diagnosis was not associated with all-cause mortality, while a statistically significant U-shaped association was found for BMI at 4.6 years post-diagnosis and all cause-mortality with increased risk observed for low BMI <21.5 kg/m2 and high BMI >35 kg/m2. It could be that the measure of BMI closer to when the event occurs has a larger impact on overall survival, or that obesity at this later time point represents women who have been obese long-term after diagnosis. The association of low BMI and increased risk of mortality may be due to underlying illness leading to unintentional weight loss. However, we did not collect information on type of weight loss and could not evaluate the reason for weight loss as a potential explanatory mechanism.23, 40
Findings for weight change and breast cancer outcomes have been inconsistent across studies.10, 24, 40, 41 To our knowledge, no studies have specifically evaluated weight change and late outcomes. In our study, we found that pre to post-diagnosis weight gain increased risk of late recurrence, but was not associated with late all-cause mortality. Although not established, potential biological pathways that may explain the association between high adiposity and recurrence/metastasis include insulin, steroid hormone, adipokine, and inflammatory pathways, which may promote breast cancer cell proliferation and tumor growth.42 Similar associations were seen when we evaluated weight gain using the second post-diagnosis weight measurement, measured on average 4.6 years after diagnosis (HRs (95% CIs) for large weight gain ≥10% were 1.52(1.21– 1.91) and 1.18 (0.98–1.42) for late recurrence and all-cause mortality, respectively). In contrast, weight loss using the second post-diagnosis weight measurement was associated with a statistically significant increased risk of all-cause mortality (HR (95% CI) for large weight loss ≥10%: 1.53, 1.23–1.90). As noted above, we did not have information on whether weight loss was intentional. As discussed in detail by Caan et al.,23 there are several mechanism that may explain an association between weight loss and increased risk of mortality, including loss of lean body mass and interactions with comorbidity status and pre-diagnosis weight, and these must be carefully considered when providing recommendations regarding weight loss among breast cancer survivors.23
Higher levels of post-diagnosis recreational physical activity were inversely associated with late all-cause mortality, with a dose-response pattern observed. Physical activity before and after diagnosis has been consistently associated with reduced risk of total and breast cancer-specific mortality.10, 43–46 However, to our knowledge, no studies have examined the association of post-diagnosis physical activity and late breast cancer outcomes overall or particularly for ER+ breast cancer survivors. Exercise has many known potential health benefits for breast cancer survivors, including reduced risk of comorbidities, improved quality of life, reduced fatigue, and enhanced immune function.47 Our results add to the literature regarding the benefits of physical activity in breast cancer survivors and specifically support that post-diagnosis recreational physical activity may reduce risk of late all-cause mortality among ER+ breast cancer survivors.
Alcohol intake was not associated with recurrence or total mortality overall in a previous report in the ABCPP among all breast cancer subtypes.30 This previous report did not consider late breast cancer outcomes. In the present study of late outcomes among ER+ breast cancer survivors, no clear association was found for alcohol and late all-cause mortality; however, alcohol intake of at least one drink per day (compared to non-drinkers) was associated with increased risk of late recurrence. One limitation of this analysis is that we did not have more than one measure of alcohol intake after diagnosis, and future studies with multiple measures of alcohol intake after diagnosis are needed.
The main strengths of our study included the large sample size, long-term follow-up beyond 10 years for breast cancer outcomes, and detailed information on post-diagnosis modifiable lifestyle-related factors and tumor characteristics. Limitations should also be considered. One limitation was that we only had binary yes/no cancer treatment information; therefore, we could not evaluate the impact of therapy adherence, in particular for long-term adjuvant hormonal therapy, on the observed associations. Another limitation was that we could only evaluate those lifestyle factors that were harmonized across cohorts in this secondary data analysis. Further, although we had pre-diagnosis information on BMI, we did not have pre-diagnosis information on alcohol or physical activity for all breast cancer survivors, and could not investigate change from pre-to-post diagnosis for these factors on long-term outcomes. Another limitation was that we only had information for the majority of lifestyle factors at one time point after diagnosis. While we did have updated weight available, the timing of measurement after diagnosis varied greatly by study, and future studies with post-diagnosis measures of lifestyle factors at multiple uniform time-points are needed. Finally, while weight and height were measured in-person in WHEL, weight and height were self-reported in other cohorts, potentially contributing to measurement error as under-reporting of weight has been observed in some studies for overweight and obese women.48 However, self-reported weight has been shown to be accurate based on comparison of self-reported and technician-measured weight in the NHS.22
In summary, we found that modifiable lifestyle factors were important predictors of late recurrence and mortality among long-term ER+ breast cancer survivors. These results set the stage for future research in this area, particularly in cohorts with long-term follow-up >10 years after diagnosis and multiple post-diagnosis lifestyle assessments, including measurements ≥5 years post-diagnosis.
Supplementary Material
Novelty & Impact Statement.
Late recurrence is a major concern for women with ER+ breast cancer, which accounts for close to two-thirds of diagnosed breast cancers. In the first study to date focusing specifically on lifestyle factors and long-term ER+ breast cancer survivors, post-diagnosis modifiable lifestyle factors, including obesity, exercise, smoking, and alcohol intake were associated with late breast cancer outcomes using pooled data from prospective cohorts.
ACKNOWLEDGEMENTS
This work was supported by the National Cancer Institute at the National Institutes of Health (NIH) (Grant number R03CA171013-01 to S.J.N.). The parent grants for each individual cohort included are as follows: WHEL Study (Susan G. Komen Foundation, #KG100988), LACE Study (NIH, R01 CA129059), and NHS (NIH, P01 CA87969).The original grant for the ABCPP establishment was from the NIH (Grant number 3R01 CA118229-03S1).
Abbreviations
- ABCPP
After Breast Cancer Pooling Project
- BMI
Body mass index
- CIs
Confidence intervals
- ER
Estrogen receptor
- HRs
Hazard ratios
- LACE
Life After Cancer Epidemiology Study
- FFQ
Food frequency questionnaire
- MET
Metabolic equivalent
- NHS
Nurses’ Health Study
- PR
Progesterone receptor
- SBCSS
Shanghai Breast Cancer Survival Study
- WHEL
Women’s Healthy Eating & Living Study
Footnotes
Disclosures
The authors have no declared conflicts of interest.
REFERENCES
- 1.Early Breast Cancer Trialists' Collaborative G. Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, McGale P, Pan HC, Taylor C, Wang YC, Dowsett M, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gray RJ. Flexible Methods for Analyzing Survival-Data Using Splines, with Applications to Breast-Cancer Prognosis. J Am Stat Assoc. 1992;87:942–951. [Google Scholar]
- 3.Hilsenbeck SG, Ravdin PM, de Moor CA, Chamness GC, Osborne CK, Clark GM. Time-dependence of hazard ratios for prognostic factors in primary breast cancer. Breast Cancer Res Treat. 1998;52:227–237. doi: 10.1023/a:1006133418245. [DOI] [PubMed] [Google Scholar]
- 4.Hess KR, Pusztai L, Buzdar AU, Hortobagyi GN. Estrogen receptors and distinct patterns of breast cancer relapse. Breast Cancer Res Treat. 2003;78:105–118. doi: 10.1023/a:1022166517963. [DOI] [PubMed] [Google Scholar]
- 5.Dignam JJ, Dukic V, Anderson SJ, Mamounas EP, Wickerham DL, Wolmark N. Hazard of recurrence and adjuvant treatment effects over time in lymph node-negative breast cancer. Breast Cancer Res Treat. 2009;116:595–602. doi: 10.1007/s10549-008-0200-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Natarajan L, Pu M, Parker BA, Thomson CA, Caan BJ, Flatt SW, Madlensky L, Hajek RA, Al-Delaimy WK, Saquib N, Gold EB, Pierce JP. Time-varying effects of prognostic factors associated with disease-free survival in breast cancer. Am J Epidemiol. 2009;169:1463–1470. doi: 10.1093/aje/kwp077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jatoi I, Anderson WF, Jeong JH, Redmond CK. Breast cancer adjuvant therapy: time to consider its time-dependent effects. J Clin Oncol. 2011;29:2301–2304. doi: 10.1200/JCO.2010.32.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Brien KM, Cole SR, Tse CK, Perou CM, Carey LA, Foulkes WD, Dressler LG, Geradts J, Millikan RC. Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina Breast Cancer Study. Clin Cancer Res. 2010;16:6100–6110. doi: 10.1158/1078-0432.CCR-10-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rock CL, Demark-Wahnefried W. Nutrition and survival after the diagnosis of breast cancer: a review of the evidence. J Clin Oncol. 2002;20:3302–3316. doi: 10.1200/JCO.2002.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patterson RE, Cadmus LA, Emond JA, Pierce JP. Physical activity, diet, adiposity and female breast cancer prognosis: a review of the epidemiologic literature. Maturitas. 2010;66:5–15. doi: 10.1016/j.maturitas.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Tr. 2010;123:627–535. doi: 10.1007/s10549-010-0990-0. [DOI] [PubMed] [Google Scholar]
- 12.Schmid D, Leitzmann MF. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta-analysis. Annals of Oncology. 2014;25:1293–1311. doi: 10.1093/annonc/mdu012. [DOI] [PubMed] [Google Scholar]
- 13.Chan DS, Vieira AR, Aune D, Bandera EV, Greenwood DC, McTiernan A, Navarro Rosenblatt D, Thune I, Vieira R, Norat T. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25:1901–1914. doi: 10.1093/annonc/mdu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahn SG, Lee HM, Cho SH, Bae SJ, Lee SA, Hwang SH, Jeong J, Lee HD. The Difference in Prognostic Factors between Early Recurrence and Late Recurrence in Estrogen Receptor-Positive Breast Cancer: Nodal Stage Differently Impacts Early and Late Recurrence. Plos One. 2013;8 doi: 10.1371/journal.pone.0063510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sestak I, Dowsett M, Zabaglo L, Lopez-Knowles E, Ferree S, Cowens JW, Cuzick J. Factors predicting late recurrence for estrogen receptor-positive breast cancer. J Natl Cancer Inst. 2013;105:1504–1511. doi: 10.1093/jnci/djt244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sgroi DC, Sestak I, Cuzick J, Zhang Y, Schnabel CA, Schroeder B, Erlander MG, Dunbier A, Sidhu K, Lopez-Knowles E, Goss PE, Dowsett M. Prediction of late distant recurrence in patients with oestrogen-receptor-positive breast cancer: a prospective comparison of the breast-cancer index (BCI) assay, 21-gene recurrence score, and IHC4 in the TransATAC study population. Lancet Oncology. 2013;14:1067–1076. doi: 10.1016/S1470-2045(13)70387-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nechuta SJ, Caan BJ, Chen WY, Flatt SW, Lu W, Patterson RE, Poole EM, Kwan ML, Chen Z, Weltzien E, Pierce JP, Shu XO. The After Breast Cancer Pooling Project: rationale, methodology, and breast cancer survivor characteristics. Cancer Causes Control. 2011;22:1319–1331. doi: 10.1007/s10552-011-9805-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shu XO, Zheng Y, Cai H, Gu K, Chen Z, Zheng W, Lu W. Soy food intake and breast cancer survival. JAMA. 2009;302:2437–2443. doi: 10.1001/jama.2009.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caan B, Sternfeld B, Gunderson E, Coates A, Quesenberry C, Slattery ML. Life After Cancer Epidemiology (LACE) study: A cohort of early stage breast cancer survivors (United states) Cancer Cause Control. 2005;16:545–556. doi: 10.1007/s10552-004-8340-3. [DOI] [PubMed] [Google Scholar]
- 20.Pierce JP, Faerber S, Wright FA, Rock CL, Newman V, Flatt SW, Kealey S, Jones VE, Caan BJ, Gold EB, Haan M, Hollenbach KA, et al. A randomized trial of the effect of a plant-based dietary pattern on additional breast cancer events and survival: the Women's Healthy Eating and Living (WHEL) Study. Control Clin Trials. 2002;23:728–756. doi: 10.1016/s0197-2456(02)00241-6. [DOI] [PubMed] [Google Scholar]
- 21.Pierce JP, Natarajan L, Caan BJ, Parker BA, Greenberg ER, Flatt SW, Rock CL, Kealey S, Al-Delaimy WK, Bardwell WA, Carlson RW, Emond JA, et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women's Healthy Eating and Living (WHEL) randomized trial. Jama. 2007;298:289–298. doi: 10.1001/jama.298.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colditz GA, Hankinson SE. The Nurses' Health Study: Lifestyle and health among women. Nat Rev Cancer. 2005;5:388–396. doi: 10.1038/nrc1608. [DOI] [PubMed] [Google Scholar]
- 23.Caan BJ, Kwan ML, Shu XO, Pierce JP, Patterson RE, Nechuta SJ, Poole EM, Kroenke CH, Weltzien EK, Flatt SW, Quesenberry CP, Jr, Holmes MD, et al. Weight change and survival after breast cancer in the after breast cancer pooling project. Cancer Epidemiol Biomarkers Prev. 2012;21:1260–1271. doi: 10.1158/1055-9965.EPI-12-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caan BJ, Kwan ML, Hartzell G, Castillo A, Slattery ML, Sternfeld B, Weltzien E. Pre-diagnosis body mass index, post-diagnosis weight change, and prognosis among women with early stage breast cancer. Cancer Cause Control. 2008;19:1319–1328. doi: 10.1007/s10552-008-9203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ainsworth BE, Haskell WL, Leon AS, Jacobs DR, Jr, Montoye HJ, Sallis JF, Paffenbarger RS., Jr Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Staten LK, Taren DL, Howell WH, Tobar M, Poehlman ET, Hill A, Reid PM, Ritenbaugh C. Validation of the Arizona Activity Frequency Questionnaire using doubly labeled water. Med Sci Sports Exerc. 2001;33:1959–1967. doi: 10.1097/00005768-200111000-00024. [DOI] [PubMed] [Google Scholar]
- 27.Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Corsano KA, Rosner B, Kriska A, Willett WC. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23:991–999. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 28.Johnson-Kozlow M, Rock CL, Gilpin EA, Hollenbach KA, Pierce JP. Validation of the WHI brief physical activity questionnaire among women diagnosed with breast cancer. Am J Health Behav. 2007;31:193–202. doi: 10.5555/ajhb.2007.31.2.193. [DOI] [PubMed] [Google Scholar]
- 29.USDHHS. Washington, DC: USDHHS; 2008. 2008 physical activity guidelines for Americans. [Google Scholar]
- 30.Kwan ML, Chen WY, Flatt SW, Weltzien EK, Nechuta SJ, Poole EM, Holmes MD, Patterson RE, Shu XO, Pierce JP, Caan BJ. Postdiagnosis alcohol consumption and breast cancer prognosis in the after breast cancer pooling project. Cancer Epidemiol Biomarkers Prev. 2013;22:32–41. doi: 10.1158/1055-9965.EPI-12-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pierce JP, Patterson RE, Senger CM, Flatt SW, Caan BJ, Natarajan L, Nechuta SJ, Poole EM, Shu XO, Chen WY. Lifetime cigarette smoking and breast cancer prognosis in the After Breast Cancer Pooling Project. J Natl Cancer Inst. 2014;106:djt359. doi: 10.1093/jnci/djt359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holmes MD, Chen WY, Li L, Hertzmark E, Spiegelman D, Hankinson SE. Aspirin intake and survival after breast cancer. J Clin Oncol. 2010;28:1467–1472. doi: 10.1200/JCO.2009.22.7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bellera CA, MacGrogan G, Debled M, de Lara CT, Brouste V, Mathoulin-Pelissier S. Variables with time-varying effects and the Cox model: Some statistical concepts illustrated with a prognostic factor study in breast cancer. Bmc Medical Research Methodology. 2010;10 doi: 10.1186/1471-2288-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brewster AM, Hortobagyi GN, Broglio KR, Kau SW, Santa-Maria CA, Arun B, Buzdar AU, Booser DJ, Valero V, Bondy M, Esteva FJ. Residual risk of breast cancer recurrence 5 years after adjuvant therapy. J Natl Cancer Inst. 2008;100:1179–1183. doi: 10.1093/jnci/djn233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 36.Smith-Warner SA, Spiegelman D, Ritz J, Albanes D, Beeson WL, Bernstein L, Berrino F, van den Brandt PA, Buring JE, Cho E, Colditz GA, Folsom AR, et al. Methods for pooling results of epidemiologic studies: the Pooling Project of Prospective Studies of Diet and Cancer. Am J Epidemiol. 2006;163:1053–1064. doi: 10.1093/aje/kwj127. [DOI] [PubMed] [Google Scholar]
- 37.Bodai BI, Tuso P. Breast cancer survivorship: a comprehensive review of long-term medical issues and lifestyle recommendations. The Permanente journal. 2015;19:48–79. doi: 10.7812/TPP/14-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kennecke HF, Olivotto IA, Speers C, Norris B, Chia SK, Bryce C, Gelmon KA. Late risk of relapse and mortality among postmenopausal women with estrogen responsive early breast cancer after 5 years of tamoxifen. Annals of Oncology. 2007;18:45–51. doi: 10.1093/annonc/mdl334. [DOI] [PubMed] [Google Scholar]
- 39.Ewertz M, Jensen MB, Gunnarsdottir KA, Hojris I, Jakobsen EH, Nielsen D, Stenbygaard LE, Tange UB, Cold S. Effect of Obesity on Prognosis After Early-Stage Breast Cancer. J Clin Oncol. 2010 Dec 01; doi: 10.1200/JCO.2010.29.7614. 2010. [DOI] [PubMed] [Google Scholar]
- 40.Bradshaw PT, Ibrahim JG, Stevens J, Cleveland R, Abrahamson PE, Satia JA, Teitelbaum SL, Neugut AI, Gammon MD. Postdiagnosis Change in Bodyweight and Survival After Breast Cancer Diagnosis. Epidemiology. 2012;23:320–327. doi: 10.1097/EDE.0b013e31824596a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caan BJ, Emond JA, Natarajan L, Castillo A, Gunderson EP, Habel L, Jones L, Newman VA, Rock CL, Slattery ML, Stefanick ML, Sternfeld B, et al. Post-diagnosis weight gain and breast cancer recurrence in women with early stage breast cancer. Breast Cancer Res Tr. 2006;99:47–57. doi: 10.1007/s10549-006-9179-y. [DOI] [PubMed] [Google Scholar]
- 42.Brown KA. Impact of obesity on mammary gland inflammation and local estrogen production. J Mammary Gland Biol Neoplasia. 2014;19:183–189. doi: 10.1007/s10911-014-9321-0. [DOI] [PubMed] [Google Scholar]
- 43.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. Jama. 2005;293:2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 44.Sternfeld B, Weltzien E, Quesenberry CP, Jr, Castillo AL, Kwan M, Slattery ML, Caan BJ. Physical activity and risk of recurrence and mortality in breast cancer survivors: findings from the LACE study. Cancer Epidemiol Biomarkers Prev. 2009;18:87–95. doi: 10.1158/1055-9965.EPI-08-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ibrahim EM, Al-Homaidh A. Physical activity and survival after breast cancer diagnosis: meta-analysis of published studies. Med Oncol. 2010 Apr 23; doi: 10.1007/s12032-010-9536-x. 2010. [DOI] [PubMed] [Google Scholar]
- 46.Beasley JM, Kwan ML, Chen WY, Weltzien EK, Kroenke CH, Lu W, Nechuta SJ, Cadmus-Bertram L, Patterson RE, Sternfeld B, Shu XO, Pierce JP, et al. Meeting the physical activity guidelines and survival after breast cancer: findings from the after breast cancer pooling project. Breast Cancer Res Treat. 2012;131:637–643. doi: 10.1007/s10549-011-1770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. Cmaj. 2006;175:34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin CJ, DeRoo LA, Jacobs SR, Sandler DP. Accuracy and reliability of self-reported weight and height in the Sister Study. Public Health Nutr. 2012;15:989–999. doi: 10.1017/S1368980011003193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.