Abstract
OBJECTIVE
To investigate the relationships between psychological and physiologic measures of stress, mood and gestational age at delivery and preterm birth (PTB).
METHODS
This prospective cohort study recruited healthy women in the early second trimester, 18–45 years of age. Validated psychological measures of perceived stress, depressive symptoms, and anxiety were completed at 16, 22, 28, 34 and 40 weeks of gestation. Cortisol concentration was measured in maternal hair at 16, 28, and 40 weeks of gestation to approximate 1st, 2nd, and 3rd trimester levels of physiologic stress. Statistical methods included: analyses of variance (ANOVA), t-tests, Chi-square, Pearson correlations, regression modeling and mediation analysis as appropriate. Hair cortisol concentrations were natural log transformed to normalize values.
RESULTS
Eleven (12%) out of 90 included women had a spontaneous preterm birth or preterm premature rupture of the membranes. Perceived stress at 16 weeks of gestation correlated with both 2nd trimester cortisol concentration (r= 0.28, p=0.007) and earlier gestational age at delivery (r= −0.30, p< 0.01). Gestational age at delivery was also negatively correlated with cortisol concentration in the 2nd trimester (r= −0.25, p=0.02) and 2nd trimester cortisol concentration was higher in preterm (2.7 ± 0.4 LN pg/mg) vs term (2.0 ± 0.7 LN pg/mg, P<0.001) delivered women. Using mediation statistics, the association between the psychological measure, the physiologic measure, and gestational age at delivery was mainly driven by increased physiologic stress (hair cortisol concentration) in the 2nd trimester (difference in coefficients (standard error)= −0.05(0.02)),.
CONCLUSION
Higher perceived stress in the 2nd trimester is associated with both elevated 2nd trimester hair cortisol concentration and gestational age at delivery. Physiologic measure of stress in the 2nd trimester appears most strongly associated with preterm birth. Identification and amelioration of early pregnancy stressors may attenuate physiologic stress and ultimately affect preterm birth.
INTRODUCTION
Chronic psychological stressors during pregnancy have long been associated with adverse pregnancy outcomes including preterm birth (PTB),1,2,3 low birth weight (LBW),4 maternal mood disorders5 and adverse child neurodevelopment.6,7,8 Yet the mechanism by which stress influences these outcomes is unclear. Cortisol, the hormonal endpoint of physiologic stress-related activation of the hypothalamic-pituitary-adrenal axis (HPA), has been associated with PTB9 and is often cited as a potential mediator between stress and pregnancy outcomes.10 Cortisol fluctuates rapidly in response to even subtle changes in the environment and single serum and saliva measurements do not reflect chronic levels. Multiple salivary samples collected across days, as representative of the varied intra-individual patterns of cortisol, have been utilized to improve stress characterization.11,12,13 While an improvement over single samples, these approaches also have limitations.12 These limitations likely contribute to the lack of clarity concerning the status of cortisol physiology in mediating relationships between stress reflected as HPA activity and adverse pregnancy outcomes such as PTB.
Cortisol is deposited in hair14 and normally increases as gestation progresses.15 Assessment of the hair cortisol concentration provides a reliable and non-invasive measure of chronic HPA activity.16,17 Differences have been noted in hair cortisol concentration in relation to chemical hair processing,18 season of delivery, and hair color at the end of the third trimester in term pregnancies.16 Taking these data into consideration, the objective of this study was to determine the relationships between psychological and physiologic measures of stress, mood, gestational age at delivery and preterm birth (PTB).
MATERIALS AND METHODS
Our study population, a prospective cohort study, consisted of women recruited during routine scheduled prenatal care visits during the first trimester of pregnancy The patients were between 18 and 45 years of age and planned to deliver at Denver Health and Hospital Authority (DHHA), Denver, Colorado. Women were excluded if they had a multiple gestation, reported daily illicit drug or alcohol abuse, had medical conditions requiring chronic corticosteroid use (topical, inhaled, or oral), had active infections such as human immunodeficiency virus, hepatitis, influenza or other clinically significant viral illnesses during pregnancy,19 or used bleach or peroxide products on their hair as these products are known to affect measured hair cortisol.18 Gestational age was a best clinical estimate based on a combination of participant-reported last menstrual period and first dating ultrasound. Hollingshead index was used to characterize socioeconomic status (SES) and is based on marital status, employment, level of education attained, and occupational prestige.20 Preterm birth was defined as delivery at less than 37 weeks of gestation and only PTB resulting from spontaneous preterm labor or preterm premature rupture of the membranes (PPROM) were included. This project was approved by the Colorado Multiple Institutional Review Board and all participants provided informed consent.
Maternal hair was cut three times during pregnancy. The first haircut occurred between 16–18 weeks (16 weeks), the second between 28–30 weeks (28 weeks), and the third between 38–42 weeks (40 weeks) of gestation. As hair provides a retrospective cortisol history and grows at 0.8 to 1cm/month,21 each 3 cm long segment was used to represent three preceding months of cumulative physiologic HPA activity, approximating first, second, and third trimester cumulative cortisol concentrations.
Hair was cut using stylist’s scissors as close to the scalp as possible along a 4cm long by 1–2mm wide strip at the posterior vertex of the scalp in the same area each time, as previously described.15 The hair samples were taped with painter’s tape to aluminum foil for protection and storage22 until all of a participant’s hair samples were available and could be processed and analyzed within the same assay to limit assay variability. New growth that emerged between hair collections allowed us to confirm that each participant’s hair growth was consistent with published rates.21 If a participant delivered at less than 34 weeks of gestation, her hair was cut on postpartum day 0 to 2 but these cortisol values were not included in 3rd trimester hair cortisol concentration analyses.
After the hair segments were measured and the proximal 3 cm from the scalp cut, the hair was placed in a pre-weighed 2 ml cryovial (Wheaton, Millville, NJ, USA) and washed three times in 100% isopropanol and dried as previously described.15 After washing, drying, and re-weighing, the hair and tubes were frozen in liquid nitrogen and ground for 4–5 minutes in the same tube using a ball mill (Retsch, Haan, Germany) with a stainless steel ball bearing added to the tube. Specially milled aluminum cassettes were designed to hold three of these cryovials. The powdered hair (5–20 mg) was extracted at room temperature in the same cryovial in 1000μl HPLC grade methanol overnight. After methanol extraction, the cryovial was centrifuged and the supernatant was removed, placed into a second microcentrifuge tube, and dried under a stream of nitrogen. The extracts were reconstituted with assay diluent based on hair weight. One hair cortisol concentration per trimester per participant was determined using a commercial high sensitivity EIA kit (Salimetrics LLC, State College, PA, USA) per manufacturer’s instructions. A pooled control of previously ground hair was extracted as above and included on each EIA plate in duplicate for determination of inter-assay coefficients of variation. Inter-assay coefficient of variation (CV) for the control hair pool was 9.2% and intra-assay CV for duplicates was 2.8%. Hair cortisol concentrations are reported as pg/mg.
At the five study time points, approximately 16, 22, 28, 34 and 40 weeks of gestation, pregnant women completed psychological self-report measures that included: a Perceived Stress Scale (PSS),23 a Center for Epidemiologic Studies-Depression Scale (CES-D),24 and the State-Trait-Anxiety Inventory, State version (STAI-S).25 The PSS is a 14-question survey with possible scores ranging from 0–56. The higher the PSS score, the higher the number and frequency of stressors, irritations, poor coping, anger, and difficulties in the preceding 4 weeks.23 The CES-D is a 20-question survey with possible scores ranging from 0–60 on which respondents rate the frequency of 20 depressive symptoms within the last week. It was designed to screen for major or clinical depression in adolescents and adults.24 The Spielberger State-Trait Anxiety Inventory, State version (STAI-S) is a 20-question self-report that measures state (short-term, situational) anxiety with scores ranging from 20–8025 and questions are anchored to the past month. All self-report measures have been validated in multiple languages and multi-cultural populations including pregnancy or the postpartum period.26,27,28 For all self-report measures, the higher the score, the greater the symptomatology.
Participant characteristics including gravidity, parity, maternal body mass index, gestational age, birth weight, mode of delivery, antepartum or intrapartum complications, medications and illicit substances (assessed throughout pregnancy), and major life events were all recorded.
Analyses of variance (ANOVA) and Chi-square analyses were used to compare groups on demographic and study characteristics including: race-ethnicity, socioeconomic status,29 level of education, marital status, tobacco use, hair cortisol concentration in the first, second and third trimesters, gestational age at delivery, birthweight, and PSS, CES-D, and STAI-S scores. Hair cortisol concentrations were natural log (LN, base e) transformed to normalize values. Pearson correlation analyses were used to examine cross-sectional associations between hair cortisol concentrations, PSS scores, CES-D scores, STAI-S scores, gestational age at delivery, and birthweight. Comparisons of hair cortisol concentration across trimesters were compared for the same subjects using repeated measures ANOVA and t-tests. Regression modeling was used to assess the relationship between gestational age at delivery and hair cortisol concentration in each trimester, controlling for race–ethnicity and any tobacco use.
To test our hypothesis that psychological stress affects physiologic stress and that both are associated with gestational age at delivery, a mediation analysis using the difference in coefficients approach was used.30 Mediation analyses clarify the relationships between an independent variable, a dependent variable, and a third explanatory variable, called the mediator. This approach compared the coefficient of the psychological measure (perceived stress) on gestational age at delivery both before and after controlling for the coefficient of the potential mediator, the physiologic measure (hair cortisol concentration). The standard error for the difference in coefficients was estimated using 5,000 bootstrap samples and used to estimate the conditional indirect effect. The association between gestational age at delivery and hair cortisol concentration was modeled using a 3rd degree polynomial. Race-ethnicity and tobacco use were included as covariates. Based on previously published studies of hair cortisol concentrations during pregnancy and a preterm birth rate of 12%, we determined that a sample size of 90 would have 80% power to detect a difference of 30% in hair cortisol concentration between preterm and term delivered groups. All data were analyzed using IBM SPSS version 22.0 (Armonk, NY, USA, 2014) and SAS version 9.4 was used for the mediation analysis (SAS Institute Inc.: Cary, NC, 2014).
RESULTS
A total of 92 women were enrolled. Two outliers were inspected to determine characteristics that may have contributed to the value. One case of maternal peroxide use (hair color) and one participant with topical corticosteroid cream was discovered and those participants’ hair cortisol concentration values were excluded. Both of these participants delivered at term. Eleven (12%) births resulting from spontaneous preterm labor or preterm premature rupture of the membranes occurred out of the 90 pregnancies remaining. Women who delivered at term and preterm are presented in Table 1. Overall, participants were predominantly multiparous, white Hispanic, non-smokers with a high-school education or less and of lower socioeconomic status. Women who delivered preterm were older, white non-Hispanic and African American, more likely to have used tobacco prior to pregnancy, and less likely to be married or living with a partner. Self-report measures of stress and mood and mean and standard deviation hair cortisol concentrations in the first, second, and third trimesters are also presented in Table 1. Women who delivered preterm (n=11) had higher mean second trimester hair cortisol concentration compared with those who delivered at term (n=79), Table 1. This difference was not seen between categories of term and preterm for first or third trimester hair cortisol concentrations
Table 1.
Participant Characteristics
| Term N=79 |
Preterm N=11 |
P-value | |
|---|---|---|---|
|
| |||
| Maternal Age (years) mean ± std | 28.2 ± 6.1 | 32.4 ± 5.8 | 0.04 |
|
| |||
| Gravidity n(%) | 0.002 | ||
| 1 | 15 (19) | 1 (9) | |
| 2–3 | 47 (60) | 1 (9) | |
| 4–5 | 12 (15) | 4 (37) | |
| ≥6 | 5 (6) | 5 (45) | |
| Parity n(%) | 0.07 | ||
| 0 | 32 (41) | 1 (9) | |
| 1–2 | 37 (46) | 8 (72) | |
| 3–4 | 10 (13) | 2 (18) | |
| Mode of Delivery n(%) | 0.06 | ||
| Vaginal delivery | 56 (71) | 6 (55) | |
| Caesarean delivery | 23 (29) | 5 (45) | |
|
| |||
| Prepregnancy Body Mass Index (kg/m2) mean ± std | 26.8 ± 6.4 | 25.3 ± 5.5 | 0.29 |
|
| |||
| Gestational Age at delivery (weeks) mean ± std | 39.5 ± 1.2 | 32.6 ± 3.9 | 0.001 |
| Birthweight (grams) mean ± std | 3289 ± 423 | 1967 ± 805 | 0.01 |
| Female fetus n(%) | 36 (46) | 7 (64) | 0.05 |
|
| |||
| Race and ethnicity n(%) | 0.03 | ||
| White Hispanic | 29 (37) | 2 (18) | |
| White non-Hispanic | 30 (38) | 4 (36) | |
| African American | 6 (8) | 4 (36) | |
| Native American | 9 (11) | 1 (9) | |
| Other | 5 (6) | 0 -- | |
|
| |||
| Years education n(%) | 0.001 | ||
| Less than high school diploma | 13 (17) | 0 -- | |
| High school diploma/G.E.D | 35 (44) | 8 (72) | |
| Associates or certificate | 8 (10) | 1 (9) | |
| Bachelors | 13 (16) | 0 -- | |
| ≥Masters | 10 (13) | 2 (18) | |
| Hollingshead Index | 12.95 ± 4.8 | 12.9 ± 2.7 | 0.78 |
| Marital status n(%) | 0.01 | ||
| Single/divorced | 3 (4) | 4 (36) | |
| Lives with partner | 29 (37) | 3 (27) | |
| Married | 47 (59) | 4 (36) | |
|
| |||
| Tobacco Use n(%) | 0.05 | ||
| None | 53 (67) | 6 (54) | |
| Prior to Pregnancy | 23 (29) | 5 (46) | |
| During Pregnancy | 3 (4) | 0 -- | |
|
| |||
| Self-report measures Perceived Stress Scale (PSS) | |||
| mean ± std | |||
| 16 weeks | 21 ± 8 | 26 ± 6 | 0.01 |
| 22 weeks | 21 ± 8 | 24 ± 6 | 0.32 |
| 28 weeks | 22 ± 9 | 22 ± 4 | 0.58 |
| 34 weeks** | 22 ± 8 | 23 ± 8 | 0.73 |
| 40 weeks | 19 ± 7 | ------------ | |
| Center for Epidemiologic Studies-Depression Scale (CES-D) | |||
| mean ± std | |||
| 16 weeks | 13 ± 9 | 15 ± 10 | 0.47 |
| 22 weeks | 12 ± 9 | 12 ± 12 | 0.86 |
| 28 weeks | 13 ± 10 | 14 ± 11 | 0.42 |
| 34 weeks** | 13 ± 10 | 16 ± 15 | 0.42 |
| 40 weeks | 10 ± 7 | ------------- | |
| State Trait Anxiety Inventory, State version (STAI-S) | |||
| mean ± std | |||
| 16 weeks | 34 ± 10 | 34 ± 10 | 0.88 |
| 22 weeks | 34 ± 11 | 34 ±13 | 0.80 |
| 28 weeks | 36 ± 11 | 37 ± 9 | 0.75 |
| 34 weeks** | 37 ± 12 | 34 ± 17 | 0.72 |
| 40 weeks | 34 ± 10 | ------------- | |
|
| |||
| Hair cortisol concentrations (natural log (LN) transformed pg/mg) | |||
| mean ± std | |||
| First trimester | 1.8 ± 0.7 | 2.3 ± 0.7 | 0.10 |
| Second trimester | 2.0 ± 0.7 | 2.7 ± 0.4 | 0.001 |
| Third trimester | 2.4 ± 0.7 | 2.3 ± 0.7 | 0.72 |
Significant comparisons in bold.
Indicates n=5 for this group as remainder of preterm participants had already delivered.
Associations between other demographic factors and hair cortisol concentration were analyzed including level of education, marital status, SES, mode of delivery, season of hair collection, and hair color, and the only significant finding was that hair cortisol concentration in African American participants was higher in the first and second trimesters (ANOVA: 1st trimester p=<0.01, 2nd trimester p= 0.01, 3rd trimester p=0.07) than white non-Hispanic and Hispanic participants.
Over the course of pregnancy hair cortisol concentration rose as expected15 and was significantly higher in the third trimester compared with the first and second trimester, Figure 1. Maternal hair cortisol concentration in the first trimester correlated with both the second and third trimester hair cortisol concentration measurements: first versus second trimester r=0.42, p<0.01 and first versus third trimester r=0.29, p=0.01.
Figure 1.
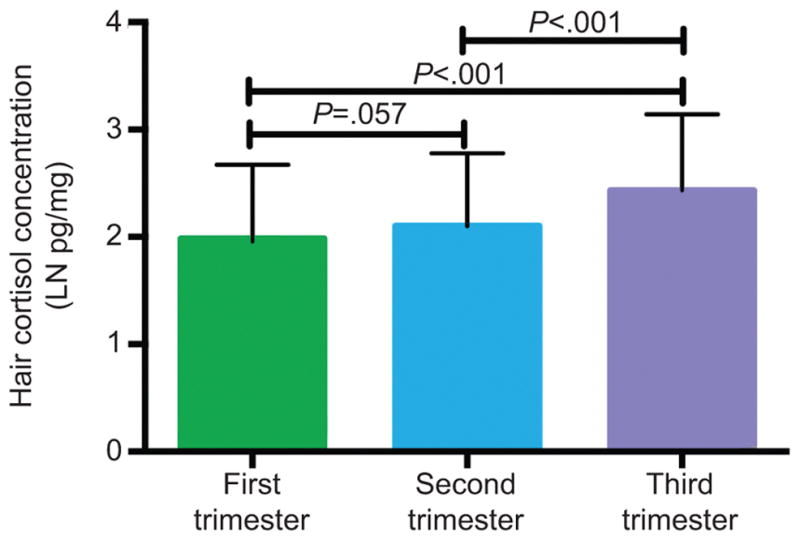
Maternal hair cortisol concentration (natural log (LN) transformed pg/mg) in the first, second, and third trimesters of pregnancy. Data presented are mean and standard deviation for the term delivered participants. Brackets and P values in figure represent paired t-tests.
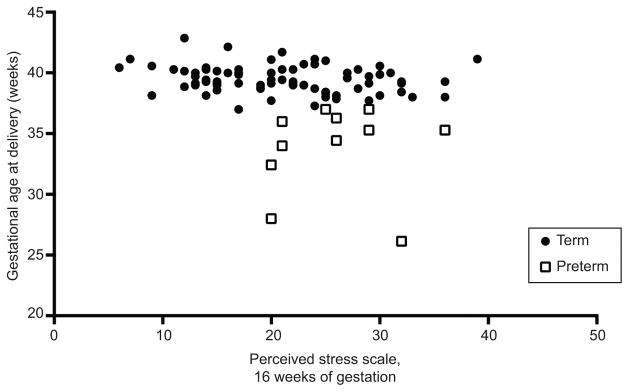
Average PSS, STAI, and CES-D scores at 16, 22, 28, 34 and 40 weeks of gestation are presented in Table 1 from both term and preterm delivered participants. There was a significant difference between PSS score in the term and preterm delivered groups at 16 weeks of gestation (p=0.01). Otherwise, PSS, CES-D, and STAI-S scores did not differ significantly over time or between groups (all p-values >0.05). Scores on the PSS, CES-D, and STAI-S were highly correlated across gestation, both within and across measures (all p-values <0.01). Of correlation analyses for all 15 self-report measures (3 measures at 5 study time-points) and gestational age at delivery, only perceived stress at 16 weeks of gestation was correlated with gestational age at delivery, Figure 2: r=−0.30, p<0.01.
Figure 2.
Perceived stress scale score at 16 weeks of gestation and gestational age at delivery (weeks). Pearson r=−0.30, P<.01.
Correlation analyses were done for all relationships at all five study time points between PSS score, CES-D score, and STAI-S score and hair cortisol concentration in the first, second and third trimesters, Table 2. The only significant relationships with first trimester hair cortisol concentration were PSS and CES-D at the 40 week study visit. In the second trimester, hair cortisol concentration was correlated with all three psychological measures at 16 weeks and CES-D and STAI-S at 28 weeks. Similar associations were noted between third trimester hair cortisol concentration and 16 and 28 week CES-D and STAIS.
Table 2.
Correlation between Perceived Stress Scale Scores, Center for Epidemiologic Studies Depression Scale Scores, and State Anxiety Inventory Scores at the five study time points.
| Perceived Stress Scale (PSS) | Center for Epidemiologic Studies Depression Scale (CES-D) | State Anxiety Inventory (STAI-S) | ||||
|---|---|---|---|---|---|---|
| Pearson r | (p-value) | Pearson r | (p-value) | Pearson r | (p-value) | |
|
| ||||||
| First trimester Hair cortisol concentration (pg/mg) | 16 weeks (0.27) | 0.11 | 16 weeks | 0.11 (0.26) | 16 weeks (0.23) | 0.12 |
| 22 weeks (0.20) | 0.13 | 22 weeks | 0.18 (0.06) | 22 weeks (0.45) | 0.08 | |
| 28 weeks (0.09) | 0.17 | 28 weeks | 0.11 (0.28) | 28 weeks (0.84) | 0.02 | |
| 34 weeks (0.39) | 0.09 | 34 weeks | 0.20 (0.06) | 34 weeks (0.25) | 0.12 | |
| 40 weeks (0.04) | 0.23 | 40 weeks | 0.27 (0.02) | 40 weeks (0.08) | 0.20 | |
|
| ||||||
| Second trimester Hair cortisol concentration (pg/mg) | 16 weeks (0.02) | 0.24 | 16 weeks (0.002) | 0.32 | 16 weeks (0.05) | 0.21 |
| 22 weeks (0.12) | 0.16 | 22 weeks | 0.08 (0.46) | 22 weeks (0.36) | 0.10 | |
| 28 weeks (0.19) | 0.14 | 28 weeks (0.02) | 0.25 | 28 weeks (0.03) | 0.23 | |
| 34 weeks (0.32) | 0.11 | 34 weeks (0.15) | 0.16 | 34 weeks (0.16) | 0.15 | |
| 40 weeks (0.07) | 0.21 | 40 weeks (0.23) | 0.14 | 40 weeks (0.91) | 0.01 | |
|
| ||||||
| Third trimester Hair cortisol concentration (pg/mg) | 16 weeks (0.06) | 0.20 | 16 weeks (0.01) | 0.27 | 16 weeks (0.05) | 0.22 |
| 22 weeks (0.09) | 0.18 | 22 weeks (0.77) | 0.03 | 22 weeks (0.27) | 0.12 | |
| 28 weeks (0.27) | 0.12 | 28 weeks (0.03) | 0.24 | 28 weeks (0.02) | 0.26 | |
| 34 weeks (0.31) | 0.11 | 34 weeks (0.21) | 0.14 | 34 weeks (0.06) | 0.21 | |
| 40 weeks (0.12) | 0.19 | 40 weeks (0.20) | 0.15 | 40 weeks (0.55) | 0.07 | |
Significant correlations are in bold.
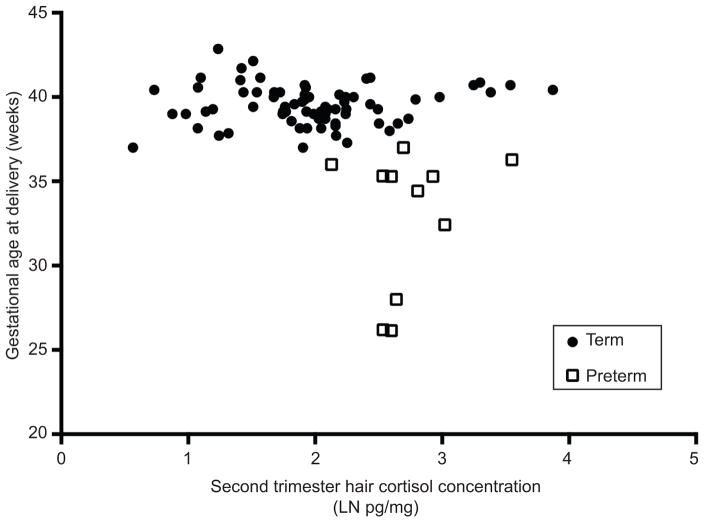
Correlation analyses assessing the relationships between hair cortisol concentration and gestational age at delivery were not significant in the first (r=−0.16, p=0.15) or third (r=0.13, p=0.25) trimesters. However, second trimester hair cortisol concentration was significantly correlated with gestational age at delivery, Figure 3: r= −0.25, p=0.02. All women who had a preterm birth <37 weeks had a second trimester hair cortisol concentration above the median, 2.3 pg/mg.
Figure 3.
Second-trimester maternal hair cortisol concentration (natural log (LN) transformed pg/mg) and gestational age at delivery (weeks) Pearson r=−0.25, P=.02.
After taking into consideration the preceding analyses, which included a significant association between 16 week PSS and gestational age at delivery (Figure 3), a significant association between 16 week PSS and second trimester hair cortisol concentration (r= 0.28, p=0.007) and a significant association between the hair cortisol concentration and gestational age at delivery (Figure 2), we used mediation analyses to assess whether gestational age at delivery was more strongly associated with the physiologic measure (second trimester hair cortisol concentration) or the psychological measure (PSS at 16 weeks). Race-ethnicity and tobacco use were included as covariates in the analyses. Gestational age at delivery was more strongly associated with second trimester hair cortisol concentration than 16 week perceived stress and the correlation between perceived stress and gestational age at delivery was no longer significant after including the mediator (hair cortisol concentration), using the difference in coefficients (indirect effect and bootstrapped standard error of −0.05(0.02), p=0.04). This indicates that hair cortisol concentration meets criteria for a mediator of the relationship between 16-week perceived stress and gestational age at delivery.
DISCUSSION
The prematurity rate in this cohort was consistent with state and national norms31 and hair cortisol levels rose across pregnancy similar to what has been published previously.15 Women who delivered preterm had a higher mean second-trimester hair cortisol concentration than those who delivered at term. Psychological measures of stressors and mood were highly correlated both across measures and across gestation. Our findings of a relationship between second, but not third, trimester psychological and physiologic stress and gestational age at delivery are congruent with previous reports indicating a relationship between second-trimester plasma cortisol and gestational age at delivery.4,32 However, this is the first report of a marker of chronic HPA physiology replicating these data and also of testing hair cortisol concentration as a statistical mediator of these relationships.
In the second trimester, but not the first or third, hair cortisol mediates the relationship between perceived stress and gestational age at delivery. Early in the first trimester, the embryo may be relatively protected from the maternal psychobiologic environment, including the influence of the maternal HPA, due to a small placental volume, low maternal blood flow to the placenta, and low oxygen tension within the fetoplacental unit.33 Conversely, in the third-trimester maternal cortisol levels are significantly increased but levels of the cortisol metabolizing enzyme, 11β-hydroxysteroid dehydrogenase type 2 are concomitantly increased and there is an overall blunted maternal response to late-pregnancy cortisol in preparation for delivery.15,34,35
Sandman et al reported higher plasma cortisol levels at 15 weeks of gestation in women who delivered preterm compared with those who delivered at term.9 The elevation seen in plasma cortisol at 15 weeks of gestation predicted elevated placental corticotrophin-releasing hormone in the third trimester, suggesting a stressor signaling pattern initiated during the second trimester that might influence earlier delivery.9 This cortisol pattern is also observed in second trimester hair cortisol concentration in our present study.
What has been proposed but has never been directly tested is how physiologic stress relates to psychological stress and, in combination, how these two measures correlate with gestational age at delivery and preterm birth. This study is unique in that a longitudinal study design was used providing repeated measures at multiple time points, allowing sufficient data to perform a mediation analysis. The strengths of this study are prospectively collected psychological and physiologic data, multiple time points of assessment over the course of pregnancy, and a non-invasive biomarker of long-term retrospective HPA status collected to represent a single trimester of maternal HPA activity. Limitations of this study include a relative lack of racial diversity, collection from a single study site and, as just recently published, an inability to account for the impact of childhood trauma on maternal hair cortisol concentrations.36
In summary, early pregnancy physiologic stress is associated with psychological stress and both are correlated with gestational age at delivery. These findings have biological plausibility and are consistent with health disparities data that relate chronic stress, dysregulated HPA function, and adverse mental and physical health outcomes including preterm birth.37,38,39 This information advances our understanding of HPA function in the presence of second trimester perceived stress, elucidating one plausible contributor to preterm birth. As stress remains a significant risk factor for preterm birth, its recognition and amelioration during early pregnancy deserves further attention and investigation.
Acknowledgments
Supported by NIH/NICHD Women’s Reproductive Health Research (WRHR) program Grant number K12HD001271-11 (MCH), NIH/NCATS Colorado CTSA Child-Maternal Health Pilot Grant Number UL1 TR001082 (MCH), and R01 MH101295 (RGR). Danielle Glenn and Patrick Benitez, University of Colorado-Anschutz Medical Campus received salary support from the Behavioral Immunology and Endocrinology Laboratory (BIEL), and Amber Americanos, RN, Kate Noonan, MSW, Jose Barron, Laura V. Karban, Michelle Six, Meredith Tittler, University of Colorado-Anschutz Medical Campus received salary support from R01 MH101295 (RGR). The contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
References
- 1.Manuck TA, Esplin MS, Biggio J, et al. The phenotype of spontaneous preterm birth: application of a clinical phenotyping tool. Am J Obstet Gynecol. 2015;212:487, e1–e11. doi: 10.1016/j.ajog.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coussons-Read ME, Lobel M, Carey JC, et al. The occurrence of preterm delivery is linked to pregnancy-specific distress and elevated inflammatory markers across gestation. Brain, behavior, and immunity. 2012;26:650–9. doi: 10.1016/j.bbi.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wadhwa PD, Entringer S, Buss C, Lu MC. The contribution of maternal stress to preterm birth: issues and considerations. Clinics in perinatology. 2011;38:351–84. doi: 10.1016/j.clp.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diego MA, Jones NA, Field T, et al. Maternal psychological distress, prenatal cortisol, and fetal weight. Psychosomatic Medicine. 2006;68:747–53. doi: 10.1097/01.psy.0000238212.21598.7b. [DOI] [PubMed] [Google Scholar]
- 5.Glynn LM, Davis EP, Sandman CA. New insights into the role of perinatal HPA-axis dysregulation in postpartum depression. Neuropeptides. 2013;47:363–70. doi: 10.1016/j.npep.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Davis EP, Sandman CA. Prenatal psychobiological predictors of anxiety risk in preadolescent children. Psychoneuroendocrinology. 2012;37:1224–33. doi: 10.1016/j.psyneuen.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis EPSC. The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. J Child Psychol Psychiatry. 2010;52:119–29. doi: 10.1111/j.1467-8624.2009.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glover V. Prenatal stress and its effects on the fetus and the child: possible underlying biological mechanisms. Advances in neurobiology. 2015;10:269–83. doi: 10.1007/978-1-4939-1372-5_13. [DOI] [PubMed] [Google Scholar]
- 9.Sandman CAGL, Schetter CD, Wadhwa P, Garite T, Chicz-DeMet A, Hobel C. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): priming the placental clock. Peptides. 2006;27:1457–63. doi: 10.1016/j.peptides.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Diego MA, Field T, Hernandez-Reif M, Schanberg S, Kuhn C, Gonzalez-Quintero VH. Prenatal depression restricts fetal growth. Early human development. 2009;85:65–70. doi: 10.1016/j.earlhumdev.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gozansky WS, Lynn JS, Laudenslager ML, Kohrt WM. Salivary cortisol determined by enzyme immunoassay is preferable to serum total cortisol for assessment of dynamic hypothalamic--pituitary--adrenal axis activity. Clinical endocrinology. 2005;63:336–41. doi: 10.1111/j.1365-2265.2005.02349.x. [DOI] [PubMed] [Google Scholar]
- 12.Vanaelst B, Huybrechts I, Bammann K, et al. Intercorrelations between serum, salivary, and hair cortisol and child-reported estimates of stress in elementary school girls. Psychophysiology. 2012;49:1072–81. doi: 10.1111/j.1469-8986.2012.01396.x. [DOI] [PubMed] [Google Scholar]
- 13.Yokokawa A, Takasaka T, Shibasaki H, et al. The effect of water loading on the urinary ratio of cortisone to cortisol in healthy subjects and a new approach to the evaluation of the ratio as an index for in vivo human 11beta-hydroxysteroid dehydrogenase 2 activity. Steroids. 2012;77:1291–7. doi: 10.1016/j.steroids.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Meyer JS, Novak MA. Minireview: Hair cortisol: a novel biomarker of hypothalamic-pituitary-adrenocortical activity. Endocrinology. 2012;153:4120–7. doi: 10.1210/en.2012-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Anna-Hernandez KL, Ross RG, Natvig CL, Laudenslager ML. Hair cortisol levels as a retrospective marker of hypothalamic-pituitary axis activity throughout pregnancy: comparison to salivary cortisol. Physiology & behavior. 2011;104:348–53. doi: 10.1016/j.physbeh.2011.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braig S, Grabher F, Ntomchukwu C, et al. Determinants of maternal hair cortisol concentrations at delivery reflecting the last trimester of pregnancy. Psychoneuroendocrinology. 2015;52:289–96. doi: 10.1016/j.psyneuen.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Yamada J, Stevens B, de Silva N, et al. Hair cortisol as a potential biologic marker of chronic stress in hospitalized neonates. Neonatology. 2007;92:42–9. doi: 10.1159/000100085. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman MC, Karban LV, Benitez P, Goodteacher A, Laudenslager ML. Chemical processing and shampooing impact cortisol measured in human hair. Clinical and investigative medicine Medecine clinique et experimentale. 2014;37:E252–7. doi: 10.25011/cim.v37i4.21731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson DP, Klein SL. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Hormones and behavior. 2012;62:263–71. doi: 10.1016/j.yhbeh.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pols H. August Hollingshead and Frederick Redlich: poverty, socioeconomic status, and mental illness. American journal of public health. 2007;97:1755. doi: 10.2105/AJPH.2007.117606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wosu AC, Gelaye B, Valdimarsdottir U, et al. Hair cortisol in relation to sociodemographic and lifestyle characteristics in a multiethnic US sample. Annals of epidemiology. 2015;25:90–5. e2. doi: 10.1016/j.annepidem.2014.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wennig R. Potential problems with the interpretation of hair analysis results. Forensic Science International. 2000;107:5–12. doi: 10.1016/s0379-0738(99)00146-2. [DOI] [PubMed] [Google Scholar]
- 23.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of health and social behavior. 1983;24:385–96. [PubMed] [Google Scholar]
- 24.Radloff LS. The use of the Center for Epidemiologic Studies Depression Scale in adolescents and young adults. Journal of youth and adolescence. 1991;20:149–66. doi: 10.1007/BF01537606. [DOI] [PubMed] [Google Scholar]
- 25.Spielberger CD, Gorssuch RL, Lushene PR, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. 1983. [Google Scholar]
- 26.Kalra S, Einarson A, Karaskov T, Van Uum S, Koren G. The relationship between stress and hair cortisol in healthy pregnant women. Clinical and investigative medicine Medecine clinique et experimentale. 2007;30:E103–7. doi: 10.25011/cim.v30i2.986. [DOI] [PubMed] [Google Scholar]
- 27.Nicholson WK, Setse R, Hill-Briggs F, Cooper LA, Strobino D, Powe NR. Depressive symptoms and health-related quality of life in early pregnancy. Obstet Gynecol. 2006;107(4):798–806. doi: 10.1097/01.AOG.0000204190.96352.05. [DOI] [PubMed] [Google Scholar]
- 28.Tamaki R, Murata M, Okano T. Risk factors for postpartum depression in Japan. Psychiatry and clinical neurosciences. 1997;51:93–8. doi: 10.1111/j.1440-1819.1997.tb02368.x. [DOI] [PubMed] [Google Scholar]
- 29.Hollingshead AB, Redlich FC. Social class and mental illness: a community study. 1958. American journal of public health. 2007;97:1756–7. doi: 10.2105/ajph.97.10.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacKinnon DP, Dwyer JH. Estimation of mediated effects in prevention studies. Eval Rev. 1993;17:144–58. [Google Scholar]
- 31.http://www.marchofdimes.com/peristats
- 32.Sandman CA, Glynn L, Schetter CD, et al. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): priming the placental clock. Peptides. 2006;27:1457–63. doi: 10.1016/j.peptides.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Burton GJ, Jaunaiux E. Maternal vascularisation of the human placenta: does the embryo develop in a hypoxic environment? Gynecologie, obstetrique & fertilite. 2001;29:503–8. doi: 10.1016/s1297-9589(01)00179-5. [DOI] [PubMed] [Google Scholar]
- 34.Mastorakos G, Ilias I. Maternal and fetal hypothalamic-pituitary-adrenal axes during pregnancy and postpartum. Annals of the New York Academy of Sciences. 2003;997:136–49. doi: 10.1196/annals.1290.016. [DOI] [PubMed] [Google Scholar]
- 35.Murphy VE, Clifton VL. Alterations in human placental 11beta-hydroxysteroid dehydrogenase type 1 and 2 with gestational age and labour. Placenta. 2003;24:739–44. doi: 10.1016/s0143-4004(03)00103-6. [DOI] [PubMed] [Google Scholar]
- 36.Schreier HM, Enlow MB, Ritz T, Gennings C, Wright RJ. Childhood abuse is associated with increased hair cortisol levels among urban pregnant women. Journal of epidemiology and community health. 2015 doi: 10.1136/jech-2015-205541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blumenshine P, Egerter S, Barclay CJ, Cubbin C, Braveman PA. Socioeconomic disparities in adverse birth outcomes: a systematic review. American journal of preventive medicine. 2010;39:263–72. doi: 10.1016/j.amepre.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 38.Jackson JS, Knight KM, Rafferty JA. Race and unhealthy behaviors: chronic stress, the HPA axis, and physical and mental health disparities over the life course. American journal of public health. 2010;100:933–9. doi: 10.2105/AJPH.2008.143446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berger M, Sarnyai Z. “More than skin deep”: stress neurobiology and mental health consequences of racial discrimination. Stress. 2015;18:1–10. doi: 10.3109/10253890.2014.989204. [DOI] [PubMed] [Google Scholar]