Abstract
Purpose
To identify null mutations in novel genes associated with early-onset high myopia using whole exome sequencing.
Methods
Null mutations, including homozygous and compound heterozygous truncations, were selected from whole exome sequencing data for 298 probands with early-onset high myopia. These data were compared with those of 507 probands with other forms of eye diseases. Null mutations specific to early-onset high myopia were considered potential candidates. Candidate mutations were confirmed with Sanger sequencing and were subsequently evaluated in available family members and 480 healthy controls.
Results
A homozygous frameshift mutation (c.39dup; p.L14Afs*21) and a compound heterozygous frameshift mutation (c.39dup; p.L14Afs*21 and c.594delG; p.Q199Kfs*35) in LOXL3 were separately identified in two of the 298 probands with early-onset high myopia. These mutations were confirmed with Sanger sequencing and were not detected in 1,974 alleles of the controls from the same region (507 individuals with other conditions and 480 healthy control individuals). These two probands were singleton cases, and their parents had only heterozygous mutations. A homozygous missense mutation in LOXL3 was recently reported in a consanguineous family with Stickler syndrome.
Conclusions
Our results suggest that null mutations in LOXL3 are likely associated with autosomal recessive early-onset high myopia. LOXL3 is a potential candidate gene for high myopia, but this possibility should be confirmed in additional studies. LOXL3 null mutations in human beings are not lethal, providing a phenotype contrary to that in mice.
Introduction
In the human genome, null mutations in protein-coding genes can lead to a wide range of phenotypic effects, ranging from invisible to severe phenotypes [1]. Null mutations are typically responsible for recessive diseases such as Duchenne muscular dystrophy [2] and erythropoietic protoporphyria [3] in which approximately 70% to 80% of the detected mutations are null mutations. Analyzing null mutations specific to certain diseases among the millions of genomic variants captured by whole exome sequencing is crucial for the identification of novel disease genes.
We previously analyzed several known myopia genes and myopia-associated genes based on whole exome sequencing data obtained from samples of 298 probands with early-onset high myopia (eoHM) [4,5]. However, we identified one null mutation in one proband that was associated with high myopia as well as other variants in a small proportion of probands, which had undetermined pathogenicity [4,5]. The cause of the remaining majority of this cohort is unknown. These findings suggest that variants in novel genes might cause this disease. Therefore, in the current study, our aim was to identify null mutations in novel genes associated with eoHM using whole exome sequencing.
Methods
Subjects
This study is part of a project established to investigate genetic defects associated with eoHM. Our aim was to identify novel genes responsible for eoHM using the same eoHM cohort that was used in our previous study [5]. Briefly, probands were recruited from the clinic at the Zhongshan Ophthalmic Center according to the following inclusion criteria: 1) spherical refraction in each meridian of ≤–6.00 D in both eyes, 2) development of high myopia before the age of 7 years, and 3) no other known ocular or related systemic diseases. The 507 controls were unrelated probands with genetic eye diseases other than myopia, including retinal degeneration and glaucoma. The 480 healthy controls had bilateral refraction of between −0.50 and +1.0 D spherical equivalents without a family history of high myopia and had a best unaided visual acuity of 1.0 or better without another known eye or systemic disease. Written informed consent was obtained from the participants or their guardians following the tenets of the Declaration of Helsinki. This study was approved by the Institutional Review Board of the Zhongshan Ophthalmic Center.
Sequencing analysis
Whole exome sequencing was performed with an Agilent SureSelect Human All Exon Enrichment Kit V4 array (51,189,318 bp; Agilent, Santa Clara, CA) that covered more than 20,000 genes (approximately 334,000 exons). DNA fragments were sequenced using an Illumina HiSeq 2000 system (Illumina, San Diego, CA). The average sequencing depth was 125-fold. Reads were mapped against UCSC hg19 (GenomeUCSC) using Burrows-Wheeler Aligner (BWA). The parameters used for whole exome sequencing have been previously described [5].
Null mutations, including homozygous and compound heterozygous truncation variants, were selected from whole exome sequencing data on the 298 probands with eoHM. These data were compared with those of the 507 probands with other forms of eye disease. Null mutations specific to eoHM were considered potential candidates. The minor allele frequency of each variant was obtained from public databases, including dbSNP, 1000 Genomes, Exome Variation Server, and Exome Aggregation Consortium (EXAC). Null variants with a minor allele frequency of >0.01 were excluded, and the remaining variants were further confirmed using Sanger sequencing and subsequently validated in available family members and 480 healthy controls. Primers were designed using the Primer3 online tool and are listed in Appendix 1. The methods used to perform Sanger sequencing, including amplification, sequencing, and analysis of the target fragments, have been previously described [4]. The variants are described according to the Human Genome Variation Society (HGVS).
Results
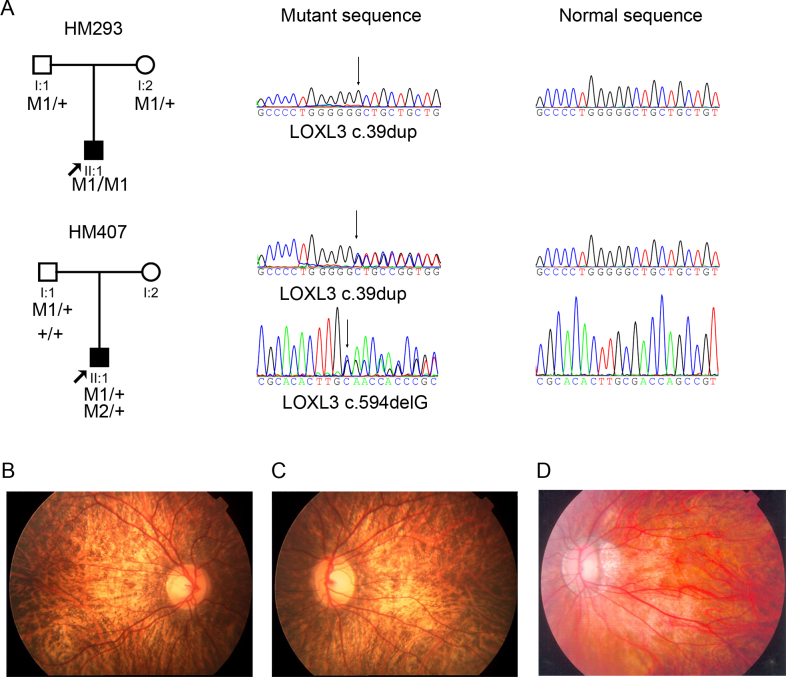
Evaluation of the whole exome sequencing data on 298 probands with eoHM revealed the presence of millions of variants targeting approximately 20,000 genes and null mutations in a few genes, LRPAP1 (Gene ID 4043; OMIM 104225) and LOXL3 (Gene ID 84695; OMIM 607163), that appeared to associate with high myopia after a series of bioinformatic filters. Null mutations in LRPAP1 have been associated with high myopia in humans [6]. Previously, we identified an additional null mutation (c.199delC) in LRPAP1 in a consanguineous family that has been reported in our previous study of known myopia genes [4]. Here, the null mutations detected in LOXL3 included a homozygous frameshift mutation (c.39dup; p.L14Afs*21) and a compound heterozygous frameshift variant (c39dup; p.L14Afs*21 and c.594delG; p.Q199Kfs*35), which were identified in two of the 298 probands with eoHM (Table 1, Figure 1A). These mutations in LOXL3 were confirmed with Sanger sequencing and were absent in 1,974 alleles of ethnicity-matched controls from the same region (507 individuals with other conditions and 480 healthy control individuals; Table 1). These null mutations were also not present in the 1000 Genomes, Exome Variant Server, and Exome Aggregation Consortium databases. The two probands were singleton cases, and their parents carried only heterozygous mutations (Figure 1A). These null mutations in LOXL3 were predicted to result in degradation of the transcript by nonsense-mediated mRNA decay [7,8]. The mutation frequencies and spectra in different types of variants of LOXL3 are shown in Appendix 2. Other less likely pathogenic heterozygous variants in LOXL3 are listed in Appendix 3.
Table 1. LOXL3 mutations identified in families with early-onset high myopia.
| Family | Exon | Position | DNA change | Protein change | Status | Co-segregation | Note | Allele frequency |
||
|---|---|---|---|---|---|---|---|---|---|---|
| Normal control | Others§ | Databases# | ||||||||
| HM293 |
E2 |
74779723 |
c.39dup |
p.L14Afs*21 |
Homo |
Yes |
Novel |
0/960 |
0/1014 |
None |
| HM407 |
E2 |
74779723 |
c.39dup |
p.L14Afs*21 |
Hetero |
Yes |
Novel |
0/960 |
0/1014 |
None |
| HM407 | E4 | 74776594 | c.594delG | p.Q199Kfs*35 | Hetero | Yes | Novel | 0/960 | 0/1014 | None |
Note: Homo, homozygous; Hetero, heterozygous. §, Samples from patients with other eye diseases, including glaucoma and retinal degeneration. #, Databases including 1000Genomes, Exome Variant Server, dbSNP, and Exome Aggregation Consortium.
Figure 1.
Null mutations in LOXL3 identified in two probands with early-onset high myopia. A: Sequence chromatography and pedigrees of HM293 and HM407. Sequence changes detected in the patients with early-onset high myopia are presented in the left column, whereas healthy sequences appear in the right column. The sample from the mother in family HM407 was not available. M1, c.39dup; M2, c.594delG; +, wild-type. B, C, D: Fundus photos for both eyes of HM293II1 (B, C) and the left eye of HM427II1 (D) revealed myopic fundus with crescent and tigroid forms. The fundus photo for the right eye of HM427II1 is not available.
The two probands with a LOXL3 mutation developed high myopia before reaching 7 years of age. One proband had a refractive error of −18.50 DS for the right eye and −18.00 DS for the left eye, and the other had a refractive error of −23.00 DS for the left eye and retinal detachment in the right eye (Table 2). Examination with an ophthalmoscope revealed myopic fundus with crescent and tigroid forms in the two probands (Figure 1B-D).
Table 2. Clinical information for patients with LOXL3 mutations.
| Patient | Gender | Age at |
First |
BCVA |
Refraction |
Axial length (mm) |
Fundus |
|
|---|---|---|---|---|---|---|---|---|
| exam (years) | synptom | right/left | right | left | right/left | right/left | ||
| HM293II1 |
Male |
3 |
PV |
NA/NA |
−18.50DS-1.25DC |
−18.00DS-2.00DC |
NA/NA |
Myopic/Myopic |
| HM407II1 | Male | 15 | PV | HM/0.04 | NA# | −23.00DS-3.50DC | 27.15/33.58 | RD/Myopic |
Note: PV, poor vision; NA, not available; HM, hand move; RD, retinal detachment; #, refraction was not available due to retinal detachment.
Discussion
In the current study, we revealed homozygous frameshift (c.39dup, p.L14Afs*21) and compound heterozygous frameshift (c.39dup, p.L14Afs*21; c.594delG, p.Q199Kfs*35) mutations in LOXL3 in two of the 298 probands with eoHM. These null mutations cosegregated with high myopia and were absent in the 1,974 alleles of the controls.
High myopia is a leading cause of visual impairment worldwide. Several lines of evidence indicated that excess elongation of eye size and axial length is due to abnormal extracellular matrix (ECM) remodeling in the sclera mediated by the transforming growth factor (TGF)-beta pathway [9-14]. LOXL3, a member of the lysyl oxidase gene family, encodes an extracellular copper-dependent amine oxidase. Loxl3 expression is enriched in the retina and the central nervous system [15,16]. The encoded protein is induced through the TGF-beta pathway [17,18] and plays a critical role in the covalent cross-linking of collagen and elastin in the ECM, which is essential for ECM integrity in connective tissues [15,16,19-21].
Abnormal LOXL3 function has been reported to be the cause of multiple types of defects in humans as well as in animals. A recent study identified a homozygous missense mutation (c.2027G>A, p.C676Y) in exon 12 of the LOXL3 gene as the cause of autosomal recessive Stickler syndrome in a consanguineous family [22], with high myopia a constant feature in this family [22]. This missense mutation was located in an evolutionarily conserved region and was predicted to be pathogenic [22]. In animal studies, knockdown of lox3b in zebrafish led to craniofacial abnormalities [21], and Loxl3−/− mice demonstrated craniofacial and spinal defects and smaller lungs at the embryonic stage (E18.5) [23]. The structure and axial length of the eyes in Loxl3- knockout mice were hard to determine, as all the knockout mice showed perinatal lethality [23]. We have generated a heterozygous Loxl3-knockout mouse model but we were unable to get any homozygous Loxl3-knockout mouse (unpublished data), also suggesting embryonic lethal in mice on complete absence of Loxl3. In the current study, the two unrelated patients with null mutations in LOXL3 exhibited high myopia without other known ocular or related systemic diseases, representing milder phenotypes than observed in previous studies in zebrafish or mouse [21,23]. Although the mechanism by which different LOXL3 mutations cause variable phenotypes is unclear, high myopia is a common symptom present in syndromic diseases such as congenital night blindness, caused by mutations in NYX [24], and Bornholm eye disease, caused by OPN1LW [25]. Mutations in NYX and OPN1LW have also been reported to cause high myopia alone due to mutations in different locations [26-28]. The null mutations in LOXL3 were determined to be located in exons 2 and 4, which is distinct from the location of the previously reported mutation in Stickler syndrome. Because of the lack of follow-up visits to further confirm the phenotypic information, we can only assume that mutations located in different locations in LOXL3 might have independent effects on patient phenotypes.
In conclusion, our results reveal the presence of null mutations in LOXL3 in families with eoHM. Due to limited phenotypic information and a lack of functional studies, these findings only indicate that null mutations in LOXL3 are likely to be associated with autosomal recessive eoHM. Meanwhile, our current approach may miss other types of variants if they are associated with high myopia. Our upcoming study will be designed to solve this issue by examining all other variants across the whole exome between cases and controls. The molecular mechanism underlying the role of LOXL3 in high myopia, as well as in Stickler syndrome, will be the subject of further study.
Acknowledgments
The authors thank all of the patients and controls for their participation in this study. Supported by the National Natural Science Foundation of China (U1201221), the Natural Science Foundation of Guangdong (S2013030012978), and the Fundamental Research Funds of the State Key Laboratory of Ophthalmology.
Appendix 1. Primers used for polymerase chain reaction
To access the data, click or select the words “Appendix 1.”
Appendix 2. The variant frequencies and the proportion of variants types of LOXL3 in the 298 patients with early-onset high myopia.
To access the data, click or select the words “Appendix 2.” In this study, 1.68% (5/298) of patients with eoHM harbored variants in LOXL3, in which 0.67% (2/298) of patients carried two null variants, 0.67% (2/298) of patients carried two heterozygous missense variants, and 0.34% (1/298) of patients carried one splicing change.
Appendix 3. Rare variants identified in 298 patients with eoHM and 507 controls
To access the data, click or select the words “Appendix 3.” Note: NA, not available; Hetero, heterozygous; §, Samples from patients with other eye diseases, including glaucoma and retinal degeneration; #, Databases including 1000Genomes, Exome Variant Server, dbSNP, and Exome Aggregation Consortium. ⱡ, Allele frequency found in Exome Aggregation Consortium database but not found in any other databases.
References
- 1.MacArthur DG, Balasubramanian S, Frankish A, Huang N, Morris J, Walter K, Jostins L, Habegger L, Pickrell JK, Montgomery SB, Albers CA, Zhang ZD, Conrad DF, Lunter G, Zheng H, Ayub Q, DePristo MA, Banks E, Hu M, Handsaker RE, Rosenfeld JA, Fromer M, Jin M, Mu XJ, Khurana E, Ye K, Kay M, Saunders GI, Suner MM, Hunt T, Barnes IH, Amid C, Carvalho-Silva DR, Bignell AH, Snow C, Yngvadottir B, Bumpstead S, Cooper DN, Xue Y, Romero IG. Genomes Project C, Wang J, Li Y, Gibbs RA, McCarroll SA, Dermitzakis ET, Pritchard JK, Barrett JC, Harrow J, Hurles ME, Gerstein MB, Tyler-Smith C. A systematic survey of loss-of-function variants in human protein-coding genes. Science. 2012;335:823–8. doi: 10.1126/science.1215040. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22344438&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tuffery-Giraud S, Beroud C, Leturcq F, Yaou RB, Hamroun D, Michel-Calemard L, Moizard MP, Bernard R, Cossee M, Boisseau P, Blayau M, Creveaux I, Guiochon-Mantel A, de Martinville B, Philippe C, Monnier N, Bieth E, Khau Van Kien P, Desmet FO, Humbertclaude V, Kaplan JC, Chelly J, Claustres M. Genotype-phenotype analysis in 2,405 patients with a dystrophinopathy using the UMD-DMD database: a model of nationwide knowledgebase. Hum Mutat. 2009;30:934–45. doi: 10.1002/humu.20976. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19367636&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 3.Parera VE, Koole RH, Minderman G, Edixhoven A, Rossetti MV, Batlle A, de Rooij FW. Novel null-allele mutations and genotype-phenotype correlation in Argentinean patients with erythropoietic protoporphyria. Mol Med. 2009;15:425–31. doi: 10.2119/molmed.2009.00006. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19693296&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang D, Li J, Xiao X, Li S, Jia X, Sun W, Guo X, Zhang Q. Detection of Mutations in LRPAP1, CTSH, LEPREL1, ZNF644, SLC39A5, and SCO2 in 298 Families With Early-Onset High Myopia by Exome Sequencing. Invest Ophthalmol Vis Sci. 2015;56:339–45. doi: 10.1167/iovs.14-14850. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25525168&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 5.Li J, Jiang D, Xiao X, Li S, Jia X, Sun W, Guo X, Zhang Q. Evaluation of 12 myopia-associated genes in Chinese patients with high myopia. Invest Ophthalmol Vis Sci. 2015;56:722–9. doi: 10.1167/iovs.14-14880. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25587058&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 6.Aldahmesh MA, Khan AO, Alkuraya H, Adly N, Anazi S, Al-Saleh AA, Mohamed JY, Hijazi H, Prabakaran S, Tacke M, Al-Khrashi A, Hashem M, Reinheckel T, Assiri A, Alkuraya FS. Mutations in LRPAP1 are associated with severe myopia in humans. Am J Hum Genet. 2013;93:313–20. doi: 10.1016/j.ajhg.2013.06.002. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23830514&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagy E, Maquat LE. A rule for termination-codon position within intron-containing genes: when nonsense affects RNA abundance. Trends Biochem Sci. 1998;23:198–9. doi: 10.1016/s0968-0004(98)01208-0. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=9644970&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 8.Karam R, Wengrod J, Gardner LB, Wilkinson MF. Regulation of nonsense-mediated mRNA decay: implications for physiology and disease. Biochim Biophys Acta. 2013;xxx:624–33. doi: 10.1016/j.bbagrm.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McBrien NA. Regulation of scleral metabolism in myopia and the role of transforming growth factor-beta. Exp Eye Res. 2013;114:128–40. doi: 10.1016/j.exer.2013.01.014. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23399866&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 10.Chen BY, Wang CY, Chen WY, Ma JX. Altered TGF-beta2 and bFGF expression in scleral desmocytes from an experimentally-induced myopia guinea pig model. Graefes Arch Clin Exp Ophthalmol. 2013;251:1133–44. doi: 10.1007/s00417-013-2269-8. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23381656&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 11.Jobling AI, Wan R, Gentle A, Bui BV, McBrien NA. Retinal and choroidal TGF-beta in the tree shrew model of myopia: isoform expression, activation and effects on function. Exp Eye Res. 2009;88:458–66. doi: 10.1016/j.exer.2008.10.022. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19046968&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 12.Seko Y, Shimokawa H, Tokoro T. Expression of bFGF and TGF-beta 2 in experimental myopia in chicks. Invest Ophthalmol Vis Sci. 1995;36:1183–7. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=7730028&dopt=Abstract [PubMed] [Google Scholar]
- 13.Harper AR, Summers JA. The dynamic sclera: extracellular matrix remodeling in normal ocular growth and myopia development. Exp Eye Res. 2015;133:100–11. doi: 10.1016/j.exer.2014.07.015. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25819458&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McBrien NA, Gentle A. Role of the sclera in the development and pathological complications of myopia. Prog Retin Eye Res. 2003;22:307–38. doi: 10.1016/s1350-9462(02)00063-0. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12852489&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 15.Coral K, Angayarkanni N, Madhavan J, Bharathselvi M, Ramakrishnan S, Nandi K, Rishi P, Kasinathan N, Krishnakumar S. Lysyl oxidase activity in the ocular tissues and the role of LOX in proliferative diabetic retinopathy and rhegmatogenous retinal detachment. Invest Ophthalmol Vis Sci. 2008;49:4746–52. doi: 10.1167/iovs.07-1550. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18566459&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 16.Jourdan-Le Saux C, Tomsche A, Ujfalusi A, Jia L, Csiszar K. Central nervous system, uterus, heart, and leukocyte expression of the LOXL3 gene, encoding a novel lysyl oxidase-like protein. Genomics. 2001;74:211–8. doi: 10.1006/geno.2001.6545. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11386757&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 17.Sethi A, Wordinger RJ, Clark AF. Gremlin utilizes canonical and non-canonical TGFbeta signaling to induce lysyl oxidase (LOX) genes in human trabecular meshwork cells. Exp Eye Res. 2013;113:117–27. doi: 10.1016/j.exer.2013.05.011. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23748100&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sethi A, Mao W, Wordinger RJ, Clark AF. Transforming growth factor-beta induces extracellular matrix protein cross-linking lysyl oxidase (LOX) genes in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2011;52:5240–50. doi: 10.1167/iovs.11-7287. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21546528&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang Y, Dai J, Tang R, Zhao W, Zhou Z, Wang W, Ying K, Xie Y, Mao Y. Cloning and characterization of a human lysyl oxidase-like 3 gene (hLOXL3). Matrix Biol. 2001;20:153–7. doi: 10.1016/s0945-053x(01)00124-x. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11334717&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 20.Lucero HA, Kagan HM. Lysyl oxidase: an oxidative enzyme and effector of cell function. Cell Mol Life Sci. 2006;63:2304–16. doi: 10.1007/s00018-006-6149-9. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16909208&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Boxtel AL, Gansner JM, Hakvoort HW, Snell H, Legler J, Gitlin JD. Lysyl oxidase-like 3b is critical for cartilage maturation during zebrafish craniofacial development. Matrix Biol. 2011;30:178–87. doi: 10.1016/j.matbio.2010.12.002. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21244857&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alzahrani F, Al Hazzaa SA, Tayeb H, Alkuraya FS. LOXL3, encoding lysyl oxidase-like 3, is mutated in a family with autosomal recessive Stickler syndrome. Hum Genet. 2015;134:451–3. doi: 10.1007/s00439-015-1531-z. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25663169&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Yang R, Liu Z, Hou C, Zong W, Zhang A, Sun X, Gao J. Loss of lysyl oxidase-like 3 causes cleft palate and spinal deformity in mice. Hum Mol Genet. 2015;24:6174–85. doi: 10.1093/hmg/ddv333. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26307084&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeitz C, Robson AG, Audo I. Congenital stationary night blindness: an analysis and update of genotype-phenotype correlations and pathogenic mechanisms. Prog Retin Eye Res. 2015;45:58–110. doi: 10.1016/j.preteyeres.2014.09.001. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25307992&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 25.McClements M, Davies WI, Michaelides M, Young T, Neitz M, MacLaren RE, Moore AT, Hunt DM. Variations in opsin coding sequences cause x–linked cone dysfunction syndrome with myopia and dichromacy. Invest Ophthalmol Vis Sci. 2013;54:1361–9. doi: 10.1167/iovs.12-11156. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23322568&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Q, Xiao X, Li S, Jia X, Yang Z, Huang S, Caruso RC, Guan T, Sergeev Y, Guo X, Hejtmancik JF. Mutations in NYX of individuals with high myopia, but without night blindness. Mol Vis. 2007;13:330–6. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17392683&dopt=Abstract [PMC free article] [PubMed] [Google Scholar]
- 27.Yip SP, Li CC, Yiu WC, Hung WH, Lam WW, Lai MC, Ng PW, Fung WY, Chu PH, Jiang B, Chan HH, Yap MK. A novel missense mutation in the NYX gene associated with high myopia. Ophthalmic Physiol Opt. 2013;33:346–53. doi: 10.1111/opo.12036. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23406521&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 28.Li J, Gao B, Guan L, Xiao X, Zhang J, Li S, Jiang H, Jia X, Yang J, Guo X, Yin Y, Wang J, Zhang Q. Unique Variants in OPN1LW Cause Both Syndromic and Nonsyndromic X–Linked High Myopia Mapped to MYP1. Invest Ophthalmol Vis Sci. 2015;56:4150–5. doi: 10.1167/iovs.14-16356. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26114493&dopt=Abstract [DOI] [PubMed] [Google Scholar]