Abstract
Purpose
To identify the lineage that contributes to the morphogenesis of the meibomian gland.
Methods
To examine which cell lineage gives rise to the meibomian gland, the expression of Pax6 as well as that of various cytokeratin markers, including keratin 14 (Krt14), Krt15, Krt4, and Krt10, was examined with immunofluorescent staining of C57BL/6J mouse eyelids from P2 to P11 pups and adult mice.
Results
Pax6 was localized to the cytoplasm within the acinar region of the meibomian glands during morphogenesis but was absent in the fully developed gland. Keratin 14 was expressed throughout the gland at all stages whereas keratin 15 was absent at all stages. Keratin 4, a marker of mucosal lineage, was present throughout the gland and was colocalized with keratin 10 (epidermal lineage marker) in the developing duct at P4. This colocalization region decreased as the gland developed becoming restricted to the central duct near the opening to the acini in the fully developed gland.
Conclusions
We identified a unique cell lineage that expresses markers characteristic of mucosal and epidermal epithelia during meibomian gland morphogenesis. This unique group of cells was located in the central duct with a concentration near the ductule orifice. The expression of these cells reduced during meibomian gland morphogenesis and may play a role in the development and homeostasis of the gland.
Introduction
The tear film is a critical component of the eye needed to maintain proper eye health. Compromise of this layer can result in dry eye disease, which currently inflicts approximately 30 million people in the United States alone [1,2]. The tear film is composed of three layers. The innermost layer, closest to the anterior surface of the cornea, is the mucous layer that is produced from goblet cells located in the conjunctiva and membrane mucins from the cornea and the conjunctiva. The second layer is the aqueous layer, which is the largest layer of the tear film and is produced by the lacrimal gland. The outermost layer is the lipid layer, which is produced by the meibomian glands. The lipid layer aids in protecting the eye from environmental insults and preventing the aqueous layer from evaporating [3,4]. In the past, dry eye disease was thought to be primarily a result of an aqueous layer deficiency; however, evidence suggests that 76% to 86% of dry eye cases are actually a result of a deficiency in the lipid layer [5-7].
The meibomian glands develop while the eyelids are closed, at which time the lid mesoderm differentiates into the tarsal plate, orbicularis and Riolan’s muscles, blood vessels, and connective tissue. The glands themselves develop from the ectodermal sheet sealing the eyelids [8-10]. Inside the meibomian anlage, lipids are produced that eventually form the central duct. Extending from the central duct are numerous secretory acini that are connected via small ductules. The entire meibomian gland ductile system is lined by stratified squamous epithelial cells [11,12]. The secretory acini are modified sebaceous glands that secrete in a holocrine fashion. The cells lining the base of each acinus, termed meibocytes, accumulate lipids and eventually shrink and disintegrate releasing the lipid and whole cell content (meibum) into the central duct [13-15].
The terminal portion of the central duct is lined by an ingrowth of epidermis forming an excretory duct that opens at the free lid margin anterior to the mucocutaneous junction (MCJ). The MCJ is a region of the eye in which the mucosa of the conjunctiva transitions to the epidermis of the skin. As stated earlier, more than 77% of the cases of dry eye are a result of a deficiency in the lipid layer of the tear film. This often results from what has been termed meibomian gland dysfunction (MGD) [16]. Meibomian gland dysfunction is defined as a chronic, diffuse abnormality of the meibomian glands characterized by terminal duct obstruction and/or qualitative or quantitative changes in secretion [17].
To characterize the morphogenesis of the meibomian gland, a panel of cytokeratin markers was used. Using a panel of cytokeratin markers to identify various tissue lineages has been done in the past on the adult mouse and primate tissue to characterize the mucocutaneous junction [18,19]. It has largely been thought that the meibomian gland is derived from an ingrowth of epidermal tissue; however, these studies focused largely on the study of adult tissue [16-19]. We hypothesized that the gland may arise from a lineage of cells from an epidermal and a mucosal lineage. In this study, we examined mouse eyelids during morphogenesis of the meibomian gland examining contribution from the mucosa, e.g., conjunctiva (keratin 4, Krt4) and epidermal lineage (keratin 10, Krt10) through the use of cytokeratin markers. Our observations suggest that the meibomian gland is derived from a population of surface ectoderm cells that simultaneously expresses Krt4 and Krt10.
Methods
Eyelid isolation
Animal care and use conformed to the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research. All animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Cincinnati and adhered to the guidelines established in the Guide for the Care and Use of Laboratory Animals. Tissue was harvested from C57BL/6J mice (The Jackson Laboratory stock #000664; Bar Harbor, ME) at P2, P4, P7, P9, P11, and adult (8 weeks old). Upper and lower eyelids were dissected from the mice and fixed in 4% paraformaldehyde in 0.1 M phosphate buffer, pH7.4 at 4 °C overnight. Following fixation, the eyelids were washed extensively in PBS (1X: 130 mM NaCl, 2.6 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.4) before proceeding with the staining.
Histology and immunofluorescence
Ten-micrometer cryosections were washed twice in PBS containing 0.15% glycine and 0.5% bovine serum albumin (BSA) for 5 min followed by three 5 min washes in PBS. Sections were blocked with 2% BSA at room temperature followed by incubation with the primary antibody in PBS with 0.03% Tween-20 (PBST) containing 0.5% BSA overnight at 4 °C. The primary antibodies used were as follows: Krt15 (1:500, Covance; Princeton, NJ), Krt14 (1:500, Covance), Pax6 (1:300, Covance), Krt4 (1:150, Novus Biologicals, LLC; Littleton, CO), Krt10 (1:500, Covance), and Ki67 (1:100, eBioscience, Inc.; San Diego, CA). Proteins were visualized with indirect immunofluorescence with appropriate secondary antibody conjugates containing AlexaFluor® 555, AlexaFluor® 488, or AlexaFluor®647 (Life Technologies; Grand Island, NY). All samples were counterstained with 4’6-diamidino-2-phenylindole (DAPI) for nuclei and observed using a Zeiss Observer Z1 microscope (Carl Zeiss; Oberkochen, Germany).
Sequential double and triple immunofluorescent staining
Ten-micrometer sections were prepared as above using the Krt4 and Ki67 (for triple immunofluorescent staining) primary antibodies followed by donkey anti-rabbit immunoglobulin G (IgG) AlexaFluor® 555 and donkey anti-rat IgG2a AlexaFluor®647 (triple staining only) secondary antibodies. This was followed by incubation with the Krt10 antibody, which was conjugated with AlexaFluor® 488 using the Alexa Fluor® 488 Protein Labeling Kit (Life Technologies; Grand Island, NY), overnight at 4 °C. Fifty-micrometer cryosections were washed in PBS containing 0.5% dimethyl sulfoxide (DMSO), 0.5% Triton X-100, and 2.5% Dextran 40 for 30 min at room temperature followed by three 5 min washes in PBS. Sections were blocked in 2% BSA at room temperature followed by sequential staining as described above. Thick (50 µm) sections were visualized using a Zeiss Observer Z1 microscope with an apotome attachment.
Image analysis
To determine the percentage of colocalization, the Coloc 2 plug-in for the Fiji software package was used. Ten randomly selected regions from three independent mouse eyelids (n = 6) were analyzed. Mander’s overlap coefficients were then determined. Mander’s overlap coefficient is a measure of colocalization and generates a number ranging from 0 to 1; 1 is high colocalization, and zero is low [20]. M1 is defined as the ratio of the “summed intensities of pixels from the green image for which the intensity in the red channel is above zero” to the “total intensity in the green channel.” M2 is defined conversely for red. Therefore, M1 (or M2) is a good indicator of the proportion of the green signal coincident with a signal in the red channel over its total intensity, which may apply even if the intensities in the two channels are distinct from one another [20,21].
Results
Expression of epithelial cell markers
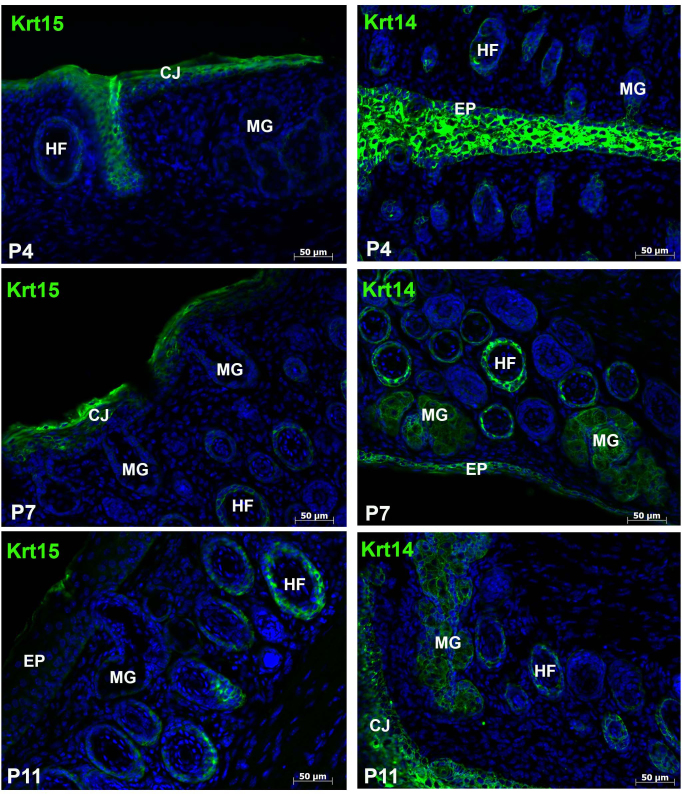
The search for meibomian gland stem cells and markers to define the development and cell type specificity of the mucocutaneous junction have used the expression of various cytokeratins. Two such keratins, Krt14 and Krt15, are type I keratins present in stratified epithelia and some progenitor basal cells within complex epithelia [22,23]. To gain a perspective on the expression of these keratins during meibomian gland development, we examined various postnatal stages of morphogenesis. As expected, Krt15 was expressed within the hair follicles as well as the conjunctiva at all stages analyzed (P4–P11) and was absent from all stages of meibomian gland morphogenesis (Figure 1). Krt14 was present in all stratified epithelia within the eyelid, including the conjunctiva, epidermis, hair follicles, and the acini and central duct of the meibomian gland (Figure 1).
Figure 1.
Expression of Krt14 but not Krt15 within the developing meibomian gland. Ten-micron horizontal cryosections of the eyelid were examined for keratin 15 (Krt15) and Krt14 expression. Krt15 was found within the conjunctiva as well as the hair follicles at P4, P7, and P11. As expected, Krt14 was expressed in all stratified epithelia, including the epidermis, conjunctiva, hair follicles, and epithelia lining the central duct and acini of the meibomian glands. MG = meibomian gland; HF = hair follicle; EP = epidermis; CJ = conjunctiva.
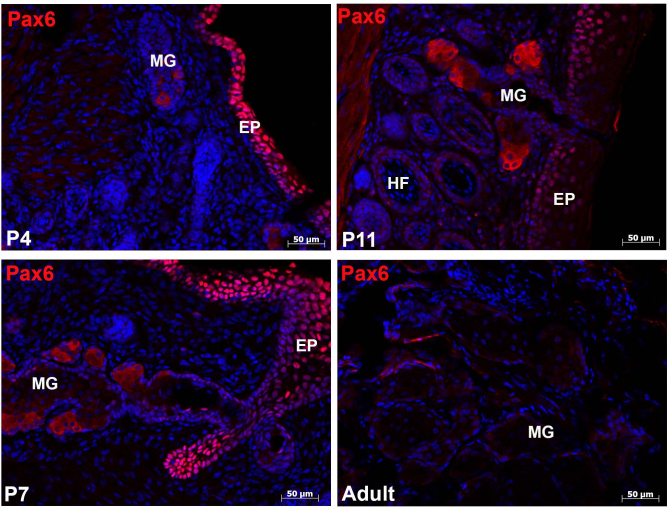
Pax6 is a transcription factor expressed by ectodermal tissue and plays a critical role in the development and morphogenesis of many ocular tissues. Cytoplasmic Pax6 expression was present in the acinar cells throughout all stages of meibomian gland morphogenesis, but this expression was significantly reduced in the adult meibomian glands (Figure 2).
Figure 2.
Differential expression of Pax6. Ten-micron horizontal cryosections of the eyelid were examined for Pax6 expression. The developing meibomian gland expressed cytoplasmic Pax6 as early as P4 in the elongating epithelial cord. At P7 and P11, expression was observed within the acinar region of the gland. Once the gland was fully developed (adult), Pax6 expression was significantly reduced within the acini. MG = meibomian gland; HF = hair follicle; EP = epidermis.
Colocalization of Krt4 and Krt10 within the central duct
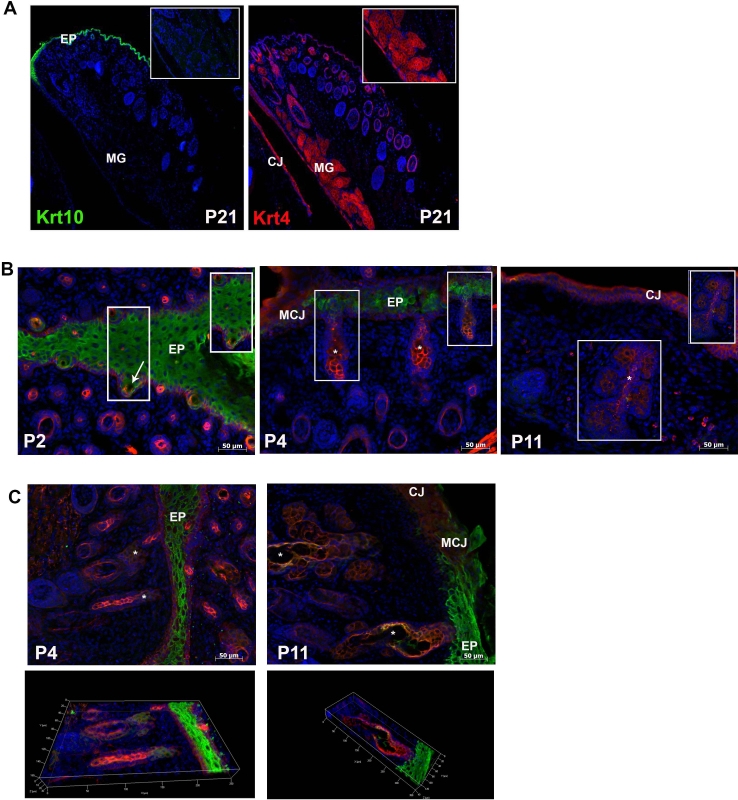
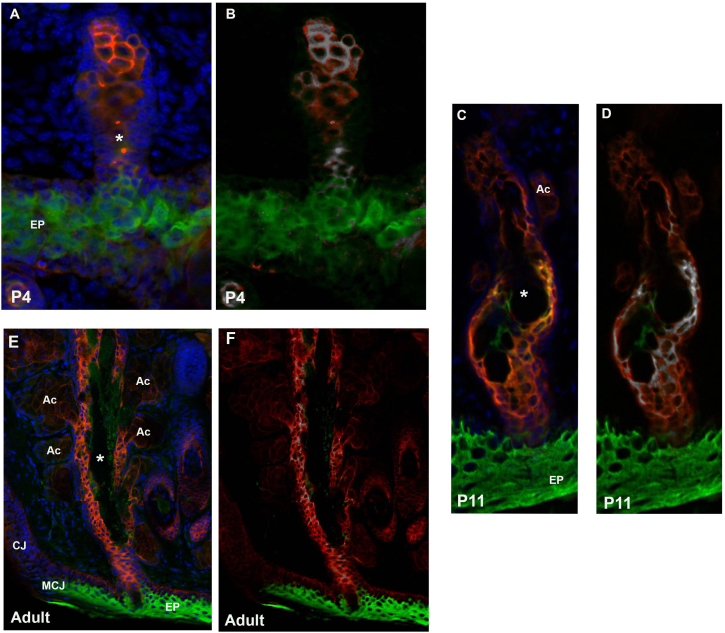
To examine more specifically the cell lineage giving rise to the meibomian gland and to expand on past research examining keratin expression only in the adult gland, we focused on two markers, Krt4 and Krt10, which stain mucosa epithelia and epidermal epithelia, respectively. In the fully developed meibomian gland (P21), Krt4 expression was present within the conjunctiva, hair follicles, and meibomian gland whereas Krt10 was restricted to the epidermis (Figure 3A). Given the exclusive nature of these two keratins in the fully developed meibomian gland, we set out to examine their expression pattern during development of the gland. Double immunofluorescent staining at P2 revealed expression of Krt4 and Krt10 within the elongated epidermal invagination (Figure 3B). Colocalization analysis using Fiji revealed that only 8% of the Krt10-positive cells colocalized with the Krt4-positive cells (Mander’s overlap coefficient: M1 = 0.1992; M2 = 0.0841). These cells were located at the base of the invaginating epidermis (Figure 3B). At P4, the meibomian gland continued to elongate with Krt10 expression predominantly in the epidermis and the invaginating central duct and distal region of the gland. Krt4 was expressed throughout the developing gland with regions of colocalization (Mander’s overlap coefficient: M1 = 0.993; M2 = 0.909) in the central canal as well as the distal gland region (Figure 3B–C, Figure 4A,B). By P11, Krt10 expression was restricted to the central duct and was absent from the acini. As in the previous two stages, Krt4 expression was present throughout the gland (Figure 3B–C). The Krt4 and Krt10 colocalization region was also restricted to the cells lining a portion of the central duct (Mander’s overlap coefficient: M1 = 0.981; M2 = 0.993; Figure 4C,D). The colocalization region became further restricted in the fully developed meibomian gland of an adult, with only a few cells expressing Krt4 and Krt10 (Mander’s overlap coefficient: M1 = 0.728; M2 = 0.732). The central duct of the adult meibomian gland expressed low levels of Krt10 with Krt4 expression being predominant. The small ductules and acini expressed Krt4 but lacked Krt10 expression (Figure 4E,F). To determine whether cells that expressed Krt4 and Krt10 were also undergoing proliferation, Ki67 immunostaining was performed on P9 and P11 eyelids. Ki67 staining was present in the cells surrounding the central duct and acini but was absent in the Krt4- and Krt10-positive cells (Figure 5).
Figure 3.
Colocalization of Krt4 and Krt10. A: Sagittal section of the eye immunostained for either keratin 10 (Krt10) or Krt4 in a P21 mouse. Note the distinct expression pattern of these two lineage markers; Krt4 was expressed in the meibomian gland (inset) and the conjunctiva, and Krt10 was restricted to the epidermis. B: Ten-micron horizontal cryosections double stained for Krt4 (red) and Krt10 (green). Inset shows the meibomian gland with a small colocalization region at P2 and a larger degree of colocalization at P4 in the developing duct. By P11, the areas of colocalization were reduced with only a small region present within the central duct. C: Fifty-micron sections double stained for Krt4 (red) and Krt10 (green) at P4 and P11 with a three-dimensional composite (bottom panel). MG = meibomian gland, EP = epidermis; MCJ = mucocutaneous junction; CJ = conjunctiva; asterisk = central duct; arrow = elongated epidermal invagination.
Figure 4.
A unique cell lineage expressing Krt4 and Krt10. Horizontal sections of eyelids double stained with keratin 4 (Krt4; red) and Krt10 (green) at P4 (A, B), P11 (C, D), and adult (E, F). Regions of colocalization were determined using the Coloc 2 plug-in for the Fiji software package. Colocalized regions are shown in white at P4 (B), P11 (D) and adult (F). At P4, colocalization of Krt4 and Krt10 is found in the developing central duct as well as the distal gland region. By P11, the colocalization region is restricted to the central duct and is absent from the acini. In the fully developed meibomian gland (adult), areas of colocalization are found within the central duct near the ductule openings. EP = epidermis; MCJ = mucocutaneous junction; CJ = conjunctiva; AC = acinus; asterisk = central duct.
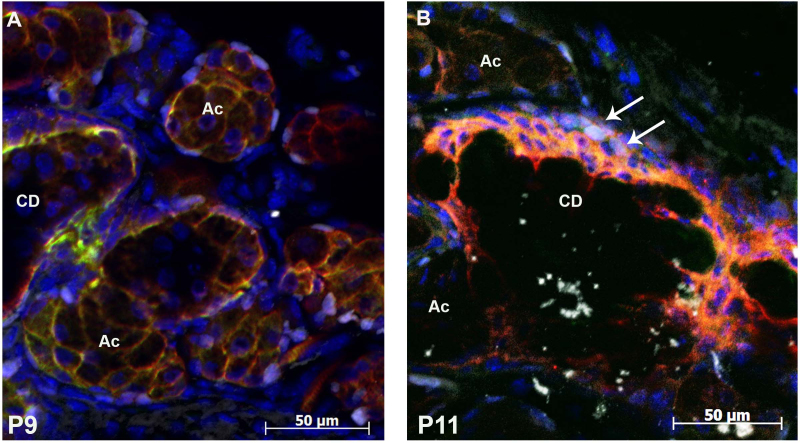
Figure 5.
Krt4- and Krt10-expressing cells do not proliferate. Ten-micron sections of eyelids were triple stained for Ki67 (white), keratin 4 (Krt4; red), and Krt10 (green) at P9 (A) and P11 (B). Ki67-positive cells were present in the cells surrounding the acini. A few Ki67-positive cells were also found outside the central duct (arrows). Ki67 was not present in the Krt4 and Krt10 cells. CD = central duct; Ac = acinus.
Discussion
In this paper, we used the expression pattern of epithelial cell markers during morphogenesis to gain insight into the cell lineage that gives rise to the mouse meibomian gland. In the mouse, the meibomian gland begins to develop at embryonic day 18.5 (E18.5) with the formation of epithelial condensations within the fused lid margin. At birth (P0), the epidermis begins to invaginate with progressive elongation through P4. By P5, early branching begins with distinct differentiation into ductal and acinar regions by P8. The gland develops an adult morphology by P15 [24]. With these morphological features previously characterized, we set out to examine the contribution from the epidermal and mucosal lineage to the developing gland.
We began by examining the expression of two keratin markers Krt15 and Krt14, which have been shown to be primitive cell markers for the hair follicle and epithelial tissue, respectively [22,23]. As of late, research has focused on characterizing cell markers that could be used to identify the location of a stem cell pool that could give rise to the conjunctiva and/or the meibomian gland. In the primate, the ductal epithelium located at the orifice and the MCJ serves as a reservoir for Krt14-positive cells. It was also found that the number of Krt14-positive cells decreases along the palpebral conjunctiva suggesting that progeny cells of the meibomian gland or the MCJ migrate toward the fornix [18]. We found Krt14 expression throughout the murine epithelia, in the conjunctiva and the meibomian gland, with no discernible differences noted at the various stages of morphogenesis. Krt15 was expressed in the conjunctiva and hair follicles but was absent from the meibomian gland at all stages examined. Krt15 expression has been observed in sebaceous glands of the sheep [25] as well as in adult human meibomian glands [26,27]. These differences raise the possibility that Krt15 expression is species-specific and/or age-dependent.
Expression of Pax6, a paired domain-homeobox transcription factor, was also examined given its role in the development of many ocular tissues, including the lens, retina, cornea, conjunctiva, and lacrimal gland [28,29]. Despite Pax6’s known role in these tissues, the role of Pax6 in the development of the meibomian gland has not yet been fully explored [30]. We found that Pax6 was differentially expressed in the meibomian gland, with expression seen during the morphogenesis of the gland but significantly reduced in the adult gland. Interestingly, Pax6 was localized to the cytoplasm within the acinar regions of the meibomian gland. This may provide some clues to its role in the morphogenesis of the meibomian gland. Previous research has indicated a role of cytoplasmic shuffling in the development of several tissue types. For example, it has been found that in Merkel cells, which are mechanoreceptors found throughout the vertebrate skin, Pax6 is primarily localized in the cytoplasm at birth but at postnatal stages is also present in the nuclei. This localization could be altered by changing the redox potential. Pax6 expression in Merkel cells is seen as a nucleocytoplasmic shuttling protein required for normal differentiation [31]. Additionally, Pax6 has been shown to undergo nucleocytoplasmic shuttling via interaction with Smad3 and secreted protein acidic and rich in cysteine (SPARC) in the murine eye [32]. Given this, it is unusual that Pax6 was never observed in the nucleus at the developmental time points analyzed, highlighting the need for future studies into the expression and function of Pax6 during the morphogenesis of the meibomian gland.
To identify the cell type responsible for giving rise to the meibomian gland, we focused on two cytokeratins that allow for the demarcation of the MCJ: Krt4, which is specific to mucosal (conjunctival) epithelia, and Krt10, which is specific for the epidermal epithelia. Unlike previous reports that examined cytokeratin expression in the adult meibomian gland, which suggest a contribution solely from the epidermal epithelia, we found that mucosal cells also contributed to the developing gland with staining of Krt10 and Krt4 present at P2 and P4. By P11, Krt10 expression was restricted to the central duct whereas Krt4 was seen throughout the gland in the central duct and the acini. Additionally, we identified a distinct population of cells that expresses Krt4 and Krt10. This population of cells was seen at P4 within the developing central duct and distal gland regions. As the gland continued to develop, the population of Krt4- and Krt10-expressing cells became limited to a small region lining the central duct (P11) and continued to become restricted, with only a few cells expressing both Krt10 and Krt4 in the adult gland. These cells were found within the central duct with a concentrated number of colocalized cells present at the base of the ductules. This region is similar in location to the small population of transient amplifying (TA) cells (Ki67+/Krt6+), which are thought to arise from a stem cell pool located in the periphery of the acini or in the central duct [33-35]. However, in this study we found that the population of cells expressing Krt4 and Krt10 was not Ki67+. As Krt4 and Krt10 are differentiation markers, this finding is not surprising. Given the proximity of these cells to the transient amplifying cells (Ki67+/Krt6+), it can be speculated that the TA cells give rise to this small population of Krt4 and Krt10 cells. More work is needed to determine the exact role of these cells during the morphogenesis of the meibomian gland.
In conclusion, we have found that during mouse meibomian gland morphogenesis there is a contribution from a unique cell lineage that expresses markers characteristic of mucosal and epidermal epithelia. As the gland matures, Krt4 expression is seen throughout the meibomian gland while Krt10 expression becomes restricted to the central duct. A small population of cells exist in the central duct with a concentration near the ductule orifice that coexpress both Krt4 and Krt10. To our knowledge, this is the first report of such a cell population that is a mucocutaneous cell population, and has features of mucosa and epidermal epithelia.
Acknowledgments
This work was supported in part by an Unrestricted Grant from Research to Prevent Blindness, Inc., New York, NY, to the Department of Ophthalmology, University of Cincinnati College of Medicine (Karl Golnik, MD, Chairman) and by NIH grant NIH/NEI R01EY013755 to WWYK.
References
- 1.Subcommittee of the International Dry Eye Workshop. The epidemiology of dry eye disease: report of the epidemiology subcommittee of the international dry eye workshop. Ocul Surf. 2007;5:93–107. doi: 10.1016/s1542-0124(12)70082-4. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17508117&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 2.Moss SE, Klein R, Klein BE. Prevalence of and risk factors for dry eye syndrome. Arch Ophthalmol. 2000;118:1264. doi: 10.1001/archopht.118.9.1264. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10980773&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 3.Green-Church KB, Butovich I, Wilcox M, Borchman D, Paulsen F, Barabino S, Glasgow BJ. The international workshop on meibomian gland dysfunction: report of the subcommittee on tear film lipids and lipid-protein interactions in health and disease. IOVS 2011; 1979–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Craig J, Tomlinson A. Importance of the lipid layer in human tear film stability and evaporation. Optom Vis Sci. 1997;74:8–13. doi: 10.1097/00006324-199701000-00014. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=9148269&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 5.Heiligenhaus A, Koch JM, Kemper D, Kruse FE, Waubke TN. [Therapy in tear film deficiencies]. Therapie von Benetzungsstӧrungen. Klin Monatsbl Augenheilkd. 1994;204:162–8. doi: 10.1055/s-2008-1035514. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=8196302&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 6.Shimazaki J, Sakata M, Tsubota K. Ocular surface changes and discomfort in patients with meibomian gland dysfunction. Arch Ophthalmol. 1995;113:1266–70. doi: 10.1001/archopht.1995.01100100054027. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=7575257&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 7.Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31:472–8. doi: 10.1097/ICO.0b013e318225415a. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22378109&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 8.Mann I. The development of the human eye. Cambridge UK: Cambridge University Press; 1928. [Google Scholar]
- 9.Barber AN. Embryology of the human eye St. Louis: C.V. Mosby Co.; 1955:193–195. [Google Scholar]
- 10.Andersen H, Ehlers N, Matthiessen Me. Histochemistry and development of the human eyelids. Acta Ophthalmol (Copenh) 1965;43:642–68. doi: 10.1111/j.1755-3768.1965.tb00335.x. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=4159407&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 11.Bron AJ, Tripathi DM, Tripathi BJ. Wolff’s anatomy of the eye and orbit. London: Chapman and Hall Medical 1997; 231. [Google Scholar]
- 12.Knop E, Knop N. [Meibomian glands. Part II. Physiology, characteristics, distribution and function of the meibomian oil] Meibom-drϋsen Teil II: Physiologie, Eigenschaften, Verteilung und Funktion des Meibom-ӧls. Ophthalmologe. 2009;106:884–92. doi: 10.1007/s00347-009-2019-9. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19856011&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 13.Nicoaides N, Kaitaranta JK, Rawdah TN, Macy JI, Boswell FM, Smith RE. Meibomian gland studies: comparison of steer and human lipids. IOVS. 1981;20:522–36. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=7194326&dopt=Abstract [PubMed] [Google Scholar]
- 14.Gorgas K. Vӧlkl A. Peroxisomes in sebaceous glands. IV. Aggregates of tubular peroxisomes in the mouse meibomian gland. Histochem J. 1984;16:1079–98. doi: 10.1007/BF01002896. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=6500992&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 15.Sirigu P, Shen RL. Pinto ds. Human meibomian glands: the ultrastructure of acinar cells as viewed by thin section and freeze-fracture transmission electron microscopies. IOVS. 1992;33:2284–92. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=1607240&dopt=Abstract [PubMed] [Google Scholar]
- 16.Knop E, Knop N, Millar T, Obata H, Sullivan DA. The International workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology and pathophysiology of the meibomian gland. IOVS 2011; 52:1938–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson JD, Shimazaki J, Benitez-del-Castillo JM, Craig JP, McCulley JP, Den S, Foulks GN. The international workshop on meibomian gland dysfunction: report of the definition and classification subcommittee. IOVS. 2011;52:1930–7. doi: 10.1167/iovs.10-6997b. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21450914&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu S, Li J, Tan DTH, Beuerman RW. The eyelid margin a transitional zone for 2 epithelial phenotypes. Arch Ophthalmol. 2007;125:523–32. doi: 10.1001/archopht.125.4.523. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17420373&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 19.Riau AK, Barathi VA, Beuerman RW. Mucocutaneous junction of eyelid and lip: a study of the transition zone using epithelial cell markers. Curr Eye Res. 2008;33:912–22. doi: 10.1080/02713680802485147. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19085373&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 20.Manders EMM, Verbeek FJ, Aten JA. Measurement of colocalization of objects in dual-color confocal images. J Microsc. 1993;169:375–82. doi: 10.1111/j.1365-2818.1993.tb03313.x. [DOI] [PubMed] [Google Scholar]
- 21.Costes SV, Daelemans D, Cho EH, Dobbin Z, Pavlakis G, Lockett S. Automatic and quantitative measurement of protein-protein colocalization in live cells. Biophys J. 2004;86:3993–4003. doi: 10.1529/biophysj.103.038422. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15189895&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vasioukhin V, Degenstein L, Wise B, Fuchs E. The magical touch: genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proc Natl Acad Sci USA. 1999;96:8551–6. doi: 10.1073/pnas.96.15.8551. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10411913&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Lyle S, Yang Z, Cotsarelis G. Keratin 15 promoter targets putative epithelial stem cells in the hair follicle bulge. J Invest Dermatol. 2003;121:963–8. doi: 10.1046/j.1523-1747.2003.12600.x. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=14708593&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 24.Nien CJ, Massei S, Lin M, Liu H, Paugh JR, Liu CY, Kao WWY, Brown DJ, Jester JV. The development of meibomian glands in mice. Mol Vis. 2010;16:1132–40. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20664693&dopt=Abstract [PMC free article] [PubMed] [Google Scholar]
- 25.Whitbread LA, Powell BC. Expression of the intermediate filament keratin gene, K15, in the basal cell layers of the epithelia and the hair follicle. Exp Cell Res. 1998;244:448–59. doi: 10.1006/excr.1998.4217. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=9806795&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 26.Liu S, Richards SM, Lo K, Hatton M, Fay A, Sullivan DA. Changes in gene expression in human meibomian gland dysfunction. IOVS. 2011;52:2727–40. doi: 10.1167/iovs.10-6482. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21372006&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tektas OY, Yadav A, Garreis F. Schlӧtzer-Schrehardt U, Schicht M, Hampel U, Brӓuer L, Paulsen F. Characterization of the mucocutaneous junction of the human eyelid margin and meibomian glands with different biomarkers. Ann Anat. 2012;194:436–45. doi: 10.1016/j.aanat.2012.07.004. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22877886&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 28.Walther C, Gruss P. Pax6, a murine paired box gene, is expressed in the developing CNS. Dev. 1991;113:1435–49. doi: 10.1242/dev.113.4.1435. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=1687460&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 29.Nishina S, Kohsaka S, Yamaguchi Y, Handa H, Kawakami A, Fujisawa H, Azuma N. Pax6 expression in the developing human eye. Br J Ophthalmol. 1999;83:723–7. doi: 10.1136/bjo.83.6.723. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10340984&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swamynathan SK. Ocular surface development and gene expression. J Ophthal 2013: 103947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parisi I, Colllinson M. Regulation of Merkel cell development by Pax6. Int J Dev Biol. 2012;56:341–50. doi: 10.1387/ijdb.113406ip. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22811268&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 32.Shubham K, Mishra R. Pax6 interacts with SPARC and TGF-β in murine eyes. Mol Vis. 2012;18:951–6. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22539874&dopt=Abstract [PMC free article] [PubMed] [Google Scholar]
- 33.Parfitt GJ, Xie Y, Geyfman M, Brown DJ, Jester JV. Absence of ductal hyper-keratinization in mouse age-related meibomian gland dysfunction (ARMGD). Aging. 2013;5:825–34. doi: 10.18632/aging.100615. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24259272&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olami Y, Zajicek G, Cogan M, Gnessin H, Pe’er J. Turnover and migration of meibomian gland cells in rats’ eyelids. Ophthalmic Res. 2001;33:170–5. doi: 10.1159/000055665. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11340409&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 35.Lavker Rea. IOVS. ARVO Annual Meeting; 2003 May 4-9; Fort Lauderdale, FL. [Google Scholar]