Abstract
Background
Schizophrenia is associated with poor theory of mind (ToM), particularly in the attribution of intentions to others. It is also associated with abnormal gaze behaviours and contextual processing. This study investigated to what extent impaired ToM in patients with schizophrenia is related to abnormal processing of social context.
Methods
We evaluated ToM using a nonverbal intention attribution task based on comic strips depicting social/nonsocial and contextual/noncontextual events while eye movements were recorded. Eye-tracking was used to assess processing time dedicated to visual cues contained in regions of interest identified in a pilot study. We measured cognitive contextual control on a separate task.
Results
We tested 29 patients with schizophrenia and 29 controls. Compared with controls, patients were slower in intention attribution but not in physical reasoning. They looked longer than controls at contextual cues displayed in the first 2 context pictures of the comic strips, and this difference was greater for intention attribution than for physical reasoning. We found no group difference in time spent looking at noncontextual cues. Patients’ impairment in contextual control did not explain their increased reaction time and gaze duration on contextual cues during intention attribution.
Limitations
Difficulty may not have been equivalent between intention attribution and physical reasoning conditions.
Conclusion
Overall, schizophrenia was characterized by a delay in intention attribution related to a slowdown of social context processing that was not explained by worse executive contextual control.
Introduction
Schizophrenia is characterized by impaired performance in mentalization or theory of mind (ToM),1 including the attribution of goals,2,3 intentions4 and beliefs.1,5,6 A better understanding of poor ToM performance in patients with schizophrenia is particularly important as it is one of the strongest predictors of functional outcome among other social as well as nonsocial cognitive domains7 and mediates the association between neurocognition and social competence.8 Despite its apparent exhaustiveness, research on mentalization in patients with schizophrenia leaves a number of questions opened regarding the explanation of the patients’ abnormal performances. Successive theoretical accounts emphasized the role of impaired contextual processing in the genesis of social cognitive impairments.9–11 More recently, the integrative nature of ToM processing was emphasized considering that inferring others’ mental states is a very efficient means, used by the human cognitive system, to bring together social cues and contextual information within a unified and coherent representation of the social environment.12 The present study aimed to better understand the difficulties in intention attribution (IA) in patients with schizophrenia by investigating visual exploration of intentional/nonintentional and contextual/noncontextual cues and by assessing contextual control aspects of executive functioning.
The first question concerns the contribution of abnormal visual scanpaths to mentalization deficits in patients with schizophrenia. The impact of inappropriate visual explorations on social cognition in these patients has received increased interest in recent studies, particularly for emotions13–15 and biological motion perception.16 Recently, mentalization in patients with schizophrenia was investigated with eye-tracking paradigms: studies have found that inappropriate visual exploration strategies impair performance in social cognition tasks. A first study used an animated cartoon false belief paradigm with object translocation. Patients’ deficits in attributing beliefs and goals to the animated character were entirely explained by a decreased visual attention toward the character’s gaze.17 A second study used an intentional motion detection paradigm involving several moving circles among which one circle was chasing another one. Patients’ decrease in chasing detection perceptual sensitivity was explained by a suboptimal centre-looking strategy characterized by a greater visual attention devoted to the barycentre of all moving circles rather than to the moving circles themselves.18 To further examine the association between visual processing and mentalization, the present study compared visual scanpaths of participants with and without schizophrenia while performing a nonverbal IA task based on short comic strips depicting social or nonsocial events.19 We hypothesized that differences in scan-paths between controls and patients would be associated with abnormal IA in patients with schizophrenia. A previous study reported a decreased visual processing of social context in individuals with schizophrenia performing a mental state perception task.10 However, it was not possible to distinguish, in that study, a specific decrease of attention toward social context from a more generally restricted scanning ability. In the present study, we hypothesized that schizophrenia was characterized by an abnormal social context processing measured by the quantity of gaze devoted to intentional contextual cues as opposed to nonintentional and noncontextual cues. Two equally likely predictions may be made about the abnormal processing of intention in patients with schizophrenia. First, patients might ignore social context, leading to impaired IA. Thus, IA accuracy and the duration of gaze devoted to intentional contextual cues would be decreased in patients with this disorder. The alternative prediction states that patients might be slower in their social context processing, thus leading to a preserved but delayed IA. Thus, IA reaction time and the duration of gaze devoted to intentional contextual cues should be greater in patients with schizophrenia than in controls.
This study also tackles the issue of whether ToM deficits in patients with schizophrenia might be due to other more general cognitive deficits, such as deficits in executive functions. Mentalizing is a complex cognitive function that requires the integration of information from multiple sources and may elicit heavy executive demands to select, inhibit, sequence and contextualize representations of social cues. Failure in ToM tasks may thus reflect, at least partially, impairments in executive functioning. Within the executive function domain, contextual control can be defined as the ability to maintain and use information to mediate later task-appropriate behaviour. A significant association between contextual processing and social inference has been shown in patients with schizophrenia9; it thus appears useful to take into account general contextual control skills within the executive function domain while assessing the association between social context processing and ToM in such patients. The present study tested to what extent patients’ poor performance in IA and social context processing might be explained by poor executive contextual control, as measured using a validated paradigm20 that has demonstrated a deficit in patients with schizophrenia.21
Methods
Participants
We recruited patients with schizophrenia and healthy control participants for this study. Patients were stable and were recruited from community mental health centres and outpatient clinics in the Versailles area. Exclusion criteria for both groups comprised substance or alcohol dependence within the past 6 months and current or prior history of untreated significant medical illness or of neurologic illness. All diagnoses in the schizophrenia group were confirmed by 2 licensed psychiatrists (one of whom was P.R.) according to the DSM-IV-R criteria for schizophrenia. The control group was screened for current or past psychiatric illness, and participants were excluded if they met criteria for any axis I disorder of the DSM-IV-TR. The experiment was approved by the local medical ethics committee (Comité de Protection des Personnes Paris Ile de France XI). All participants received a complete description of the study verbally and in a written form. We checked whether patients were capable of giving fully informed consent through specific interviews (focused on the ability able to comprehend and retain information about the research and to use and weigh this information to make an appropriate decision). Written informed consent was then obtained from each participant.
Cognitive and clinical measures
We estimated general intelligence using 4 Wechsler Adult Intelligence Scale (WAIS)-III subtests: the Vocabulary and Similarities subtests for verbal intelligence and the Pictures Completion and Matrices subtests for nonverbal intelligence. We rated the severity of schizophrenic symptoms in all patients using the Positive and Negative Syndrome Scale (PANSS).22
Contextual control paradigm
Participants were presented a series of coloured letters and had to respond by pressing 1 of 2 response buttons. Each participant underwent 4 blocks including 16 sequences of 12 stimuli (duration 500 ms; onset asynchrony 3500 ms) preceded by an instruction. In contrast with the original paradigm, participants controlled the duration of instruction presentation in order to ensure that they had enough time to read and understand the instructions. Participants performed 2 tasks according to the colour of the letter (Appendix 1, available at jpn.ca): a lower-/uppercase discrimination task when the letter was green and a consonant/vowel discrimination task when the letter was red. In each task, participants had to ignore the stimulus when it was white. In low-contextual control sequences, participants were presented with only red/white or only green/white letters and performed only 1 task during a given sequence. In high-contextual control sequences, participants were presented with a mixture of red, green and white letters and had to select between tasks 1 and 2 according to the colour of the letter.
Stimuli were pseudorandomized. Two successive stimuli were never identical, there were no more than 3 consecutive identical responses, and the ratio of left:right:no responses and the ratio of congruent:incongruent letters (same v. different responses for task 1 and task 2) were equal to 1.
Participants were trained on low- (1 sequence of task 1, 1 sequence of task 2) and high-contextual control sequences (2 task 1/task 2 sequences). Participants had to obtain a minimal score of 2 out of 3 correct responses on every sequence, otherwise the training went on until this criterion was reached.
Intention inference task
The intention interference task presented a character performing a very simple action in a short black and white comic strip. The comic strips drawn by a professional illustrator were inspired from previous works that demonstrated abnormal behavioural and brain response in patients with schizophrenia.23–25 The type of causality involved in the comic strips was varied through 2 experimental conditions. In the IA condition, the participants were presented with 29 scenarios depicting a character whose behaviour was driven by a specific intention; participants had to infer the character’s intention to understand the scenario. In the physical causality with characters (PCCh) condition, the participants were presented another set of 29 scenarios depicting a character whose movement was determined by a physical causality; participants had to reason on the mechanical properties of human bodies (e.g., gravity) in order to understand the scenario.
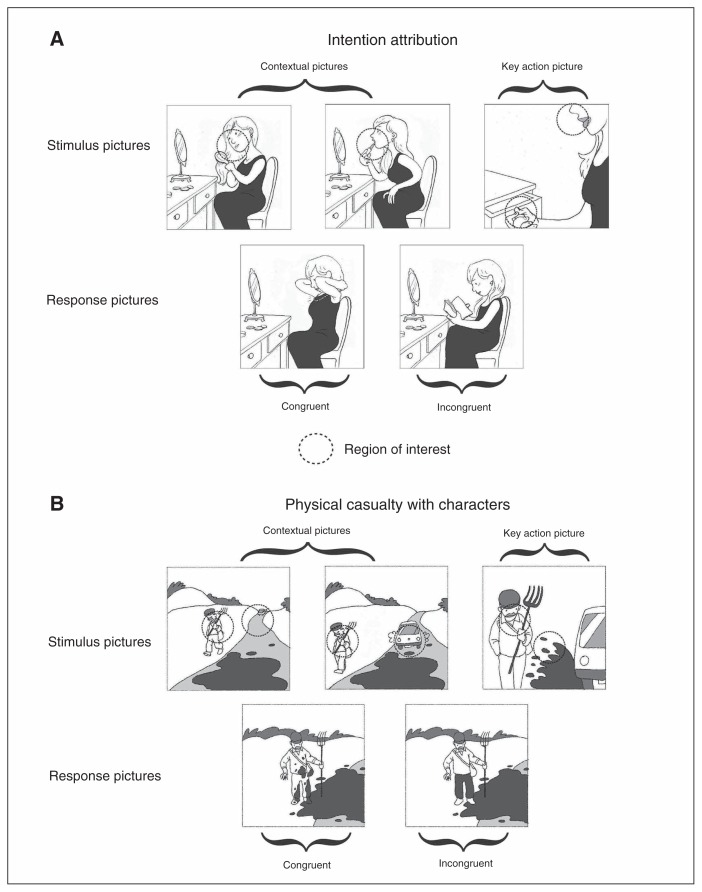
The comic strip display was divided into 2 phases. During the first phase, 3 pictures were simultaneously displayed in the upper part of the screen for 3000 ms. These 3 pictures presented the scenario of the comic strip; the first 2 pictures gave contextual information, whereas the third one focused on the key action (Fig. 1). In the second phase, 2 response images (congruent v. incongruent) were presented in the lower part of the screen while the 3 story pictures remained in the upper part of the screen. This procedure allowed the participants to look at the story pictures during the presentation of response pictures. Compared with a sequential presentation of images, their simultaneous presentation avoids a load on working memory, which is impaired in patients with schizophrenia.26 The participants were instructed to choose as quickly as possible which of the 2 response pictures was the most logical to complete the story told in the first 3 pictures by pressing 1 of 2 keyboard buttons during a 6500 ms time window. Trials were presented in a pseudorandomized order: 3 successive stimuli never belonged to the same condition or required participants to select the correct answer on the same side.
Fig. 1.
Example of an item in the intention attribution and in the physical causality conditions of the task. Dotted circles represent the regions of interest.
All pictures were squares of 9.7° (visual angle) presented on a 17-inch display with a 60 Hz refresh rate and a 1280 × 1024 pixel resolution, viewed from a distance of 60 cm in a dimly lit room. Eye movements were recorded monocularly with a video-based desktop-mounted eye-tracker during stimulus and response phases (see Appendix 1 for a description of the eye-tracking apparatus).
Statistical analysis
Participants
We compared group characteristics using Student t or χ2 tests as appropriate. Antipsychotic doses were expressed in chlorpromazine equivalents.27
Contextual control
A repeated-measures analysis of variance (ANOVA) was run on error rate using group (patient v. control) as a between-subjects factor and contextual demand (high v. low) as a within-subjects factor. For all ANOVAs in this study, when the group × condition interactions were not significant, we omitted the interaction term from the model to assess the marginal group and condition effects.
Intention attribution
For technical reasons, the forced-choice response data of 1 patient were lost. We discarded nonresponses from the forced-choice response analysis (see Appendix 1 for an analysis of nonresponses), and errors and reaction time below 100 ms were discarded from the reaction time analysis. We first ran repeated-measures analyses of covariance (ANCOVA) on error rate and reaction time with group (patient v. control) as the between-subjects factor, condition (IA v. PCCh) as the within-subjects factor and IQ as the covariate. We then ran the same ANCOVAs, adding contextual control as an additional covariate in order to investigate whether contextual control fully explained group differences.
Eye movements
Nonresponses and errors were kept in the eye movement analysis, and the ocular measures of the patient for whom the explicit responses data were lost was included in the eye movement analysis. Trials were discarded from the analysis when the percentage of filtered ocular samples was below 70% (blink, artifact or other technical reasons).
We defined 1 or 2 regions of interest (ROI) for each stimulus picture (see Fig. 1 for an example of ROI): they were discs whose centres’ localization were determined in a pilot study (see Appendix 1 for a complete description of the pilot study). The ROIs’ diameter was 25% of the picture width (2.4°).
We computed an ocular measure reflecting the ability to focus one’s attention on relevant contextual cues before the main character’s action: the contextual ROI looking time (ratio of ocular samples that fell into ROIs of the first or second contextual image). We also computed the action ROI looking time (ratio of ocular samples that fell into ROIs of the third action image), which reflects the tendency to focus one’s attention toward relevant noncontextual cues during the main character’s action.
We ran repeated-measures ANCOVAs on contextual and action ROI looking times with group as the between-subjects factor, condition as the within-subjects factor and IQ as the covariate. We then ran the same ANCOVAs adding contextual control as another covariate in order to investigate whether contextual control fully explained group differences.
Supplementary analyses
We investigated whether accuracy and reaction times were predicted by ocular measures. We also investigated correlations between chlorpromazine equivalents and patients’ performances in IA (2-alternative forced-choice and ocular responses; Appendix 1).
Results
Participants
We tested 29 patients with schizophrenia and 29 controls. The demographic and clinical characteristics of participants are shown in Table 1. All participants had normal or corrected-to-normal vision. At the time of testing, all patients with schizophrenia were taking antipsychotics. Patients had a PANSS positive mean score in the average range (70th percentile), a PANSS negative mean score in the average range (65th percentile) and a PANSS general psychopathology mean score in the high average range (75th percentile). Patients had a marginally lower general intelligence than controls (justifying the inclusion of IQ as a covariate in the different ANCOVAs) but were comparable with controls on all other variables. Results obtained in other experiments with the same participants have been reported previously.17,18
Table 1.
Demographic and clinical characteristics of study participants
| Group; mean ± SD* | ||||
|---|---|---|---|---|
|
|
||||
| Characteristic | Schizophrenia n = 29 |
Control n = 29 |
Statistic | p value |
| Sex, male:female | 21:8 | 19:10 | χ2 = 0.1 | 0.78 |
| Visual correction, contact lenses: glasses† | 1:12 | 3:9 | χ2 = 0 | > 0.99 |
| Age, yr | 39 ± 12.5 | 40.7 ± 13.5 | t56 = 0.5 | 0.63 |
| Education, yr | 12 ± 2.3 | 12.4 ± 1.5 | t56 = 0.9 | 0.39 |
| Estimated general intelligence‡ | 8.3 ± 2.1 | 9.3 ± 2.1 | t56 = 1.8 | 0.08 |
| Paranoia§ | 53.6 ± 14.9 | 42.5 ± 11.2 | t56 = −3.2 | 0.002 |
| Illness duration, yr | 18 ± 11.1 | — | — | — |
| Hospitalization duration, yr | 16.5 ± 19.3 | — | — | — |
| Cpz equivalents, mg/24h | 544.2 ± 364.3 | — | — | — |
| PANSS total | 90.6 ± 12 | — | — | — |
| PANSS positive scale | 21.8 ± 4 | — | — | — |
| PANSS negative scale | 24.3 ± 4.9 | — | — | — |
| PANSS general symptoms scale | 44.5 ± 6.8 | — | — | — |
Cpz = chlorpromazine; PANSS = Positive and Negative Syndrome Scale.
Unless otherwise indicated
For the χ2 test, contact lenses and glasses were counted as one category owing to small sample size.
Mean scaled scores from 1 to 19. Wechsler Adult Intelligence Scale scores have a mean of 10 ± 3 in the general population.
Mean scores on the Paranoia Scale from 20 to 100.
Contextual control
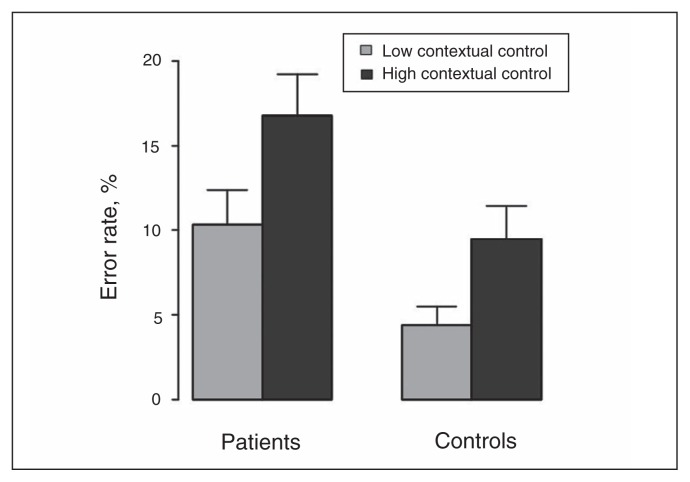
One patient was unable to perform the task because she did not reach the criterion in the high-contextual control sequences during the training phase, whereas she managed to reach this criterion for task 1 and task 2 low-contextual control sequences. The missing measure for this participant was replaced with the highest error rate in the high-control condition obtained in the patient group (37.5%) in order to avoid excluding a patient with low-contextual control ability who was able to perfom all other tasks.
The group × contextual demand interaction was not significant (F1,55 = 0.8, p = 0.38). There were significant effects of group (F1,55 = 6.3, p = 0.015) and contextual demand (F1,56 = 59.7, p < 0.001). Figure 2 illustrates that patients were impaired for both low- and high-contextual control conditions compared with controls. In this study, we were mainly interested in a measure of contextual control in patients with schizophrenia in order to check whether contextual control could influence IA. We thus selected the accuracy rate in the high-contextual control condition as the measure of contextual control.
Fig. 2.
Mean error rates for the low and high contextual control conditions. Error bars represent the standard error of the mean.
Intention attribution
Two-alternative forced-choice responses
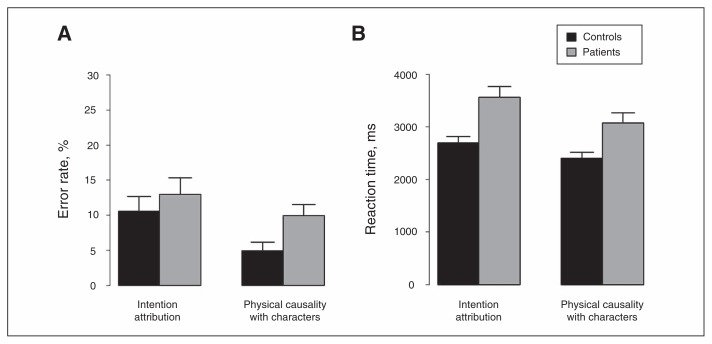
The ANCOVA run-on error rate with IQ as a covariate revealed no significant group × condition interaction (F1,55 = 1.1, p = 0.31). There was a significant effect of condition (F1,55 = 12.2, p < 0.001), but no significant effect of group (F1,55 = 1.6, p = 0.21). Figure 3A illustrates that error rate was greater for IA than PCCh.
Fig. 3.
Mean (A) error rates and (B) reaction times computed from forced-choice responses. Error bars represent the standard error of the mean.
The ANCOVA run-on error rate with IQ and contextual control as covariates revealed that the group effect remained nonsignificant (F1,55 = 0.003, p = 0.95).
The ANCOVA run-on reaction time with IQ as a covariate revealed a significant group × condition interaction (F1,55 = 8.7, p = 0.005). Planned post hoc ANOVAs revealed that the group effect was significant for IA (F1,55 = 12.5, p < 0.001) and for PCCh (F1,55 = 7.5, p = 0.008). Figure 3B illustrates that reaction times were greater for patients than controls in both conditions, but the group difference was greater for IA than PCCh. The condition effect was significant for patients (F1,28 = 77.9, p < 0.001) and controls (F1,27 = 53.3, p < 0.001). Figure 3B illustrates that reaction time was greater for IA than PCCh in both groups.
The ANCOVA run-on reaction time with IQ and contextual control as covariates revealed that the group effect remained significant for IA (F1,55 = 6.7, p = 0.012) but became only marginally significant for PCCh (F1,55 = 3.7, p = 0.06). This suggests that the slowdown found for patients in PCCh was mainly explained by the patients’ lower contextual control than controls’, contrary to the patients’ slowdown in IA.
Eye movements
Eight trials were excluded owing to the low quality of the eye-tracking recording (6 for patients, 2 for controls).
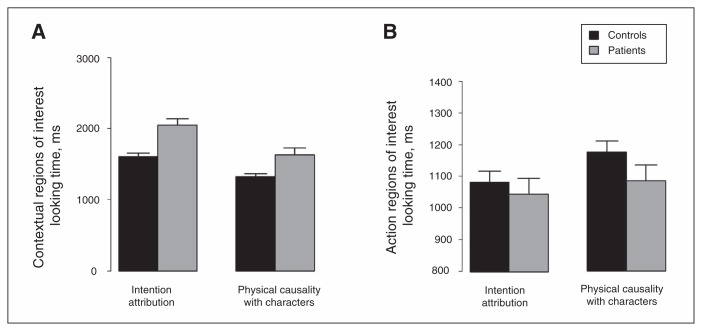
The ANCOVA run-on contextual ROI looking time with IQ as a covariate revealed a significant group × condition interaction (F1,56 = 11.4, p = 0.001). Planned post hoc ANOVAs revealed that the group effect was significant for IA (F1,56 = 16.8, p < 0.001) and for PCCh (F1,56 = 10.5, p = 0.002). Figure 4A illustrates that contextual ROI looking time was greater for patients than controls in both conditions, but the group difference was greater for IA than PCCh. The condition effect was significant for patients (F1,28 = 192, p < 0.001) and controls (F1,28 = 102.8, p < 0.001). Figuxre 4A illustrates contextual ROI looking time was greater for IA than PCCh in both groups.
Fig. 4.
Mean (A) contextual and (B) action region of interest looking times. Error bars represent the standard error of the mean.
The ANCOVA run-on contextual ROI looking time with IQ and contextual control as covariates revealed that the group effect remained significant for IA (F1,56 = 14.5, p < 0.001) and PCCh (F1,56 = 4.9, p = 0.003).
The ANCOVA run-on action ROI looking time with IQ as a covariate revealed a nonsignificant group × condition interaction (F1,56 = 2.6, p = 0.11). There was a significant effect of condition (F1,56 = 17.4, p < 0.001), but no significant effect of group (F1,56 = 1.1, p = 0.30). Figure 4B illustrates that action ROI looking time was greater for PCCh than for IA.
The ANCOVA run-on action ROI looking time with IQ and contextual control as covariates revealed that the group effect remained nonsignificant (F1,56 = 2.4, p = 0.13).
Discussion
The main aim of this study was to characterize the associations between the abnormal attribution of intentions to others and the dysfunctional contextual processing in patients with schizophrenia. While the main behavioural impairment could be described as a delay in IA in patients with schizophrenia, eye-tracking data provided richer insights into the deficit. Abnormal scanpaths were found in patients with schizophrenia, with an increased looking time for contextual cues, which was more pronounced for IA than physical reasoning. This suggests a delay in social context processing in patients with schizophrenia that was not explained by a more general deficit in executive contextual control.
Impaired contextual control in patients with schizophrenia
Patients were equally impaired in low- and high-cognitive control conditions, which was not expected because a previous study21 found that patients with schizophrenia were selectively impaired in the high-contextual control condition. This discrepancy might be explained by the fact that participants were much more trained to the general procedure of this task in the earlier study compared with the present study, which may have helped them achieve normal performance in the easier condition.
Slowdown of intention processing in patients with schizophrenia
No group difference was found for the accuracy of IA with the present version of the task, which contrasts with results obtained with previous versions of this task. The version used in the present study may have been easier than that used previously because of a simplification in the procedure: in the present version, only 2 response pictures were presented instead of 3,23 and instead of a sequential presentation in a previous version24 all the pictures in the present study were displayed on the screen until the participants responded. The present version may also have been easier because of an improvement in the quality of drawings. This result also contrasts with a large number of other tasks.5,28 Some patients had benefited from a remediation program targeting ToM:29 this intervention may have led to an improvement in the rate of correct responses. Patients might also have used a cognitive compensatory strategy favouring accuracy over speed to maintain performance; this strategy has been involved in the improvement of several cognitive processes in patients with schizophrenia.30–32
However, our results suggest that mentalizing was slowed down in patients with schizophrenia, even when executive contextual control was taken into account. Many studies have reported increased response times during ToM tasks in patients with schizophrenia,33–35 although the specificity of this slowdown to the domain of social cognition has remained unclear. In the present study, this slowdown in patients’ responses was more pronounced for IA than for physical reasoning, thus suggesting at least a certain degree of specificity to the social domain.
Our eye-tracking analysis revealed that this slowdown did not affect the processing of all visual cues equally. Patients looked longer than controls at contextual cues specifically (as opposed to action cues), and this increase was more pronounced for social cues displayed in the IA condition than for nonsocial cues displayed in the physical reasoning condition. Furthermore, patients’ increased looking time was not explained by a general executive impairment in contextual control. This result clearly suggests that the slowdown of mentalizing found in patients with schizophrenia specifically involves the processing of social context as opposed to non-contextual and nonsocial cues. These results suggest that individuals with schizophrenia are delayed in their mentalization, particularly in social context processing. This interpretation is in line with the results of an fMRI study showing delayed activations in ToM-related brain areas in individuals with schizophrenia while mentalizing.36
One might suggest that IA required more eye movements to faces than PCCh, thus explaining patients’ slowdown in IA. This would be consistent with the results of previous reports suggesting that schizophrenia is characterized by an impaired visual attention toward faces,37–41 leading to ToM deficits.17 In the analysis reported in Appendix 1, we found that there were as many contextual ROIs including a face in IA as in PCCh conditions, but there were marginally more action ROIs including a face in IA than in PCCh. However, an additional analysis revealed that patients and controls did not differ in the amount of eye movements toward action ROIs including a face both in the IA and PCCh conditions (Appendix 1). Thus it seems that the delayed mentalization in patients with schizophrenia cannot be entirely explained by a failure to focus attention on faces in our paradigm.
The prediction that patients would spend less time on social contextual cues and would therefore be less accurate was not borne out. Our results are more consistent with the alternative prediction that patients take more time to process contextual cues, particularly when these cues convey intentional information, perhaps reflecting a difficulty to integrate this context into a meaningful representation of the story displayed by the comic strip.
Limitations
Two limitations require consideration to interpret the present results. First, it is not clear whether the delay found for the intentional scenarios truly reflects a specific impairment of IA in patients with schizophrenia or whether it is a consequence of an unequal matching in the level of difficulty between IA and PCCh. Several behavioural and ocular data suggested that IA trials were more difficult to resolve than PCCh trials. First, error rates and reaction times were greater for IA than for PCCh. Then, contextual ROI looking time was greater for IA than for PCCh, whereas action ROI looking time was greater for PCCh than for IA. This suggests that IA required a longer processing of the context than physical reasoning. However, it seems unlikely that this longer processing of contextual cues in IA compared with PCCh might be explained by an unequal matching of visual complexity between the 2 conditions (Appendix 1). Intention attribution might be intrinsically more cognitively demanding than physical reasoning. Further studies should address this question by trying to develop an experiment in which intentional and physical reasoning are strictly matched on their degree of complexity. Second, all patients were taking antipsychotic medication. However, we did not find any significant correlation between chlorpromazine equivalents and reaction time or contextual ROI or looking time (Appendix 1), thus suggesting that the delay in IA and in social context processing found in patients with schizophrenia was not explained by a psychomotor slowdown induced by medication. In contrast, antipsychotic dosage was associated with a faster processing of noncontextual action cues.
Conclusion
The present study showed that IA was not lacking, but was delayed in patients with schizophrenia. This delay was not explained by lower intelligence or an executive deficit in contextual control. Most interestingly, the delayed mentalization was associated with an abnormal visual exploration: patients took more time to process contextual cues, particularly when they had to attribute intentions. This might reflect a slowdown of contextual processing involved in IA. Speed is a particularly important variable for social cognition because real social situations require rapid judgments about others’ mental states with frequent adjustment. Mentalizing is thought to rely on fast cognitive processes that require little or no mental effort.42–44 Thus, a disruption in the subtle timing of mentalizing processes might be compensated by effortful cognitive functioning when individuals with schizophrenia are confronted with others’ complex social behaviour. These results may have some practical implications for the existing programs of ToM remediation in patients with schizophrenia.29,45,46 First, cognitive remediation therapies targeting mentalization should give patients enough time to find the correct interpretation of others’ mental states. Second, mentalization might be improved by training patients to speed up the processing of social context.
Acknowledgments
This work was supported by Agence Nationale de la Recherche (ANR-09-BLAN-0327, ANR-11-IDEX-0001-02 PSL* and ANR-10-LABX-0087) and Assistance Publique Hôpitaux de Paris–Centre National de la Recherche Scientifique. We thank the psychiatrists at Versailles hospital for their help in recruiting patients. We are also grateful to the members of the Laboratoire de Sciences Cognitives et Psycholinguistique for the recruitment of control participants and for their assistance with eye-tracking data collection and processing. The authors thank the Centre Hospitalier de Versailles for editorial assistance.
Footnotes
Competing interests: None declared.
Contributors: All authors designed the study. P. Roux acquired and analyzed the data, which E. Brunet-Gouet and F. Ramus also analyzed. E. Brunet-Gouet and F. Ramus wrote the article, which all authors reviewed and approved for publication.
References
- 1.Brüne M. “Theory of mind” in schizophrenia: a review of the literature. Schizophr Bull. 2005;31:21–42. doi: 10.1093/schbul/sbi002. [DOI] [PubMed] [Google Scholar]
- 2.Liepelt R, Schneider JC, Aichert DS, et al. Action blind: disturbed self-other integration in schizophrenia. Neuropsychologia. 2012;50:3775–80. doi: 10.1016/j.neuropsychologia.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 3.Zalla T, Verlut I, Franck N, et al. Perception of dynamic action in patients with schizophrenia. Psychiatry Res. 2004;128:39–51. doi: 10.1016/j.psychres.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 4.Koelkebeck K, Pedersen A, Suslow T, et al. Theory of mind in first-episode schizophrenia patients: correlations with cognition and personality traits. Schizophr Res. 2010;119:115–23. doi: 10.1016/j.schres.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 5.Bora E, Yucel M, Pantelis C. Theory of mind impairment in schizophrenia: meta-analysis. Schizophr Res. 2009;109:1–9. doi: 10.1016/j.schres.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 6.Langdon R, Ward PB, Coltheart M. Reasoning anomalies associated with delusions in schizophrenia. Schizophr Bull. 2010;36:321–30. doi: 10.1093/schbul/sbn069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fett AK, Viechtbauer W, Dominguez MD, et al. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci Biobehav Rev. 2011;35:573–88. doi: 10.1016/j.neubiorev.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Couture SM, Granholm EL, Fish SC. A path model investigation of neurocognition, theory of mind, social competence, negative symptoms and real-world functioning in schizophrenia. Schizophr Res. 2011;125:152–60. doi: 10.1016/j.schres.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung YS, Mathews JR, Barch DM. The effect of context processing on different aspects of social cognition in schizophrenia. Schizophr Bull. 2011;37:1048–56. doi: 10.1093/schbul/sbq012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green MJ, Waldron JH, Simpson I, et al. Visual processing of social context during mental state perception in schizophrenia. J Psychiatry Neurosci. 2008;33:34–42. [PMC free article] [PubMed] [Google Scholar]
- 11.Hardy-Baylé MC, Sarfati Y, Passerieux C. The cognitive basis of disorganization symptomatology in schizophrenia and its clinical correlates: toward a pathogenetic approach to disorganization. Schizophr Bull. 2003;29:459–71. doi: 10.1093/oxfordjournals.schbul.a007019. [DOI] [PubMed] [Google Scholar]
- 12.Brunet-Gouet E, Achim AM, Vistoli D, et al. The study of social cognition with neuroimaging methods as a means to explore future directions of deficit evaluation in schizophrenia? Psychiatry Res. 2011;190:23–31. doi: 10.1016/j.psychres.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 13.Marsh PJ, Luckett G, Russell T, et al. Effects of facial emotion recognition remediation on visual scanning of novel face stimuli. Schizophr Res. 2012;141:234–40. doi: 10.1016/j.schres.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Simpson C, Pinkham AE, Kelsven S, et al. Emotion recognition abilities across stimulus modalities in schizophrenia and the role of visual attention. Schizophr Res. 2013;151:102–6. doi: 10.1016/j.schres.2013.09.026. [DOI] [PubMed] [Google Scholar]
- 15.Zhu XL, Tan SP, Yang FD, et al. Visual scanning of emotional faces in schizophrenia. Neurosci Lett. 2013;552:46–51. doi: 10.1016/j.neulet.2013.07.046. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto Y, Takahashi H, Murai T, et al. Visual processing and social cognition in schizophrenia: relationships among eye movements, biological motion perception, and empathy. Neurosci Res. 2015;90:95–100. doi: 10.1016/j.neures.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 17.Roux P, Forgeot d’Arc B, Passerieux C, et al. Is the theory of mind deficit observed in visual paradigms in schizophrenia explained by an impaired attention toward gaze orientation? Schizophr Res. 2014;157:78–83. doi: 10.1016/j.schres.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 18.Roux P, Passerieux C, Ramus F. An eye-tracking investigation of intentional motion perception in patients with schizophrenia. J Psychiatry Neurosci. 2015;40:118–25. doi: 10.1503/jpn.140065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vistoli D, Passerieux C, El Zein M, et al. Characterizing an ERP correlate of intentions understanding using a sequential comic strips paradigm. Soc Neurosci. 2015;10:391–407. doi: 10.1080/17470919.2014.1003272. [DOI] [PubMed] [Google Scholar]
- 20.Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302:1181–5. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- 21.Chambon V, Franck N, Koechlin E, et al. The architecture of cognitive control in schizophrenia. Brain. 2008;131:962–70. doi: 10.1093/brain/awn032. [DOI] [PubMed] [Google Scholar]
- 22.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 23.Brunet E, Sarfati Y, Hardy-Bayle MC. Reasoning about physical causality and other’s intentions in schizophrenia. Cogn Neuropsychiatry. 2003;8:129–39. doi: 10.1080/13546800244000256. [DOI] [PubMed] [Google Scholar]
- 24.Vistoli D, Brunet-Gouet E, Lemoalle A, et al. Abnormal temporal and parietal magnetic activations during the early stages of theory of mind in schizophrenic patients. Soc Neurosci. 2011;6:316–26. doi: 10.1080/17470919.2010.530870. [DOI] [PubMed] [Google Scholar]
- 25.Brunet E, Sarfati Y, Hardy-Bayle MC, et al. A PET investigation of the attribution of intentions with a nonverbal task. Neuroimage. 2000;11:157–66. doi: 10.1006/nimg.1999.0525. [DOI] [PubMed] [Google Scholar]
- 26.Barch DM, Carter CS, Braver TS, et al. Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Arch Gen Psychiatry. 2001;58:280–8. doi: 10.1001/archpsyc.58.3.280. [DOI] [PubMed] [Google Scholar]
- 27.Andreasen NC, Pressler M, Nopoulos P, et al. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67:255–62. doi: 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bora E, Pantelis C. Theory of mind impairments in first-episode psychosis, individuals at ultra-high risk for psychosis and in first-degree relatives of schizophrenia: systematic review and meta-analysis. Schizophr Res. 2013;144:31–6. doi: 10.1016/j.schres.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 29.Kayser N, Sarfati Y, Besche C, et al. Elaboration of a rehabilitation method based on a pathogenetic hypothesis of “theory of mind” impairment in schizophrenia. Neuropsychol Rehabil. 2006;16:83–95. doi: 10.1080/09602010443000236. [DOI] [PubMed] [Google Scholar]
- 30.John CH, Hemsley DR. Gestalt perception in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 1992;241:215–21. doi: 10.1007/BF02190256. [DOI] [PubMed] [Google Scholar]
- 31.Moustafa AA, Keri S, Somlai Z, et al. Drift diffusion model of reward and punishment learning in schizophrenia: modeling and experimental data. Behav Brain Res. 2015;291:147–54. doi: 10.1016/j.bbr.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 32.Salgado-Pineda P, Fakra E, Delaveau P, et al. Differential patterns of initial and sustained responses in amygdala and cortical regions to emotional stimuli in schizophrenia patients and healthy participants. J Psychiatry Neurosci. 2010;35:41–8. doi: 10.1503/jpn.090017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Achával D, Villarreal MF, Costanzo EY, et al. Decreased activity in right-hemisphere structures involved in social cognition in siblings discordant for schizophrenia. Schizophr Res. 2012;134:171–9. doi: 10.1016/j.schres.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 34.Walter H, Ciaramidaro A, Adenzato M, et al. Dysfunction of the social brain in schizophrenia is modulated by intention type: an fMRI study. Soc Cogn Affect Neurosci. 2009;4:166–76. doi: 10.1093/scan/nsn047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anselmetti S, Bechi M, Bosia M, et al. ‘Theory’ of mind impairment in patients affected by schizophrenia and in their parents. Schizophr Res. 2009;115:278–85. doi: 10.1016/j.schres.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 36.Pedersen A, Koelkebeck K, Brandt M, et al. Theory of mind in patients with schizophrenia: Is mentalizing delayed? Schizophr Res. 2012;137:224–9. doi: 10.1016/j.schres.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 37.Davison PS, Frith CD, Harrison-Read PE, et al. Facial and other non-verbal communicative behaviour in chronic schizophrenia. Psychol Med. 1996;26:707–13. doi: 10.1017/s0033291700037727. [DOI] [PubMed] [Google Scholar]
- 38.Choi SH, Ku J, Han K, et al. Deficits in eye gaze during negative social interactions in patients with schizophrenia. J Nerv Ment Dis. 2010;198:829–35. doi: 10.1097/NMD.0b013e3181f97c0d. [DOI] [PubMed] [Google Scholar]
- 39.Delerue C, Laprevote V, Verfaillie K, et al. Gaze control during face exploration in schizophrenia. Neurosci Lett. 2010;482:245–9. doi: 10.1016/j.neulet.2010.07.048. [DOI] [PubMed] [Google Scholar]
- 40.Green MJ, Williams LM, Davidson D. Visual scanpaths to threat-related faces in deluded schizophrenia. Psychiatry Res. 2003;119:271–85. doi: 10.1016/s0165-1781(03)00129-x. [DOI] [PubMed] [Google Scholar]
- 41.Loughland CM, Williams LM, Gordon E. Schizophrenia and affective disorder show different visual scanning behavior for faces: a trait versus state-based distinction? Biol Psychiatry. 2002;52:338–48. doi: 10.1016/s0006-3223(02)01356-2. [DOI] [PubMed] [Google Scholar]
- 42.Butterfill SA, Apperly IA. How to construct a minimal theory of mind. Mind Lang. 2013;28:606–37. [Google Scholar]
- 43.Schneider D, Bayliss AP, Becker SI, et al. Eye movements reveal sustained implicit processing of others’ mental states. J Exp Psychol Gen. 2012;141:433–8. doi: 10.1037/a0025458. [DOI] [PubMed] [Google Scholar]
- 44.Kovács AM, Teglas E, Endress AD. The social sense: susceptibility to others’ beliefs in human infants and adults. Science. 2010;330:1830–4. doi: 10.1126/science.1190792. [DOI] [PubMed] [Google Scholar]
- 45.Peyroux E, Franck N. RC2S: A cognitive remediation program to improve social cognition in schizophrenia and related disorders. Front Hum Neurosci. 2014;8:400. doi: 10.3389/fnhum.2014.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bechi M, Riccaboni R, Ali S, et al. Theory of mind and emotion processing training for patients with schizophrenia: preliminary findings. Psychiatry Res. 2012;198:371–7. doi: 10.1016/j.psychres.2012.02.004. [DOI] [PubMed] [Google Scholar]