Abstract
Background
We examined the blood-oxygen level–dependent (BOLD) activation in brain regions that signal errors and their association with intraindividual behavioural variability and adaptation to errors in children with attention-deficit/hyperactivity disorder (ADHD).
Methods
We acquired functional MRI data during a Flanker task in medication-naive children with ADHD and healthy controls aged 8–12 years and analyzed the data using independent component analysis. For components corresponding to performance monitoring networks, we compared activations across groups and conditions and correlated them with reaction times (RT). Additionally, we analyzed post-error adaptations in behaviour and motor component activations.
Results
We included 25 children with ADHD and 29 controls in our analysis. Children with ADHD displayed reduced activation to errors in cingulo-opercular regions and higher RT variability, but no differences of interference control. Larger BOLD amplitude to error trials significantly predicted reduced RT variability across all participants. Neither group showed evidence of post-error response slowing; however, post-error adaptation in motor networks was significantly reduced in children with ADHD. This adaptation was inversely related to activation of the right-lateralized ventral attention network (VAN) on error trials and to task-driven connectivity between the cingulo-opercular system and the VAN.
Limitations
Our study was limited by the modest sample size and imperfect matching across groups.
Conclusion
Our findings show a deficit in cingulo-opercular activation in children with ADHD that could relate to reduced signalling for errors. Moreover, the reduced orienting of the VAN signal may mediate deficient post-error motor adaptions. Pinpointing general performance monitoring problems to specific brain regions and operations in error processing may help to guide the targets of future treatments for ADHD.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is the most frequent disorder in child and adolescent mental health services, affecting 3%–5% of children.1 Several pathways and processes have been identified in the multicausal emergence of the typical symptoms of the disorder, such as inattention, hyperactivity and impulsivity and deficits in self-regulatory control.2–4 Successful self-regulation requires the capacity to monitor and adjust behaviour to current demands, with the aim of optimizing outcomes.5 Previous studies in children with ADHD have measured activation patterns to errors;6–10 however, it is still unclear which elements in the process of error monitoring — monitoring of ongoing actions, signalling the need for cognitive control11 or subsequent behavioural adaptation12 — are most impaired.13
A decreased ability to monitor conflicts and errors may be a central factor underlying the high degree of reaction time (RT) variability consistently seen in children with ADHD.14 Several perspectives for the association between anterior cingulate cortex (ACC) signal and performance have been put forward,15,16 indicating that fluctuations of behaviour, including response variability, might reflect the state of cognitive control, which is signalled by the conflict signals in the ACC on a trial-by-trial basis.15 Stronger activation of the ACC via reinforcement of increased top–down cognitive control (e.g., in the dorsolateral cortex) results in more focused and less variable behaviour.17 Reaction time variability better differentiates between children with ADHD and healthy controls than group mean differences in RT, accuracy and even error rates.14,18–21 The few studies that have addressed the underlying biological correlates of RT variability in children with ADHD have implicated the ACC8,22,23 as well as temporal regions.24
We aimed to examine different aspects of performance monitoring in children with ADHD using the Flanker task, a speeded forced-choice task with an interference condition. Specifically, we investigated the blood-oxygen level–dependent (BOLD) signal at conflict monitoring (in the incompatible condition) and at error monitoring (during an error) as well as the association between these BOLD activations and RT variability (behaviourally). Finally, we also examined post-error adaptations (behavioural changes and changes in the BOLD signal of the relevant motor component after an error). We focused on brain networks that are involved in moment-to-moment processing of stimulus/response conflict and errors.8,22,23 Specifically, we considered the cingulo-opercular network (CON), given its role in salience detection and signalling the need for greater control,11,25,26 as well as networks involved in allocating and maintaining attentional resources, including the dorsal attention network (DAN), for its central role in top–down control of attention27,28 and the right-lateralized ventral attention network (VAN),27–29 given its crucial function for bottom–up, stimulus-driven reorienting, which is important for adaptation to environmental or intrinsic changes.
We tested the hypotheses that 1) children with ADHD will exhibit decreased activation in networks responsible for error monitoring on trials requiring greater control, 2) children exhibiting decreased activation to errors will show greater RT variability and 3) children with ADHD will display deficits in post-error adaptation.
Methods
Participants were recruited as part of a broader investigation into regulatory disorders that included 92 children aged 8–12 years referred from primary care physicians to mental health services owing to symptoms of ADHD or Tourette syndrome (TS). Typically developing children were invited for participation via 5 schools nearest to the geographical areas of the referred children.
We used the Schedule for Affective Disorders and Schizophrenia for School-Age Children — Present and Lifetime Version30 and the Children Global Assessment Scale (CGAS).31 A best-estimate consensus procedure was performed by a child and adolescent psychiatrist and a psychologist using all available clinical and investigational materials,32 including the DuPaul ADHD Rating Scale33 to establish diagnoses and the Wechsler Intelligence Scale for Children-IV34 to assess IQ. All children were white, native Norwegian speakers and medication-naive, and they had received no prior treatment for ADHD. Exclusion criteria were any prior seizure, previous head trauma with loss of consciousness, previous major neurologic injury or illness, current or prior use of psychotropic medication, IQ below 75, premature birth (< 36 wk), dyslexia or other developmental disorder, or any Axis II disorder. Among controls, further exclusion criteria were an Axis I psychiatric disorder other than phobia or elimination disorder. The West Norway Regional Committee for Medical Research and the Norwegian Social Science Data Service approved our study protocol, and we obtained written informed consent and assent after describing the study to participants and their parents.
Experimental task
We used a modified version of the Erikson Flanker task.35 Participants were instructed to respond as accurately and quickly as possible to the direction of a central target stimulus. Flanking stimuli were presented for 100 ms before the onset of the target. In compatible trials (50%), flankers pointed in the same direction as the target, whereas in incompatible trials they pointed in the opposite direction, creating an interference effect. Stimuli were presented on screen until a response was registered or until 1400 ms after target onset, when the trial was terminated. The trial sequence was pseudorandomized and consisted of 100 trials with an average intertrial interval of 5 s (1.5-s jitter) interspersed with 25 additional null events. We provided performance feedback in the form of an exclamation point on the screen if responses were erroneous or if the individual’s RT was slower than the mean RT ± 1.5 standard deviations (SD). The children were instructed as follows: “Sometimes, after having clicked on the mouse, you may see an exclamation point appearing below the fixation point. This exclamation point can indicate 2 things: either it appears because you responded too slowly compared with how fast you responded before, or it means that you answered incorrectly because you pressed the button on the wrong side. The exclamation point is to remind you that you should respond correctly as quickly as possible.”
Data acquisition and processing
Functional images were obtained using a 3.0 T GE Signa MRI scanner with a standard quadrature head coil and an echo-planar pulse sequence with full coverage using the following parameters: repetition time (TR) 2.3 s, echo time (TE) 23 ms, flip angle 90°, single excitation per image, field of view (FOV) 200 mm, 3.125 × 3.125 mm in-plane resolution, slice thickness 3.3 mm. During each experiment 270 volumes were acquired (approximately 10.3-min duration). We excluded images in 19 cases owing to incomplete scans (n = 2), continuous movement (median framewise displacement > 0.20 mm; n = 11), low signal-to-noise ratio (SNR0; < 120; n = 1), or > 4 Fourier spikes (n = 5), as determined using the Data Quality tool (http://cbi.nyu.edu/software/dataQuality.php). Quality criteria procedures were blind to participant diagnoses. The remaining data sets were free of artifacts.
We used SPM 8 (http://www.fil.ion.ucl.ac.uk/spm/) and AFNI (http://afni.nimh.nih.gov/afni/) for image preprocessing. Data underwent despiking, slice-time correction, realignment with unwarping, nonlinear spatial normalization to a study-specific echo planar imaging template,36 reslicing to 3 mm isotropic voxels, spatial smoothing with a 6 mm full-width at half-maximum kernel and variance normalization of the voxel time series. Preprocessed data were decomposed into 100 components using group independent components analysis, as implemented in the GIFT toolbox (http://mialab.mrn.org/software/gift/). We estimated participant-specific time courses (TCs) using regression of participant data sets onto group components.37
Following our a priori hypotheses, we considered components implicated in performance monitoring, salience and attention. Based on spatial properties, we identified 2 CONs, 2 (lateralized) DANs, and a right-lateralized VAN. In our analysis of post-error adaptations, we also included 2 lateralized motor networks. The TCs of selected components underwent additional post-processing, including regression of low-frequency trends and motion-related trends (6 realignment parameters, their derivatives and the squares of these terms), replacement of remaining spikes with a third-order spline fit, and low-pass filtering with a high-frequency cutoff of 0.18 Hz. Single trial amplitude estimation largely followed the procedure outlined by Eichele and colleagues.25 Cleaned TCs were first deconvolved with respect to target onsets to estimate hemodynamic response functions (HRFs) specific to participants and components. Separate HRFs for compatible and incompatible trials were estimated in a single model, wherein each HRF was modelled with 38 FIR basis functions spanning from −2.3 s to 26 s around the onset of the Flanker stimuli. All trials were included in the model regardless of the response accuracy to keep the number of estimated parameters consistent across participants.
To estimate a time series of trial × trial amplitudes, we assumed a single HRF shape for all trial types and identified the task condition (i.e., compatible or incompatible flankers) that yielded the largest response at the group level. Specifically, for each component, we performed 1-sample t tests on the HRF amplitude averaged from 5 s to 10 s, and the condition with the largest absolute t statistic was selected. We then estimated single trial amplitudes using the method described by Mumford and colleagues,38 in which each trial’s amplitude was estimated through a general linear model including a regressor for that trial as well as another regressor for all other trials. Here, regressors are simply the convolution of the estimated HRF and the target onset(s). Prior to single trial amplitude estimation, participant HRFs were normalized to have a peak of 1 so the resulting amplitudes could be compared fairly across components and participants.
Group inferences
Statistical analyses of behavioural and imaging data were limited to children with ADHD and controls and excluding children with a diagnosis of TS. Behavioural and imaging variables were residualized with respect to age, sex and mean framewise displacement (imaging variables only) before subsequent modelling.
Primary behavioural measures included the mean RT in correct trials, percent omitted errors, percent committed errors and RT variability, which was estimated as the standard deviation of RTs and denoted as σ(RT). Each variable was modelled with a repeated-measures analysis of variance (rmANOVA), considering compatibility as the within-subjects repeated factor, diagnosis as the between-subjects factor and the 2-way interaction between compatibility and diagnosis. Post-error slowing (PES) was calculated as the mean post-error RT (after errors of commission) minus the mean post-correct RT, normalized by the mean post-correct RT to account for the group difference in mean RT. We had to exclude 6 participants (3 controls) from this analysis because they made no errors of commission; 1 additional control participant was removed owing to an unusually large PES (> 3.5 SD above the mean).
Primary imaging measures were the component single trial amplitudes, averaged separately over compatible correct, incompatible correct and error conditions. Component amplitudes were also modelled with an rmANOVA, considering trial condition, diagnosis and their interaction. Three participants (2 controls) had perfect performances and thus were not included in these models. We investigated post-error adaptations in activation for components corresponding to the lateralized motor systems; we calculated post-error motor adaptation (PEMA) as the difference in activation between post-error trials and post-correct trials on the relevant side. We considered only errors of commission, eliminating 6 participants from the analysis. In a follow-up analysis, we calculated the cross-correlation (i.e., lagged correlations) between trial × trial amplitudes. Prior to computing cross-correlations, component amplitudes were residualized with respect to Flanker compatibility, errors and compatibility × error interactions to remove task-driven variance.
We studied associations between imaging variables and behavioural variables using regression models with backward elimination, as implemented in the MANCOVAN toolbox (http://www.mathworks.com/matlabcentral/fileexchange/27014-mancovan). We modelled behavioural variability, s(RT), as a function of component amplitudes during error trials. The initial model included error amplitudes from the 5 selected components as predictors, and a reduced model was determined in a stepwise fashion until remaining predictors were significant at the α = 0.05 level. Identical stepwise regression procedures were applied to model PES and PEMA. Modelling was always performed first on all participants and then on ADHD and control groups separately.
Results
Our final sample included 25 children with ADHD (mean age 10.75 ± 1.09 yr) and 29 controls (mean age 10.15 ± 1.04 yr), with a small group difference in mean age (t52 = 2.08, p = 0.042) and well-matched sex distributions (68% boys in the ADHD group, 52% boys in the control group, χ2 = 1.47, p = 0.23). Children with ADHD had a diagnosis of ADHD, combined type (n = 17); ADHD, inattentive type (n = 6); or ADHD, hyperactive impulsive type (n = 2). Groups differed between the ADHD and control groups on their ADHD-RS total scores (29.34 ± 7.1 v. 2.93 ± 2.9), total inattention scores (16.12 ± 3.4 v. 1.86 ± 2.2) and total hyperactivity/impulsivity scores (13.22 ± 5.1 v. 1.07 ± 1.5). In line with other reports7 children with ADHD had a lower IQ than controls (92.4 ± 5.6 v. 106.4 ± 11.4, t52 = 5.56, p < 0.001) and a lower score than controls on the CGAS (55.5 ± 7.0 v. 85.6 ± 7.4 [n = 26], t49 = 14.8, p < 0.001). As expected, children with ADHD had co-morbidities; twelve had oppositional defiant disorder, and 2 of these children also had conduct disorders. Moreover, children in both groups fulfilled criteria for phobia (4 ADHD, 1 control), separation anxiety disorder (1 ADHD), chronic tics (1 ADHD) and elimination disorder (1 ADHD, 1 control). The ADHD and control groups did not differ significantly with respect to framewise displacement during fMRI acquisition (0.12 ± 0.07 mm v. 0.10 ± 0.05 mm, t52 = 1.12, p = 0.24).
Behavioural performance
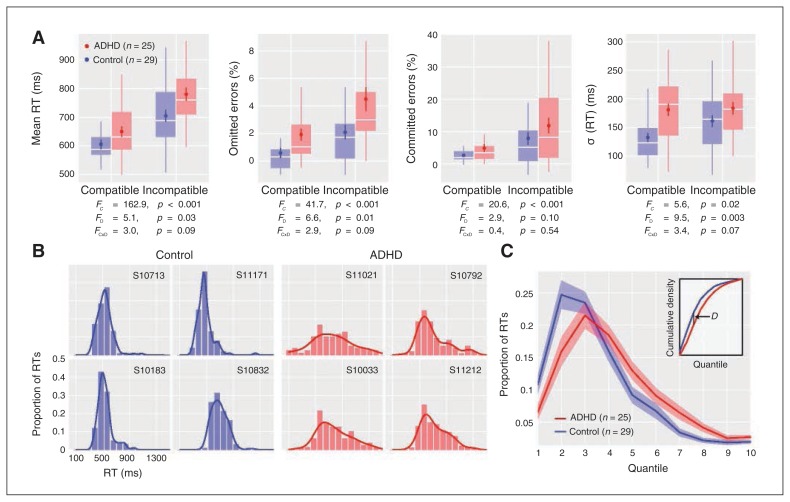
Both groups showed Flanker compatibility effects, with slower responses and more errors on incompatible trials (Fig. 1A). Children with ADHD showed slightly slower RTs and more omitted responses than controls, without significant group × compatibility interactions. Children with ADHD also showed higher σ (RT), particularly for compatible trials. Examination of RT distributions suggested that controls had more positively skewed distributions, with the bulk of trials tightly clustered at shorter RTs and relatively few trials distributed over longer RTs, whereas children with ADHD showed broad RT distributions (Fig. 1B). Qualitative observations were confirmed when comparing the quantized RT distributions between groups with the Kolmogorov–Smirnov statistic, which indicated a significant difference in distributions across groups (p = 0.014; Fig. 1C).
Fig. 1.
Behavioural data. (A) Behavioural variables as a function of Flanker compatibility and group (attention-deficit/hyperactivity disorder [ADHD] = red; control = blue). The distribution of each variable is summarized with a boxplot; the mean ± 1 standard error of the mean (SEM) are also displayed to show group differences. These plots display residualized variables (see Methods section), thus variables with only positive values may be transformed to (slightly) negative values after residualization. Repeated-measures analysis of variance (ANOVA) F1,52 stat-tistics for Flanker compatibility, diagnosis and their interaction are provided as FC, FD, and FC × D, respectively. (B) Examples of reaction time (RT) distributions over all trials in 4 control (left) and ADHD (right) participants. Histograms are overlayed with kernel density estimates. (C) Average RT distributions ± 1 SEM for each group. To fairly compare distributions between groups, RTs for each participant were divided into 10 quantiles and normalized to compute probability densities. Differences in the average RT distribution were determined using the Kolmogorov–Smirnov statistic, D, which considers the maximum absolute difference between the cumulative distribution functions (inset). Significance of D was determined by comparing the observed value to a null distribution of D′ values, which were created by permuting group labels (10 000 permutations). The distributions differed significantly across groups (D = 0.15, p = 0.014).
Imaging results
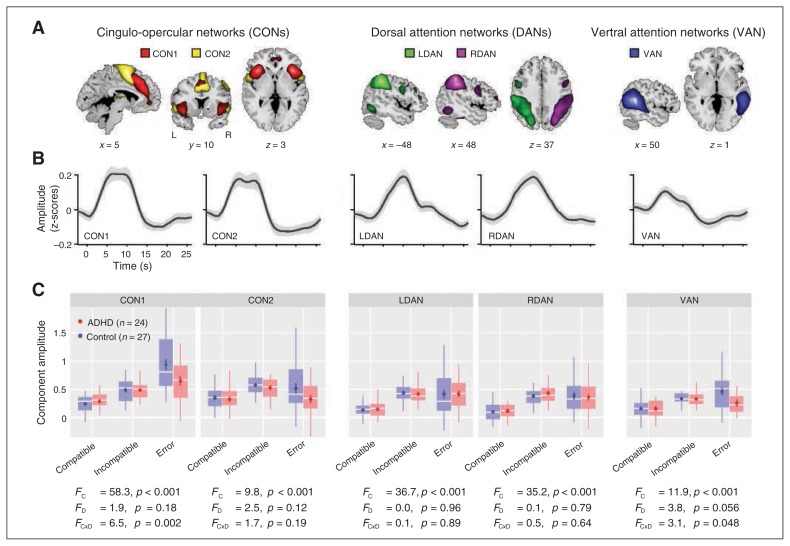
We identified 5 different independent components (Fig. 2A), with spatial properties that matched the primary networks of interest. Two components represented different aspects of the cingulo-opercular network (CON1 and CON2). The CON1 component peaked in the dorsal ACC and the bilateral insula, whereas CON2 peaked in the presupplementary motor area (preSMA) and the more posterior aspects of the insula, with additional peaks in bilateral precentral gyri. Although we refer to the network as cingulo-opercular, it also includes smaller clusters in the cerebellum, consistent with reports of cerebellar activation to errors and conflict.39,40 Two components represented the left and right dorsal attention networks (LDAN and RDAN, respectively), with primary peaks in the intraparietal sulci. The fifth component corresponded to the right-lateralized ventral attention network (VAN), with a primary peak in the right temporoparietal junction. Peak coordinates and cluster extents of each component are provided in Table 1.
Fig. 2.
Component activation to different task conditions. (A) Component maps of cingulo-opercular networks (CONs; left), dorsal attention networks (DANs; middle) and the right-lateralized ventral attention network (VAN; right). Component t maps are thresholded at t > 8 (1-sample t test) and displayed at the most informative slices. See Table 1 for peak coordinates and cluster extents of each component. (B) Average hemodynamic response functions (HRFs) were estimated from incompatible trials, the condition that evoked the strongest response for these components (see Methods section). Error bands indicate ± 1 SEM. (C) Component amplitudes to compatible, incompatible and error trials as a function of group (attention-deficit/hyperactivity [ADHD] = red; control = blue). Repeated-measures analysis of variance F statistics for Flanker compatibility, diagnosis, and their interaction are provided as FC2,98, FD1,98 and FC × D2,98, respectively.
Table 1.
Anatomic regions and peak coordinates of selected components
| MNI coordinate | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Component | Vol, mm3 | Max t statistic | x | y | z | Region | BA |
| CON1 (Component 21) | |||||||
| 22815 | 44.8 | −33 | 27 | −6 | L insula | L BA 47 | |
| 24273 | 42.25 | 36 | 21 | 0 | R insula | R BA 47 | |
| 38016 | 32.11 | 0 | 27 | 48 | L/R cingulate gyrus | L/R BA 32 | |
| 621 | 11.09 | 39 | −60 | −33 | R cerebellum (Crus 1) | — | |
| 324 | 10.5 | −39 | −57 | −33 | L cerebellum (Crus 1) | — | |
| CON2 (Component 91) | |||||||
| 37098 | 33.84 | 3 | 0 | 66 | L/R SMA | L/R BA 6 | |
| 12744 | 28.38 | 54 | 0 | 51 | R precentral gyrus | R BA 6 | |
| 7587 | 23.02 | −48 | −9 | 54 | L precentral gyrus | L BA6 | |
| 5886 | 21.56 | 54 | 9 | 0 | R insula | R BA 13 | |
| 9261 | 18.72 | −54 | 9 | 0 | L insula | L BA 13 | |
| 2835 | 15.94 | 3 | −18 | 12 | L/R thalamus | — | |
| 5508 | 15.41 | −21 | −57 | 45 | L SPL | L BA 7 | |
| 3753 | 13.47 | 33 | −72 | 54 | R SPL | R BA 7 | |
| 783 | 10.84 | 15 | −6 | 21 | R caudate | — | |
| 297 | 10.75 | −6 | 54 | −12 | L medial frontal gyrus | L BA 11 | |
| 540 | 10.11 | −30 | 42 | 21 | L middle frontal gyrus | L BA 10 | |
| LDAN (Component 27) | |||||||
| 61101 | 41.63 | −42 | −45 | 48 | L IPL | L BA 40 | |
| 4563 | 17.89 | −51 | −63 | −9 | L ITG | L BA 19 | |
| 6021 | 14.87 | 45 | −39 | 51 | R IPL | R BA 40 | |
| 3024 | 14.37 | −51 | 3 | 33 | L precentral gyrus | L BA 9 | |
| 2565 | 13.78 | −24 | −6 | 57 | L middle frontal gyrus | L BA 6 | |
| 2889 | 13.13 | 15 | −72 | 54 | R SPL | R BA 7 | |
| RDAN (Component 36) | |||||||
| 61992 | 46.63 | 42 | −45 | 48 | R IPL | R BA 40 | |
| 11718 | 21.57 | 54 | −57 | −12 | R ITG | R BA 19 | |
| 5562 | 19.8 | 54 | 9 | 27 | R precentral gyrus | R BA 9 | |
| 2781 | 11.71 | 30 | −3 | 54 | R middle frontal gyrus | R BA 6 | |
| 1620 | 11.45 | −36 | −48 | 42 | L IPL | L BA 40 | |
| 756 | 10.35 | −21 | −72 | 54 | L SPL | L BA 7 | |
| VAN (Component 33) | |||||||
| 69741 | 46.15 | 48 | −51 | 15 | R MTG | R BA 22, 39 | |
| 4293 | 12.8 | −54 | −63 | 12 | L MTG | L BA 22, 39 | |
| 513 | 10.81 | 45 | 3 | 51 | R middle frontal gyrus | R BA 6 | |
| 675 | 10.58 | 54 | 27 | 0 | R IFG (p. triangularis) | R BA 47 | |
| RMOT (Component 1) | |||||||
| 44226 | 49.64 | 42 | −24 | 66 | R precentral gyrus | R BA 4 | |
| 8640 | 25.58 | −24 | −54 | −24 | L cerebellum (VI) | — | |
| 3591 | 21.19 | 33 | −3 | −6 | R putamen | — | |
| 2376 | 17.63 | 15 | −21 | 9 | R thalamus | — | |
| 3267 | 14.11 | 48 | −21 | 18 | R insula | R BA 13 | |
| 837 | 12.47 | −24 | −63 | −54 | L cerebellum (VIII) | — | |
| LMOT (Component 2) | |||||||
| 47223 | 50.22 | −39 | −30 | 69 | L precentral gyrus | L BA 3 | |
| 6588 | 22.56 | 21 | −57 | −21 | R cerebellum (VI) | — | |
| 2322 | 19.69 | −12 | −21 | 0 | L thalamus | — | |
| 3321 | 19.04 | −30 | −9 | −3 | L putamen | — | |
| 1863 | 12.27 | −48 | −24 | 21 | L insula | L BA 13 | |
| 540 | 9.79 | 12 | −72 | −48 | R cerebellum (VIII) | — | |
BA = Brodmann area; CON = cingulo-opercular network; IFG = inferior frontal gyrus; IPL = inferior parietal lobule; ITG = inferior temporal gyrus; L = left; LDAN/RDAN = left/right dorsal attention network; LMOT/RMOT = lateralized left/right motor systems; MNI = Montreal Neurological Institute; MTG = middle temporal gyrus; R = right; SMA = supplementary motor area; SPL = superior parietal lobule; VAN = ventral attention network.
All components showed prominent effects of Flanker compatibility (incompatible > compatible; Fig. 2C). The CON1 component showed a strong group × trial type interaction, resulting from little difference on correct compatible and incompatible trials, but reduced activation in the ADHD group on error trials. A post hoc 1-way ANOVA on error trials confirmed a significant group difference (F1,49 = 5.22, p = 0.027). The VAN showed a similar interaction effect (reduced activation in ADHD group on error trials), confirmed with a 1-way ANOVA (F1,49 = 4.73, p = 0.034). The CON2 component showed no significant group or interaction effects, although activation in children with ADHD was lower on error trials. Both DANs showed similar activations between groups for all conditions.
Associations between activation and behavioural variability
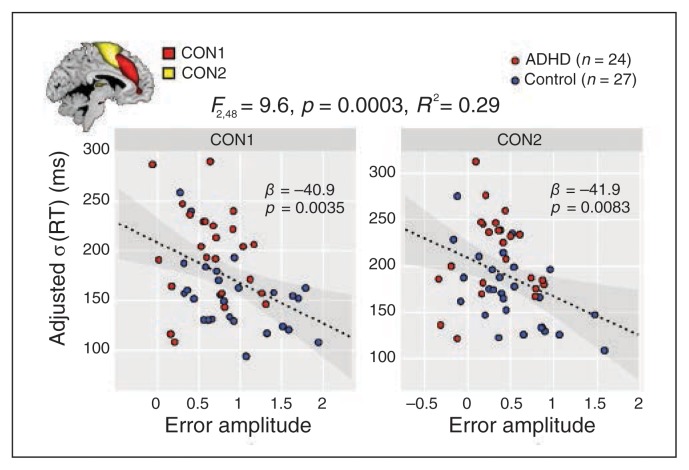
To better understand the behavioural implication of deficient performance monitoring, we tested the prediction that greater s(RT) is related to reduced amplitude on error trials. Our initial model considered s(RT) as the dependent variable and the error amplitudes of CON1, CON2, LDAN, RDAN and VAN as predictors. We used s(RT) during compatible trials unless noted otherwise, given that these trials are easier to perform and thus should be more sensitive to differences in variability. Backward elimination identified CON1 and CON2 as significant predictors (Fig. 3). Both components showed negative slopes with s(RT), meaning that greater activation on error trials was related to less response variability on correct, compatible trials. Error amplitudes of CON1 and CON2 showed no covariation with each other (r49 = 0.11, p = 0.44). Follow-up models within controls showed nearly identical associations between error amplitudes and the s(RT) of compatible trials (Appendix 1, Fig. S1, available at jpn.ca) and similar findings for incompatible trials (Appendix 1, Fig. S1C). We found no significant error amplitude predictors of s(RT) of either trial type within the ADHD group.
Fig. 3.
Association between error amplitudes and behavioural variability. Multiple regression model predicting reaction time (RT) from the cingulo-operculum network (CON1; left) and CON2 (right) error amplitudes. The model was determined with backward selection (see Methods). Each scatterplot displays the association between a single predictor and the response variable (sRT) adjusted for the remaining predictors (i.e., the remaining terms of the model are subtracted from the response vector). Grey contours indicate the 95% confidence interval for the regression line (dashed black line). ADHD = attention-deficit/hyperactivity disorder.
In a control analysis, we included the number of errors made as a term in the regression model, given the previously observed negative associations between error frequency and error amplitude.41 While this term accounted for significant additional variance in s(RT), it did not change the main results regarding CON 1 and CON 2 (Appendix 1, Fig. S1A). We also found no correlation between the number of errors and error amplitude (p > 0.1 across and within groups), suggesting that the association between the error amplitude and s(RT) was not significantly confounded by between-group differences in numbers of errors.
Associations with symptoms
We correlated symptom scores with s(RT) and with component error amplitudes in all participants (n = 54). Total symptom load was positively correlated with s(RT) (r51 = 0.54, p < 0.001). Symptom subcategories for inattention, hyperactivity/impulsivity and emotional lability showed similar correlations with s(RT) (r = 0.48–0.53), with no suggestion of specificity.
Post-error adaptations
We found little evidence of PES across all participants (mean 3.1 ± 14.1%, t46 = 1.49, p = 0.07, 1-tailed t test) or within groups (control: 2.1 ± 11.5%, t24 = 0.91, p = 0.19; ADHD: 4.2 ± 16.8, t21 = 1.17, p = 0.13), nor did we find a between-group difference (t45 = 0.50, p = 0.69, 2-sample 1-tailed t test). Post-error improvements in accuracy and post-error reductions in interference,42 were not present and did not differ across groups (p > 0.1 for all tests).
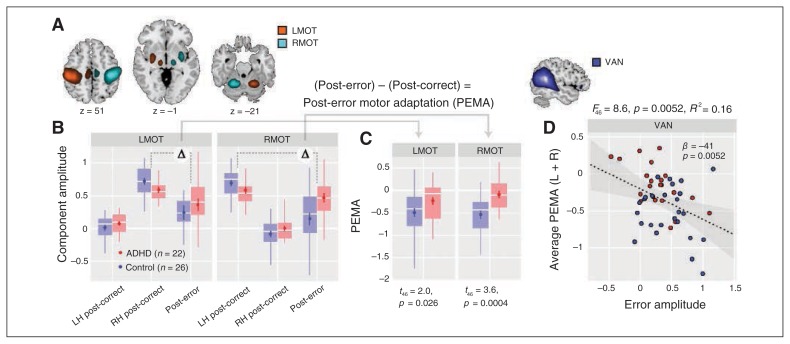
We investigated post-error changes in motor network activation43 in 2 components representing the lateralized left (LMOT) and right (RMOT) motor systems, with peaks in the motor cortex, SMA, thalamus, putamen and cerebellum (Fig. 4A, Table 1). We found that PEMA was significantly reduced in children with ADHD, particularly for the RMOT network (Fig. 4B and C). To determine whether PEMA was related to the detection/signalling of an error, we used a multiple regression model where PEMA (averaged over LMOT and RMOT) was the dependent variable and the error amplitudes of cingulo-opercular and attention networks were predictors. Backward elimination identified the VAN as a single significant predictor, with higher error amplitudes in the VAN associated with greater adaptation (Fig. 4D). The same association was present when considering the ADHD group alone, but absent when considering the control group alone (Appendix 1, Fig. S2).
Fig. 4.
Post-error motor adaption (PEMA) and its association with the ventral attention network (VAN). (A) Component maps of lateralized left and right motor (MOT) systems. Component t maps are thresholded at t > 8 (1-sample t test) and displayed at the most informative slices. See Table 1 for peak coordinates and cluster extents of each component. (B) The MOT component activations on post-correct trials (divided by response laterality) and post-error trials as a function for each group. Only errors of commission are considered. (C) Post-error motor adaption for MOT components as a function of group. We calculated PEMA as the difference in activation between post-error trials and post-correct trials on the relevant side. Group differences in PEMA are indicated with t statistics and p values (1-tailed). (D) Scatterplot showing the association between VAN error amplitude and PEMA, averaged over right and left MOT networks. The simple regression model was determined with backward selection (see Methods section). Grey contours indicate the 95% confidence interval for the regression line (dashed black line).
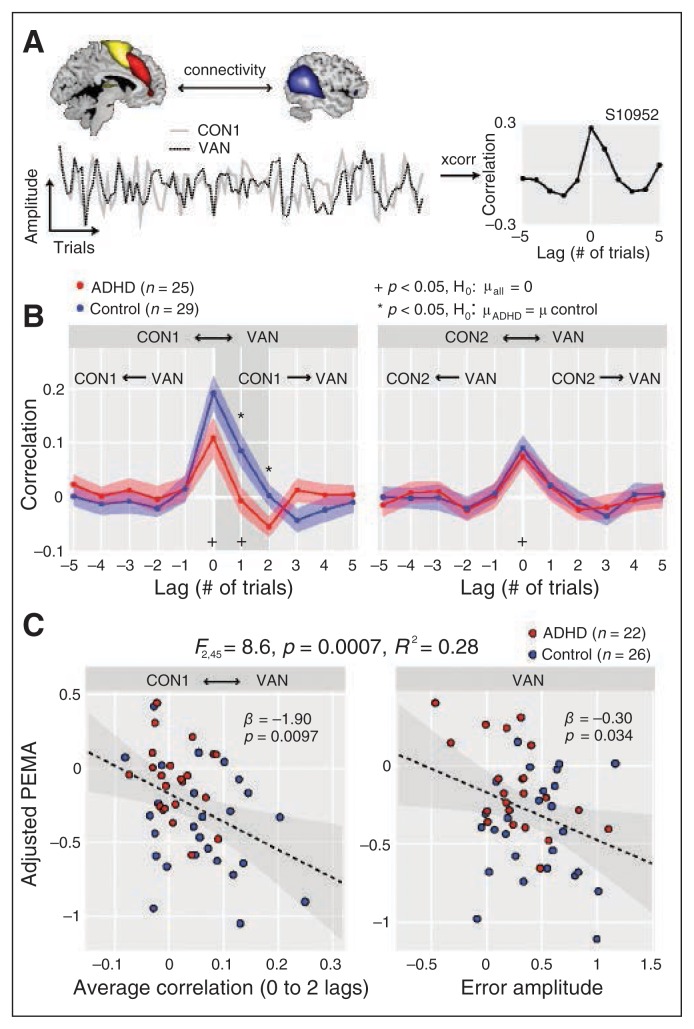
Finally, we explored whether the VAN acts downstream of the cingulo-opercular system to mediate PEMA. We computed the lagged correlations between the trial amplitudes of the CONs and VAN (Fig. 5A and B). Correlations between both CONs and VAN were significantly greater than zero at lag 0, but only the correlation between CON1 and VAN was significant at nonzero lags (lag 1), indicating that activity in CON1 at a given trial predicts activity in the VAN in the following trial (Fig. 4B). Between-group comparisons showed reduced connectivity between the CON1 and VAN in the ADHD group specific to positive lags 1 (t52 = 2.36, p = 0.022) and 2 (t52 = 2.14, p = 0.037; Fig. 4A), and with a trend in the same direction at lag 0 (t52 = 1.74, p = 0.09).
Fig. 5.
Task-driven connectivity analysis between cingulo-opercular (CON) and ventral attention (VAN) networks. (A) Example of the cross-correlation analysis performed between trial × trial amplitudes of CON1 (solid line) and VAN for a single control participant. Positive lags denote the VAN lagging behind the CON1, while negative lags denote the VAN leading. (B) Average cross-correlations (± 1 SEM) between CON1 and VAN (left), and CON2 and VAN (right), as a function of group (attention-deficit/hyperactivity disorder [ADHD] = red; control = blue). †Significantly different from zero across the entire sample at p < 0.05, uncorrected; *significant difference between groups (p < 0.05, uncorrected). The CON1–VAN connectivity was significantly different from zero at lag 0 (t53 = 6.3, p < 0.001) and lag 1 (t53 = 2.11, p = 0.039). The CON2–VAN connectivity was significantly different from zero at lag 0 (t53 = 5.41, p < 0.001). Group differences were found only for CON1–VAN at positive lags 1 (t52 = 2.4, p = 0.022) and 2 (t = 2.1, p = 0.037). (C) Regression model predicting post-error motor adaption (PEMA) from CON1–VAN connectivity (left) and VAN (right) error amplitude. The model was determined with backward selection (see Methods section). Inclusion of CON1–VAN connectivity in the model significantly increases the fraction of accounted variance (see Fig. 4D). The CON1–VAN connectivity was averaged over 0 to 2 lags (shaded region in B). Grey contours indicate the 95% confidence interval for the regression line (dashed black line).
To determine whether task driven connectivity contributes to observed differences in PEMA and RT variability, we repeated the stepwise regression analyses including CON1–VAN connectivity as a predictor. The correlation between the CON1 and VAN was retained as a significant predictor of PEMA (along with VAN error amplitude), with stronger correlations predicting greater post-error adjustments (Fig. 5C). Similarly, CON1–VAN connectivity significantly predicted σ (RT) (in addition to CON1 and CON2 error amplitudes), with stronger connectivity related to reduced RT variability (Appendix 1, Fig. S1D).
Discussion
We investigated aspects of performance monitoring in children with ADHD and focused on associations between behavioural variability and activation in cingulo-opercular and attention networks. Medication-naive children with ADHD compared with a control group demonstrated reduced BOLD activation to errors in cingulo-opercular networks and the right-lateralized VAN. These findings indicate reduced signalling for errors, supported by our finding that RT variability was inversely related to cingulo-opercular error activation. Furthermore, reduced task-driven connectivity from the CON to the VAN in children with ADHD suggested a deficit in activating the orienting system, which also was inversely associated with RT variability and PEMA. Our findings pinpoint impaired interactions of the signalling components that may lead to a deficit in error monitoring and an increase in RT variability in children with ADHD, as well as reduced motor adaptation after an error.
Diminished BOLD response to errors and RT variability
Children with ADHD displayed reduced activation on error trials in the CON1 and VAN. Numerous studies document the significance of ACC activation during error trials as a monitor for conflict and a signal for the need of greater control, triggering subsequent behavioural adaptation.15,17 Several studies have reported lower volumes of the ACC in children6 and adults44 with ADHD, less activation,6,22,45,46 and finally a normalization of ACC BOLD signal after administration of methylphenidate.7 A recent meta-analysis47 revealed that children with ADHD compared with controls most reliably exhibited reduced brain activation in the ACC during inhibitory tasks, such as the go/no-go task. Our findings extend these data, suggesting that diminished ACC activation may represent reduced recognition or response to the error itself, rather than a mere lack of inhibition. Because participants were trained on the task and received performance feedback, problems with error awareness should be minimal. However, we can certainly not exclude brief lapses of attention, reduced alertness/vigilance that would interfere with error detection. Through pre-task training and a post-task briefing it was ascertained that children understood that the same exclamation point feedback signal was provided for slow performance and errors. Based on the present data, however, we cannot entirely exclude that specific feedback responses may have been misinterpreted by the children.
We aimed to validate the significance of the reduced activation to errors in children with ADHD by evaluating the association between activation and RT variability, which we regard as a behavioural end point for an individual’s capacity to monitor performance and adapt after an error. The inverse association between BOLD response in the CON and response variability in the whole sample and controls alone confirmed this association. The lack of significant associations in the ADHD group alone may be due to the smaller range of error amplitudes or greater variability in this group, in line with other reports.8 Prior studies using oddball or inhibitory tasks have also identified associations between functional brain activation and RT variability in children with ADHD in the ACC/preSMA and temporal regions.8,22–24 A recent study22 also reported that improvement of ADHD symptoms during nonpharmacological intervention was associated with a reduction of RT variability and an increase in BOLD signal in the ACC and dorsolateral prefrontal cortex.
Notably, even though we found that children with ADHD were both significantly slower and had more errors of omission, the interaction between group and stimulus congruency did not exceed the trend level for either the error rate or RT. This suggests that children with ADHD did not exhibit deficient interference suppression, which has been found in several other prior studies in children with ADHD, although inconsistently (for a review see the study by Mullane and colleagues,48 and for the underlying neural response see the study by Hart and colleagues47).
Post-error adaptations
Several quantities can reflect behavioural changes after an error, most prominently PES.42,49 Post-error slowing may reflect the equivalent inhibitory top–down control, which ultimately leads to a motor slowing,50 or alternatively the behavioural correlate of an orienting response, expressing the surprise effect due to the typically relative infrequency of an error.51 Participants in the present study did not manifest PES, which may reflect the long interstimulus intervals used to accommodate an event-related fMRI task design;42,52 notably, PES was observed for the same participants during an electroencephalography version of the task with faster pacing (data not shown).
Despite the absence of post-error behavioural adaptions, we observed a decrease of the amplitude of motor components after an error in both groups (PEMA), which we regard as analogous for the aforementioned behavioural changes.43 A smaller difference between post-correct and post-error amplitudes in the motor component in individuals with ADHD relative to controls may be an expression for a reduced adaptation after an error, even in the absence of behavioural changes. However, we cannot exclude other explanations for this correlational finding within the present data set (e.g., group differences of the general level of motor inhibition or differences in processing feedback, engagement of a different number of neurons). The spatial and temporal limitations of the BOLD signal as well as its indirect association with neural activity precluded investigations at the mechanistic level in this study, though future studies designed for this purpose (e.g., pharmacological imaging, combined EEG-fMRI) may be able to provide illumination in this area. To our knowledge this is the first study reporting these phenomena in children with ADHD, though several studies have documented reduced PES in children with ADHD.53
Higher error amplitudes in the VAN were associated with greater PEMA, both in the whole sample and in the ADHD group alone. This observed association between VAN activity and PEMA agrees well with previous reports that VAN activation is related to PES.54 Identification of the VAN, implicated in bottom–up reorienting, as a predictor of PEMA is consistent with the “orienting hypothesis” of PES (and PEMA), which posits that post-error adjustments are part of a general reorienting response that occurs because of the relative infrequency of errors rather than a response to the error per se.51 Because the VAN may be viewed as a mediator (rather than initiator) in the process of post-error adaptations, it is consistent that VAN error activation also was reduced in children with ADHD and that the directed task-driven connectivity from CON1 to the VAN accounts for additional variance in RT variability and PEMA. We interpret this finding to suggest that the VAN signal helps to mediate post-error motor adaptations and that reduced VAN signalling may contribute to deficient post-error adaptations in children with ADHD; however, this hypothesis will require support from replications in larger samples.
Limitations
Notable study limitations include a modest sample size, imperfect matching between groups, the potential of motion artifacts, low number of errors in some participants and study scope. Because, to our knowledge, no prior studies have applied these specific analyses in this age group and patient group, it was not feasible to perform a power calculation a priori. Independent replication of the present findings in a larger sample would strengthen the evidence and allow examination of ADHD subgroups. Owing to sampling constraints, we could not match the groups for age, and the ADHD group was slightly older than the control group. To help control for this difference we residualized dependent variables with respect to age. Although the groups also differed with respect to IQ, in line with other investigations in children with ADHD, we chose not to covary for this factor, not only for statistical reasons,55 but also because IQ reductions are central to neurodevelopmental disorders.56 The effect of motion on imaging variables is always a concern, especially in children. In our sample there was fortunately no group difference in motion summary statistics, and we took additional precautions to minimize the impact of motion artifacts (eliminating participants with extreme movement, regressing motion terms from component TCs, residualizing fMRI-derived variables with respect to motion statistics). Several of the findings presented here are related to the error amplitude (i.e., the average of single trial amplitudes across error trials). Given the low error rate for many participants (ADHD: median 9 ± interquartile range [IQR] 14.3; control: median 8 ± IQR 8.5) and noise inherent to the BOLD signal,57 we highlight the following: the ADHD and control groups included a similar number of participants with very few error trials, thus there is no reason to expect bias in the group distributions or group differences shown; increased noise in error amplitudes would diminish associations with independent variables rather than create them; supplementary analyses that included the number of committed errors as an additional term in stepwise regression models did not affect the predictive association between cingulo-opercular error amplitudes and response variability. Increasing the task duration would result in more error trials, but would also present a substantial challenge in this pediatric sample, resulting in a more selected population. It is thus important to replicate these findings in larger participant samples and to investigate similar associations in tasks that yield a larger number of errors per participant. Because responses to errors can be strongly affected by both the error frequency and task timing,42 the results presented here may not generalize to all tasks and performance scenarios. By focusing on networks implicated in performance monitoring and attention, we likely failed to identify important group differences elsewhere in the brain, such as the basal ganglia and cerebellum.58 However, the selected components represent important networks for performance monitoring that have previously shown deficiencies in children and adults with ADHD. Given our sample size, we believe the conservative approach is reasonable; larger studies are better equipped to tackle a full-brain analysis that considers interactions of these key areas with other regions, which may be important to move beyond the frontostriatal model of ADHD.59
Conclusion
Our findings are concurrent with earlier evidence, confirming that medication-naive children with ADHD display a deficit in cingulo-opercular BOLD activation. Associations of BOLD amplitudes in the CON with behavioral RT variability suggest that this deficit could relate to reduced signalling for errors. Moreover, the reduced orienting of the VAN signal may mediate deficient post-error motor adaptions in children with ADHD compared with controls. Pinpointing general performance monitoring problems to specific brain regions and operations in error processing may help to guide the targets of future treatments for children with ADHD.
Acknowledgements
We thank Roger Barndon for his technical assistance. This work was supported by grants from the Research Council of Norway (190544/H110), the Western Norway Health Authority (MoodNet and the Network for Anxiety Disorders; 911435, 911607, 911827) and the National Norwegian ADHD network to K. Plessen and from the K.G. Jebsen Foundation to E. Allen, K. Hugdahl and T. Eichele.
Footnotes
Competing interests: K. Hugdahl own shares in NordicNeuroLab Inc., which produces the LCD goggles and audio system used for presenting the stimuli to the participants in this study. No other competing interests declared.
Contributors: K. Plessen, E. Allen, H. Eichele, L. Sørensen, K. Hugdahl and T. Eichele designed the study. K. Plessen, H. Eichele, H. van Wageningen, M. Høvik, L. Sørensen, M. Worren and T. Eichele acquired the data, which K. Plessen, E. Allen, K. Hugdahl and T. Eichele analyzed. K. Plessen, E. Allen and T. Eichele wrote the article, which all authors reviewed and approved for publication.
References
- 1.Polanczyk G, de Lima MS, Horta BL, et al. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164:942–8. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- 2.Bush G, Frazier JA, Rauch SL, et al. Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the counting stroop. Biol Psychiatry. 1999;45:1542–52. doi: 10.1016/s0006-3223(99)00083-9. [DOI] [PubMed] [Google Scholar]
- 3.Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nat Rev Neurosci. 2002;3:617–28. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- 4.Rubia K, Overmeyer S, Taylor E, et al. Hypofrontality in attention deficit hyperactivity disorder during higher-order motor control: a study with functional MRI. Am J Psychiatry. 1999;156:891–6. doi: 10.1176/ajp.156.6.891. [DOI] [PubMed] [Google Scholar]
- 5.Ullsperger M, Danielmeier C, Jocham G. Neurophysiology of performance monitoring and adaptive behavior. Physiol Rev. 2014;94:35–79. doi: 10.1152/physrev.00041.2012. [DOI] [PubMed] [Google Scholar]
- 6.Pliszka SR, Glahn DC, Semrud-Clikeman M, et al. Neuroimaging of inhibitory control areas in children with attention deficit hyperactivity disorder who were treatment naive or in long-term treatment. Am J Psychiatry. 2006;163:1052–60. doi: 10.1176/ajp.2006.163.6.1052. [DOI] [PubMed] [Google Scholar]
- 7.Rubia K, Halari R, Mohammad AM, et al. Methylphenidate normalizes frontocingulate underactivation during error processing in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;70:255–62. doi: 10.1016/j.biopsych.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubia K, Smith AB, Brammer MJ, et al. Temporal lobe dysfunction in medication-naive boys with attention-deficit/hyperactivity disorder during attention allocation and its relation to response variability. Biol Psychiatry. 2007;62:999–1006. doi: 10.1016/j.biopsych.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 9.Spinelli S, Joel S, Nelson TE, et al. Different neural patterns are associated with trials preceding inhibitory errors in children with and without attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2011;50:705–715. doi: 10.1016/j.jaac.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suskauer SJ, Simmonds DJ, Fotedar S, et al. Functional magnetic resonance imaging evidence for abnormalities in response selection in attention deficit hyperactivity disorder: differences in activation associated with response inhibition but not habitual motor response. J Cogn Neurosci. 2008;20:478–93. doi: 10.1162/jocn.2008.20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ullsperger M, Volz KG, von Cramon DY. A common neural system signaling the need for behavioral changes. Trends Cogn Sci. 2004;8:445–6. doi: 10.1016/j.tics.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 12.Ridderinkhof KR, Ullsperger M, Crone EA, et al. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–7. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- 13.Cortese S, Kelly C, Chabernaud C, et al. Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies. Am J Psychiatry. 2012;169:1038–55. doi: 10.1176/appi.ajp.2012.11101521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein C, Wendling K, Huettner P, et al. Intra-subject variability in attention-deficit hyperactivity disorder. Biol Psychiatry. 2006;60:1088–97. doi: 10.1016/j.biopsych.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8:539–46. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Verguts T, Notebaert W. Hebbian learning of cognitive control: dealing with specific and nonspecific adaptation. Psychol Rev. 2008;115:518–25. doi: 10.1037/0033-295X.115.2.518. [DOI] [PubMed] [Google Scholar]
- 17.Kerns JG, Cohen JD, MacDonald AW, III, et al. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–6. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- 18.Christakou A, Halari R, Smith AB, et al. Sex-dependent age modulation of frontostriatal and temporoparietal activation during cognitive control. Neuroimage. 2009;48:223–36. doi: 10.1016/j.neuroimage.2009.06.070. [DOI] [PubMed] [Google Scholar]
- 19.Rubia K, Halari R, Christakou A, et al. Impulsiveness as a timing disturbance: neurocognitive abnormalities in attention-deficit hyperactivity disorder during temporal processes and normalization with methylphenidate. Philos Trans R Soc Lond B Biol Sc. 2009;364:1919–1931. doi: 10.1098/rstb.2009.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubia K, Noorloos J, Smith A, et al. Motor timing deficits in community and clinical boys with hyperactive behavior: the effect of methylphenidate on motor timing. J Abnorm Child Psychol. 2003;31:301–13. doi: 10.1023/a:1023233630774. [DOI] [PubMed] [Google Scholar]
- 21.Karalunas SL, Geurts HM, Konrad K, et al. Annual research review: reaction time variability in ADHD and autism spectrum disorders: measurement and mechanisms of a proposed trans-diagnostic phenotype. J Child Psychol Psychiatry. 2014;55:685–710. doi: 10.1111/jcpp.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siniatchkin M, Glatthaar N, von Muller GG, et al. Behavioural treatment increases activity in the cognitive neuronal networks in children with attention deficit/hyperactivity disorder. Brain Topogr. 2012;25:332–44. doi: 10.1007/s10548-012-0221-6. [DOI] [PubMed] [Google Scholar]
- 23.Suskauer SJ, Simmonds DJ, Caffo BS, et al. fMRI of intrasubject variability in ADHD: anomalous premotor activity with prefrontal compensation. J Am Acad Child Adolesc Psychiatry. 2008;47:1141–50. doi: 10.1097/CHI.0b013e3181825b1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spinelli S, Vasa RA, Joel S, et al. Variability in post-error behavioral adjustment is associated with functional abnormalities in the temporal cortex in children with ADHD. J Child Psychol Psychiatry. 2011;52:808–16. doi: 10.1111/j.1469-7610.2010.02356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eichele T, Debener S, Calhoun VD, et al. Prediction of human errors by maladaptive changes in event-related brain networks. Proc Natl Acad Sci U S A. 2008;105:6173–8. doi: 10.1073/pnas.0708965105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Maanen L, Brown SD, Eichele T, et al. Neural correlates of trial-to-trial fluctuations in response caution. J Neurosci. 2011;31:17488–95. doi: 10.1523/JNEUROSCI.2924-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–15. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 28.Vincent JL, Kahn I, Snyder AZ, et al. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–42. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–24. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–8. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 31.Shaffer D, Gould MS, Brasic J, et al. A Children’s Global Assessment Scale (CGAS) Arch Gen Psychiatry. 1983;40:1228–31. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- 32.Leckman JF, Sholomskas D, Thompson WD, et al. Best estimate of lifetime psychiatric diagnosis: a methodological study. Arch Gen Psychiatry. 1982;39:879–83. doi: 10.1001/archpsyc.1982.04290080001001. [DOI] [PubMed] [Google Scholar]
- 33.DuPaul GJ, Power TJ, Anastopoulos AD, et al. ADHD Rating Scale-IV: Checklist, Norms, and Clinical Interpretation. New York: Guilford Press; 1998. [Google Scholar]
- 34.Wechsler D. Wechsler Intelligence Scale for Children–Fourth Edition (WISC-IV) San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- 35.Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept Psychophys. 1974;16:143–9. [Google Scholar]
- 36.Huang CM, Lee SH, Hsiao IT, et al. Study-specific EPI template improves group analysis in functional MRI of young and older adults. J Neurosci Methods. 2010;189:257–66. doi: 10.1016/j.jneumeth.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 37.Erhardt EB, Rachakonda S, Bedrick EJ, et al. Comparison of multi-subject ICA methods for analysis of fMRI data. Hum Brain Mapp. 2011;32:2075–95. doi: 10.1002/hbm.21170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mumford JA, Turner BO, Ashby FG, et al. Deconvolving BOLD activation in event-related designs for multivoxel pattern classification analyses. Neuroimage. 2012;59:2636–43. doi: 10.1016/j.neuroimage.2011.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Becerril KE, Barch DM. Conflict and error processing in an extended cingulo-opercular and cerebellar network in schizophrenia. Neuroimage Clin. 2013;3:470–80. doi: 10.1016/j.nicl.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dosenbach NU, Visscher KM, Palmer ED, et al. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Polli FE, Barton JJ, Thakkar KN, et al. Reduced error-related activation in two anterior cingulate circuits is related to impaired performance in schizophrenia. Brain. 2008;131:971–86. doi: 10.1093/brain/awm307. [DOI] [PubMed] [Google Scholar]
- 42.Danielmeier C, Ullsperger M. Post-error adjustments. Front Psychol. 2011;2:233. doi: 10.3389/fpsyg.2011.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Danielmeier C, Eichele T, Forstmann BU, et al. Posterior medial frontal cortex activity predicts post-error adaptations in task-related visual and motor areas. J Neurosci. 2011;31:1780–9. doi: 10.1523/JNEUROSCI.4299-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seidman LJ, Valera EM, Makris N, et al. Dorsolateral prefrontal and anterior cingulate cortex volumetric abnormalities in adults with attention-deficit/hyperactivity disorder identified by magnetic resonance imaging. Biol Psychiatry. 2006;60:1071–80. doi: 10.1016/j.biopsych.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 45.Bush G. Cingulate, frontal, and parietal cortical dysfunction in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;69:1160–7. doi: 10.1016/j.biopsych.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Konrad K, Neufang S, Hanisch C, et al. Dysfunctional attentional networks in children with attention deficit/hyperactivity disorder: evidence from an event-related functional magnetic resonance imaging study. Biol Psychiatry. 2006;59:643–51. doi: 10.1016/j.biopsych.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 47.Hart H, Radua J, Nakao T, et al. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. 2013;70:185–98. doi: 10.1001/jamapsychiatry.2013.277. [DOI] [PubMed] [Google Scholar]
- 48.Mullane JC, Corkum PV, Klein RM, et al. Interference control in children with and without ADHD: a systematic review of Flanker and Simon task performance. Child Neuropsychol. 2009;15:321–42. doi: 10.1080/09297040802348028. [DOI] [PubMed] [Google Scholar]
- 49.Rabbit PMA. Sequential reactions. In: Holding D, editor. Human skills. New York: Wiley; 1981. pp. 153–175. [Google Scholar]
- 50.Gehring WJ, Fencsik DE. Functions of the medial frontal cortex in the processing of conflict and errors. J Neurosci. 2001;21:9430–7. doi: 10.1523/JNEUROSCI.21-23-09430.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Notebaert W, Houtman F, Opstal FV, et al. Post-error slowing: an orienting account. Cognition. 2009;111:275–9. doi: 10.1016/j.cognition.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 52.Jentzsch I, Dudschig C. Why do we slow down after an error? Mechanisms underlying the effects of posterror slowing. Q J Exp Psychol (Hove) 2009;62:209–18. doi: 10.1080/17470210802240655. [DOI] [PubMed] [Google Scholar]
- 53.Balogh L, Czobor P. Post-error slowing in patients with ADHD: a meta-analysis. J Atten Disord. 2014 doi: 10.1177/1087054714528043. 1087054714528043. [DOI] [PubMed] [Google Scholar]
- 54.Danielmeier C, Allen E, Jocham G, et al. Acetylcholine mediates behavioral and neural post-error control. Curr Biol. 2015;25:1461–8. doi: 10.1016/j.cub.2015.04.022. [DOI] [PubMed] [Google Scholar]
- 55.Miller GA, Chapman JP. Misunderstanding analysis of covariance. J Abnorm Psychol. 2001;110:40–8. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- 56.Dennis M, Francis DJ, Cirino PT, et al. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. J Int Neuropsychol Soc. 2009;15:331–43. doi: 10.1017/S1355617709090481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huettel SA, McCarthy G. The effects of single-trial averaging upon the spatial extent of fMRI activation. Neuroreport. 2001;12:2411–6. doi: 10.1097/00001756-200108080-00025. [DOI] [PubMed] [Google Scholar]
- 58.Valera EM, Faraone SV, Murray KE, et al. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;61:1361–9. doi: 10.1016/j.biopsych.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 59.Castellanos FX, Proal E. Large-scale brain systems in ADHD: beyond the prefrontal-striatal model. Trends Cogn Sci. 2012;16:17–26. doi: 10.1016/j.tics.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]