Abstract
Background
Acute diarrhea accounts for a huge burden of infectious diseases in under-five children.
Objective
This systematic review was carried out to study the prevalence and associated risk factors of diarrhea among Indian children aged <5 years.
Methods
Papers were identified by a comprehensive electronic search of relevant medical subject heading (MeSH) terms in PubMed. Identified articles were independently reviewed against inclusion/exclusion criteria and rated for quality. 15 articles were abstracted and reviewed to identify the reported prevalence and risk factors for childhood diarrhea. Meta-analysis was done for calculating the pooled prevalence of diarrhea and point estimates of risk factors using random effects model with use of appropriate population weights, and depicted using forest plot.
Results
The overall prevalence of diarrhea between 2002 and 2013 was 21.70% (95% confidence interval [CI]: 11.24–34.46). The significantly associated risk factors were malnutrition (odds ratio [OR]: 1.73, 95% CI: 1.53–1.96) and anemia (OR: 1.71, 95% CI: 1.29–2.28) in child, and low socioeconomic status (OR: 7.14, 95% CI: 2.19–23.32). Age of the child <24 months, not breastfeeding, mothers’ low literacy status and untreated drinking water did not show a significant association. Sex of the child, religion, higher education of mothers, and seasonality were found to be inconsistently associated in single studies.
Conclusion
It was concluded that there is sufficient evidence on the association of childhood diarrhea with socio-demographic factors, but evidence on other contributory factors including breastfeeding and vaccination is inconclusive. There is need to conduct more analytical studies on lesser known risk factors of diarrhea to establish their risk factors in Indian children.
Keywords: Diarrhea, India, Meta-analysis, Prevalence, Systematic review, Under-five children, Risk factors
Children under 5 years of age frequently suffer from acute infectious diseases. Childhood diarrhea causes a huge burden killing approximately 7 lakh children under the age of 5 years constituting almost 16% of child deaths globally [1]. Literature from the developing countries shows a high burden of morbidity with an estimated 1.7 billion episodes (2.9 episodes per child per year), concentrated in Southeast Asia and Sub Saharan Africa [2]. UNICEF, while quoting World Health Organization data has reported about 3.8 lakh deaths due to diarrhea among under-five children annually in India in 2004, the highest in the world. Published literature from India suggests the higher prevalence of childhood diarrhea in rural areas compared to the urban [3], a finding supported by National Family Health Survey 3 (NFHS) data (2005–06) that reported about 8.3% of slum children aged under 5 years suffering from diarrhea during 2 weeks preceding the survey. Most studies also indicate the higher prevalence of multiple risk factors for diarrhea in urban slum areas [3,4], similar to NFHS 3 findings.
Numerous studies conducted worldwide indicate that most of the childhood diarrhea, which is usually worsened in severity and duration due to a multitude of sociocultural and environmental factors [4], can be controlled by instituting simple control measures at the household or primary health care level. The Government of India introduced novel interventions including low osmolarity oral rehydration salts, zinc and ciprofloxacin for diarrhea treatment in addition to the existing measures that include adequate nutritional support and personal and community sanitation that reduced the risk for diarrhea [5].
The control of childhood infections has been advocated as a major strategy to reduce child deaths under Millennium Development Goals-4 [6]. New strategies were implemented to achieve this goal through innumerate child survival innovations and interventions encompassing management with local resources at peripheral level, widespread availability of low-cost reverse osmosis drinking water, sanitation improvements, increasing awareness through the use of mobile technology and media to increase diarrhea related vaccines and techniques to alleviate poverty [1,5], all of which have been shown to reduce mortality by almost 50% [6]. In spite of the effective interventions, diarrheal diseases are still prevalent. The most recent systematic review on diarrhea prevention interventions identified a gap pertaining to knowledge of etiological factors and prevalence trends over the last decade, thus making designing control interventions with substantial impact difficult [5].
Current literature is abound with region specific examples of harmful practices and other socio-cultural factors that may have led to this situation of not only high mortality from diarrheal diseases, but also an unexplainable high burden of morbidity among Indian children [4,7]. Moreover, the burden of childhood diarrhea in India in the past 10 years has not changed in spite of the implementation of multiple efficacious interventions. We also hypothesized that there might be a change in the risk factors over the past decade. The present systematic review was carried out to study the prevalence and the associated risk factors of diarrhea to generate evidence for initiating need-based action in this field.
METHODS
Search Strategy
We sought articles with original data on the risk factors associated with diarrheal disease among children younger than 5 years in India. Outcomes of interest included diarrhea prevalence and its risk factors in children. Articles were identified from a systematic search of PubMed. The search covered articles published from January 1, 2002, to December 31, 2013. PubMed was searched using the following algorithm containing MeSH terms combined with text words: “Child, preschool” (MeSH terms) AND “morbidity” (MeSH terms) AND “India” (MeSH terms) AND “epidemiologic factors” (MeSH terms) AND “diarrhea” (MeSH terms) OR “diarrhea, infantile” (MeSH terms).
Selection Criteria and Data Extraction
Studies were eligible for inclusion if full text was available in English, and if the prevalence of diarrhea or gastrointestinal disease was reported and if the association of risk factors in relation to childhood diarrhea on Indian children 5 years of age and younger was reported. Systematic reviews, review articles, meta-analyses, editorials, case reports, duplicate publications, withdrawn publications, conference proceedings, studies published before 2001, studies among children older than 5 years of age, studies from outside India, studies not reporting risk factors, not conducted on humans and not community-based studies were excluded.
Study Selection
The search identified 228 articles potentially involving childhood diarrhea. Two independent reviewers (EG and PS) reviewed the titles and abstracts and narrowed the list of relevant articles to 26. If the abstract indicated that the study fulfilled the eligibility criteria or the abstract did not provide sufficient information for selection decision, the reviewers assessed the full texts of articles for eligibility. If necessary, supplementary files were also reviewed for additional information. Reading of the full text resulted in the inclusion of 15 articles which had data on childhood diarrhea in children <5 years old (Fig. 1).
Figure 1.
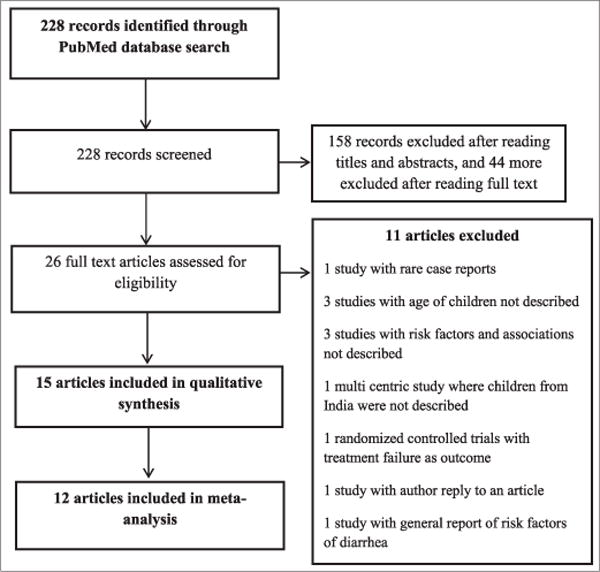
PRISMA diagram showing selection of studies for inclusion in systematic review and meta-analysis of determinants of diarrhea among under-five children in India
Data Extraction
A data collection form, that was designed prior to the implementation of the search strategy, was used by the reviewers to extract the relevant information from the selected studies. The form included questions on identifying study author, year of publication, study design, geographic origin and study setting, patient samplings, information on the frequency of the reported outcomes and association of diarrhea with risk factors.
Statistical Analysis
Data analysis was done using Comprehensive Meta-Analysis software (version 2.2 Engelwood, NJ, Biostat Inc.) [8] and Stats Direct (version 2.7.8) software. Using the reported percentage prevalence and sample size for each of the included studies, standard error (SE) of prevalence was calculated using the formula √[p×(1−p)/n], where p is the proportion of prevalence and n is the reported sample size. Pooled proportions with 95% confidence intervals (CI) were calculated using the DerSimonian and Laird method (random effects model) and depicted using Forest plots. Urban and rural pooled estimate for the prevalence of childhood diarrhea weighted by population size in each study was also calculated. Pooling of the selected risk factors of diarrhea was also done using the random effects model. Heterogeneity was assessed using I2 (% residual variation due to heterogeneity), Cochran Q (describing the percentage of total variation across studies due to heterogeneity) and τ2 (method of moments estimate of between-study variance in a random effects meta-analysis) for each of the pooled proportions. The values of I2 (p value) and Cochran Q (df), and τ2 (standard error) were calculated for pooled prevalence of diarrhea in urban and rural areas, and for its risk factors as well (Supplementary File [Supplementary files are available with editor]).
Quality Rating of Studies
A composite quality construct of methodology using the STROBE Statement [9] for reporting observational studies was devised to draw conclusions about the strength of evidence drawn from these studies. The authors adopted a simplified rating procedure; one point was assigned to each relevant subhead in the methods section of the checklist (Supplementary File 1). The checklist was validated by two independent reviewers who rated ten observational articles (unrelated) each and interobserver agreement was calculated using weighted kappa statistic. The maximum total score was ten points. Articles securing 1–4 points were rated poor, 5–7 as fair and 8–10 as good quality articles. The other sections of the checklist were not used for quality rating. The stated objectives of the paper were also matched to the reporting of outcomes within the paper before assigning the final quality. Thus, an article which was methodologically robust and secured 10 points according to the quality construct, but did not report the outcomes of stated objectives in the results section was still rated poor. Although each included study was rated for quality, the quality scores were not incorporated in the meta-analysis weights.
RESULTS
15 studies met the criteria to be included in this review. The quality rating and characteristics of the studies are shown in Table 1.
Table 1.
Characteristics of the included studies
| Authors | Year | Study design | Setting | Quality |
|---|---|---|---|---|
| Ahmed et al. [7] | 2008 | Cross-sectional | Kashmir | Fair |
| Nandy et al. [10] | 2005 | Cohort, retrospective, data analysis | All India | Good |
| Gladstone et al. [11] | 2010 | Cohort | Vellore, Tamil Nadu | Poor |
| Deshmukh et al. [12] | 2009 | Cross-sectional | Wardha, Maharashtra | Good |
| Phadke et al. [13] | 2003 | Observational (case control) | Pune, Maharashtra | Good |
| Gladstone et al. [14] | 2008 | Cohort study | Vellore, Tamil Nadu | Good |
| Mazumder et al. [15] | 2010 | Cluster randomized trial | Faridabad, Haryana | Fair |
| Joshi et al. [16] | 2011 | Two stage stratified cluster survey | Uttar Pradesh | Good |
| Sur et al. [17] | 2004 | Period prevalence, surveillance study | Kolkata | Fair |
| Banerjee et al. [18] | 2004 | Prospective (observational) | West Bengal (Urban) | Fair |
| Rose et al. [19] | 2006 | Case control study | Vellore, Tamil Nadu | Good |
| Mishra et al. [20] | 2010 | Cross sectional study | Lucknow, Uttar Pradesh | Good |
| Bandyopadhyay and Banerjee [21] | 2005 | Cross sectional study | Kolkata | Poor |
| Khush et al. [22] | 2013 | Cohort study | Tiruchirapalli, Tamil Nadu | Good |
| Dhingra et al. [23] | 2009 | Randomized controlled trial | New Delhi | Fair |
1–4: Poor, 5–7: Fair, 8–10: Good
Burden of Childhood Diarrhea in India
Burden of diarrhea varied widely depending on the population studied. Among children aged <5 years, 12 studies [7,11–19,21,22] reported the prevalence of diarrhea to be 2.2% [17] to 55.6% [16]. The overall pooled prevalence of diarrhea between 2002 and 2013 was 21.70% (95% CI: 11.24–34.46) (Fig, 2). The prevalence in rural areas (14.04%) was found to be lower compared to urban 23.89% (Table 2). p<0.0001 for the I2 values and Cochran Q suggest gross heterogeneity in the literature included in meta-analysis for calculating rural, urban and combined pooled prevalence, whereas Kendall’s tau (τ) suggests low power. The bias assessment plot for pooled prevalence is shown as Fig. 3.
Figure 2.
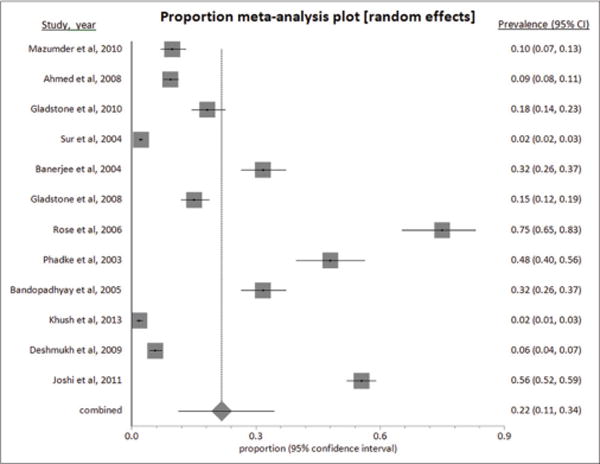
Pooled prevalence of childhood diarrhea
Table 2.
Point estimates for diarrhea prevalence among under-five children in India
| Place | Pooled prevalence (95% CI) | I2 (95% CI) | Cochran Q (df) (p value) | Kendall’s τ (p value) |
|---|---|---|---|---|
| Rural (n=4) | 14.04 (0.43–41.74) | 99.72 (99.63–99.78) | 1054.79 (3) (p<0.0001) | 1 (p=0.0833) |
| Urban (n=9) | 23.89 (12.45–37.66) | 99.2 (99.1–99.3) | 989.28 (8) (p<0.0001) | 0.94 (p=0.0007) |
| Overall (n=12) | 21.70 (11.24–34.46) | 99.5 (99.4–99.5) | 2102.20 (11) (p<0.0001) | 0.81 (p=0.0003) |
CI: Confidence interval
Figure 3.
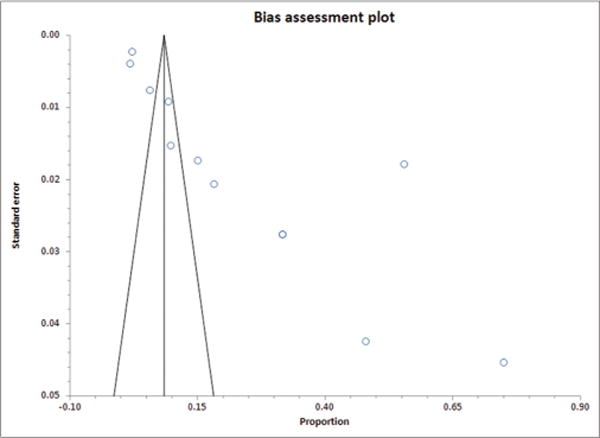
Funnel plot for bias assessment of included studies for calculating pooled prevalence of diarrhea
Risk Factors of Diarrhea
Risk factors are categorized into three categories-significantly associated, non-associated and non-consistently associated.
Diarrhea associated risk factors: Young age (<23 months) has been cited as the predominant risk factor positively associated with all types of diarrhea [7,11,12,14,16], whereas age <60 months has been found to be significantly positively associated to diarrhea by two studies [12,17]. Strong positive association with anemia [12,16], severe malnutrition with three anthropometric failures [10,12] and mothers’ education up to high school compared with mothers having a higher education was also reported in few studies [12,14]. Children belonging to low socioeconomic strata classified according to modified Prasad’s classification [18], also identified by characteristics as households taken loan [16], household income below 2000 INR per month [17] or involved in small occupations like beedi work [14] were significantly positively associated with childhood diarrhea. Single studies also reported significant positive association to adverse season [7,11,14], water supply from non-community sources [17], non-use of solar disinfected water [19], kutcha house [17], another member having diarrhea [17], use of toilets [17] or no hand washing after defecation [17]. The role of zinc in diarrhea prevention has been highlighted by two studies, one on imparting education to caregivers about zinc supplementation [15], and second on the study of low plasma zinc levels [23], both of which were reported significantly associated. Hindu religion [17] and female sex of the child [13,14] were reported significant positive association too (Table 3).
Table 3.
Summary of studies enumerating risk factors of diarrhea in children
| Authors (year) |
Place | Number of participants |
Age (months) |
Out come | Burden reported |
Risk factors | Test of association |
Strength of association |
CI |
|---|---|---|---|---|---|---|---|---|---|
| Joshi et al. [16] | Rural | 800 children (in 40 clusters) | 6–59 | Diarrhea | 55.6% | Age 6–17 months | OR | – | p<0.001 |
| Family taken loan | OR | – | p=0.002 | ||||||
| No anemia | OR | Ref. | – | ||||||
| Anemia | OR | 2.03 | 1.26–3.25 | ||||||
| Mazumder et al. [15] | Urban and rural | 414 children (int) 410 children (con) |
<6 | Diarrhea (in preceding 24 h) | 7.7% (int) | Educating caregivers to give zinc and ORS during diarrhea | OR | 0.34 | 0.18–0.63 |
| 9.8% (con) | |||||||||
| Ahmed et al. [7] | Urban | 1055 | 0–59 | Diarrhea | 9.3% | Age 6–11 months Summer season | |||
| Gladstone et al. [11] | Urban slum | 373 children | 0–36 | Acute diarrheal disease | 18.4% | First year of life Hot/dry season (March to June) | – | ||
| Sur et al. [17] | Urban | 4389 children | <60 | Diarrhea | 2.2% | No household member with diarrhea | OR | Ref. | |
| Another household member with diarrhea | OR | 3.8 | 3.3–4.4 | ||||||
| Age under 60 months | OR | 3.7 | 3–4.7 | ||||||
| Muslim | OR | Ref. | – | ||||||
| Hindu religion | OR | 2.1 | 1.7–2.6 | ||||||
| Living in pucca house | OR | Ref. | – | ||||||
| Living in a semipucca or kuccha house | OR | 1.6 | 1.2–2.2 | ||||||
| Not using latrine | OR | Ref. | – | ||||||
| Using latrine | OR | 1.5 | 1–2.3 | ||||||
| Household expenditure >2000 INR per month | OR | Ref. | – | ||||||
| Below 2,000 INR per month | OR | 1.3 | 1–1.6 | ||||||
| Handwashing after defecation | OR | Ref. | – | ||||||
| No regular handwashing after defecation | OR | 1.3 | 1–1.7 | ||||||
| Water from community tap | OR | Ref. | – | ||||||
| Water from other sources | OR | 1.3 | 1–1.7 | ||||||
| Banerjee et al. [18] | Urban | 300 children | 0–59 | Diarrhea Dysentery |
31.67% 7.67% |
Lower socio economic class (compared to upper middle class) |
Z | 2.406 | p<0.005 |
| Lower class compared with lower middle class | Z | 1.78 | p<0.05 | ||||||
| Deshmukh et al. [12] | Rural | 990 children | 0–36 | Acute | 5.7% | Children aged 0–11 months | OR | Ref. | – |
| morbidities | 0.3% | Children aged 12–23 months | OR | 1.62 | 1.16–2.22 | ||||
| Diarrhea | Mother educated more than 10 years | OR | Ref. | – | |||||
| Dysentery | Mothers having 1–10 years of education | OR | 1.43 | 1.06–1.93 | |||||
| No anemia | OR | Ref. | – | ||||||
| Mild anemia | OR | 1.56 | 1.09–2.23 | ||||||
| Moderate anemia | OR | 1.57 | 1.14–2.17 | ||||||
| Severe Anemia | OR | 8.9 | 1.17–70.83 | ||||||
| No anthropometric failure | OR | Ref. | – | ||||||
| Children with three anthropometric failures | OR | 2.27 | 1.13–4.64 | ||||||
| Nandy et al. [10] | Urban and rural | 24,396 children | 0–36 | Diarrhea | No failure | OR | Ref. | – | |
| Wasting and underweight | OR | 1.45 | 1.27–1.65 | ||||||
| Wasting, stunting and underweight | OR | 1.72 | 1.52–1.95 | ||||||
| Stunting and underweight | OR | 1.54 | 1.42–1.67 | ||||||
| Underweight only | OR | 1.19 | 1.03–1.37 | ||||||
| Severe diarrhea | Wasting, stunting and underweight | OR | 1.95 | 1.45–2.61 | |||||
| Stunting and underweight | OR | 2.03 | 1.66–2.49 | ||||||
| Underweight | OR | 1.64 | 1.17–2.29 | ||||||
| Gladstone et al. [14] | Urban | 452 children | GI illness-diarrhea or vomiting | 15.2% (3.6 episodes per child-year) | Age 0–2 months | RR | Ref. | – | |
| Age 9–11 months | RR | 0.8 | 0.7–0.9, p=0.001 | ||||||
| Male | RR | Ref. | – | ||||||
| Female | RR | 0.8 | 0.7–1.0, p=0.02 | ||||||
| Hot/dry season | RR | Ref. | – | ||||||
| Cold/wet season | RR | 0.9 | 0.8–1.0, p=0.004 | ||||||
| No beedi work | RR | Ref. | – | ||||||
| Beedi work | RR | 1.3 | 1.1–1.5, p=0.003 | ||||||
| Mother’s education none | RR | Ref. | – | ||||||
| High school/college | RR | 0.8 | 0.6–0.8 | ||||||
| Rose et al. [19] | Urban slum | 100 cases and 100 controls | 6–59 | Diarrhea | Incidence 1.7 per child year (int) 2.7 (con) | Use of solar disinfected drinking water | IRR | 0.64 | 0.48–0.86 |
| Severe diarrhea | Incidence 0.6 per child year (int) 1.3 (con) | Use of solar disinfected drinking water | IRR | 0.45 | 0.28–0.72 | ||||
| Phadke et al. [13] | Urban | 148 infants born to mothers with HIV | <60 | Acute gastroenteritis Jaundice | 48.1% | Breast fed | OR | Ref. | – |
| 11.1% | Replacement feeding | OR | 0.093 | 0.062–0.136 | |||||
| Bandyopadhyay and Banerjee [21] | Peri-Urban | 300 | <5 | Diarrhea | 31.67% | Increasing educational level of mother | p<0.005 | ||
| Increase in family income | p<0.05 | ||||||||
| Working mothers | p<0.001 | ||||||||
| Untrained outsider caregiver | p<0.005 | ||||||||
| Fed using hand | p<0.01 | ||||||||
| Khush et al. [22] | Rural | 1284 | <5 | Diarrhea | 1.8% | H2S positive (fecal contamination of water) | PR | 1.17 | 0.54–2.56 |
| Total coliforms >10,000 | PR | 0.73 | 0.45–1.17 | ||||||
| E. coli 101–1000 | PR | 1.14 | 0.58–1.23 | ||||||
| E. coli >1000 | PR | 1.66 | 0.65–4.21 | ||||||
| Mishra et al. [20] | Urban | 412 | 1–36 | Diarrhea-rotavirus | Not currently breast fed | OR | 2.2 | 1.13–4.64 | |
| Children ≤7 months of age | OR | 1.1 | 0.58–1.23 | ||||||
| Children with severe dehydration | OR | 1.8 | 1.52–1.95 | ||||||
| Dhingra et al. [23] | Urban | 940 | 6–35 | Diarrhea | Plasma zinc levels <56.0 mcg/dl | Adjusted RR | 1.02 | 0.81–1.29 | |
| Plasma zinc levels <56.0 mcg/dl | Adjusted RR | 1.32 | 1.18–1.49 | ||||||
| Plasma zinc levels <56.0 mcg/dl | Adjusted RR | 1.25 | 0.75–2.07 |
OR: Odds ratio, RR: Risk ratio, PR: Prevalence ratio, IRR: Incidence rate ratio, ORS: Oral rehydration salts
Diarrhea non-associated risk factors: Few studies found no significant association of following factors with diarrhea – low SES classified according to modified Prasad’s classification [12], other backward caste [12], child with one or two anthropometric failures [10,12], mild or moderate malnutrition [16], uneducated mothers [12], and mothers studied up to primary or middle school [14]. Only one study [13] conducted to explore the role of maternal antenatal and postnatal characteristics including parity, maternal anemia, gestational age, male sex, APGAR scores and low birth weight of newborn, reported all parameters to be non-significant except no breastfeeding that was positively associated, whereas another by Gladstone et al. [14] found breastfeeding for 4 or more months to be non-significant. Previous acute respiratory infection episodes, previous treatment history and vitamin A deficiency in children was reported to be non-significant risk factor by Joshi et al. [16]. Two other studies reported that age more than 24 months [12] and age between 3–5 months and 6–8 months [14] did not have significant association (Table 3).
Non-consistent risk factors: Inconsistent findings were generated from the present review on the following factors: Age of the child [12–17], sex [13,14], anemia [12,13], mother’s education [12,14], socioeconomic status [16–18,21] and breastfeeding [13,14].
The point estimates of association, generated from meta-analyses of common risk factors listed in the included studies, is shown in Table 4. Child malnutrition (odds ratio [OR]: 1.73; 95% CI: 1.53–1.96), anemia (OR: 1.71; 95% CI: 1.29–2.28) and low socioeconomic status (OR: 7.14; 95% CI: 2.19–23.32) were found to be significantly associated with childhood diarrhea. The high values of I2 and τ2 indicate a large variation due to heterogeneity across and between the studies included for meta-analysis for each of the risk factors.
Table 4.
Point estimates of reported risk factors for diarrhea in children
| Risk factor | Point estimate (95% CI) | p value | I2 (p) | τ2 (SE) |
|---|---|---|---|---|
| Age of child<24 months (n=5) | 1.54 (0.94–2.51) | 0.08 | 95.37 (0.000) | 0.28 (0.26) |
| Malnutrition (n=2) | 1.73 (1.53–1.96) | 0.000 | 0.00 (0.44) | 0.00 (0.09) |
| Anemia (n=2) | 1.71 (1.29–2.28) | 0.000 | 0.00 (0.38) | 0.00 (0.06) |
| Not breastfed currently (n=2) | 0.44 (0.02–10.06) | 0.61 | 98.23 (0.00) | 5.01 (7.22) |
| Mother’s schooling up to/less than 10 years (n=3) | 1.18 (0.96–1.44) | 0.10 | 85.22 (0.001) | 0.18 (0.21) |
| Low socioeconomic status (n=5) | 7.14 (2.19–23.32) | 0.001 | 98.40 (0.000) | 1.73 (1.46) |
| Unsafe drinking water (n=3) | 0.98 (0.57–1.66) | 0.41 | 71.52 (0.03) | 0.15 (0.22) |
CI: Confidence interval, SE: Standard error
DISCUSSION
Two important risk factors, malnutrition, and anemia, that are associated with multiple problems related to growth and development in older children, emerged as significant risk factors for acquiring infection in early years of life. This has been similarly reported from other studies conducted earlier [3,5]. In the light of these findings, it may be advocated that controlling anemia and malnutrition in children may bring down the morbidity and resultant mortality due to severe diarrhea. This may be a novel approach to diarrhea prevention since most available interventions are targeted to safe drinking water and sanitation measures nonetheless necessary [5]. Low socioeconomic status, which goes hand in hand with poor sanitation in low and middle-income countries, also emerged as a significant positively associated risk factor in the present meta-analysis.
Based on our findings, on the policy and public health practice front, it would be reasonable to advocate intensifying control of malnutrition and anemia in children through population-based (non-pharmacological) and pharmacological interventions, while continuing to institute measures for poverty alleviation and behavior change for sanitation. The existing nutritional and child health programs in India (e.g. Integrated Child Development Services and mid-day meal) can play a pivotal role through improving the provision of good quality nutrition and educating children for proper hand washing and safe drinking water and introducing new initiatives to curb malnutrition.
The pooled prevalence of diarrhea was found to be lower in rural areas compared to urban slums contrary to popular belief, indicating wider presence of risk factors amongst the urban children. Gupta et al. have reported the presence of multiple risk factors for diarrhea among under-five children in an urban slum, similar to our finding [4,24]. This may be due to the increased emphasis placed on providing benefits of preventive services under the national health programs and other welfare programs to the rural population and neglect of slums at large. Moreover, the studies on slum children lacked robustness to elicit all the prevalent risk factors for childhood diarrhea.
The evidence generated from systematic review of literature is conclusive on following risk factors of acute childhood diarrhea; young age of the child (usually <24 months), low socioeconomic status and mothers’ low educational status. These findings are consistent with other large reports on risk factors of diarrhea among under-five children [25]. Poor sanitation standards and hand washing practices in the community were found to have a significant association with childhood diarrhea in the current systematic review, as also reported significant in multiple other reports [25,26]. We found inconclusive evidence on poor breastfeeding among children as a determinant for diarrhea. Since the protective role of breastfeeding is well established [27] and advocated [5,28], we propose this may be re-examined with more research studies.
Shah et al. [5], in their systematic review on childhood diarrhea, reported that diarrheagenic Escherichia coli, especially Enteroaggregative E. coli were the most common bacterial pathogen isolated in most studies. The same study reported the point prevalence to vary from 9% to 20%, and identified exclusive breastfeeding, hand washing and point-of-use water treatment to be effective strategies for diarrhea reduction. These findings, although concordant to ours on a systematic review, were not found to be significant in our meta-analysis [13,17,19]. An independent study [29] used District Level Household Survey-3 data to quantify the impact of access to improved sanitation on diarrheal morbidity for under-five children, and they found that access to improved sanitation reduced the risk of contracting diarrhea. They, however, did not find the risk among children in the poor household, for girls, boys and high socioeconomic status children to be statistically significant.
Reviews have described the common environmental risk factors [3,4,30]; but have also reported heterogeneity in the articles, similar to the present meta-analysis, which may exert profound effect on the interpretation of the result obtained. The authors understand that ideally, a meta-analysis of heterogeneous studies should be avoided, but the present meta-analysis was warranted since good quality studies demonstrating the associations of risk factors with childhood diarrhea in India are lacking. This reinstates that studies with good methodology and outcomes are still unavailable from different parts of the country.
We also expected to find an association of diarrhea with certain biochemical parameters such as serum zinc, magnesium, sodium, and potassium. However, we did not find studies that explored the role of biomarkers in causation of diarrheal diseases in children except for one [31] (not included in review). In addition, we also did not find any studies that reported risk factors specific to diarrhea of bacterial or viral origin. It may be timely to shift focus from the study of socio-demographic characteristics of population to exploring relations between different biochemical markers and determining their role in the causation of diarrheal diseases [31] in children. The association of diarrhea with reference to specific strains of the causative agent, status of rotavirus and measles vaccination [1,5,25], nutritional status [5,32], specific exposures during intrauterine life, and association with co-morbidities, although documented, could not be identified in the present review. These need to be addressed in research to fill the gap as there is an acute dearth of studies on these risk factors of childhood diarrhea in the country.
The major strength of this systematic review is the independent literature search and rating of the methodological quality of studies; hence, considering the evidential basis of the included studies. We have also reported the point estimates and heterogeneity associated with each risk factor reviewed which no other study has done as far as our knowledge goes. However, we attempted to include all original research journal articles that were published in English and indexed in PubMed, including studies with inappropriate power which rated low on quality score that increased heterogeneity of literature. Furthermore, no expert consultation was undertaken to ensure that all relevant articles were included. Finally, we could not distinguish between low reporting and low methodological quality of the included studies using our quality scoring method. Hence, low scorings of methodological quality may reflect either weak reporting or weak study designs. Before undertaking the systematic review, we were expectant that some widely documented risk factors like unsafe drinking water and poor sanitation [1,5] may be positively associated that we couldnot find. We explored and discussed such risk factors in the light of available evidence.
CONCLUSION
The present systematic review yielded sufficient evidence on association of socio-demographic factors with diarrhea among under-five children. However, evidence on other contributory factors including breastfeeding and vaccination is inconclusive. Analytical studies on emerging and less studied risk factors of diarrhea are warranted to understand the reasons behind the persistence of high prevalence of diarrhea morbidity among Indian children.
Supplementary Material
Acknowledgments
Research reported in this publication was conducted by scholars in the Fogarty International Center of the National Institutes of Health training program under Award Number D43 TW 009078. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding: None
Footnotes
Conflict of Interest: None Stated.
References
- 1.WHO. Diarrhea: Why Children are Still Dying and What Can Be Done. Geneva: The United Nations Children’s Fund (UNICEF)/World Health Organization (WHO); 2009. Available from: http://www.unicef.org/health/files/Final_Diarrhea_Report_October_2009_final.pdf. [Last accessed on 2014 Nov 09] [Google Scholar]
- 2.Hutton G, Haller L. Evaluation of the Costs and Benefits of Water and Sanitation Improvements at the Global Level. Geneva: World Health Organization; 2004. [Google Scholar]
- 3.Kumar SG, Subita L. Diarrheal diseases in developing countries: A situational analysis. Kathmandu Univ Med J. 2012;38(2):83–8. doi: 10.3126/kumj.v10i2.7351. [DOI] [PubMed] [Google Scholar]
- 4.Singh A, Singh MN. Diarrhea and acute respiratory infections among under-five children in slums: Evidence from India. Available from: https://www.peerj.com/preprints/208.pdf. [Last accessed on 2014 Nov 09]
- 5.Shah D, Choudhury P, Gupta P, Mathew JL, Gera T, Gogia S, et al. Promoting appropriate management of diarrhea: A systematic review of literature for advocacy and action: UNICEF-PHFI series on newborn and child health, India. Indian Pediatr. 2012;49(8):627–49. doi: 10.1007/s13312-012-0134-1. [DOI] [PubMed] [Google Scholar]
- 6.PATH. Diarrheal Disease: Solutions to Defeat a Global Killer. 2009 May; Available from: http://www.path.org/publications/files/IMM_solutions_global_killer. [Last accessed on 2015 Oct 03]
- 7.Ahmed SF, Farheen A, Muzaffar A, Mattoo GM. Prevalence of diarrhoeal disease, its seasonal and age variation in under- fives in Kashmir, India. Int J Health Sci (Qassim) 2008;2(2):126–33. [PMC free article] [PubMed] [Google Scholar]
- 8.Bax L, Yu LM, Ikeda N, Moons KG. A systematic comparison of software dedicated to meta-analysis of causal studies. BMC Med Res Methodol. 2007;7:40. doi: 10.1186/1471-2288-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.STROBE Statement. Available from: http://www.strobe-statement.org/PDF%20hochladen/index. [Last accessed on 2013 Nov 14]
- 10.Nandy S, Irving M, Gordon D, Subramanian SV, Smith GD. Poverty, child undernutrition and morbidity: New evidence from India. Bull World Health Organ. 2005;83(3):210–6. [PMC free article] [PubMed] [Google Scholar]
- 11.Gladstone BP, Das AR, Rehman AM, Jaffar S, Estes MK, Muliyil J, et al. Burden of illness in the first 3 years of life in an Indian slum. J Trop Pediatr. 2010;56(4):221–6. doi: 10.1093/tropej/fmp116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deshmukh PR, Dongre AR, Sinha N, Garg BS. Acute childhood morbidities in rural Wardha: Some epidemiological correlates and health care seeking. Indian J Med Sci. 2009;63(8):345–54. [PubMed] [Google Scholar]
- 13.Phadke MA, Gadgil B, Bharucha KE, Shrotri AN, Sastry J, Gupte NA, et al. Replacement-fed infants born to HIV-infected mothers in India have a high early postpartum rate of hospitalization. J Nutr. 2003;133(10):3153–7. doi: 10.1093/jn/133.10.3153. [DOI] [PubMed] [Google Scholar]
- 14.Gladstone BP, Muliyil JP, Jaffar S, Wheeler JG, Le Fevre A, Iturriza-Gomara M, et al. Infant morbidity in an Indian slum birth cohort. Arch Dis Child. 2008;93(6):479–84. doi: 10.1136/adc.2006.114546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazumder S, Taneja S, Bhandari N, Dube B, Agarwal RC, Mahalanabis D, et al. Effectiveness of zinc supplementation plus oral rehydration salts for diarrhoea in infants aged less than 6 months in Haryana state, India. Bull World Health Organ. 2010;88(10):754–60. doi: 10.2471/BLT.10.075986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joshi PC, Kaushal S, Aribam BS, Khattri P, D’Aoust O, Singh MM, et al. Recurrent floods and prevalence of diarrhea among under five children: Observations from Bahraich district, Uttar Pradesh, India. Glob Health Action. 2011;4 doi: 10.3402/gha.v4i0.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sur D, Manna B, Deb AK, Deen JL, Danovaro-Holliday MC, von Seidlein L, et al. Factors associated with reported diarrhoea episodes and treatment-seeking in an urban slum of Kolkata, India. J Health Popul Nutr. 2004;22(2):130–8. [PubMed] [Google Scholar]
- 18.Banerjee B, Hazra S, Bandyopadhyay D. Diarrhea management among under fives. Indian Pediatr. 2004;41(3):255–60. [PubMed] [Google Scholar]
- 19.Rose A, Roy S, Abraham V, Holmgren G, George K, Balraj V, et al. Solar disinfection of water for diarrhoeal prevention in southern India. Arch Dis Child. 2006;91(2):139–41. doi: 10.1136/adc.2005.077867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mishra V, Awasthi S, Nag VL, Tandon R. Genomic diversity of group A rotavirus strains in patients aged 1–36 months admitted for acute watery diarrhoea in northern India: A hospital-based study. Clin Microbiol Infect. 2010;16(1):45–50. doi: 10.1111/j.1469-0691.2009.02772.x. [DOI] [PubMed] [Google Scholar]
- 21.Bandyopadhyay D, Banerjee B. Influence of child care practices on prevalence of diarrheal diseases. Indian Pediatr. 2005;42(5):497–8. [PubMed] [Google Scholar]
- 22.Khush RS, Arnold BF, Srikanth P, Sudharsanam S, Ramaswamy P, Durairaj N, et al. H2S as an indicator of water supply vulnerability and health risk in low-resource settings: A prospective cohort study. Am J Trop Med Hyg. 2013;89(2):251–9. doi: 10.4269/ajtmh.13-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dhingra U, Hiremath G, Menon VP, Dhingra P, Sarkar A, Sazawal S. Zinc deficiency: Descriptive epidemiology and morbidity among preschool children in peri-urban population in Delhi, India. J Health Popul Nutr. 2009;27(5):632–9. doi: 10.3329/jhpn.v27i5.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta A, Sarker G, Rout AJ, Mondal T, Pal R. Risk correlates of diarrhea in children under 5 years of age in slums of bankura, west bengal. J Glob Infect Dis. 2015;7(1):23–9. doi: 10.4103/0974-777X.150887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.UNICEF. Pneumonia and Diarrhea: Tackling the Deadliest Diseases for the World’s Poorest Children. New York: United Nations Children’s Fund; Jun, 2012. Available from: http://www.unicef.org/media/files/UNICEF_P_D_complete_0604.pdf. [Last accessed on 2013 Jul 25] [Google Scholar]
- 26.Snilstveit B, Waddington H. Effectiveness and sustainability of water, sanitation, and hygiene interventions in combating diarrhea. J Dev Eff. 2009;1(3):295–335. [Google Scholar]
- 27.Effect of breastfeeding on infant and child mortality due to infectious diseases in less developed countries: A pooled analysis. WHO Collaborative Study Team on the Role of Breastfeeding on the Prevention of Infant Mortality. Lancet. 2000;355(9202):451–5. [PubMed] [Google Scholar]
- 28.WHO & MOHFW. Students’ Handbook for IMNCI Integrated Management of Neonatal and Childhood Illness. Ministry of Health and Family Welfare; India: 2003. [Google Scholar]
- 29.Kumar S, Vollmer S. Does improved sanitation reduce diarrhea in children in rural India? doi: 10.1002/hec.2809. Available from: http://www.mpra.ub.uni-muenchen.de/id/eprint/31808 [Last accessed on 2013 Dec 12] [DOI] [PubMed]
- 30.Fewtrell L, Kaufmann RB, Kay D, Enanoria W, Haller L, Colford JM., Jr Water, sanitation, and hygiene interventions to reduce diarrhoea in less developed countries: A systematic review and meta-analysis. Lancet Infect Dis. 2005;5(1):42–52. doi: 10.1016/S1473-3099(04)01253-8. [DOI] [PubMed] [Google Scholar]
- 31.Carcillo JA, Planquois JM, Goldstein B. Early markers of infection and sepsis in newborns and children. Adv Sepsis. 2006;5(4):118–25. Available from: http://www.advancesinsepsis.com/pdfs/4134.pdf. [Last accessed on 2015 Nov 21] [Google Scholar]
- 32.Ramachandran P, Gopalan HS. Undernutrition & risk of infections in preschool children. Indian J Med Res. 2009;130(5):579–83. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


