Abstract
Activation of visceral nociceptors by inflammatory mediators contributes to visceral hypersensitivity and abdominal pain associated with many gastrointestinal disorders. Purine and pyrimidine nucleotides (e.g., ATP and UTP) are strongly implicated in this process following their release from epithelial cells during mechanical stimulation of the gut, and from immune cells during inflammation. Actions of ATP are mediated through both ionotropic P2X receptors and metabotropic P2Y receptors. P2X receptor activation causes excitation of visceral afferents; however, the impact of P2Y receptor activation on visceral afferents innervating the gut is unclear. Here we investigate the effects of stimulating P2Y receptors in isolated mouse colonic sensory neurons, and visceral nociceptor fibers in mouse and human nerve-gut preparations. Additionally, we investigate the role of Nav1.9 in mediating murine responses. The application of UTP (P2Y2 and P2Y4 agonist) sensitized colonic sensory neurons by increasing action potential firing to current injection and depolarizing the membrane potential. The application of ADP (P2Y1, P2Y12, and P2Y13 agonist) also increased action potential firing, an effect blocked by the selective P2Y1 receptor antagonist MRS2500. UTP or ADP stimulated afferents, including mouse and human visceral nociceptors, in nerve-gut preparations. P2Y1 and P2Y2 transcripts were detected in 80% and 56% of retrogradely labeled colonic neurons, respectively. Nav1.9 transcripts colocalized in 86% of P2Y1-positive and 100% of P2Y2-positive colonic neurons, consistent with reduced afferent fiber responses to UTP and ADP in Nav1.9−/− mice. These data demonstrate that P2Y receptor activation stimulates mouse and human visceral nociceptors, highlighting P2Y-dependent mechanisms in the generation of visceral pain during gastrointestinal disease.
SIGNIFICANCE STATEMENT Chronic visceral pain is a debilitating symptom of many gastrointestinal disorders. The activation of pain-sensing nerves located in the bowel wall and their sensitization to physiological stimuli, including bowel movements, underpins the development of such pain, and is associated with mediators released during disease. This work addresses the unstudied role of purine and pyrimidine nucleotides in modulating colonic nociceptors via P2Y receptors using a combination of electrophysiological recordings from human ex vivo samples and a detailed functional study in the mouse. This is the first report to identify colonic purinergic signaling as a function of P2Y receptor activation, in addition to established P2X receptor activity, and the results contribute to our understanding of the development of visceral pain during gastrointestinal disease.
Keywords: ATP, Nav1.9, nociceptor, P2Y receptors, purinergic signaling, visceral pain
Introduction
Visceral pain is a principal symptom of many gastrointestinal disorders and is strongly associated with inflammation and mechanical stimulation of the gut (Knowles and Aziz, 2009). One endogenous mediator of bowel discomfort and pain is ATP, which is released from the mucosal epithelium during distension (Burnstock, 2009), particularly during colitis (Shinoda et al., 2009). Both ionotropic P2X and metabotropic P2Y receptors are activated by extracellular ATP. Of the seven reported P2X receptors (P2X1–P2X7), P2X2 and P2X3 receptor subunits are widely expressed in gut-projecting sensory neurons and contribute to the transduction of nociceptive visceral signals in the bowel (Shinoda et al., 2009). By contrast, the role of P2Y receptors in the processing of visceral pain has not been studied in detail. Eight members of the P2Y receptor family (P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11–14) have been identified responding to endogenous purine and pyrimidine nucleotides (ATP and UTP; Lazarowski and Harden, 1999; Lazarowski and Boucher, 2001; Gerevich and Illes, 2004). Each P2Y receptor binds a heterotrimeric G-protein, the activation of which results in intracellular signaling cascades, including the release of intracellular Ca2+ stores (Sanada et al., 2002) capable of sensitizing sensory neurons (Yousuf et al., 2011). P2Y1, P2Y2, P2Y4, and P2Y6 receptors are Gq/11 coupled and excitatory in function. P2Y12-14 receptors are Gi coupled and are inhibitory in function. No rodent ortholog of P2Y11 exists (von Kügelgen, 2006). P2Y receptor agonists cause pain in human blister-base preparations, directly activate mouse cutaneous afferents, and can regulate their sensitivity to mechanical and thermal stimuli (Bleehen and Keele, 1977; Tominaga et al., 2001; Stucky et al., 2004).
Multiple ion channels act as downstream effectors for P2Y receptor-mediated changes in neuronal excitability. For example, P2Y1 receptor activation potentiates transient receptor potential vanilloid 1 currents, leading to hyperalgesia in rodents (Tominaga et al., 2001). M-currents (mediated by KV7) that contribute to resting membrane potentials are also modulated by P2Y receptors (Zaika et al., 2007; Yousuf et al., 2011). Recently, we have shown that voltage-gated sodium channel subtype Nav1.9 is a major regulator of visceral afferent excitability to noxious mechanical and inflammation stimuli, including ATP and mediators derived from patients with inflammatory bowel disease (IBD; Hockley et al., 2014). Under certain conditions, we have shown that Nav1.9 currents can be enhanced by ATP (Baker, 2005), suggesting that P2Y receptor activation can enhance Nav1.9 currents, stimulating visceral afferents. This is supported by in vivo observations that UTP-induced paw hypersensitivity is lost in Nav1.9−/− mice (Amaya et al., 2006). It is therefore of considerable interest to understand whether P2Y receptor activation stimulates visceral nociceptors and whether Nav1.9 contributes to these effects.
The goals of the study were (1) to determine the effects of P2Y receptor activation on the excitability of colonic sensory neurons, (2) to investigate P2Y receptor activation of mouse afferent fibers including visceral nociceptors, (3) to demonstrate the translation of these effects from mouse to human, and (4) to understand the role for Nav1.9 as a common transducer of visceral afferent excitability to P2Y receptor signaling.
Materials and Methods
Experiments were performed using male C57BL/6 mice (10–12 weeks of age) and Nav1.9 knock-out (Nav1.9−/−) or wild-type (Nav1.9+/+) mice bred on a C57BL/6 background (for original characterization, see Ostman et al., 2008). Mice were housed in a 12 h light/dark cycle with ad libitum access to food and water. All protocols were performed in accordance with the UK Animal (Scientific Procedures) Act 1986. Human tissue was collected and used with approval of the East London and The City HA Local Research Ethics Committee (NREC 10/H0703/71).
Retrograde labeling of colonic sensory neurons.
Male mice were anesthetized with 1.5% isoflurane, and a midline 1.5 cm laparotomy was performed. Five injections of 0.2 μl Fast Blue (FB: 2% in saline; Polysciences GmbH) per site, were made into the wall of the distal colon using a fine pulled-glass needle and microinfusion pump (0.4 μl/min). The abdomen was flushed with saline solution to remove any excess FB, and the peritoneal muscle layer was sutured and the skin secured with Michel clips. Postoperative analgesia (buprenorphine 0.05–0.1 mg/kg daily) and care (monitoring body weight and soft diet) was provided.
Primary culture of mouse dorsal root ganglia neurons.
Three to five days after surgery, mice were humanely killed by concussion of the brain and cervical dislocation of the neck, and thoracolumbar (TL; T10–L1) dorsal root ganglia (DRGs) were harvested for electrophysiological whole-cell recordings. Dissected ganglia were washed in Dulbecco's PBS and then incubated at 37°C (in 5% CO2) in Lebovitz L-15 Glutamax (Invitrogen) media containing 1 mg/ml collagenase type 1A (Sigma-Aldrich) and 6 mg/ml bovine serum albumin (BSA; Sigma-Aldrich) for 15 min. Ganglia were incubated in L-15 media containing 1 mg/ml trypsin (Sigma-Aldrich) and 6 mg/ml BSA for 30 min. The ganglia were gently triturated using fire-polished pipettes and collected by brief centrifugation at 500 × g. The supernatant (containing dissociated cells) was collected, and gentle trituration and centrifugation of ganglia were repeated five times. Cells were pelleted and resuspended in Lebovitz L-15 Glutamax media containing 2% penicillin/streptomycin, 24 mm NaHCO3, 38 mm glucose, and 10% fetal bovine serum. Cells were plated onto poly-d-lysine/laminin-coated coverslips (BD Biosciences) and incubated at 37°C in 5% CO2. All electrophysiological recordings were performed within 24 h of plating.
Patch-clamp electrophysiology.
Coverslips were transferred to a recording chamber continually perfused with the following extracellular solution (in mm): 140 NaCl, 4 KCl, 1.8 CaCl2, 1 MgCl2, 10 HEPES, and 5 glucose, pH adjusted to 7.4 with NaOH at room temperature. Whole-cell patch-clamp recordings were made using a Molecular Devices Axopatch 200B amplifier, digitized at 10 kHz (Digidata 1440), and controlled by pClamp 9 software. Glass borosilicate pipettes were used with a typical pipette resistance of 2–3 MΩ, resulting in series resistance of < 10 MΩ. Pipettes were filled with the following intracellular solution (in mm): 140 KCl, 1.6 MgCl2, 2.5 MgATP, 0.5 NaGTP, 2 EGTA, and 10 HEPES, and pH adjusted to 7.3. After the formation of whole-cell recordings, cells were clamped at −60 mV, and membrane excitability was tested by increasing current injections at 50 pA increments to 650 pA, with each lasting 500 ms. Only Fast-Blue-positive colonic sensory neurons with resting membrane potentials more negative than −40 mV were studied. The total number of action potentials elicited by current injections was quantified before and after the application of nucleotide for 2 min by rapid exchange of the recording chamber perfusate. The selective P2Y1 receptor antagonist MRS2500 was applied for 2 min before and during the application of ADP (Hechler et al., 2006).
Mouse colonic splanchnic afferent preparations.
Mice of either sex were humanely killed by a rising concentration of CO2, and the distal colon with associated lumbar splanchnic nerves (LSNs) was removed. Tissues were superfused (7 ml/min; 32–34°C) with carbogenated Krebs buffer (in mm: 124 NaCl, 4.8 KCl, 1.3 NaH2PO4, 2.5 CaCl2, 1.2 MgSO4.7H2O, 11.1 glucose, and 25 NaHCO3) supplemented with nifedipine (10 μm) and atropine (10 μm) to block smooth muscle contraction, and indomethacin (3 μm) to block endogenous prostanoid production. In whole-nerve studies, the colon was cannulated and luminally perfused (100 μl/min) with the same supplemented Krebs buffer. For teased nerve fiber studies, the colon was opened along the antimesenteric border and pinned with the flat mucosal side up.
Human mesenteric afferent preparations.
Resected human bowel tissues were obtained after full written consent from 28 patients undergoing surgery at Barts Health NHS Trust, London. All applicable patients were invited to participate in the study, and pharmacological treatment regimens were not accounted for in patient selection. Descending colon, appendix, and ileum were obtained from patients undergoing colectomy as part of their normal surgical treatment for bowel cancer, inflammatory bowel disease, stricture, trauma, and slow-transit constipation (Table 1). For cancer resections, only tissue that was not required for histopathological assessment (e.g., tissue taken away from the tumor or resection margins or lymphatic drainage field) was used. Tissues from inflammatory bowel disease resections were derived from both chronically inflamed and resolving regions of bowel; however, all patients possessed active disease that was unresponsive to medical treatment. Once removed, tissues were immediately placed in cold Krebs buffer before sharp dissection of full-thickness colonic wall (∼4 cm square with the mesenteric fan down the center), and, depending on length, appendix specimens were split in two or used in their entirety. Tissue was superfused with Krebs buffer (7 ml/min; 32–34°C) supplemented with 10 μm nifedipine and 10 μm atropine. Under a dissection microscope, the neurovascular bundles in the associated mesentery were blunt dissected, and mesenteric nerves were identified and subsequently cleared of connective tissue (Peiris et al., 2011).
Table 1.
Details of patients from which human tissue was used in the present studies
| Patient | Disease | Tissue | Operation | Age (years) | Sex |
|---|---|---|---|---|---|
| 1 | Cancer | Descending colon | Anterior resection | 52 | M |
| 2 | Cancer | Descending colon | Laparoscopic anterior resection | 50 | F |
| 3 | Cancer | Descending colon | Anterior resection | 76 | M |
| 4 | Cancer | Descending colon | Anterior resection | 65 | F |
| 5 | Cancer | Descending colon | Anterior resection | 64 | M |
| 6 | Cancer | Descending colon | Subtotal colectomy | 47 | M |
| 7 | Cancer | Descending colon | Anterior resection | 73 | M |
| 8 | Cancer | Descending colon | Anterior resection | 61 | M |
| 9 | Cancer | Descending colon | Anterior resection | 57 | M |
| 10 | Cancer | Descending colon | Laparoscopic colectomy | 54 | F |
| 11 | Cancer | Descending colon | Right hemicolectomy | 48 | F |
| 12 | Cancer | Ileum | Laparoscopic right hemicolectomy | 76 | M |
| 13 | Cancer | Appendix | Right hemicolectomy | 62 | F |
| 14 | Cancer | Appendix | Subtotal colectomy | 57 | M |
| 15 | Cancer | Appendix | Laparoscopic right hemicolectomy | 81 | F |
| 16 | Cancer | Appendix | Right hemicolectomy | 62 | M |
| 17 | Cancer | Appendix | Right hemicolectomy | 72 | M |
| 18 | Slow Transit Constipation | Descending colon | Subtotal colectomy | 50 | F |
| 19 | Stricture | Descending colon | Sigmoid colectomy | 64 | F |
| 20 | Trauma | Ileum | Ileotransverse anastomosis and abdominal wall repair | 60 | M |
| 21a | Stricture | Appendix | Right hemicolectomy | 63 | F |
| 22 | Ulcerative colitis | Descending colon | Subtotal colectomy | 21 | M |
| 23 | Ulcerative colitis | Descending colon | Completion proctectomy | 37 | F |
| 24 | Crohn's disease | Ileum | Small bowel resection | 39 | F |
| 25 | Crohn's disease | Ileum | Right hemicolectomy | 30 | F |
| 26 | Crohn's disease | Ileum | Right hemicolectomy | 41 | F |
| 27 | Crohn's disease | Ileum | Right hemicolectomy | 21 | M |
| 28b | Crohn's disease | Appendix | Right hemicolectomy | 16 | M |
Tissue from 28 patients was collected and used in electrophysiological mesenteric nerve recordings. Patients undergoing surgery were characterized by disease process into two groups, noninflamed (consisting of macroscopically normal tissues collected from bowel cancer, slow transit constipation, stricture and trauma resections) and inflamed (tissues isolated from inflammatory bowel disease resections). F, Female; M, male.
aFor patients 1–21: mean age, 62 years; male/female ratio, 1:0.75.
bFor patients 22–28: mean age, 29 years; male/female ratio, 1:1.33.
Electrophysiological recordings of mouse and human visceral nerves.
Multiunit activity or single-unit activity (from teased fibers) from whole LSNs (rostral to the inferior mesenteric ganglia) of mouse, or from mesenteric nerves of human bowel tissues, was recorded using borosilicate glass suction electrodes. Signals were amplified, bandpass filtered (gain 5K; 100–1300 Hz; Neurolog, Digitimer Ltd), digitally filtered for 50 Hz noise (Humbug, Quest Scientific), and digitized at 20 kHz (micro1401, Cambridge Electronic Design). In whole-nerve multiunit recordings, a threshold of twice the background noise (typically, 100 μV) was used to determine action potential firing counts and the signal displayed on a PC using Spike 2 software. In mouse flat sheet preparations, receptive fields were characterized by von Frey hair (VFH) probing (1 g; applied three times for a period of 3 s), circumferential stretch (5 g; applied for 1 min), and mucosal stroking with a light von Frey hair (0.16 g; applied 10 times). A claw attached to the tissue adjacent to the receptive field was used to apply circumferential stretch by addition of a 5 g weight in a cantilever system. Comparable methodology was used to characterize receptive fields from human mesenteric nerve recordings. This included von Frey hair probing (2 g; applied 3 times for a period of 3 s), circumferential and longitudinal stretch (curved forceps were used to apply stretch across the receptive field repeated three times; 5 min interval), and mucosal stroking with a rod (three times; 5 min interval). Afferent fibers were characterized into the following four previously established classes based on these responses: mesenteric (those responding to focal compression of the mesentery); serosal (those responding to focal compression of the colon wall, but not mucosal stroke or stretch); muscular (those responding to stretch, but not fine mucosal stroking); and mucosal (those responding to mucosal stroking; Brierley et al., 2004).
Mouse single-cell qRT-PCR.
Fast Blue-positive colonic sensory neurons were individually harvested from primary cultures of retrogradely labeled DRG (TL: T10–L1) by pulled glass pipette. The cells were collected in PBS, and the pipette tip containing the cell was broken into a tube containing preamplification mastermix (5 μl of CellDirect 2× reaction buffer (Invitrogen), 2.5 μl of 0.2× primer/probe mix, 0.1 μl of SUPERase-in (Ambion), 1.2 μl of TE buffer (Applichem), and 0.2 μl of Superscript III Reverse Transcriptase/Platinum Taq mix (Invitrogen); briefly centrifuged; frozen immediately; and stored at ×80°C. Only individual Fast Blue-positive neurons free from debris were collected. Samples of the bath solution were collected in the absence of cells for no-template control experiments. Preamplification of cDNA was achieved with the following protocol: 50°C for 30 min, 95°C for 2 min, then 21 cycles of 95°C for 15 s and 60°C for 4 min). The cDNA product was diluted 1:5 in TE buffer and TaqMan quantitative PCR assays run for each gene of interest (TaqMan Assay ID: SCN11A, Mm00449367_m1; P2Y1, Mm02619947_s1; P2Y2, Mm02619978_s1; P2Y4, Mm00445136_s1; GAPDH, Mm99999915_g1; Applied Biosystems) with the following cycling protocol: 50°C for 2 min, 95°C for 10 min, then 40 cycles of 95°C for 15 s and 60°C for 1 min on a Bio-Rad C1000 Thermal Cycler and CFX96 Real-Time System. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) acted as an internal positive control. Quantitative assessment of gene expression was determined by quantification cycle values less than the threshold of 35 being considered as positive. All single-cell RT-PCR products expressed GAPDH, while bath control samples did not.
Extracellular electrophysiology protocols.
In whole-nerve recordings, following a stabilizing period of 30 min, drugs were applied by bath superfusion of a 20 ml volume, with a minimum 60 min interval maintained between the sequential concentrations applied. In the mouse, increasing concentrations of nucleotides, ADP, and UTP (0.1, 1.0, and 3.0 mm; in human, 1 and 10 mm) were applied in separate colonic preparations. In some human preparations, ADP and UTP (1 and 10 mm) were applied sequentially with a minimum 60 min interval. In teased-fiber studies on mesenteric and serosal units, the effect of applying 100 μl of either ADP or UTP (3–10 mm; 2 min) to receptive fields isolated by a metal ring was also examined on ongoing nerve discharge.
Data analysis.
Peak changes in electrophysiological nerve activity in multiunit experiments were determined by subtracting baseline firing (3 min before drug application) from increases in nerve activity following nucleotide superfusion. Changes in nerve activity were compared between Nav1.9+/+ and Nav1.9−/− animals using two-way ANOVA with Bonferroni post hoc test, as appropriate. In teased single-fiber experiments, the average number of spikes per second per stimulus were quantified, and fibers were considered responsive if a maintained >25% increase in firing discharge above basal levels occurred (Brierley et al., 2005). Expression data were visualized using R and the ggplot2 package (Wickham, 2009). Statistical significance was set at p < 0.05. Data are displayed as the mean ± SEM.
Drugs.
Stock concentrations of ADP, UTP, and uridine 5-diphosphoglucose disodium salt (UDP; 300 mm; water); atropine (10 mm; ethanol); indomethacin (3 mm; DMSO); and nifedipine (10 mm; DMSO) were all purchased from Sigma-Aldrich and prepared as described. MRS2500 (10 mm; water), pyridoxalphosphate-6-azophenyl-2-disulfonic acid (PPADS; water), 2′,3′-O-(2,4,6-trinitrophenyl)adenosine-5′-triphosphate tetre(triethylammonium) salt (TNP-ATP; water), and CGS15943 (DMSO) were all purchased from Tocris Bioscience. All compounds were diluted to working concentrations in buffer on the day of experimentation.
Results
UTP and ADP increase visceral sensory neuron excitability
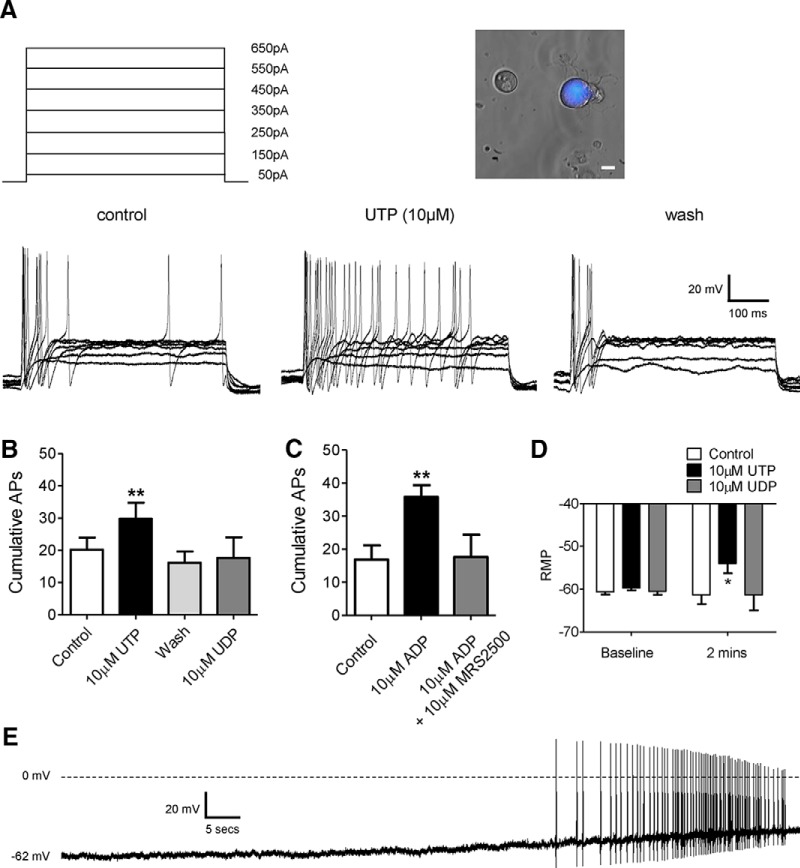
We first assessed the ability for the purine and pyrimidine nucleotides UTP (P2Y2 and P2Y4 receptor agonist), UDP (P2Y6 receptor agonist), and ADP (P2Y1, P2Y12 and P2Y13 receptor agonist) to sensitize the response of colon projecting sensory neurons (31.4 ± 1.8 pF; n = 53) to repeat 500 ms current injections from 50 to 650 pA applied in 50 pA increments. For example, following 100 pA current injection neurons fired 1.1 ± 0.2 action potentials per 500 ms (n = 53; Fig. 1A). With increasing current injection, the frequency of firing also increased with 650 pA injections evoking 2.7 ± 0.4 action potentials per 500 ms. The application of UTP or ADP resulted in a significant increase in the total number of action potentials fired during current injections in 50% or 40% of cells tested, respectively (p < 0.01, n = 10, paired t test vs pre-UTP application; p < 0.01, n = 6, paired t test vs pre-ADP application; Fig. 1B,C). After the removal of UTP, the number of action potentials elicited to current injection returned to baseline levels (Fig. 1A) in all but one cell tested, by contrast neuronal excitability remained potentiated for the duration of the wash period (5 min) following the removal of ADP (cumulative, 55.0 ± 12.4 APs; n = 6). Preincubation with the selective P2Y1 receptor antagonist MRS2500 inhibited the sensitizing effects of ADP (10 μm; p < 0.05, n = 6, unpaired t test 10 μm ADP vs 10 μm ADP plus 10 μm MRS2500; Fig. 1C). In addition, the application of UTP, but not UDP, depolarized the membrane potential from −59.7 ± 0.5 to −53.9 ± 2.3 mV (p < 0.05, n = 6–7, two-way ANOVA vs control buffer; Fig. 1D), an effect that was not observed following ADP incubation (pre-ADP, −61.7 ± 1.1 mV; post-ADP, −63.4 ± 3.6 mV; n = 6). The application of UDP failed to increase action potential firing in any cell tested (n = 6, p = 0.91, paired t test vs pre-UDP application; Fig. 1B). In addition, in 4 of 20 cells tested, the application of UTP also evoked sustained spontaneous activity (an example is shown in Fig. 1E). Spontaneous activity was observed in 1 of 15 cells following the application of ADP.
Figure 1.
Increased excitability of mouse colonic sensory neurons by purine and pyrimidine nucleotides. A, Membrane potential of thoracolumbar colonic neurons was observed during incrementally increasing (50 pA) current injections before and after nucleotide application (10 μm; 2 min). Traces were obtained before (control), during (UTP; 10 μm), and after (wash) application of 10 μm UTP (for clarity, only traces at 100 pA intervals are shown). Inset, Example bright-field image of dissociated dorsal root ganglia neurons overlaid with colonic Fast Blue retrograde labeling. Scale bar, 10 μm. B, Cumulative action potentials fired in response to current injections before (n = 10), in the presence of (n = 10), and after application of 10 μm UTP (n = 6), and in the presence of 10 μm UDP (n = 6). **p < 0.01, paired t test, 10 μm UTP vs control. C, Cumulative action potentials fired in response to current injections before and in the presence of 10 μm ADP (n = 6), and in the presence of 10 μm ADP and 10 μm MRS2500 (n = 6). **p < 0.01, paired t test, 10 μm ADP vs control. D, Membrane potential before and following the application of either control buffer or nucleotide (2 min; 10 μm). *p < 0.05, n = 6–7, two-way ANOVA repeated-measures with Bonferroni post hoc test, 10 μm UTP vs control. E, Example trace of spontaneous action potential firing during the application of UTP (10 μm).
These data show that agonist-driven activation of P2Y receptors can sensitize visceral colonic sensory neurons. To understand how these effects manifest at the level of the sensory nerve ending, we next investigated the ability of UTP and ADP to directly excite visceral colonic afferents.
Activation of mouse visceral colonic afferents by UTP and ADP, but not UDP
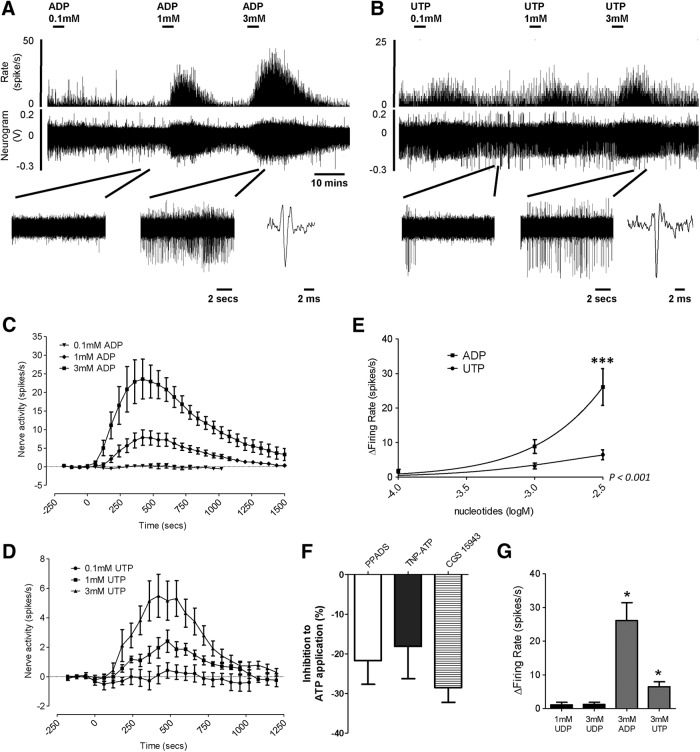
Initial studies used multiunit recordings of LSN activity to observe whole-nerve firing following P2Y receptor activation. This was later refined to study the discrete effect of applying P2Y agonists to characterized receptive fields of visceral nociceptors. The application of increasing concentrations of UTP or ADP (100 μm, 1 and 3 mm) produced robust and concentration-dependent increases in afferent firing (Fig. 2A–D), with the magnitude of the response to ADP significantly greater than that to UTP (p < 0.0001, n = 6, two-way ANOVA; Fig. 2E). In agreement with our patch-clamp observations, the application of UDP (1 and 3 mm) had no effect on colonic afferent fiber activity (Fig. 2G).
Figure 2.
Effect of increasing concentrations of ADP and UTP on colonic splanchnic nerve activity in mouse. A, B, Example neurogram and rate histogram response to 0.1, 1, and 3 mm ADP (A) and UTP (B). Below, in both instances, are expanded traces at baseline and following the addition of 3 mm nucleotide, and an example action potential. C, D, Average response profiles to the addition of increasing concentrations of ADP (C; n = 6) and UTP (D; n = 6). E, Peak increase in nerve activity above baseline following the addition of increasing concentrations of ADP and UTP. ***p < 0.001, n = 6, two-way ANOVA with Bonferroni post hoc test, ADP vs UTP. F, Reduction in peak activity to the application of 3 mm ATP following preincubation with 30 μm PPADS (n = 3), 10 μm TNP-ATP (n = 5), and 3 μm CGS 15943 (n = 3). G, Effect of P2Y6 agonist UDP on colonic splanchnic nerve activity in mouse, the peak increase in nerve activity above baseline in response to the addition of 1 and 3 mm UDP (n = 3) compared with 3 mm ADP and UTP (both n = 6, *p < 0.05, unpaired t test vs 3 mm UDP). No direct excitation by UDP was observed in any preparation.
To further investigate the contribution of P2Y receptors to purinergic signaling by ATP, we next sought to determine the residual afferent response to ATP following pretreatment with broad-spectrum and selective P2X receptor or P1 (adenosine) receptor antagonists as an indication of the degree of P2Y receptor activation by ATP. Repeat application of ATP (3 mm) evoked stable, reproducible whole-nerve responses. Pretreatment with the P2X antagonist PPADS (30 μm), the selective P2X2/3 antagonist TNP-ATP (10 μm), or the pan-P1 (adenosine) receptor antagonist CGS15943 (3 μm) resulted in a reduction of ∼25% in afferent response to ATP (p < 0.05, paired t test; Fig. 2F), confirming not only that P2X receptors are involved, but also indicating that a substantial proportion of the response to exogenous application of ATP is likely to be P2Y receptor mediated in our preparation. Together with the observed increases in afferent activity following the application of selective P2Y agonists UTP and ADP, the data demonstrate that the activation of P2Y receptors has a profound stimulatory effect on visceral afferent activation.
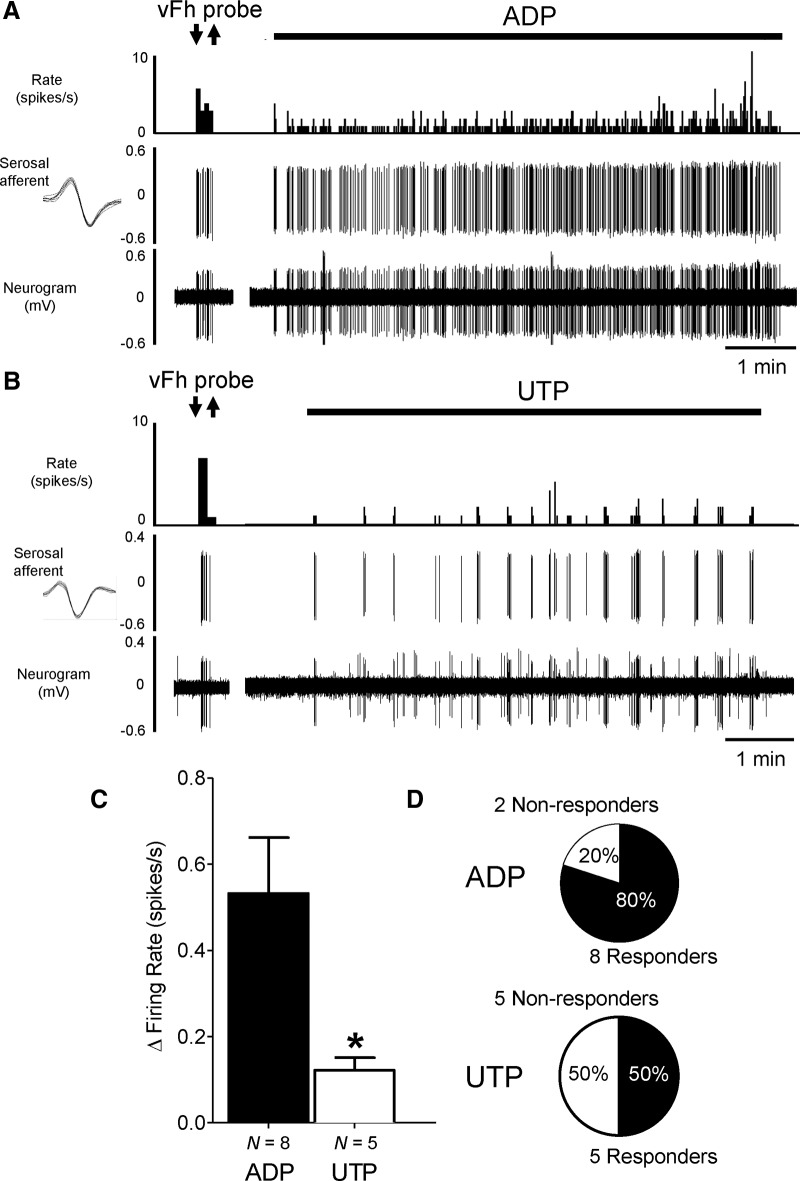
Our recordings were made from the LSN innervating the distal colon, which has been shown to be predominantly composed of visceral nociceptors with endings located on the mesenteric and serosal blood vessels of the colon (Brierley et al., 2004). These nociceptors transduce noxious mechanical and chemical stimuli, and are thought to contribute significantly to the visceral pain and hypersensitivity associated with many gastrointestinal disorders. To confirm that P2Y receptor activation stimulates visceral nociceptors within the LSN, we used teased-fiber recordings to study the effects of P2Y agonists on defined populations of nociceptors with receptive fields isolated in the serosa or mesentery of flat sheet preparations. Using a small chamber to isolate the receptive field, we applied UTP (3–10 mm) or ADP (3–10 mm) directly to nociceptor endings. Both UTP and ADP stimulated nociceptor activity (Fig. 3A–C). However, consistent with the whole-nerve studies, ADP activated a greater proportion of units tested (80% vs 50%; Fig. 3D). There were significantly greater increases in activity with ADP compared with UTP in serosal units (p < 0.05, n = 5–8, unpaired t test; Fig. 3C). By contrast, responses to ADP and UTP were comparable in mesenteric units (ADP, 4 of 10 units responded with a response of 0.59 ± 0.30 spikes/s; UTP, 6 of 12 units responded with a response of 0.56 ± 0.30 spikes/s).
Figure 3.
Excitation of isolated mechanoreceptive fields in mouse colon by purine and pyrimidine nucleotides. A, B, Example colonic nerve activity to ring application of ADP (A) and UTP (B; 2 min; 10 mm) over receptive fields located in the serosa. Mechanically sensitive afferents are shown by response to von Frey hair probe (2 g) before application of nucleotide. Inset, The average spike shape of the serosal afferents responsive to ADP and UTP. Top trace shows spike rate histogram, middle trace shows serosal afferent activity, and bottom trace shows raw neurogram data. C, Response to nucleotide ring application in serosal receptive fields. *p < 0.05, n = 5–8, unpaired t test, UTP vs ADP. D, Proportions of responsive vs nonresponsive serosal units following the application of ADP and UTP.
Overall, we have found that UTP and ADP directly excite a significant subset of visceral nociceptors, and that, in parity with whole-nerve data, ADP is capable of evoking greater excitation than UTP. As such, this demonstrates that, in addition to the established excitatory effect of P2X receptor activation, ATP will also stimulate visceral nociceptors through the activation of P2Y receptors.
Excitation of human colonic afferents by UTP and ADP
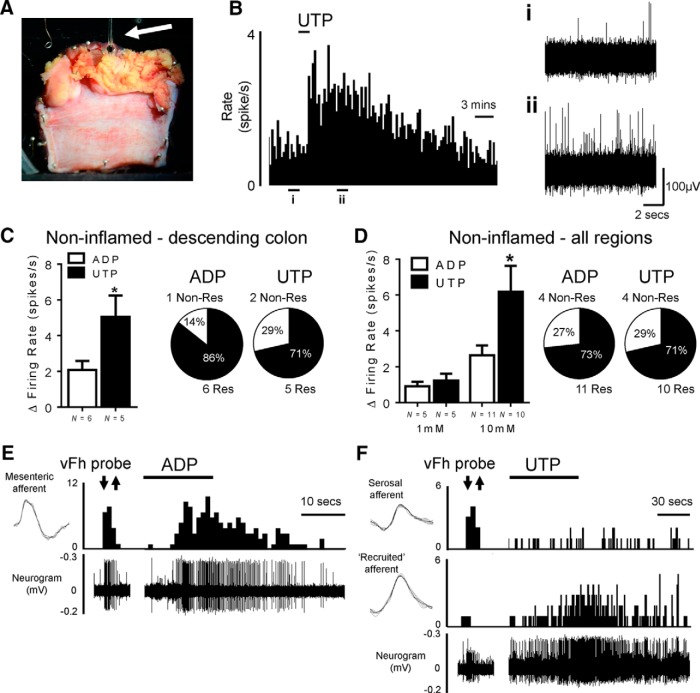
Having established an excitatory effect of P2Y receptor activation on mouse visceral nociceptors, we next sought to confirm the translation of this effect from mouse to human visceral nociceptors. To do this, we recorded mesenteric nerve activity in resected bowel tissues obtained from patients with full written consent (Table 1, Fig. 4A). As described previously, recordings were made from the following three gross tissue regions: ileum, descending colon, and appendix (Peiris et al., 2011). Studies concentrated on noninflamed descending colon tissues obtained from patients undergoing resection for bowel cancer (n = 11). These tissues were taken away from cancer tumors, resection margins, and lymphatic drainage fields, and were considered noninflamed by macroscopic assessment. Recordings from descending colon tissues are also likely to be predominantly populated by LSN activity and are therefore directly translatable to our murine investigation. In noninflamed descending colon, bath superfusion of UTP (10 mm) led to robust activation of human visceral afferents (Fig. 4B, example trace), with 71% of tissues tested responding with an increase in afferent firing rates (Fig. 4C). Similarly, ADP (10 mm) also produced an increase in human visceral afferent activity with responses observed in 86% of preparations tested (Fig. 4C). In contrast to mouse tissue, where ADP produced greater responses than UTP, the visceral afferent response in this defined gut region and tissue type to UTP was consistently greater in magnitude than those of ADP (p < 0.05, unpaired t test; Fig. 4C). Baseline firing rates were comparable between preparations tested with UTP or ADP. Having established the ability of ADP and UTP to activate human visceral afferents in the descending colon, we extended this observation by including tissues obtained from other gut regions (including ileum, appendix, and descending colon) and from resections necessitated by other noninflammatory disease processes (including stricture, slow transit constipation, and trauma). The application of UTP and ADP (1 and 10 mm) in these tissues led to concentration-dependent increases in visceral afferent activity in 71% and 73% of tissues tested with 10 mm UTP and 10 mm ADP, respectively (Fig. 4D; Table 2). Furthermore, the magnitude of the UTP responses was greater than that observed for ADP in these tissues (p < 0.05, unpaired t test; Fig. 4D). Comparison of baseline firing rates between different regions of responding noninflamed tissues showed no significant difference [e.g., ileum (0.05 spikes/s; n = 1), descending colon (2.50 ± 0.80 spikes/s; n = 11), and appendix (5.79 ± 5.00 spikes/s; n = 4; p = 0.31, unpaired t test, descending colon vs appendix], suggesting that the nerves recorded from were consistent in ectopic background activity and the number of nerve fibers regardless of origin along the gastrointestinal tract; however, this does not exclude that regional pharmacology may exist.
Figure 4.
Effect of purine and pyrimidine nucleotides on human mesenteric nerve activity from resected bowel tissues. A, Example image of ileum pinned serosa upward in the recording chamber. The mesenteric nerve within the mesenteric fan is located at the top of the image. The white arrow highlights the glass suction electrode recording mesenteric afferent activity from the mesenteric nerve. B, Example rate histogram of nerve activity following the addition of 1 mm UTP. Expanded traces of nerve activity at baseline (i) and after application of UTP (ii). C, Peak firing rates over baseline following the application of 10 mm ADP and UTP to noninflamed descending colon tissues isolated from bowel cancer resections only. *p < 0.05, n = 5–6, unpaired t test. Right, Proportions of responsive vs nonresponsive noninflamed descending colon tissue preparations. D, Peak firing rates over baseline following the application of 1 and 10 mm ADP and UTP to tissues (including descending colon, ileum, and appendix) isolated from noninflammatory pathologies (bowel cancer, slow transit constipation, trauma, and stricture). *p < 0.05, n = 5–11, unpaired t test. Right, Proportions of responsive vs nonresponsive noninflamed tissue preparations. E, Example of a human mechanically sensitive mesenteric afferent responding to von Frey hair probe (2 g) and application of ADP (30 mm). F, Example of a human mechanically sensitive serosal afferent (small-amplitude) that was responsive to von Frey hair probe (2 g) but was unresponsive to UTP (10 mm). However, the application of UTP recruited a mechanically insensitive afferent (large-amplitude). Insets, Average spike shapes of serosal and mesenteric afferents and the recruited mechanically insensitive afferent. The top traces show the spike rate, and the bottom traces show raw neurogram data.
Table 2.
Responsiveness of human tissues to P2Y receptor agonist applications split by disease and gut region
| Disease | Descending colon |
Ileum |
Appendix |
|||
|---|---|---|---|---|---|---|
| Responders | Nonresponders | Responders | Nonresponders | Responders | Nonresponders | |
| Cancer | ||||||
| ADP | 6 | 1 | 1 | 0 | 2 | 1 |
| UTP | 5 | 2 | 1 | 0 | 2 | 0 |
| Stricture | ||||||
| ADP | 1 | 0 | 0 | 0 | 0 | 1 |
| UTP | 1 | 0 | 0 | 0 | 0 | 1 |
| Constipation | ||||||
| ADP | 1 | 0 | 0 | 0 | 0 | 0 |
| UTP | 1 | 0 | 0 | 0 | 0 | 0 |
| Trauma | ||||||
| ADP | 0 | 0 | 0 | 1 | 0 | 0 |
| UTP | 0 | 0 | 0 | 1 | 0 | 0 |
| Ulcerative colitis | ||||||
| ADP | 2 | 0 | 0 | 0 | 0 | 0 |
| UTP | 0 | 0 | 0 | 0 | 0 | 0 |
| Crohn's disease | ||||||
| ADP | 0 | 0 | 2 | 1 | 1 | 0 |
| UTP | 0 | 0 | 2 | 3 | 1 | 0 |
In a limited number of experiments, tissues were collected from IBD patients to confirm that visceral afferents present in chronically inflamed tissues remained sensitive to purine and pyrimidine nucleotides (Table 1). Of those tissues tested, 83% (five of six tissues) responded to the application of 10 mm ADP with an increase in visceral afferent firing (1.62 ± 0.53 spikes/s), while superfusion of 10 mm UTP led to increased visceral afferent activity (3.30 ± 0.97 spikes/s) in 50% of IBD tissues (three of six tissues) tested (Table 2). The heterogeneity of gut regions investigated (descending colon, n = 2; ileum, n = 4; and appendix, n = 1) limit the conclusions that can be drawn on the relative effects of nucleotide agonists and the sensitivity of tissue during disease, other than highlighting the ability for ADP and UTP to excite human visceral afferents during chronic inflammatory disease.
Human bowel tissues were also routinely and systematically probed by VFH to identify mechanically sensitive receptive fields. Once identified, these were characterized by location and response to VFH probe and circumferential stretch in a comparable way to teased-fiber recordings of the LSN from the distal colon of mouse. In human bowel tissues, mechanically sensitive receptive fields were identified in relative low frequency (4 units of 30 recordings from 28 patients), restricting our ability to comprehensively characterize the exact afferent subtypes responsive to purine and pyrimidine nucleotides in human tissues. Four units were identified that were responsive to VFH probe of the mesentery or colon wall, but not to circumferential stretch or mucosal stroking, a pattern of mechanosensitivity that likely is representative of sensory afferents associated with the intermural and mesenteric vasculature, in contrast to the mucosa or intramuscular myenteric plexi (Brookes et al., 2013). Based on these mechanosensitive properties and the location of the receptive field, one unit was classified as mesenteric, and responded to both ADP and UTP (for the response to ADP, see Fig. 4E). The remaining three units were characterized as serosal by their response to VFH probing of the colon wall and lack of response to tissue stretch. The application of ADP or UTP had no effect on activity in any of the three mechanosensitive serosal units; however, in all recordings previously “silent” units were recruited, and responded to sequential addition of both ADP and UTP (for an example, see Fig. 4F). Mechanosensitive receptive fields were sought for these recruited units by subsequent von Frey hair probing; however, none were found, suggesting that these units may represent examples of mechanically insensitive silent afferents. No units fulfilling the criteria for muscular or mucosal units were identified in the present study. These findings demonstrate that human visceral afferents, including those with a visceral nociceptor phenotype, can be activated by P2Y receptor agonists. This suggests that the release of purine and pyrimidine nucleotides, for example during inflammation, could act via P2Y receptors to trigger visceral pain in humans.
Localization of P2Y1, P2Y2, and P2Y4 receptors in mouse colonic sensory neurons
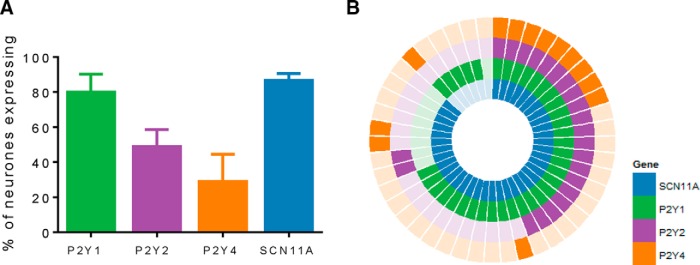
Next we confirmed the expression of P2Y receptors in mouse colonic sensory neurons. We examined the expression of P2Y1, P2Y2, and P2Y4 receptors in thoracolumbar gut-projecting sensory neurons by single-cell qRT-PCR (Fig. 5). Data from our patch-clamp experiments have shown that the effects of ADP were mediated by P2Y1 receptors, while previous studies (Chen et al., 2010; Yousuf et al., 2011) show that P2Y2 receptors are predominantly responsible for the activation of sensory nerves by UTP. We chose to examine expression in DRGs T10 and L1 levels as these possess the greatest number of sensory neurons projecting to the distal colon via the LSN, the nerve in which our electrophysiological data were recorded (Robinson et al., 2004). In total, 15 single cells per mouse (n = 3) were isolated and processed for qRT-PCR. Analysis of qRT-PCR products from colonic sensory neurons revealed greater expression of P2Y1 transcripts (80.0 ± 10.2%) compared with P2Y2 (48.9 ± 9.7%) and P2Y4 transcripts (28.9 ± 15.6%; Fig. 5A).
Figure 5.
Expression of P2Y receptors and Nav1.9 mRNA transcripts in mouse colon-projecting sensory neurons by single-cell qRT-PCR. A, Proportion of colon-projecting sensory neurons expressing transcripts for P2Y1, P2Y2, P2Y4, and SCN11A. B, Coexpression analysis of P2Y1, P2Y2, P2Y4, and SCN11A transcripts in colonic sensory neurones. Each segment represents a single cell with a filled colored block signifying positive expression.
Coexpression of P2Yreceptors with Nav1.9 in mouse colonic sensory neurons
The underlying mechanism by which P2Y receptor activation alters neuronal excitability and hypersensitivity has been attributed to multiple ion channels expressed by sensory neurons (Gerevich and Illes, 2004). We have previously shown that voltage-gated sodium channel Nav1.9 currents are significantly enhanced by ATP (Baker, 2005), and, consistent with this observation, colonic afferent responses to ATP are greatly diminished in Nav1.9−/− mice (Hockley et al., 2014). These data strongly suggest that the modulation of Nav1.9 is an important mechanism by which P2Y receptor activation stimulates visceral afferent activity. To support this hypothesis, the coexpression of Nav1.9 with P2Y1, P2Y2, and P2Y4 was examined in single-cell qRT-PCR products. Overall, 86.7 ± 3.8% of thoracolumbar colonic sensory neurons expressed Nav1.9 mRNA transcripts, which was greater than the proportion observed by isotopic in situ hybridizations from a previous study (50.5 ± 3.3%; Hockley et al., 2014), perhaps reflecting the greater sensitivity of the qRT-PCR technique. Of the P2Y1 transcript-positive colonic neurons (Fig. 5B), 86% expressed Nav1.9 transcripts, while 100% of P2Y2 transcript-positive neurons and 92% of P2Y4 transcript-positive neurons also expressed Nav1.9 transcripts. In addition, substantial coexpression of multiple P2Y receptor subtypes was observed, with 20% of colonic neurons coexpressing all three P2Y receptor transcripts (P2Y1, P2Y2, and P2Y4), and 44% expressing both P2Y1 and P2Y2 transcripts.
Loss of Nav1.9 significantly reduces afferent sensitivity to UTP and ADP
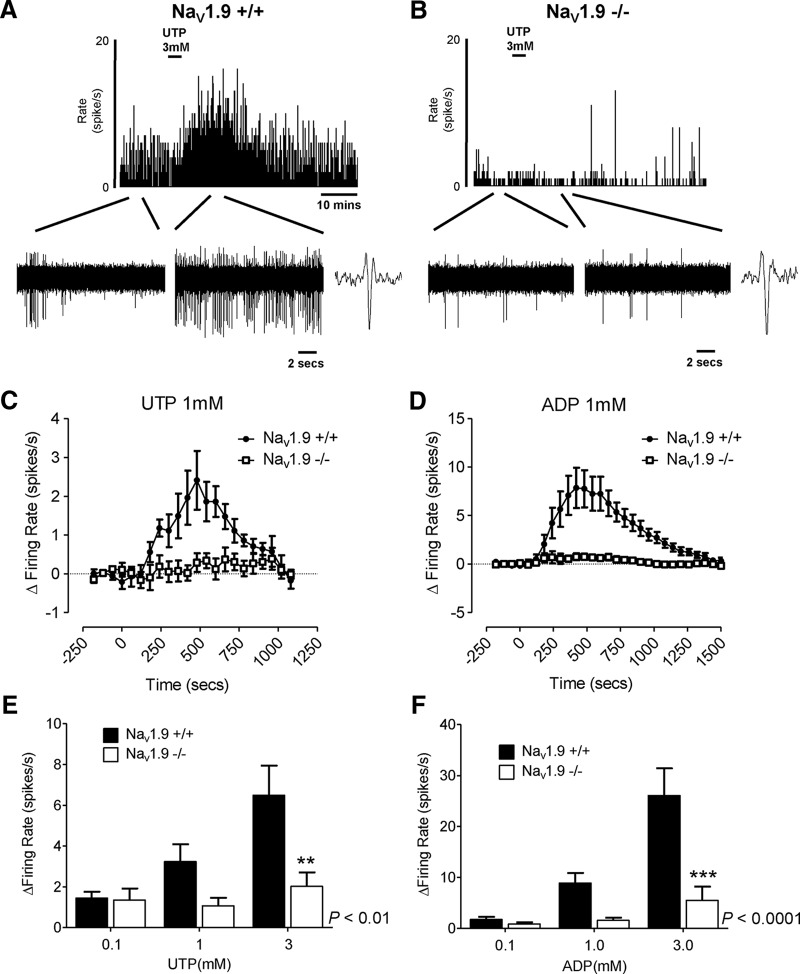
Finally, to confirm that Nav1.9 is required for P2Y receptor-mediated visceral afferent activation, we examined the effect of UTP or ADP application in whole-nerve recordings of LSN activity from Nav1.9−/− and wild-type (Nav1.9+/+) mice (Fig. 6). Responses to both UTP (p < 0.01, two-way ANOVA; Fig. 6E) and ADP (p < 0.0001, two-way ANOVA; Fig. 6F) were significantly reduced in tissue from Nav1.9−/− mice, confirming that P2Y receptor-mediated activation of visceral afferents is strongly coupled to Nav1.9 channels. These data provide further evidence in support of Nav1.9 as a regulator of excitability in visceral afferents to stimuli involved during chronic gastrointestinal inflammation.
Figure 6.
Effect of the deletion of Nav1.9 on colonic splanchnic nerve activity to purine and pyrimidine nucleotide agonists. A, B, Example rate histogram of nerve activity following addition of 3 mm UTP in Nav1.9+/+ mice (A) and Nav1.9−/− mice (B). Below, Expanded traces of nerve activity at baseline and after the application of UTP, with representative action potentials. C, D, Response profile to the addition of 1 mm UTP (C) and 1 mm ADP (D) in both Nav1.9+/+ and Nav1.9−/− mice (n = 6 for each group). E, F, Peak increase in nerve activity in Nav1.9+/+ and Nav1.9−/− mice following the addition of 0.1, 1, and 3 mm nucleotide agonists (E, UTP, p < 0.01, n = 6 each group; and F, ADP, p < 0.0001, n = 6 each group; two-way ANOVA with Bonferroni post hoc test). **p < 0.01, ***p < 0.001.
Discussion
Our data demonstrate that P2Y1, P2Y2, and P2Y4 receptors are expressed on gut-projecting sensory neurons and that agonist stimulation of these receptors sensitizes these neurons. We have shown that the application of UTP and ADP directly stimulate both mouse and human visceral afferents, and the expression of Nav1.9 is required for nucleotide activation of colonic afferents. We show that activation of P2Y receptors is capable of exciting mouse visceral nociceptors, an effect that translates to human nociceptors and, therefore, represents an understudied mechanism in the development of visceral pain. As a consequence, P2Y receptors are likely an important regulator of visceral pain and hypersensitivity in disease states where purinergic transmission is enhanced, such as with inflammation and during distension of the gut.
Purine and pyrimidine nucleotides, ATP and UTP, are released from tissues into the extracellular space, where their levels are regulated by active transport and enzymatic hydrolysis (Ralevic and Burnstock, 1998; Burnstock, 1999; Lazarowski and Boucher, 2001). In the gastrointestinal tract, endogenous ATP is released from the mucosal epithelium during distension, activating subepithelial afferents through purinoceptors, most notably P2X3 (Chen et al., 1995; Burnstock, 1999; Shinoda et al., 2009). In addition to mechanosensory transduction, the release of ATP from immune cells and following cell damage implicates purinergic signaling in nociception during inflammation, injury, and infection (North, 2002; Burnstock, 2009). In colitis, the contribution of ATP to mechanosensitive transduction is enhanced (Wynn et al., 2004). By contrast, the role of UTP in sensory signaling from the colon has not been extensively studied. Here we show that UTP is capable of sensitizing colonic sensory neurons. The same is not true of its metabolite and P2Y6 agonist UDP, which failed to sensitize gut-projecting neurons. Our data are consistent with studies showing increased action potential firing in sensory neurons by UTP and excitation of cutaneous afferent fibers (Molliver et al., 2002; Stucky et al., 2004; Lechner and Lewin, 2009). Indeed, thoracolumbar sensory neurons that project to the bladder via the LSN are sensitized by UTP acting on P2Y2 receptors (Chen et al., 2010). The present study builds on these findings, showing that UTP also sensitizes colonic sensory neurons, and that ADP acting at P2Y1 is sensitizing in these cells. Further, we demonstrate that nucleotide-dependent changes in sensory neuron excitability are consistent with increased afferent firing in visceral nociceptors of both mouse and human tissue. This suggests that P2Y receptors are a likely mechanism by which purine and pyrimidine nucleotides mediate visceral pain in human disease states.
Our findings are supported by indirect reports suggesting that P2Y receptors contribute to purinergic activation of visceral afferents and the production of visceral pain. Studies using P2X3 knock-out mice and P2X2/3 dual knock-out mice indicate that P2X2 and P2X3 receptors contribute to transduction of noxious stimuli, especially during inflammatory hypersensitivity (Cockayne et al., 2005; Rong et al., 2009; Shinoda et al., 2009). However, in the colon at least, residual responses to ATP in these mice suggest that afferent firing may be induced by pathways other than direct activation of P2X receptors (Rong et al., 2009; Shinoda et al., 2009). In agreement with this, we observe only partial reduction in afferent firing in response to ATP following the inhibition of P2X receptors by the application of selective antagonists TNP-ATP and PPADS, with these effects being comparable in magnitude to previous studies in P2X2/3 dual knock-out mice (Rong et al., 2009). Furthermore, given that adenosine is an ectonucleotidase breakdown product of ATP/ADP and can directly excite afferents in its own right, we show that the pan-P1 receptor antagonist CGS15943 only partially inhibits afferent discharge to ATP, which is consistent with previous reports using P1 antagonist 8p-SPT (Kirkup et al., 1998). In light of our findings that UTP and ADP stimulate visceral afferent activity, these data collectively support a role for P2Y receptors as a mechanism of visceral afferent activation alongside P2X receptors, although complex interactions may be occurring (Mo et al., 2013). Furthermore, this strongly suggests that the excitatory effects of UTP or ADP cannot be solely explained by either the activation or facilitation of P2X channels, or by P1 receptors by UTP/ADP or their metabolites.
Of the eight P2Y receptors, P2Y1, P2Y2, and P2Y6 receptors are coupled to stimulatory Gq/11-protein signaling cascades and are expressed in sensory neurons (Gerevich and Illes, 2004). The application of UDP had no effect on colonic afferent activity, suggesting that P2Y6 receptors are not involved in the sensitization of colonic neurons. To confirm that the effects of ADP and UTP on colonic neurons could be mediated by the expression of P2Y1 and P2Y2 receptors, respectively, we demonstrated the presence of P2Y1 transcripts and P2Y2 transcripts in 80% and 49% of colonic neurons, respectively, using qRT-PCR. P2Y4 transcripts were also observed in a smaller proportion (30%) of colonic neurons. No previous studies have examined the expression of P2Y receptors in colonic sensory neurons; however, the expression levels of P2Y2 are similar to those found in thoracolumbar sensory neurons that project to the bladder (Chen et al., 2010). The proportions of colonic sensory neurons expressing P2Y1 and P2Y2 were similar to proportions sensitized by the application of ADP or UTP in teased-fiber afferent studies (80% or 50%, respectively), providing support for the direct activation of visceral afferents by P2Y receptors.
One important consideration is the extensive expression of P2Y receptors by both enteric neurons and smooth muscle (Gallego et al., 2006; Zizzo et al., 2012), where they are involved in neuromuscular transmission and control of gastrointestinal motility. P2Y1 and P2Y6 receptors have been shown to mediate relaxation of intestinal smooth muscle, while P2Y2 and P2Y4 receptors are linked to contraction in the small intestine (Johnson and Hourani, 1994; Gallego et al., 2006; Zizzo et al., 2012). To mitigate this possible source of indirect afferent fiber activation, our preparations were treated with nifedipine to prevent resultant changes in gut contractility and atropine to inhibit any confounding cholinergic-mediated responses to enteric purinoceptor activation (Duarte-Araújo et al., 2009).
Significantly, we demonstrate that our observations in the distal colon of mouse translate to human colonic afferents by using ex vivo electrophysiological recordings of surgically resected descending colon. Visceral pain is associated with hyperexcitability and increased firing of colonic sensory neurons to gut distension and contraction, typically during, or following, an inflammatory insult (Knowles and Aziz, 2009). Our data suggest that purine and pyrimidine nucleotides acting at P2Y receptor subtypes can sensitize human visceral afferents to evoke increased action potential firing. Indeed, the ability for P2Y agonists to excite human visceral afferents was also observed in chronically inflamed tissues from IBD patients, suggesting that the activation of P2Y receptor subtypes cannot be discounted as a potential mechanism contributing to the subset of IBD patients in which chronic visceral pain represents a significant problem (Isgar et al., 1983; Grover et al., 2009). In contrast to murine afferents, however, the magnitude of response to UTP is significantly greater than ADP in human tissues. As we and others have shown, ADP and UTP can evoke comparable sensitization of mouse sensory neurons (Fig. 1; Yousuf et al., 2011), suggesting that any switch in potency may be mediated by changes in the downstream coupling of P2Y1 and P2Y2 receptors between mouse and human, or by the difference in binding affinities between species. The functional consequence of this is not clear; however, this effect was observed in both noninflamed and, to a lesser extent, inflamed bowel tissues, indicating that the effect is not attributable to specific disease pathology.
Finally, we sought to examine the importance of the voltage-gated sodium channel subtype Nav1.9 in the P2Y receptor-mediated activation of visceral afferents. We have previously shown that ATP can enhance Nav1.9 currents (Baker, 2005) and that Nav1.9 enhancement occurs via a G-protein-coupled mechanism (Baker et al., 2003), suggesting that Nav1.9 could be a downstream effector for P2Y receptor-mediated increases in neuronal excitability. We found that 86% of P2Y1-positive, 100% of P2Y2-positive, and 92% of P2Y4-positive mouse colonic sensory neurons coexpressed Nav1.9 transcripts, demonstrating that Nav1.9 is likely to be a significant effector for P2Y receptor-expressing visceral afferents. Indeed, we observe substantial reductions in the afferent response to UTP and ADP in Nav1.9−/− mice, which were greatest at the lower concentrations of the nucleotides examined. The role of Nav1.9 in prolonging subthreshold depolarizing stimuli and as a threshold channel regulating neuronal excitability may confer increased sensitivity to nucleotides in afferents that coexpress Nav1.9 and P2Y receptors. The observed importance of Nav1.9 in visceral afferent activation by nucleotides is of great significance in light of recently identified human Nav1.9 channelopathies associated with multiple pain disorders (including episodic pain syndrome and painful neuropathy), providing evidence that Nav1.9-expressing afferents function as nociceptors in humans (Zhang et al., 2013; Huang et al., 2014; Han et al., 2015).
To conclude, our data demonstrate that P2Y receptors are expressed in visceral sensory neurons. Activation of P2Y receptors by selective agonists sensitizes visceral neurons and directly excites both rodent and human colonic nociceptors, indicating an important role for P2Y receptors in the generation of visceral pain during gastrointestinal diseases where purinergic signaling is enhanced.
Footnotes
This work was supported by The Medical Research Council (D.C.B.), a Wellcome Trust University Award (L.A.B.), Neusentis (D.C.B.), The Royal College of Surgeons of England (G.B.), The Biotechnology and Biological Sciences Research Council (J.R.F.H.), Bowel & Cancer Research (M.A.T.), Pain Relief Foundation and Crohn's in Childhood Research Association (V.C.-G.), and The Dr Hadwen Trust for Humane Research (C.M.). We thank Anna Wilbrey and Ben Sidders for expert technical assistance.
The authors declare no competing financial interests.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License Creative Commons Attribution 4.0 International, which permits unrestricted use, distribution and reproduction in any medium provided that the original work is properly attributed.
References
- Amaya F, Wang H, Costigan M, Allchorne AJ, Hatcher JP, Egerton J, Stean T, Morisset V, Grose D, Gunthorpe MJ, Chessell IP, Tate S, Green PJ, Woolf CJ. The voltage-gated sodium channel Nav1.9 is an effector of peripheral inflammatory pain hypersensitivity. J Neurosci. 2006;26:12852–12860. doi: 10.1523/JNEUROSCI.4015-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker MD. Protein kinase C mediates up-regulation of tetrodotoxin-resistant, persistent Na+ current in rat and mouse sensory neurones. J Physiol. 2005;567:851–867. doi: 10.1113/jphysiol.2005.089771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker MD, Chandra SY, Ding Y, Waxman SG, Wood JN. GTP-induced tetrodotoxin-resistant Na+ current regulates excitability in mouse and rat small diameter sensory neurones. J Physiol. 2003;548:373–382. doi: 10.1113/jphysiol.2003.039131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleehen T, Keele CA. Observations on the algogenic actions of adenosine compounds on the human blister base preparation. Pain. 1977;3:367–377. doi: 10.1016/0304-3959(77)90066-5. [DOI] [PubMed] [Google Scholar]
- Brierley SM, Jones RC, 3rd, Gebhart GF, Blackshaw LA. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology. 2004;127:166–178. doi: 10.1053/j.gastro.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Brierley SM, Carter R, Jones W, 3rd, Xu L, Robinson DR, Hicks GA, Gebhart GF, Blackshaw LA. Differential chemosensory function and receptor expression of splanchnic and pelvic colonic afferents in mice. J Physiol. 2005;567:267–281. doi: 10.1113/jphysiol.2005.089714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes SJ, Spencer NJ, Costa M, Zagorodnyuk VP. Extrinsic primary afferent signalling in the gut. Nat Rev Gastroenterol Hepatol. 2013;10:286–296. doi: 10.1038/nrgastro.2013.29. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Release of vasoactive substances from endothelial cells by shear stress and purinergic mechanosensory transduction. J Anat. 1999;194:335–342. doi: 10.1046/j.1469-7580.1999.19430335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purinergic mechanosensory transduction and visceral pain. Mol Pain. 2009;5:69. doi: 10.1186/1744-8069-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Akopian AN, Sivilotti L, Colquhoun D, Burnstock G, Wood JN. A P2X purinoceptor expressed by a subset of sensory neurons. Nature. 1995;377:428–431. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- Chen X, Molliver DC, Gebhart GF. The P2Y2 receptor sensitizes mouse bladder sensory neurons and facilitates purinergic currents. J Neurosci. 2010;30:2365–2372. doi: 10.1523/JNEUROSCI.5462-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockayne DA, Dunn PM, Zhong Y, Rong W, Hamilton SG, Knight GE, Ruan HZ, Ma B, Yip P, Nunn P, McMahon SB, Burnstock G, Ford AP. P2X2 knockout mice and P2X2/P2X3 double knockout mice reveal a role for the P2X2 receptor subunit in mediating multiple sensory effects of ATP. J Physiol. 2005;567:621–639. doi: 10.1113/jphysiol.2005.088435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte-Araújo M, Nascimento C, Timóteo MA, Magalhães-Cardoso MT, Correia-de-Sá P. Relative contribution of ecto-ATPase and ecto-ATPDase pathways to the biphasic effect of ATP on acetylcholine release from myenteric motoneurons. Br J Pharmacol. 2009;156:519–533. doi: 10.1111/j.1476-5381.2008.00058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego D, Hernández P, Clavé P, Jiménez M. P2Y1 receptors mediate inhibitory purinergic neuromuscular transmission in the human colon. Am J Physiol Gastrointest Liver Physiol. 2006;291:G584–G594. doi: 10.1152/ajpgi.00474.2005. [DOI] [PubMed] [Google Scholar]
- Gerevich Z, Illes P. P2Y receptors and pain transmission. Purinergic Signal. 2004;1:3–10. doi: 10.1007/s11302-004-4740-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover M, Herfarth H, Drossman DA. The functional-organic dichotomy: postinfectious irritable bowel syndrome and inflammatory bowel disease-irritable bowel syndrome. Clin Gastroenterol Hepatol. 2009;7:48–53. doi: 10.1016/j.cgh.2008.08.032. [DOI] [PubMed] [Google Scholar]
- Han C, Yang Y, de Greef BT, Hoeijmakers JG, Gerrits MM, Verhamme C, Qu J, Lauria G, Merkies IS, Faber CG, Dib-Hajj SD, Waxman SG. The domain II S4–S5 linker in Nav1.9: a missense mutation enhances activation, impairs fast inactivation, and produces human painful neuropathy. Neuromolecular Med. 2015;17:158–169. doi: 10.1007/s12017-015-8347-9. [DOI] [PubMed] [Google Scholar]
- Hechler B, Nonne C, Roh EJ, Cattaneo M, Cazenave JP, Lanza F, Jacobson KA, Gachet C. MRS2500 [2-iodo-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate], a potent, selective, and stable antagonist of the platelet P2Y1 receptor with strong antithrombotic activity in mice. J Pharmacol Exp Ther. 2006;316:556–563. doi: 10.1124/jpet.105.094037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockley JR, Boundouki G, Cibert-Goton V, McGuire C, Yip PK, Chan C, Tranter M, Wood JN, Nassar MA, Blackshaw LA, Aziz Q, Michael GJ, Baker MD, Winchester WJ, Knowles CH, Bulmer DC. Multiple roles for NaV1.9 in the activation of visceral afferents by noxious inflammatory, mechanical, and human disease-derived stimuli. Pain. 2014;155:1962–1975. doi: 10.1016/j.pain.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Han C, Estacion M, Vasylyev D, Hoeijmakers JG, Gerrits MM, Tyrrell L, Lauria G, Faber CG, Dib-Hajj SD, Merkies IS, Waxman SG. Gain-of-function mutations in sodium channel NaV1.9 in painful neuropathy. Brain. 2014;137:1627–1642. doi: 10.1093/brain/awu079. [DOI] [PubMed] [Google Scholar]
- Isgar B, Harman M, Kaye MD, Whorwell PJ. Symptoms of irritable bowel syndrome in ulcerative colitis in remission. Gut. 1983;24:190–192. doi: 10.1136/gut.24.3.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CR, Hourani SM. Contractile effects of uridine 5′-triphosphate in the rat duodenum. Br J Pharmacol. 1994;113:1191–1196. doi: 10.1111/j.1476-5381.1994.tb17123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkup AJ, Eastwood C, Grundy D, Chessell IP, Humphrey PP. Characterization of adenosine receptors evoking excitation of mesenteric afferents in the rat. Br J Pharmacol. 1998;125:1352–1360. doi: 10.1038/sj.bjp.0702202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles CH, Aziz Q. Basic and clinical aspects of gastrointestinal pain. Pain. 2009;141:191–209. doi: 10.1016/j.pain.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Lazarowski ER, Boucher RC. UTP as an extracellular signaling molecule. News Physiol Sci. 2001;16:1–5. doi: 10.1152/physiologyonline.2001.16.1.1. [DOI] [PubMed] [Google Scholar]
- Lazarowski ER, Harden TK. Quantitation of extracellular UTP using a sensitive enzymatic assay. Br J Pharmacol. 1999;127:1272–1278. doi: 10.1038/sj.bjp.0702654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner SG, Lewin GR. Peripheral sensitisation of nociceptors via G-protein-dependent potentiation of mechanotransduction currents. J Physiol. 2009;587:3493–3503. doi: 10.1113/jphysiol.2009.175059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo G, Peleshok JC, Cao CQ, Ribeiro-da-Silva A, Séguéla P. Control of P2X3 channel function by metabotropic P2Y2 utp receptors in primary sensory neurons. Mol Pharmacol. 2013;83:640–647. doi: 10.1124/mol.112.082099. [DOI] [PubMed] [Google Scholar]
- Molliver DC, Cook SP, Carlsten JA, Wright DE, McCleskey EW. ATP and UTP excite sensory neurons and induce CREB phosphorylation through the metabotropic receptor, P2Y2. Eur J Neurosci. 2002;16:1850–1860. doi: 10.1046/j.1460-9568.2002.02253.x. [DOI] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Ostman JA, Nassar MA, Wood JN, Baker MD. GTP up-regulated persistent Na+ current and enhanced nociceptor excitability require NaV1.9. J Physiol. 2008;586:1077–1087. doi: 10.1113/jphysiol.2007.147942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris M, Bulmer DC, Baker MD, Boundouki G, Sinha S, Hobson A, Lee K, Aziz Q, Knowles CH. Human visceral afferent recordings: preliminary report. Gut. 2011;60:204–208. doi: 10.1136/gut.2010.221820. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Robinson DR, McNaughton PA, Evans ML, Hicks GA. Characterization of the primary spinal afferent innervation of the mouse colon using retrograde labelling. Neurogastroenterol Motil. 2004;16:113–124. doi: 10.1046/j.1365-2982.2003.00456.x. [DOI] [PubMed] [Google Scholar]
- Rong W, Keating C, Sun B, Dong L, Grundy D. Purinergic contribution to small intestinal afferent hypersensitivity in a murine model of postinfectious bowel disease. Neurogastroenterol Motil. 2009;21:665–671. e32. doi: 10.1111/j.1365-2982.2008.01259.x. [DOI] [PubMed] [Google Scholar]
- Sanada M, Yasuda H, Omatsu-Kanbe M, Sango K, Isono T, Matsuura H, Kikkawa R. Increase in intracellular Ca(2+) and calcitonin gene-related peptide release through metabotropic P2Y receptors in rat dorsal root ganglion neurons. Neuroscience. 2002;111:413–422. doi: 10.1016/S0306-4522(02)00005-2. [DOI] [PubMed] [Google Scholar]
- Shinoda M, Feng B, Gebhart GF. Peripheral and central P2X receptor contributions to colon mechanosensitivity and hypersensitivity in the mouse. Gastroenterology. 2009;137:2096–2104. doi: 10.1053/j.gastro.2009.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucky CL, Medler KA, Molliver DC. The P2Y agonist UTP activates cutaneous afferent fibers. Pain. 2004;109:36–44. doi: 10.1016/j.pain.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Wada M, Masu M. Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proc Natl Acad Sci U S A. 2001;98:6951–6956. doi: 10.1073/pnas.111025298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kügelgen I. Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther. 2006;110:415–432. doi: 10.1016/j.pharmthera.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Wickham H. Ggplot2: elegant graphics for data analysis. New York: Springer; 2009. [Google Scholar]
- Wynn G, Ma B, Ruan HZ, Burnstock G. Purinergic component of mechanosensory transduction is increased in a rat model of colitis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G647–G657. doi: 10.1152/ajpgi.00020.2004. [DOI] [PubMed] [Google Scholar]
- Yousuf A, Klinger F, Schicker K, Boehm S. Nucleotides control the excitability of sensory neurons via two P2Y receptors and a bifurcated signaling cascade. Pain. 2011;152:1899–1908. doi: 10.1016/j.pain.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaika O, Tolstykh GP, Jaffe DB, Shapiro MS. Inositol triphosphate-mediated Ca2+ signals direct purinergic P2Y receptor regulation of neuronal ion channels. J Neurosci. 2007;27:8914–8926. doi: 10.1523/JNEUROSCI.1739-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XY, Wen J, Yang W, Wang C, Gao L, Zheng LH, Wang T, Ran K, Li Y, Li X, Xu M, Luo J, Feng S, Ma X, Ma H, Chai Z, Zhou Z, Yao J, Zhang X, Liu JY. Gain-of-function mutations in SCN11A cause familial episodic pain. Am J Hum Genet. 2013;93:957–966. doi: 10.1016/j.ajhg.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zizzo MG, Mastropaolo M, Grählert J, Mulè F, Serio R. Pharmacological characterization of uracil nucleotide-preferring P2Y receptors modulating intestinal motility: a study on mouse ileum. Purinergic Signal. 2012;8:275–285. doi: 10.1007/s11302-011-9281-4. [DOI] [PMC free article] [PubMed] [Google Scholar]