Abstract
Background
Frontostriatal and frontoamygdalar connectivity alterations in patients with obsessive–compulsive disorder (OCD) have been typically described in functional neuroimaging studies. However, structural covariance, or volumetric correlations across distant brain regions, also provides network-level information. Altered structural covariance has been described in patients with different psychiatric disorders, including OCD, but to our knowledge, alterations within frontostriatal and frontoamygdalar circuits have not been explored.
Methods
We performed a mega-analysis pooling structural MRI scans from the Obsessive–compulsive Brain Imaging Consortium and assessed whole-brain voxel-wise structural covariance of 4 striatal regions (dorsal and ventral caudate nucleus, and dorsal-caudal and ventral-rostral putamen) and 2 amygdalar nuclei (basolateral and centromedial-superficial). Images were preprocessed with the standard pipeline of voxel-based morphometry studies using Statistical Parametric Mapping software.
Results
Our analyses involved 329 patients with OCD and 316 healthy controls. Patients showed increased structural covariance between the left ventral-rostral putamen and the left inferior frontal gyrus/frontal operculum region. This finding had a significant interaction with age; the association held only in the subgroup of older participants. Patients with OCD also showed increased structural covariance between the right centromedial-superficial amygdala and the ventromedial prefrontal cortex.
Limitations
This was a cross-sectional study. Because this is a multisite data set analysis, participant recruitment and image acquisition were performed in different centres. Most patients were taking medication, and treatment protocols differed across centres.
Conclusion
Our results provide evidence for structural network–level alterations in patients with OCD involving 2 frontosubcortical circuits of relevance for the disorder and indicate that structural covariance contributes to fully characterizing brain alterations in patients with psychiatric disorders.
Introduction
Structural covariance, or the volume correlations across distant brain regions, is a relatively novel measurement that can be derived from the analysis of structural MRI.1,2 It is considered a brain connectivity measurement, because the existence of significant structural covariance indicates that inter-individual differences in regional volumes are coordinated within brain networks that vary together in size. In this sense, while global brain volume is largely genetically determined, regional brain volumes, and therefore structural covariance measurements, are more flexibly determined by a number of factors.3,4 Such factors range from genetic5 and other developmental influences6–8 to aging effects.9,10
Other aspects related to the basic principles of brain organization, such as the existence of functional connectivity, or correlated spontaneous activity across time between distant structures, may also influence the patterns of structural covariance.2 Structural covariance is observed between the regions of the different resting state functional networks.6 Importantly, within the context of activity-dependent structural plasticity,11,12 this association between functional connectivity and structural covariance suggests that interindividual variability in functional brain networks should result in similarly variable patterns of structural covariance.13 In agreement with this, brain disorders selectively affecting nodal regions within functional networks simultaneously disrupt both functional connectivity and structural covariance patterns.14 Nevertheless, functional connectivity and structural covariance are only partially correlated.13 Therefore, structural covariance provides a specific and distinctive measurement that should aid in the comprehensive characterization of network-level brain features. Specifically, in comparison with functional connectivity, structural covariance assesses brain connectivity on a different time scale; while functional connectivity reflects a state feature, which may oscillate across different states (i.e., at rest v. task performance), structural covariance may better represent more stable (e.g., maturational or trait-like) connectivity features.2
Normal structural covariance patterns have been shown to be altered in patients with different brain disorders. Among those with psychiatric conditions, such alterations have been described mostly in patients with schizophrenia,15,16 although there have also been reports in those with autism17,18 and obsessive–compulsive disorder (OCD). In patients with OCD, Pujol and colleagues19 described positive volume correlations between cortical areas (dorsomedial prefrontal, medial orbitofrontal and insular cortices) shown to be reduced in volume in comparison with healthy controls, suggesting that volume alterations in patients with OCD were coordinated in patterns of structural covariance. Nevertheless, despite theoretical accounts suggesting that frontostriatal and frontolimbic circuits are crucially involved in OCD symptomatology, with dysfunction of specific subcircuits underpinning core symptoms of the disorder,20 abnormal structural covariance patterns have not been reported within these frontosubcortical circuits.
Neuroimaging studies in patients with OCD have widely characterized functional connectivity alterations involving both frontostriatal21–25 and frontoamygdalar26 circuits, although results have been somewhat heterogeneous. Thus, while functional connectivity increases between ventral striatal and orbitofrontal regions have been reported,21,22,27 such results depend on sample characteristics,25 analysis methods24,28 or on the assessment of resting-state versus task- related connectivity.23 Frontolimbic connectivity has been less explored, and studies have also provided conflicting findings, ranging from decreased connectivity at rest29 to functional connectivity increases during the performance of a cognitive task.26 In this context, the assessment of structural covariance should inform about the existence of stable inter-regional connectivity alterations, probably stemming from maturational abnormalities or persistent and enduring functional connectivity changes that should underpin the expression of OCD symptoms across different scenarios. The normal patterns of structural covariance within frontostriatal circuits have been recently described,13 showing partial overlap with the functional connectivity patterns that characterize these circuits.30 Likewise, structural covariance of the amygdala has been previously explored;31 however, in contrast to findings reported in functional connectivity studies,32,33 there have been no reports of specific structural covariance patterns associated with different amygdala subregions.
The present study aimed to assess putative OCD-related alterations in the structural covariance patterns of 4 distinct striatal territories (dorsal [DC] and ventral caudate [VC] nucleus, dorsal-caudal [DCP] and ventral-rostral [VRP] putamen) and 2 distinct amygdalar subregions (basolateral [BLA] and centromedial-superficial [CMS]). To this end, we used multi-centre structural MRI data from the OCD Brain Imaging Consortium (OBIC)34 and performed a mega-analysis with a very large series of patients with OCD and healthy controls carefully matched for age, sex, handedness, race and education level. We hypothesized structural covariance increases involving the ventral striatal and orbitofrontal regions as well as disruptions of structural covariance within the corticolimbic system in patients with OCD. In addition, we explored the effects on such structural covariance patterns of clinical and sociodemographic variables. Disorder severity21 and the presence of specific co-morbidities35 have been associated with particular changes in frontosubcortical connectivity in OCD samples. Likewise, sociodemographic variables, especially age, have been found to modulate regional volumes within striatal regions in patients with OCD19,34 and structural covariance patterns.13 Assessment of age effects was of particular interest for the purpose of this study, as it may help to discriminate alterations of maturational origin from causes associated with the course of the disorder (i.e., shared history of coactivation between 2 regions).
Methods
Participants
We recruited patients with OCD from 6 research centres participating in the OBIC. We used a standardized structured interview and the Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-IV) to confirm the OCD diagnosis. Sociodemographic and clinical data, such as age at onset, OCD severity, symptom dimension scores and current medication use, were collected at each center. Exclusion criteria for patients with OCD included age younger than 18 years or older than 65 years, presence of a current psychotic disorder, a recent history of psychoactive substance abuse or dependence, mental retardation, any severe organic or neurological pathology except tic disorder, and the presence of any contraindication to MRI scanning. Comorbidity with other Axis I disorders was not considered an exclusion criterion provided that OCD was the main diagnosis and the reason for seeking medical assistance. Healthy controls were also recruited from the OBIC centres, and exclusion criteria were the same as those for patients with OCD. In addition, we excluded individuals with current or past psychiatric disorders. Written informed consent was obtained from all participants after a complete description of the study performed at each centre, which, in all cases, was performed in accordance with the Declaration of Helsinki and approved by the local ethical review board of each centre (the Bellvitge University Hospital Ethical Committee, Barcelona, Spain; the Medical Ethics Review Committee of the VU University Medical Center, Amsterdam, the Netherlands; the Kyoto Prefectural University of Medicine Research Ethics Committee, Kyoto, Japan; the Ethics Committee (Research) of the Maudsley Hospital and Institute of Psychiatry, King’s College, London, UK; the Ethics Committee of the University of Sao Paulo Medical School, Sao Paulo, Brazil; and the Institutional Review Board of Seoul National University Hospital, Seoul, South Korea).
Data acquisition and preprocessing
A 1.5 T structural T1-weighted MRI scan was locally acquired for each participant at 1 of the 6 contributing centres. Further details regarding imaging acquisition and preprocessing are described in Appendix 1, available at jpn.ca.
Seed volumes extraction
We first extracted individual grey matter volumes from 8 striatal (4 per hemisphere) and 4 amygdalar (2 per hemisphere) seed regions of interest (ROIs). Based on previous functional connectivity21,30 and structural covariance13 studies, all of the striatal seeds were defined using the dorsoventral boundaries of caudate and putamen nuclei initially proposed by Postuma and Dagher.36 Striatal seeds of interest were the DC, VC, DCP and VRP. Amygdala seeds were defined according to Baur and colleagues,33 dividing the amygdala region into the BLA and CMS seeds of interest.
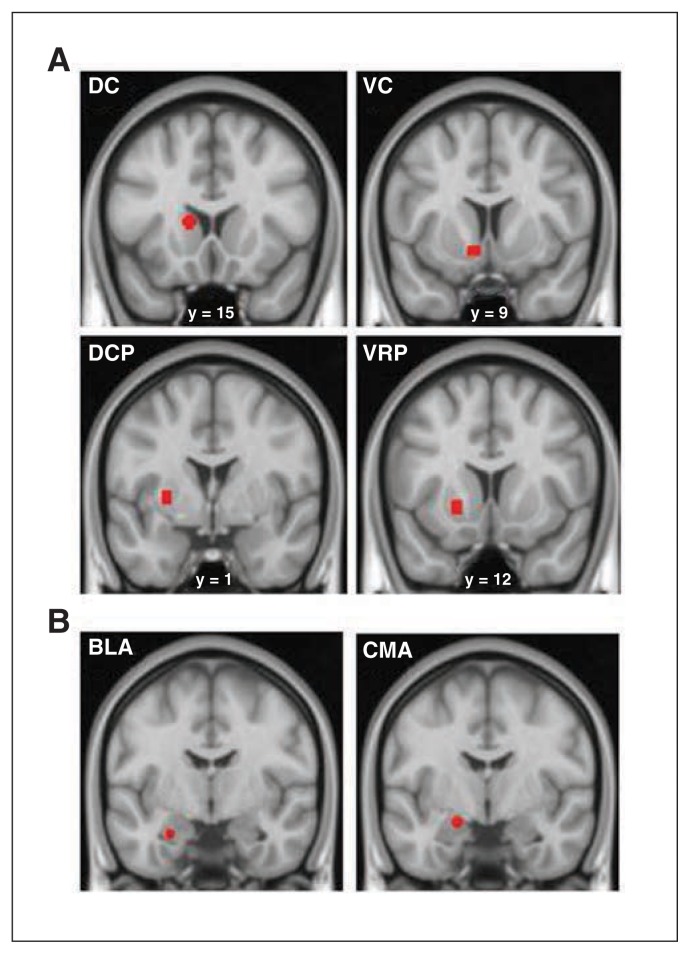
Each of these seeds were defined using the MarsBar ROI toolbox37 as 3.5 mm radial spheres centred at bilateral Montreal Neurological Institute (MNI) coordinates. Specifically, striatal seeds were symmetrically located as follows: x, y, z = ±13, 15, 9 for the DC; x, y, z = ±9, 9, −8 for the VC, involving the nucleus accumbens; x, y, z = ±28, 1, 3 for the DCP; and x, y, z = ±20, 12, −3 for the VRP. Amygdala seed locations were as follows: x, y, z = −26, −5, −23 for the left BLA; x, y, z = 29, −3, −23 for the right BLA; x, y, z = −19, −5, −15 for the left CMS; and x, y, z = 23, −5, −13 for the right CMS (Fig. 1). Importantly, to account for the potential between-seed volumetric covariance induced by spatial smoothing, we checked that all striatal and amygdala seeds were spatially separated by at least 10 mm (1 mm full-width at half-maximum) according to the formula √(x1 − x2)2 + (y1 − y2)2 + (z1 − z2)2, where (x1, y1, z1, x2, y2 and z2) refer to the coordinates of any 2 voxels in MNI space. We calculated global grey matter volume by integrating all the modulated voxel values of grey matter segments.
Fig. 1.
(A) Striatal seed placements (dorsal caudate [DC], ventral caudate [VC], dorso-caudal putamen [DCP] and ventro-rostral putamen [VRP]) and (B) amygdalar seed placements (basolateral amygdala [BLA] and centromedial-superficial amygdala [CMS]) corresponding to the left hemisphere, overlaid on high-resolution coronal sections. The “y” denotes the anterior–posterior coordinate in standard Montreal Neurological Institute space.
Statistical analysis
In order to calculate the whole-brain structural covariance patterns of our seeds of interest (4 striatal and 2 amygdalar seeds per hemisphere), we estimated 12 SPM models, 1 for each seed region. In all these analyses, we maximized statistical sensitivity by including only relevant within-brain voxels using an absolute threshold masking of 0.2. The different models included the variable group (patient v. control) and the individual value of the seed volume of interest × group interaction as well as the following confounding covariates: scan sequence (corresponding to the different acquisitions performed across centres), global grey matter volume, age, sex and the remaining seed volumes of the region where the seed of interest was located (3 striatal or 1 amygdalar seed from the same hemisphere). In addition, within each SPM model the variables were sequentially orthogonalized following an iterative Gram–Schmidt procedure. Specifically, scan sequence was always the first to be entered, followed by global grey matter volume, age, sex, the striatal or amygdalar seeds of no interest and, finally, the seed of interest. Following such an approach, we aimed to remove from the seed of interest all the variance shared with the other striatal or amygdalar seeds as well as with the general confounding factors of scan sequence, global grey matter volume, age and sex, thus avoiding the inclusion of multiple collinear measurements in the design matrix. We then generated t statistic maps by assessing the positive correlations of the seed region of interest with the rest of the brain (voxel-wise). The results of such analyses were expected to be maximally specific structural covariance whole-brain patterns. Significance threshold was set at p < 0.05 (voxel-level), family-wise error (FWE)–corrected for multiple comparisons, with a minimum cluster extent of 10 voxels.
To assess potential interactions with age and sex, we estimated additional SPM models similar to those already described, although patient and control groups were further divided based on age (younger v. older) or sex (male v. female). The cut-point between younger and older participants was established at age 30 years (the statistical median age), which provided a relatively balanced distribution of younger and older participants across the 4 groups. The sex distribution across groups was equally well balanced. These analyses were restricted to the regions where significant correlations with the seeds of interest were observed in the general analyses. Specifically, from these analyses, we first extracted the masks of between-group differences thresholded at p < 0.001, uncorrected voxel level. Subsequently, we assessed (diagnosis × age or sex [4 categories]) × seed volume of interest interactions at a threshold of p < 0.05, voxel-level, FWE-corrected across in-mask voxels using small volume correction (SVC) procedures. We also assessed second-order (diagnosis × age × sex [8 categories]) × seed volume of interest) interactions using similar procedures.
Finally, we assessed possible interactions with selected clinical variables. Specifically, we assessed the effects of disorder severity (with a cut-point established at a Yale–Brown Obsessive–Compulsive Scale [Y-BOCS] score of 24) and the presence of affective or anxiety comorbidities. For these analyses, we compared the interregional correlation values from the above analyses between the different subgroups of patients and also between healthy controls and each specific subgroup of patients.
Results
We included 329 patients with OCD (mean age 32.03 ± 9.39 yr, 172 men) and 316 healthy controls (mean age 31.18 ± 9.42 yr, 162 men) in our study. The sociodemographic characteristics of all participants and the clinical characteristics of patients with OCD are described in Table 1. Further details about participants’ characteristics and clinical assessments are provided in Appendix 1.
Table 1.
Sociodemographic and clinical characteristics of patients with OCD and healthy controls from the Obsessive–compulsive Brain Imaging Consortium
| Group; mean ± SD or no. (%) | ||||
|---|---|---|---|---|
|
|
||||
| Characteristic | OCD, n = 329 | Control, n = 316 | Statistic | p value |
| Age, yr | 32.03 ± 9.39 | 31.18 ± 9.42 | t = 1.139 | 0.26 |
| Male sex | 172 (52.3%) | 162 (51.3%) | χ2 = 0.066 | 0.81 |
| Race* | χ2 = 5.918 | 0.12 | ||
| White | 160 (54.6%) | 175 (58.9%) | — | — |
| Asian | 129 (44%) | 111 (37.4%) | — | — |
| Other | 4 (1.4%) | 11 (3.7%) | — | — |
| Right-handedness† | 276 (90.5%) | 280 (92.7%) | χ2 = 1.103 | 0.58 |
| Educational level, yr | 14.09 ± 2.91 | 14.37 ± 3.18 | t = −1.176 | 0.24 |
| Age at symptom onset, yr‡ | 19.76 ± 8.36 | — | — | — |
| Y-BOCS score | ||||
| Obsessions subscale§ | 12.50 ± 3.28 | — | — | — |
| Compulsions subscale§ | 12.04 ± 3.79 | — | — | — |
| Total score¶ | 24.54 ± 6.18 | — | — | — |
| Medication history§ | ||||
| Medication naive | 77 (25.9%) | — | — | — |
| Taking medication | 220 (74.1%) | — | — | — |
OCD = obsessive–compulsive disorder; SD = standard deviation; Y-BOCS = Yale–Brown Obsessive–Compulsive Scale.
Data available for 590 participants.
Data avilable for 607 participants.
Age at onset was defined as the age when symptoms became a substantial source of distress and interfered with the patient’s social functioning. Data available for 311 participants.
Data available for 297 participants.
Data available for 298 participants.
Within-group structural covariance maps for each seed ROI are presented in Appendix 1, Figs. S1–S3.
Between-group comparisons
Striatal seeds
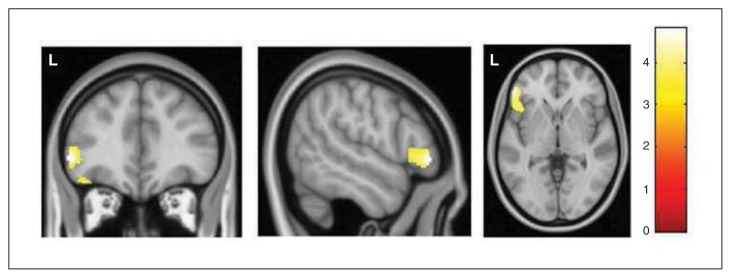
In comparison with healthy controls, patients with OCD showed a significantly increased correlation between the volume of the left VRP seed and the volume of the left inferior frontal gyrus (IFG)/frontal operculum region (x, y, z = −53, 38, −2, t = 4.63, z-score = 4.59, pFWE = 0.018, 24 voxels; Table 2 and Fig. 2). The volume of the right VRP seed was also correlated with this same frontal region, although at a trend level (x, y, z = −47, 20, −6, t = 4.42, z-score = 4.38, pFWE = 0.041, 3 voxels). The structural covariance patterns of the rest of the striatal seeds did not differ significantly between groups.
Table 2.
Regions showing significant structural covariance increases in patients with OCD compared with healthy controls
| MNI coordinates | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Seed region | x | y | z | z-score (t value) | p value* | k | Anatomic location |
| Left VRP | −53 | 38 | −2 | 4.59 (4.63) | 0.018 | 24 | Left IFG |
| Right CMS | 12 | 42 | −6 | 4.79 (4.84) | 0.008 | 145 | vmPFC |
CMS = centromedial-superficial amygdala; IFG = inferior frontal gyrus; MNI = Montreal Neurological Institute; OCD = obsessive–compulsive disorder; vmPFC = ventromedial prefrontal cortex; VRP = ventro-rostral putamen.
Family-wise error–corrected.
Fig. 2.
Regions of increased correlation in patients with obsessive–compulsive disorder, with the volume of the left ventro-rostral putamen seed. The cluster is located in the left inferior frontal gyrus. Voxels with p < 0.001 (uncorrected) are displayed (cluster extent = 1144 voxels). L indicates left hemisphere. The colour bar represents t values.
Amygdalar seeds
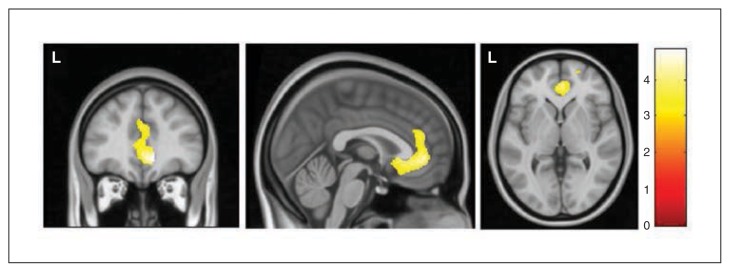
In comparison with healthy controls, patients with OCD showed a significantly increased correlation between the volumes of the right CMS amygdala and the ventromedial pre-frontal cortex (vmPFC), including peri- and subgenual regions of the anterior cingulate cortex (x, y, z = 12, 42, −6, t = 4.84, z-score = 4.79, pFWE = 0.008, 145 voxels; Table 2 and Fig. 3). No significant between-group differences were observed for the rest of the amygdalar seeds.
Fig. 3.
Regions of increased correlation in patients with obsessive–compulsive disorder with the volume of the right centromedial-superficial amygdala seed. The cluster is located in the ventromedial prefrontal cortex. Voxels with p < 0.001 (uncorrected) are displayed (cluster extent = 2500 voxels). L indicates the left hemisphere. The colour bar represents t values.
In a post hoc analysis we confirmed that the structural covariance alterations described were not due to medication effects. Specifically, we compared the structural covariance patterns of the left and right VRP and the right CMS amygdala between the 77 medication-naive patients and the 220 patients who were taking medication (data were missing for 32 patients) and found no significant results in the IFG/frontal operculum region or the vmPFC, even at a very low significance threshold (p < 0.05, uncorrected).
Interactions between age and sex
Age was equally distributed among participants: there were 154 younger and 175 older patients with OCD and 166 younger and 150 older controls (χ2 = 2.11, p = 0.15) The mean age (range) of these 4 groups was 24.14 (18–29) years for younger patients with OCD, 38.97 (30–62) years for older patients with OCD, 24.28 (19–29) years for younger controls and 38.83 (30–63) years for older controls. Sex was equally distributed among participants: there were 172 male and 157 female patients and 162 male and 154 female controls (χ2 = 0.66, p = 0.80).
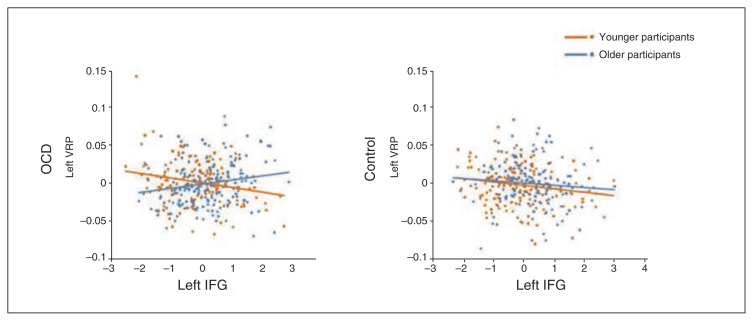
The group × age interaction analysis revealed a significant finding within the cluster of the left IFG/frontal operculum correlating with the left VRP volume. Specifically, we detected a significant difference between younger and older patients with OCD (x, y, z = −32, 39, −17, t = 3.53, pFWE-SVC = 0.049). While in older patients with OCD we observed a positive correlation between left VRP and IFG/operculum volume (r = 0.182, p = 0.018), in younger patients such correlations were negative (r = −0.191, p = 0.020) and significantly different from those of older patients with OCD (z-score = −3.38, p < 0.001). By contrast, in healthy controls, the correlations between younger and older participants did not significantly differ (younger controls: r = −0.17, p = 0.031; older controls: r = −0.108, p = 0.20; z-score = −0.56, p = 0.29), although correlations observed in older controls differed significantly from those observed in older patients with OCD (z-score = −2.6, p = 0.005; Fig. 4). We did not observe any significant age interaction in the correlation between the right CMS amygdala and vmPFC volumes. Likewise, no sex or second-order interactions were detected for any of the seeds.
Fig. 4.
Correlations between the adjusted volumes of the left ventro-rostral putamen (VRP) seed and the left inferior frontal gyrus (IFG). Linear regression fit lines are displayed for younger (orange) and older (blue) participants in the obsessive–compulsive disorder (OCD) and healthy control groups.
Effect of clinical variables
The interregional correlations described in previous sections did not differ between patients with severe and mild OCD (n = 175 and n = 123, respectively), between patients with OCD with and without affective disorders (n = 96 and n = 215, respectively), or between patients with OCD with and without anxiety disorders (n = 67 and n = 241, respectively). Likewise, such interregional correlations were significantly different in relation to healthy controls for all subgroups of patients except for patients with anxiety disorders, in whom the correlation between the right CMS amygdala and vmPFC did not differ from healthy controls (r = −0.01, p = 0.94 v. r = −0.30, p < 0.001, z-score = 1.84, p = 0.07).
Discussion
In this study we assessed potential alterations in corticostriatal and corticoamygdalar circuitry in patients with OCD using structural MRI data. Specifically, we studied the differences in the structural covariance patterns of distinct striatal and amygdalar regions between large groups of patients with OCD and healthy controls using the multicentre database of the OCD Brain Imaging Consortium (OBIC).34 Our findings are consistent with those of models describing alterations in patients with OCD as involving both corticostriatal and corticoamygdalar circuits.20 Specifically, regarding corticostriatal circuits, and in agreement with our hypotheses, we observed increased structural covariance in patients with OCD between the VRP and the left IFG/frontal operculum. Regarding corticoamygdalar circuits, we observed increased covariance in patients with OCD between the right CMS amygdala and the vmPFC. In addition, alterations in corticostriatal circuits interacted with age, suggesting that structural covariance alterations within these circuits might develop over the course of the disorder.
Our findings involving corticostriatal structures should be interpreted in the context of previous functional and structural research. In healthy individuals, the VRP and the IFG/operculum have been shown to be functionally (resting-state fMRI) and structurally (diffusion tensor imaging) connected,30,38,39 and significant structural covariance between them has also been reported.13 Results in OCD samples have shown abnormal task-related activity in both regions40–42 as well as changes in functional connectivity between them.21 Regarding morphometric assessments, different studies have detected cortical thickness43 and grey matter volume reductions in the IFG/frontal operculum region34,44,45 as well as volume enlargements in the ventral putamen.19,46 In addition, although in our previous voxel-based morphometry study34 we did not replicate this last finding, we observed a positive correlation between ventral putamen volume and age, similar to what was originally reported in the study by Pujol and colleagues.19
In relation to the putative role of these corticostriatal structures in patients with OCD, it is important to note that the IFG/frontal operculum is involved in response inhibition and emotional processing and has been consistently shown to respond to anxiety and stress situations.47 Together with dorsomedial frontal regions, it is thought to regulate the activity of subcortical regions, thus affecting control over the selection and execution of actions.48 On the other hand, compulsive behaviours have been associated with increased volume or activity in the ventral striatum (including the ventral putamen).40,49 It is thus tempting to suggest that the IFG/frontal operculum may be implicated in the (largely unsuccessful) regulation of abnormally increased ventral striatal activity in patients with OCD, though the correlational nature of the study precludes firm conclusions. In support of this idea, recent research has shown how these regions show aberrant activity in patients with OCD during tasks of cognitive control and conflict processing.41,42 Interestingly, the IFG/frontal operculum activity seems to specifically regulate behaviour in low-predictability scenarios, thus allowing for fast and accurate responding in changing environments,48 a pattern of response that is opposite to compulsive behaviour and clearly disrupted in patients with OCD.
The increased structural covariance between the IFG/frontal operculum and the VRP reported here seems therefore consistent with the postulated role of these structures in patients with OCD. Nevertheless, the opposed volumetric changes typically reported for these structures in OCD samples in combination with the decreased functional connectivity observed between the IFG/frontal operculum and the VRP21 are seemingly inconsistent with our findings. These discrepancies, however, may partially be accounted for by the interaction with aging effects. Thus, it should be emphasized that in our sample increased structural covariance was specifically observed in older participants (mean age 38.97 yr), whereas in younger participants such volume correlations were negative (although nonsignificant). In agreement with this, decreased functional connectivity between the IFG/frontal operculum and VRP21 was reported in a group of relatively young patients with OCD (mean age 28.52 yr). Moreover, orbitofrontal cortex volume alterations seem to change over time, and although volume reductions have been shown to be present from early disease stages,19 age-related volume increases have been detected in orbitofrontal cortex clusters adjacent to the IFG/frontal operculum region.34 These findings, in combination with the age-related volume increases typically observed in the ventral putamen,19,34 suggest that structural covariance increases between the IFG/frontal operculum and that the VRP may result from activity-dependent neuroplastic changes associated with the course of the disorder, probably as a consequence of a shared history of coactivation underpinning chronic compulsive behaviours (ventral putamen changes) and protracted compensatory activations of cortical regulation regions (IFG/frontal operculum changes). However, longitudinal studies will be required to confirm this hypothesis.
The increased structural covariance between the right CMS amygdala and the vmPFC should also be interpreted in relation to previous research. First, the vmPFC is structurally connected to the amygdala.50 Second, decreased functional connectivity between the CMS and the vmPFC has been associated with anxiety traits and symptoms in both controls and patients with anxiety disorders.51–53 Likewise, structural covariance between these 2 regions has also been found to be decreased in patients with more severe anxiety traits.51 Altogether, such results have been interpreted as indicative of impaired cortical regulation of limbic activity in individuals with high levels of anxiety. The increased structural covariance between the vmPFC and CMS amygdala reported here suggests that patients with OCD may differ from those with other anxiety disorders, which is consistent with a range of other data54 and points to the need for further studies of functional connectivity between the vmPFC and the amygdala in OCD samples. Importantly, in our study patients with a lifetime history of anxious disorders did not differ from controls in the correlation between these structures, which suggests that anxiety may partially compensate for the increased structural covariance between the vmPFC and the amygdala observed in patients with OCD.
Although the vmPFC has been characterized as hypoactive at rest in OCD populations,20 perhaps owing to difficulties in fear extinction,55 hyperactivation of this region has been reported in response to error processing,56 uncertainty57 and moral dilemma.58 Such findings indicate that the vmPFC may be involved in the regulation of transiently increased limbic activity when individuals experience anxiety symptoms, a hypothesis that seems to concur with our findings. Increased functional connectivity between the amygdala and prefrontal areas has been reported in patients with OCD during executive functioning as well.26 In the present study, structural covariance increases with vmPFC were limited to the CMS amygdala, which is in agreement with the specific pattern of functional connectivity of this amygdala region.32 The CMS amygdala is involved both in regulating the motor and autonomic output of amygdala activity59 and in processing socially relevant information and modulating approach-avoidant behaviour.60 Interestingly, hyperactivity in regions of the CMS amygdala has been recently shown in patients with OCD in response to emotional face processing.61
At the molecular level, structural covariance between distant structures may depend both on the mutually trophic influences mediated by the white matter tracts linking the structures31 and the release of use-related trophic factors, which may link synaptic density and neuropil mass within functionally connected regions even in the absence of direct fibre connection.14 Nevertheless, the patterns of structural covariance are typically less expanded that the functional connectivity patterns described for the same structures.13 As a consequence, the structural covariance alterations associated with OCD in our study are less extensive than those described at the functional connectivity level.21 In this respect, it should be noted that structural covariance may reflect stable, persistent and enduring connectivity alterations, leading to volume correlations between structures through structural plasticity. Transient changes in functional connectivity may be mediated by functional plasticity (i.e., Hebbian synaptic plasticity), which may change synaptic strengths without changing the anatomic connectivity between neurons.11
Limitations
This study has a number of limitations. First, the cross-sectional design of the study did not allow firm conclusions regarding possible dynamic changes in structural covariance over time. Second, although the use of a multisite data set allows exploration of a very large number of patients and controls, increasing the statistical power of our analyses, the clinical protocols and measurements used for patient characterization diverged across centres. Likewise, most patients were taking medication, and treatment protocols also differed across centres; however, we have shown that our main findings were unaffected by medication history. In any case, an exhaustive description of medication effects and the association between specific clinical characteristics and the regional morphometry measurements of this sample of participants can be found elsewhere.34 Third, scanner protocols also differed across centres, although in all cases 1.5 T magnets and customary T1-weighted anatomic sequences were used. Moreover, scan sequence was introduced as a confounding covariate in all analyses, and image preprocessing was performed simultaneously for all images. As we have previously shown,34 using common preprocessing algorithms for large groups of images permits identification of significant between-group effects despite the variance introduced by the different origin of the images. Finally, all participants were scanned in 1.5 T scanners, which provided a limited spatial resolution. As a consequence of this and the necessity of including a smoothing step in our preprocessing, we were not able to independently assess structural covariance of the CMS amygdala. Replication and extension of the present findings with higher-resolution scanning sequences is thus warranted.
Conclusion
We have described, to our knowledge for the first time, network-level alterations in the brains of patients with OCD using structural MRI. Our results support prevailing neurobiological models of OCD, which emphasize the importance of alterations in corticostriatal and corticoamygdalar connectivity for understanding the pathophysiological basis of the disorder. Moreover, our results imply that structural covariance should be considered a measurement of interest to fully characterize brain network alterations in patients with psychiatric and other disorders. Although more research is needed to fully understand the neurobiological basis of structural covariance, such measurement can provide evidence of persistent and enduring connectivity alterations between brain regions and may relevantly contribute to multimodal neuroimaging research aimed at characterizing the structural and functional underpinnings of brain disorders.
Acknowledgements
This study was supported by the Carlos III Health Institute (PI09/01331, PI12/01306, PI13/00918, PI14/00413, CP10/00604, PI13/01958, and CIBER-CB06/03/0034); FEDER funds (“A way to build Europe”); the Agency for Administration of University and Research (AGAUR, Barcelona; 2014SGR1672); a “Miguel Servet” contract from the Carlos III Health Institute (CP10/00604) to C. Soriano-Mas; a grant from the Bellvitge Biomedical Research Institute (IDIBELL) (06/IDB001) to M. Subirà; a grant from the Spanish Ministry for Education, Culture and Sport to M. Cano (FPU13/02141); the Dutch Organization for Scientific Research (NWO) (grants 912-02-050, 907-00-012, 940-37-018, and 916.86.038); grants from the Foundation for the Support of Research in the State of São Paulo (FAPESP) to M. Hoexter (2005/04206-6) and J. Sato (2013/10498-6); a National Research Foundation of Korea grant funded by the Korean government (Ministry of Education, Science, and Technology, 2012-0005150); a Ministry of Education, Culture, Sports, Science, and Technology (Japan) Grants-in-Aid for Young Scientists to J. Narumoto (23591724) and to T. Nakamae (24791223); the Medical Research Council of South Africa; Wellcome Trust project (grant 064846) and South London and Maudsley Trust.
Footnotes
Competing interests: D. Stein declares personal fees from Lundbeck, Novartis, AMBRF, Sun and Cipla outside the submitted work. O. van den Heuvel has received speaker fees from Lundbeck. No other competing interests declared.
Contributors: M. Subirà, S. de Wit, P. Alonso, N. Cardoner, D. Stein, J. Menchón, O. van den Heuvel and C. Soriano-Mas designed the study. M. Subirà, P. Alonso, M. Hoexter, J.-S. Kwon, T. Nakamae, J. Sato, W.-H. Jung, J. Narumoto, D. Stein, J. Pujol, D. Mataix-Cols, D. Veltman, J. Menchón, O. van den Heuvel and C. Soriano-Mas acquired the data, which M. Subirà, M. Cano, S. de Wit, N. Cardoner, C. Lochner, D. Veltman, O. van den Heuvel and C. Soriano-Mas analyzed. M. Subirà, M. Cano and C. Soriano-Mas wrote the article, which all authors reviewed and approved for publication.
References
- 1.Alexander-Bloch A, Giedd JN, Bullmore E. Imaging structural covariance between human brain regions. Nat Rev Neurosci. 2013a;14:322–36. doi: 10.1038/nrn3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans AC. Networks of anatomical covariance. Neuroimage. 2013;80:489–504. doi: 10.1016/j.neuroimage.2013.05.054. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy DN, Lange N, Makris N, et al. Gyri of the human neocortex: an MRI-based analysis of volume and variance. Cereb Cortex. 1998;8:372–84. doi: 10.1093/cercor/8.4.372. [DOI] [PubMed] [Google Scholar]
- 4.Allen JS, Damasio H, Grabowski TJ. Normal neuroanatomical variation in the human brain: an MRI-volumetric study. Am J Phys Anthropol. 2002;118:341–58. doi: 10.1002/ajpa.10092. [DOI] [PubMed] [Google Scholar]
- 5.Tost H, Bilek E, Meyer-Lindenberg A. Brain connectivity in psychiatric imaging genetics. Neuroimage. 2012;62:2250–60. doi: 10.1016/j.neuroimage.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Zielinski BA, Gennatas ED, Zhou J, et al. Networklevel structural covariance in the developing brain. Proc Natl Acad Sci U S A. 2010;107:18191–6. doi: 10.1073/pnas.1003109107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khundrakpam BS, Reid A, Brauer J, et al. Developmental changes in organization of structural brain networks. Cereb Cortex. 2013;23:2072–85. doi: 10.1093/cercor/bhs187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexander-Bloch A, Raznahan A, Bullmore E, et al. The convergence of maturational change and structural covariance in human cortical networks. J Neurosci. 2013b;33:2889–99. doi: 10.1523/JNEUROSCI.3554-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brickman AM, Habeck C, Zarahn E, et al. Structural MRI covariance patterns associated with normal aging and neuropsychological functioning. Neurobiol Aging. 2007;28:284–95. doi: 10.1016/j.neurobiolaging.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 10.Bergfield KL, Hanson KD, Chen K, et al. Age-related networks of regional covariance in MRI gray matter: reproducible multivariate patterns in healthy aging. Neuroimage. 2010;49:1750–9. doi: 10.1016/j.neuroimage.2009.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butz M, Wörgötter F, van Ooyen A. Activity-dependent structural plasticity. Brain Res Rev. 2009;60:287–305. doi: 10.1016/j.brainresrev.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 12.May A. Experience-dependent structural plasticity in the adult human brain. Trends Cogn Sci. 2011;15:475–82. doi: 10.1016/j.tics.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Soriano-Mas C, Harrison BJ, Pujol J, et al. Structural covariance of the neostriatum with regional gray matter volumes. Brain Struct Funct. 2013;218:697–709. doi: 10.1007/s00429-012-0422-5. [DOI] [PubMed] [Google Scholar]
- 14.Seeley WW, Crawford RK, Zhou J, et al. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitelman SA, Brickman AM, Shihabuddin L, et al. Correlations between MRI-assessed volumes of the thalamus and cortical Brodmann’s areas in schizophrenia. Schizophr Res. 2005;75:265–81. doi: 10.1016/j.schres.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 16.Modinos G, Vercammen A, Mechelli A, et al. Structural covariance in the hallucinating brain: a voxel-based morphometry study. J Psychiatry Neurosci. 2009;34:465–9. [PMC free article] [PubMed] [Google Scholar]
- 17.Sato JR, Hoexter MQ, Oliveira PP, Jr, et al. Inter-regional cortical thickness correlations are associated with autistic symptoms: a machine-learning approach. J Psychiatr Res. 2013;47:453–9. doi: 10.1016/j.jpsychires.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 18.Zielinski BA, Anderson JS, Froehlich AL, et al. scMRI reveals large-scale brain network abnormalities in autism. PLoS ONE. 2012;7:e49172. doi: 10.1371/journal.pone.0049172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pujol J, Soriano-Mas C, Alonso P, et al. Mapping structural brain alterations in obsessive-compulsive disorder. Arch Gen Psychiatry. 2004;61:720–30. doi: 10.1001/archpsyc.61.7.720. [DOI] [PubMed] [Google Scholar]
- 20.Milad MR, Rauch SL. Obsessive-compulsive disorder: beyond segregated corticostriatal pathways. Trends Cogn Sci. 2012;16:43–51. doi: 10.1016/j.tics.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harrison BJ, Soriano-Mas C, Pujol J, et al. Altered corticostriatal functional connectivity in obsessive-compulsive disorder. Arch Gen Psychiatry. 2009;66:1189–200. doi: 10.1001/archgenpsychiatry.2009.152. [DOI] [PubMed] [Google Scholar]
- 22.Harrison BJ, Pujol J, Cardoner N, et al. Brain corticostriatal systems and the major clinical symptom dimensions of obsessive-compulsive disorder. Biol Psychiatry. 2013;73:321–8. doi: 10.1016/j.biopsych.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Jung WH, Kang DH, Kim E, et al. Abnormal corticostriatal-limbic functional connectivity in obsessive-compulsive disorder during reward processing and resting-state. Neuroimage Clin. 2013;3:27–38. doi: 10.1016/j.nicl.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anticevic A, Hu S, Zhang S, et al. Global resting-state functional magnetic resonance imaging analysis identifies frontal cortex, striatal, and cerebellar dysconnectivity in obsessive-compulsive disorder. Biol Psychiatry. 2014;75:595–605. doi: 10.1016/j.biopsych.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Posner J, Marsh R, Maia TV, et al. Reduced functional connectivity within the limbic cortico-striato-thalamo-cortical loop in unmedicated adults with obsessive-compulsive disorder. Hum Brain Mapp. 2014;35:2852–60. doi: 10.1002/hbm.22371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Vries FE, de Wit SJ, Cath DC, et al. Compensatory frontoparietal activity during working memory: an endophenotype of obsessive-compulsive disorder. Biol Psychiatry. 2014;76:878–87. doi: 10.1016/j.biopsych.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 27.Sakai Y, Narumoto J, Nishida S, et al. Corticostriatal functional connectivity in non-medicated patients with obsessive-compulsive disorder. Eur Psychiatry. 2011;26:463–9. doi: 10.1016/j.eurpsy.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Beucke JC, Sepulcre J, Talukdar T, et al. Abnormally high degree connectivity of the orbitofrontal cortex in obsessive-compulsive disorder. JAMA Psychiatry. 2013;70:619–29. doi: 10.1001/jamapsychiatry.2013.173. [DOI] [PubMed] [Google Scholar]
- 29.Göttlich M, Krämer UM, Kordon A, et al. Decreased limbic and increased frontoparietal connectivity in unmedicated patients with obsessive-compulsive disorder. Hum Brain Mapp. 2014;35:5617–32. doi: 10.1002/hbm.22574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Martino A, Scheres A, Margulies DS, et al. Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex. 2008;18:2735–47. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- 31.Mechelli A, Friston KJ, Frackowiak RS, et al. Structural covariance in the human cortex. J Neurosci. 2005;25:8303–10. doi: 10.1523/JNEUROSCI.0357-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roy AK, Shehzad Z, Margulies DS, et al. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45:614–26. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baur V, Hänggi J, Langer N, et al. Resting-state functional and structural connectivity within an insula-amygdala route specifically index state and trait anxiety. Biol Psychiatry. 2013;73:85–92. doi: 10.1016/j.biopsych.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 34.de Wit SJ, Alonso P, Schweren L, et al. Multicenter voxel-based morphometry mega-analysis of structural brain scans in obsessive-compulsive disorder. Am J Psychiatry. 2014;171:340–9. doi: 10.1176/appi.ajp.2013.13040574. [DOI] [PubMed] [Google Scholar]
- 35.Cardoner N, Soriano-Mas C, Pujol J, et al. Brain structural correlates of depressive comorbidity in obsessive-compulsive disorder. Neuroimage. 2007;38:413–21. doi: 10.1016/j.neuroimage.2007.07.039. [DOI] [PubMed] [Google Scholar]
- 36.Postuma RB, Dagher A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb Cortex. 2006;16:1508–21. doi: 10.1093/cercor/bhj088. [DOI] [PubMed] [Google Scholar]
- 37.Brett MA, Anton JL, Valabregue R, et al. Region of interest analysis using an SPM toolbox. Neuroimage. 2002;16:497. [Google Scholar]
- 38.Draganski B, Kherif F, Klöppel S, et al. Evidence for segregated and integrative connectivity patterns in the human basal ganglia. J Neurosci. 2008;28:7143–52. doi: 10.1523/JNEUROSCI.1486-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greene DJ, Laumann TO, Dubis JW, et al. Developmental changes in the organization of functional connections between the basal ganglia and cerebral cortex. J Neurosci. 2014;34:5842–54. doi: 10.1523/JNEUROSCI.3069-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Menzies L, Achard S, Chamberlain SR, et al. Neurocognitive endophenotypes of obsessive-compulsive disorder. Brain. 2007;130:3223–36. doi: 10.1093/brain/awm205. [DOI] [PubMed] [Google Scholar]
- 41.de Wit SJ, de Vries FE, van der Werf YD, et al. Presupplementary motor area hyperactivity during response inhibition: a candidate endophenotype of obsessive-compulsive disorder. Am J Psychiatry. 2012;169:1100–8. doi: 10.1176/appi.ajp.2012.12010073. [DOI] [PubMed] [Google Scholar]
- 42.Marsh R, Horga G, Parashar N, et al. Altered activation in frontostriatal circuits during sequential processing of conflict in unmedicated adults with obsessive-compulsive disorder. Biol Psychiatry. 2014;75:615–22. doi: 10.1016/j.biopsych.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shin YW, Yoo SY, Lee JK, et al. Cortical thinning in obsessive compulsive disorder. Hum Brain Mapp. 2007;28:1128–35. doi: 10.1002/hbm.20338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoo SY, Roh MS, Choi JS, et al. Voxel-based morphometry study of gray matter abnormalities in obsessive-compulsive disorder. J Korean Med Sci. 2008;23:24–30. doi: 10.3346/jkms.2008.23.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piras F, Piras F, Chiapponi C, et al. Widespread structural brain changes in OCD: a systematic review of voxel-based morphometry studies. Cortex. 2015;62:89–108. doi: 10.1016/j.cortex.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 46.Radua J, Mataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br J Psychiatry. 2009;195:393–402. doi: 10.1192/bjp.bp.108.055046. [DOI] [PubMed] [Google Scholar]
- 47.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tops M, Boksem MA. A potential role of the inferior frontal gyrus and anterior insula in cognitive control, brain rhythms, and event-related potentials. Front Psychol. 2011;2:330. doi: 10.3389/fpsyg.2011.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Montigny C, Castellanos-Ryan N, Whelan R, et al. A phenotypic structure and neural correlates of compulsive behaviors in adolescents. PLoS ONE. 2013;8:e80151. doi: 10.1371/journal.pone.0080151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johansen-Berg H, Gutman DA, Behrens TE, et al. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb Cortex. 2008;18:1374–83. doi: 10.1093/cercor/bhm167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pezawas L, Meyer-Lindenberg A, Drabant EM, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–34. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- 52.Kim MJ, Gee DG, Loucks RA, et al. Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cereb Cortex. 2011;21:1667–73. doi: 10.1093/cercor/bhq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim MJ, Loucks RA, Palmer AL, et al. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav Brain Res. 2011;223:403–10. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stein DJ, Fineberg NA, Bienvenu OJ, et al. Should OCD be classified as an anxiety disorder in DSM-V? Depress Anxiety. 2010;27:495–506. doi: 10.1002/da.20699. [DOI] [PubMed] [Google Scholar]
- 55.Milad MR, Furtak SC, Greenberg JL, et al. Deficits in conditioned fear extinction in obsessive-compulsive disorder and neurobiological changes in the fear circuit. JAMA Psychiatry. 2013;70:608–18. doi: 10.1001/jamapsychiatry.2013.914. [DOI] [PubMed] [Google Scholar]
- 56.Stern ER, Welsh RC, Fitzgerald KD, et al. Hyperactive error responses and altered connectivity in ventromedial and frontoinsular cortices in obsessive-compulsive disorder. Biol Psychiatry. 2011;69:583–91. doi: 10.1016/j.biopsych.2010.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stern ER, Welsh RC, Gonzalez R, et al. Subjective uncertainty and limbic hyperactivation in obsessive-compulsive disorder. Hum Brain Mapp. 2013;34:1956–70. doi: 10.1002/hbm.22038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harrison BJ, Pujol J, Soriano-Mas C, et al. Neural correlates of moral sensitivity in obsessive-compulsive disorder. Arch Gen Psychiatry. 2012;69:741–9. doi: 10.1001/archgenpsychiatry.2011.2165. [DOI] [PubMed] [Google Scholar]
- 59.LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23:727–38. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bzdok D, Laird AR, Zilles K, et al. An investigation of the structural, connectional, and functional subspecialization in the human amygdala. Hum Brain Mapp. 2013;34:3247–66. doi: 10.1002/hbm.22138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Via E, Cardoner N, Pujol J, et al. Amygdala activation and symptom dimensions in obsessive-compulsive disorder. Br J Psychiatry. 2014;204:61–8. doi: 10.1192/bjp.bp.112.123364. [DOI] [PubMed] [Google Scholar]