Abstract
The Wnt signaling pathway is crucial for development and disease. The regulation of Wnt protein trafficking is one of the pivotal issues in the Wnt research field. Here we performed a genetic screen in Drosophila Melanogaster for genes involved in Wingless/Wnt secretion, and identified the p24 protein family members Baiser, CHOp24, Eclair and a v-SNARE protein Sec22, which are involved in the early secretory pathway of Wingless/Wnt. We provided genetic evidence demonstrating that loss of p24 proteins or Sec22 impedes Wingless (Wg) secretion in Drosophila wing imaginal discs. We found that Baiser cannot replace other p24 proteins (CHOp24 or Eclair) in escorting Wg, and only Baiser and CHOp24 interact with Wg. Moreover, we showed that the v-SNARE protein Sec22 and Wg are packaged together with p24 proteins. Taken together, our data provide important insights into the early secretory pathway of Wg/Wnt.
Keywords: Wingless/Wnt trafficking, early secretory pathway, p24, Baiser, Sec22
Introduction
The Wnt signaling pathway is involved in many biological processes during development and in disease [1,2,3]. Wnt proteins are essential morphogen and secreted signal molecules that ignite the Wnt signaling pathway and induce downstream genes expression in the receiving cells [4,5]. Thus, over the past decades, great efforts have been made to clarify the synthesis, sorting, processing, and secretion of Wnt proteins in the Wnt-producing cells.
In the ER (endoplasmic reticulum), newly synthesized Wnt proteins are lipid modified by the acyltransferase Porcupine [6,7,8]. Porcupine catalyzes palmitoylation at the conserved cysteine and serine residues of Wnt proteins, which is essential for interactions of Wnt proteins with Wntless at the Golgi [9,10]. Following palmitoylation the multipass transmembrane Wntless escorts the Wnt protein for post-Golgi trafficking to the plasma membrane for secretion [11,12,13,14,15].
One regulator of Wnt secretion is the p24 family, which sorts Wnt proteins from the ER to the Golgi. Buechling and Port first identified several p24 protein family members, such as CHOp24, Eclair, Opm and p24-1, involved in Wingless/Wnt secretion [16,17]. However, there are four sub-families of p24 proteins, and the molecular mechanisms governing sorting of Wg/Wnt by p24 proteins is still unclear.
The p24 family proteins are highly conserved Type-I transmembrane proteins of approximately 24 kDa, and can be subdivided into four subfamilies including α, β, γ and δ [18]. The p24 family consists of ten members in mammals [19,20], eight in yeast [21,22,23] and nine in Drosophila [24,25,26,27]. Investigation of the p24 protein family uncovered its role in regulating the bi-directional transportation between the ER and the Golgi [28,29,30]. In the early secretory pathway, p24 proteins form a complex which functions as cargo selector. The complex recruits the correct subsets of trafficking machinery, packages them to escort cargoes and then cycles continuously between these compartments.
Early studies in yeast have revealed that yeast p24 proteins form functionally redundant αβγδ complexes [31]. In the study of p24 proteins escorting Wg/Wnt, Buechling found that several p24 proteins may contribute in a partially redundant manner [32]. However, Port observed a fraction of Wg escapes from the ER even in the absence of two p24 proteins, providing a hint that there might be other p24 proteins that function as Wg selectors [33].
In the early secretory pathway, cargoes are packaged by cargo selectors and transported by vesicle budding, docking, and fusion. SNARE (soluble NSF attachment protein receptor) proteins are highly conserved proteins that trigger a vesicle fusion event [34,35], and can be divided into two categories: vesicle membrane localized v-SNAREs and target compartment localized t-SNAREs. Since the Wnt secretory pathway is known to be tightly controlled by trafficking machinery, we performed genetic screen in Drosophila Melanogaster to identify new genes involved in the Wg/Wnt secretory process. We found another p24δ protein Baiser, which interacts with Wg and co-localizes with Wg and the v-SNARE protein, Sec22.
We found that over-expression of Baiser cannot rescue defects caused by depleting other p24 proteins. We present both genetic and in vitro evidence that functional diversity of the p24 protein family members is essential to sort and process Wg/Wnt proteins. Furthermore, the ER-enriched v-SNARE Sec22 [36,37] is packaged together with Wg and p24.
Materials and Methods
Drosophila stocks
The RNAi line, UAS-Sec22RNAi (100766), was from the Vienna Drosophila Resource Center (http://stockcenter.vdrc.at/control/main). The following RNAi lines were generated in our laboratory: UAS-BaiserRNAi, UAS-CHOp24RNAi, UAS-EclairRNAi. Tool strains were described in Flybase including enGal4 and wg-lacZ.
shRNA against Baiser, CHOp24 and Eclair were designed from DSIR. The following primers were annealed in the annealing buffer at 95 °C for 5 min, and then cloned into pWALIUM20 with NheI and EcoRI sites to generate the shRNA constructs. Primers are:
BaiserRNAi top, 5′-ctagcagtGATCATCGACTACATTGCACGtagttatattcaagcataCGTGCAATGTAGTCGATGATCgcg -3′
BaiserRNAi bottom, 5′-aattcgcGATCATCGACTACATTGCACGtatgcttgaatataactaCGTGCAATGTAGTCGATGATCactg -3′
CHOp24RNAi top, 5′-ctagcagtGACCAGTGTCAAGCACGAACAtagttatattcaagcataTGTTCGTGCTTGACACTGGTCgcg -3′
CHOp24RNAi bottom, 5′-aattcgcGACCAGTGTCAAGCACGAACAtatgcttgaatataactaTGTTCGTGCTTGACACTGGTCactg -3′
EclairRNAi top, 5′-ctagcagtGGCAGATGCGTCATCTCAAGAtagttatattcaagcataTCTTGAGATGACGCATCTGCCgcg -3′
EclairRNAi bottom, 5′-aattcgcGGCAGATGCGTCATCTCAAGAtatgcttgaatataactaTCTTGAGATGACGCATCTGCCactg -3′
Generation of Baiser mutant and Sec22 null allele
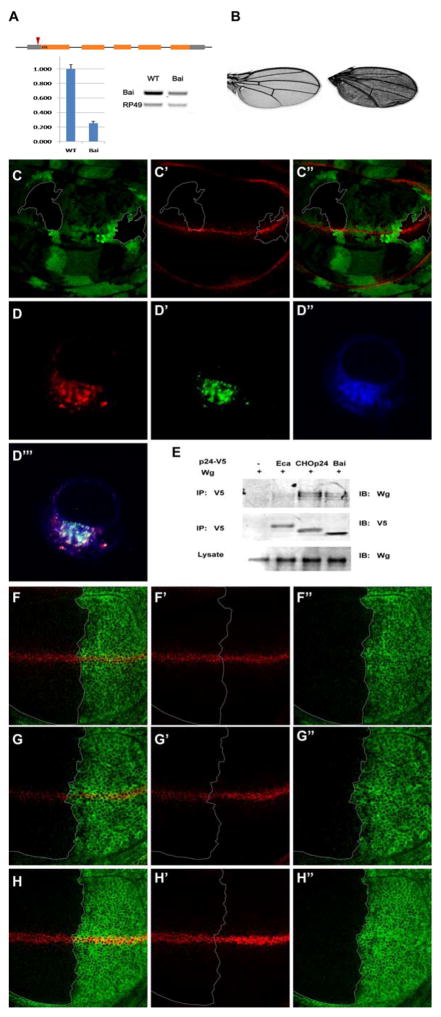
The Baiser insertion mutant (32614) was obtained from the Bloomington Drosophila Stock Center (http://flystocks.bio.indiana.edu), and recombined with w;; FRT82B/Tm6b to generate w;;FRT82BBaiser/TM6b (Fig. 2A). Homozygous mutant clones were generated by the FLP-FRT method [38]. Flies were crossed with ywhsp-Flp;; FRT82Bubi-GFP/Tm6b and at 40 hrs post egg laying, the F1 progeny were heat shocked for 90min at 37°C.
Figure 2.
Baiser is a direct cargo selector that escorts Wg.
The Baiser insertion mutant detail and the real-time quantitative PCR results of Baiser homozygotes are shown in (A). Wing blade of wt (left) and Baiser (right) homozygous escaper (B). Wg accumulates (C′) in the Wg-producing cells bearing Baiser homozygous mutant clones marked by the absence of GFP (C). Wg (D″) co-localizes with Baiser (D′) and Eclair (D) in S2 cells. Wg can be immunoprecipitated with Baiser, CHOp24 but not Eclair (E). Over-expression of Baiser-V5 driven by enGal4 can rescue the Wg secretion defect caused by UAS-BaiserRNAi (F), but not UAS-CHOp24RNAi (G) or UAS-EclairRNAi (H). Wing discs are oriented anterior left, dorsal up.
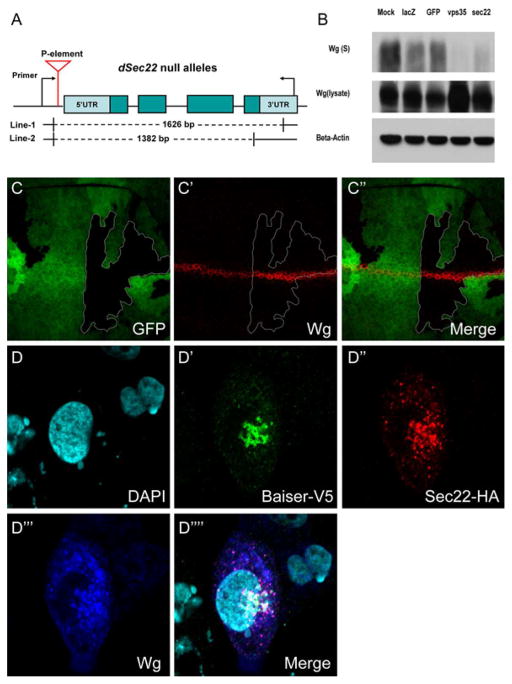
Another P-element insertion line, (14846), y1 P{EPgy2}EY00427 w67c23, residing upstream of the Sec22 gene was obtained from the Bloomington Drosophila Stock Center. We obtained two deletions that are homozygous lethal from imprecise excision of a P-element insertion (Fig. 3A). The mutant were recombined with w;; FRT101/Y to generate w;;Sec22-FRT101/FM7. Homozygous mutant clones were generated by the FLP-FRT method [38]. Flies were crossed with hs-Flp;; ubi-GFP FRT101, and at 48hrs post egg laying, the F1 progeny were heat shocked for 90min at 37°C.
Figure 3.
Sec22 regulates Wg trafficking and is packaged together with Wg and p24.
Two mutant alleles of Sec22 were generated through P-element-mediated imprecise excision (A). Drosophila S2 cell was fed with dsRNA of Sec22, Vps35, GFP and lacZ. Wg protein in the supernatant (S) and in the cell lysate (lysate) were detected (B). Wg accumulates (C′) in the Wg-producing cells bearing Sec22 homozygous mutant clones marked by the absence of GFP (C). Baiser-V5 (D′), Sec22-HA (D″) and Wg (D‴) co-localize precisely (D‴′) and DAPI is shown in (D).
Drosophila immunostaining and antibodies
Fixation and immunostaining were performed following standard protocols. Primary antibodies used in this study are as follows: mouse anti-Wg (1:4; DSHB), chicken anti-β-gal (1:1000; Abcam), mouse anti-engrailed (DSHB), rat anti-Ci (1:2; DSHB), mouse anti-E-cadherin (DSHB), rabbit anti-Hh (1:200; [39]), mouse anti-Patched (DSHB), rabbit anti-Spalt (1:200; made in our laboratory according to [40]), mouse anti-V5 (1:500; Invitrogen) and rat anti-HA (1:1000; Roche).
The primary antibodies were detected by fluorescence using Alexa Fluor 488-, Cy3- and Cy5- conjugated secondary antibodies from Jackson Immuno Research Laboratories, Inc.
The Confocal fluorescence images were collected using the Zeiss LSM 780 Laser Scanning Confocal Microscope (Carl Zeiss).
Results
Identification of Wingless (Wg) secretion regulators
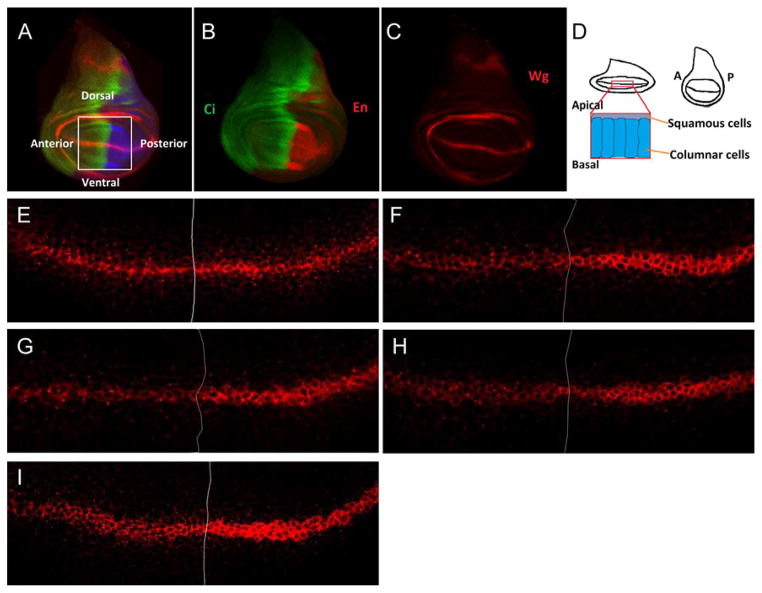
We performed a genetic RNAi screen using different tissue-specific Gal4 drivers in Drosophila wing discs (Fig. 1 A–C). Using enGal4 to induce RNAi expression to deplete Baiser (CG11785), CHOp24 (CG3564), Eclair (CG33104), or Sec22 (CG7359) in the posterior compartment of the wing disc, we found that endogenous Wg accumulated significantly in the Wg-producing cells (Fig. 1 F–I) and the adult wing blade presented slight wing margin defects in the posterior compartment (Fig. S1 F–I). However, wg transcription, as determined by wg-lacZ, was not altered (Fig. S1 A–D), indicating that Wg protein trafficking is likely to be affected in the absence of these genes.
Figure 1.
RNAi screen identify Wingless secretion regulators.
Schematic illustration of the Drosophila wing imaginal disc is shown in (A). ci (cubitusinterruptus, Green) and en (engrailed, Red) expression pattern in Drosophila wing disc are shown in (B). Wingless expression pattern is shown in (C) and a longitudinal section of Wg-producing cells from a Confocal Z-stack of Wing disc is shown in (D). Wild-type Wingless antibody staining is shown in (E). (F–I) Expression of UAS-BaiserRNAi, UAS-CHOp24RNAi, UAS-EclairRNAi and UAS-Sec22RNAi were induced by enGal4, Wg accumulates in the posterior Wg-producing cells of wing disc. Wing discs are oriented anterior left, dorsal up.
Baiser, CHOp24 and Eclair belong to different p24 protein sub-families. The p24β member CHOp24, p24α member Eclair, and p24γ member Opm and p24-1 have already been proven to escort the Wg protein from the ER to the Golgi by Buechling and Port in 2011. However, we found Baiser, the single p24δ member in Drosophila, is another p24 protein involved in Wg secretion. Thus we wanted to uncover the molecular mechanism of these p24 members escorting Wg: are they playing the same role or acting partially redundantly? In addition, we observed Sec22, an ER-enriched v-SNARE protein involved in Wg secretion. Therefore, we further examined the mechanism(s) by which the protein machinery regulates the early secretory pathway of Wg/Wnt.
Baiser is a cargo selector of Wg protein
To further confirm the above results, we obtained a Baiser mutant, with a P-element inserted in the 5′UTR of Baiser (Fig. 2 A). Over 90% of the homozygotes die before eclosion and the living escapers present a reduced Baiser expression to 25% of wild-type flies (Fig. 2 A), smaller wing blade, and slight wing margin defects (Fig. 2 B). Then we generated Baiser mutant clones by the FLP–FRT method [38] and detected significant accumulation of Wg proteins in the Wg-producing cells bearing a Baiser homozygous mutation (Fig. 2 C), which is consistent with our aforementioned RNAi screen results.
p24 protein family members are functionally diverse
To further understand the molecular mechanism in which the p24 proteins escort Wg, we transfected Drosophila S2 cells with Eclair, Baiser and Wg, and found that the two p24 proteins co-localize with the Wg protein precisely (Fig. 2 D). Moreover, upon immunoprecipitation of the Baiser-V5 protein from transfected cells, Wg was detected by western blotting in the immunoprecipitate, indicating that there is an interaction between Baiser and Wg (Fig. 2 E), and that Baiser is a cargo selector of Wg.
Surprisingly, we found that both Baiser and CHOp24 interact with Wg but Eclair does not (Fig. 2 E), suggesting that these p24 proteins are functionally diverse. Furthermore, over-expression of Baiser-V5 cannot rescue the Wg secretion defect caused by depleting CHOp24 or Eclair (Fig. 2 F–H), arguing that these p24 members are playing different roles in the process of escorting Wg, and cannot be replaced by each other.
Thus in the process of escorting the Wg protein for anterograde transportation from the ER to the Golgi, the p24 proteins interact with Wg and get the cargo packaged. Several p24 proteins, including Baiser, CHOp24, Eclair, Opm and p24-1 [16,17], are involved in this process. These p24 family members are functionally diverse and cannot be replaced by each other, probably function in a partially redundant manner and/or by forming diverse complexes [31].
Sec22 is involved in Wg secretion
Upon our screen, we identify an ER-enriched v-SNARE protein Sec22 involved in Wg trafficking, and thus we generated two Sec22 mutants (Fig. 3 A). Consistent with the RNAi results, Wg protein accumulates significantly in the Sec22 homozygous mutant clones (Fig. 3 C). We fed Drosophila S2 cells with dsRNA of Sec22 and examined the Wg protein level in the supernatant and lysate. We observed a significant reduction of the secreted Wg in the supernatant and an increased level of Wg in the cell lysate (Fig. 3 B), indicating that depletion of Sec22 impedes Wg secretion.
The v-SNARE Sec22 is packaged together with Wg and p24
To further examine the molecular mechanism by which Sec22 regulates Wg trafficking, we transfected Drosophila S2 cells with Baiser, Sec22, and Wg, and found that Sec22 co-localized with Baiser and Wg precisely, suggesting that Wg and Sec22 are packaged together with Baiser. Thus, during the early secretory phase of Wg, the v-SNARE Sec22 is packaged together with p24 proteins to escort Wg during the anterograde transportation. These data demonstrate that the molecular machinery involved in Wg trafficking are diverse.
Discussion
The Wg trafficking regulators
Wingless (Wg)/Wnt signaling plays crucial roles in development, tissue homeostasis, and diseases, thus the appropriate regulation of Wnt secretion is essential in Wnt signaling. Our genetic screening among genes of the secretory pathway organelles, identified Baiser, CHOp24, Eclair and Sec 22 as genes involved in the regulation of Wnt secretion. We conducted further experiments to clarify the molecular mechanism(s) by which these regulators escort Wg protein.
Multiple molecular machines are assembled and packaged together to transport cargoes. During the process of escorting Wg protein, p24 proteins interact with Wg, function as cargo selectors and package Wg together with the v-SNARE Sec22 for anterograde transportation.
The functional diversity of p24 family members
Previous study in yeast uncovered that the different p24 sub-family members form diverse complexes, thus playing multiple roles in escorting cargoes [31]. Consistent with the yeast work, we found that different p24 proteins play different roles and cannot be replaced by each other, indicating the functional diversity of the p24 family.
Baiser, CHOp24, Eclair, Opm and p24-1 are essential for the p24 complex activity in escorting Wg. These p24 members shuttle between ER and Golgi to facilitate the translocation of Wg. Upon our work, deletion of Baiser inhibited p24 activity and impeded Wg secretion. However, over-expression of Baiser cannot relieve the p24 activity inhibition caused by loss of other p24 proteins, arguing that p24 family members are not redundant but indispensable in escorting Wg.
The SNARE complex involved in Wg secretion
We identified Sec22 as an important regulator of Wg anterograde transportation. However, Sec22 encodes a conserved v-SNARE, which will form a SNARE complex with the cis-Golgi-localized t-SNARE. Thus it is likely that the conserved SNARE complex components Sed5, Bos1 and Bet1 [41,42,43] are involved in Wg trafficking.
Furthermore, on the pathway of Wg trafficking, docking and fusion between vesicles and the target membrane require the SNARE complex. It is reasonable to speculate that there are other SNARE complex components essential for Wg transportation. It is important to determine other molecular machinery such as Tethers, Rabs, Arfs, and COPs involved.
The synthesis, sorting, processing, and secretion of Wg proteins take place in different compartments and pathways. Multiple regulators determine Wg/Wnt trafficking and signaling. Upon our screen, we identified p24 and Sec22 as regulators in the early secretory pathway of Wg trafficking. The aforementioned experiments have provided compelling evidence that p24 proteins such as Baiser and CHOp24 sort Wg by direct interaction, and package it together with other molecular machinery, such as the v-SNARE Sec22. Then the cargo and cargo selector are assembled for anterograde transportation from the ER to the Golgi (Fig. 4).
Figure 4.
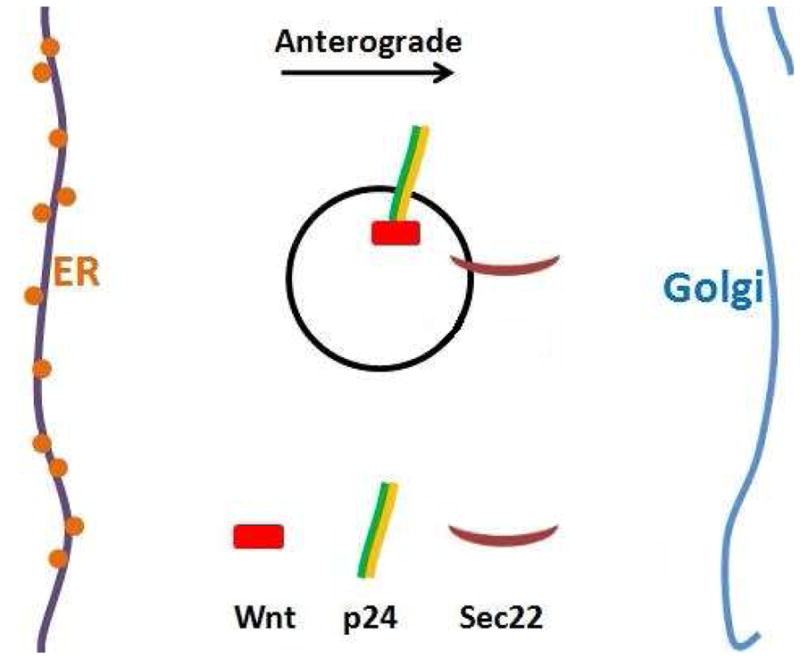
p24 and Sec22 escort Wg for anterograde transportation.
Wg, p24 and Sec22 are packaged together for Wg anterograde transportation from ER to Golgi. P24 proteins function as the cargo selector and Sec22 functions as the v-SNARE.
We demonstrate that the p24 proteins are functionally diverse by forming complexes and playing different roles in this process. Furthermore, the v-SNARE Sec22 is involved in this process and gives us a hint that other SNARE complex components are potential regulators of Wg/Wnt trafficking. Our work provides insight into the molecular machinery shuttling between ER and Golgi to escort Wg/Wnt for secretion.
Supplementary Material
Baiser, CHOp24, Eclair and Sec22 are Wg secretion regulators.
(A–D) β-gal staining representing wg expression is not altered when knocking-down Baiser, CHOp24, Eclair and Sec22 in the posterior compartment of wing disc by hhGal4. Wing blade of wt (E), enGal4>BaiserRNAi (F), enGal4>CHOp24RNAi (G), enGal4>EclairRNAi (H) and enGal4>Sec22RNAi (I) are shown.
Highlights.
p24 proteins and Sec22 are molecular machinery of Wingless/Wnt trafficking.
p24 members play different roles in escorting Wingless and are functionally diverse.
p24 and Sec22 are packaged together to escort Wingless.
Acknowledgments
We thank Stephen Cohen and Philip Ingham for reagents; the Iowa Developmental Studies Hybridoma Bank (DSHB) for antibodies; the Bloomington Stock Center; the Vienna Drosophila Resource center and the Tsinghua University Drosophila Stock Center for Drosophila stocks. This work was supported by grants from National Basic Research Program of China (2011CB943901, 2011CB943902 and 2011CB943802), the Strategic Priority Research Program of the Chinese Academy of Sciences Grant (XDA01010101), Nature Sciences foundation of China (31371464 and 31071284) and NIH grants (2R01GM063891).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annual Review of Cell and Developmental Biology. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- 2.Moon RT, Bowerman B, Boutros M, Perrimon N. The promise and perils of Wnt signaling through beta-catenin. Science. 2002;296:1644–1646. doi: 10.1126/science.1071549. [DOI] [PubMed] [Google Scholar]
- 3.MacDonald BT, Tamai K, He X. Wnt/beta-Catenin Signaling: Components, Mechanisms, and Diseases. Developmental Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zecca M, Basler K, Struhl G. Direct and long-range action of a wingless morphogen gradient. Cell. 1996;87:833–844. doi: 10.1016/s0092-8674(00)81991-1. [DOI] [PubMed] [Google Scholar]
- 5.Neumann CJ, Cohen SM. Distinct mitogenic and cell fate specification functions of wingless in different regions of the wing. Development. 1996;122:1781–1789. doi: 10.1242/dev.122.6.1781. [DOI] [PubMed] [Google Scholar]
- 6.Hofmann K. A superfamily of membrane-bound O-acyltransferases with implications for wnt signaling. Trends Biochem Sci. 2000;25:111–112. doi: 10.1016/s0968-0004(99)01539-x. [DOI] [PubMed] [Google Scholar]
- 7.Takada R, Satomi Y, Kurata T, Ueno N, Norioka S, Kondoh H, Takao T, Takada S. Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev Cell. 2006;11:791–801. doi: 10.1016/j.devcel.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Zhai L, Chaturvedi D, Cumberledge S. Drosophila wnt-1 undergoes a hydrophobic modification and is targeted to lipid rafts, a process that requires porcupine. J Biol Chem. 2004;279:33220–33227. doi: 10.1074/jbc.M403407200. [DOI] [PubMed] [Google Scholar]
- 9.Herr P, Basler K. Porcupine-mediated lipidation is required for Wnt recognition by Wls. Dev Biol. 2012;361:392–402. doi: 10.1016/j.ydbio.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Tang X, Wu Y, Belenkaya TY, Huang Q, Ray L, Qu J, Lin X. Roles of N-glycosylation and lipidation in Wg secretion and signaling. Dev Biol. 2012;364:32–41. doi: 10.1016/j.ydbio.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bänziger C, Soldini D, Schütt C, Zipperlen P, Hausmann G, Basler K. Wntless, a Conserved Membrane Protein Dedicated to the Secretion of Wnt Proteins from Signaling Cells. Cell. 2006;125:509–522. doi: 10.1016/j.cell.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 12.Bartscherer K, Pelte N, Ingelfinger D, Boutros M. Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell. 2006;125:523–533. doi: 10.1016/j.cell.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Belenkaya TY, Wu Y, Tang X, Zhou B, Cheng L, Sharma YV, Yan D, Selva EM, Lin X. The retromer complex influences Wnt secretion by recycling wntless from endosomes to the trans-Golgi network. Dev Cell. 2008;14:120–131. doi: 10.1016/j.devcel.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Port F, Kuster M, Herr P, Furger E, Bänziger C, Hausmann G, Basler K. Wingless secretion promotes and requires retromer-dependent cycling of Wntless. Nature Cell Biology. 2008;10:178–185. doi: 10.1038/ncb1687. [DOI] [PubMed] [Google Scholar]
- 15.Yang PT, Lorenowicz MJ, Silhankova M, Coudreuse DY, Betist MC, Korswagen HC. Wnt signaling requires retromer-dependent recycling of MIG-14/Wntless in Wnt-producing cells. Dev Cell. 2008;14:140–147. doi: 10.1016/j.devcel.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Buechling T, Chaudhary V, Spirohn K, Weiss M, Boutros M. p24 proteins are required for secretion of Wnt ligands. EMBO reports. 2011;12:1265–1272. doi: 10.1038/embor.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Port F, Hausmann G, Basler K. A genome-wide RNA interference screen uncovers two p24 proteins as regulators of Wingless secretion. EMBO reports. 2011;12:1144–1152. doi: 10.1038/embor.2011.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dominguez M, Dejgaard K, Fullekrug J, Dahan S, Fazel A, Paccaud JP, Thomas DY, Bergeron JJ, Nilsson T. gp25L/emp24/p24 protein family members of the cis-Golgi network bind both COP I and II coatomer. J Cell Biol. 1998;140:751–765. doi: 10.1083/jcb.140.4.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stamnes MA, Craighead MW, Hoe MH, Lampen N, Geromanos S, Tempst P, Rothman JE. An integral membrane component of coatomer-coated transport vesicles defines a family of proteins involved in budding. Proc Natl Acad Sci U S A. 1995;92:8011–8015. doi: 10.1073/pnas.92.17.8011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sohn K, Orci L, Ravazzola M, Amherdt M, Bremser M, Lottspeich F, Fiedler K, Helms JB, Wieland FT. A major transmembrane protein of Golgi-derived COPI-coated vesicles involved in coatomer binding. J Cell Biol. 1996;135:1239–1248. doi: 10.1083/jcb.135.5.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marzioch M, Henthorn DC, Herrmann JM, Wilson R, Thomas DY, Bergeron JJ, Solari RC, Rowley A. Erp1p and Erp2p, partners for Emp24p and Erv25p in a yeast p24 complex. Mol Biol Cell. 1999;10:1923–1938. doi: 10.1091/mbc.10.6.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belden WJ, Barlowe C. Erv25p, a component of COPII-coated vesicles, forms a complex with Emp24p that is required for efficient endoplasmic reticulum to Golgi transport. J Biol Chem. 1996;271:26939–26946. doi: 10.1074/jbc.271.43.26939. [DOI] [PubMed] [Google Scholar]
- 23.Ollert MW, Wende C, Gorlich M, McMullan-Vogel CG, Borg-von Zepelin M, Vogel CW, Korting HC. Increased expression of Candida albicans secretory proteinase, a putative virulence factor, in isolates from human immunodeficiency virus-positive patients. J Clin Microbiol. 1995;33:2543–2549. doi: 10.1128/jcm.33.10.2543-2549.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boltz KA, Ellis LL, Carney GE. Drosophila melanogaster p24 genes have developmental, tissue-specific, and sex-specific expression patterns and functions. Dev Dyn. 2007;236:544–555. doi: 10.1002/dvdy.21032. [DOI] [PubMed] [Google Scholar]
- 25.Bartoszewski S, Luschnig S, Desjeux I, Grosshans J, Nusslein-Volhard C. Drosophila p24 homologues eclair and baiser are necessary for the activity of the maternally expressed Tkv receptor during early embryogenesis. Mech Dev. 2004;121:1259–1273. doi: 10.1016/j.mod.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 26.Carney GE, Taylor BJ. Logjam encodes a predicted EMP24/GP25 protein that is required for Drosophila oviposition behavior. Genetics. 2003;164:173–186. doi: 10.1093/genetics/164.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strating JR, Martens GJ. The p24 family and selective transport processes at the ER-Golgi interface. Biol Cell. 2009;101:495–509. doi: 10.1042/BC20080233. [DOI] [PubMed] [Google Scholar]
- 28.Strating JR, van Bakel NH, Leunissen JA, Martens GJ. A comprehensive overview of the vertebrate p24 family: identification of a novel tissue-specifically expressed member. Mol Biol Evol. 2009;26:1707–1714. doi: 10.1093/molbev/msp099. [DOI] [PubMed] [Google Scholar]
- 29.Strating JR, Hafmans TG, Martens GJ. COP-binding sites in p24delta2 are necessary for proper secretory cargo biosynthesis. Int J Biochem Cell Biol. 2009;41:1619–1627. doi: 10.1016/j.biocel.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 30.Strating JR, Hafmans TG, Martens GJ. Functional diversity among p24 subfamily members. Biol Cell. 2009;101:207–219. doi: 10.1042/BC20080075. [DOI] [PubMed] [Google Scholar]
- 31.Hirata R, Nihei C, Nakano A. Isoform-selective oligomer formation of Saccharomyces cerevisiae p24 family proteins. J Biol Chem. 2013;288:37057–37070. doi: 10.1074/jbc.M113.518340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buechling T, Chaudhary V, Spirohn K, Weiss M, Boutros M. p24 proteins are required for secretion of Wnt ligands. EMBO Rep. 2011;12:1265–1272. doi: 10.1038/embor.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Port F, Hausmann G, Basler K. A genome-wide RNA interference screen uncovers two p24 proteins as regulators of Wingless secretion. EMBO Rep. 2011;12:1144–1152. doi: 10.1038/embor.2011.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sollner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE. A Protein Assembly-Disassembly Pathway in-Vitro That May Correspond to Sequential Steps of Synaptic Vesicle Docking, Activation, and Fusion. Cell. 1993;75:409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- 35.Sollner T, Whitehart SW, Brunner M, Erdjumentbromage H, Geromanos S, Tempst P, Rothman JE. Snap Receptors Implicated in Vesicle Targeting and Fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- 36.Lewis MJ, Rayner JC, Pelham HR. A novel SNARE complex implicated in vesicle fusion with the endoplasmic reticulum. EMBO J. 1997;16:3017–3024. doi: 10.1093/emboj/16.11.3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y, Barlowe C. Analysis of Sec22p in endoplasmic reticulum/Golgi transport reveals cellular redundancy in SNARE protein function. Mol Biol Cell. 2002;13:3314–3324. doi: 10.1091/mbc.E02-04-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- 39.Taylor AM, Nakano Y, Mohler J, Ingham PW. Contrasting distributions of patched and hedgehog proteins in the Drosophila embryo. Mech Dev. 1993;42:89–96. doi: 10.1016/0925-4773(93)90101-3. [DOI] [PubMed] [Google Scholar]
- 40.Teleman AA, Cohen SM. Dpp gradient formation in the Drosophila wing imaginal disc. Cell. 2000;103:971–980. doi: 10.1016/s0092-8674(00)00199-9. [DOI] [PubMed] [Google Scholar]
- 41.Flanagan JJ, Barlowe C. Cysteine-disulfide cross-linking to monitor SNARE complex assembly during endoplasmic reticulum-Golgi transport. J Biol Chem. 2006;281:2281–2288. doi: 10.1074/jbc.M511695200. [DOI] [PubMed] [Google Scholar]
- 42.Volchuk A, Ravazzola M, Perrelet A, Eng WS, Di Liberto M, Varlamov O, Fukasawa M, Engel T, Sollner TH, Rothman JE, Orci L. Countercurrent distribution of two distinct SNARE complexes mediating transport within the Golgi stack. Mol Biol Cell. 2004;15:1506–1518. doi: 10.1091/mbc.E03-08-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parlati F, McNew JA, Fukuda R, Miller R, Sollner TH, Rothman JE. Topological restriction of SNARE-dependent membrane fusion. Nature. 2000;407:194–198. doi: 10.1038/35025076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Baiser, CHOp24, Eclair and Sec22 are Wg secretion regulators.
(A–D) β-gal staining representing wg expression is not altered when knocking-down Baiser, CHOp24, Eclair and Sec22 in the posterior compartment of wing disc by hhGal4. Wing blade of wt (E), enGal4>BaiserRNAi (F), enGal4>CHOp24RNAi (G), enGal4>EclairRNAi (H) and enGal4>Sec22RNAi (I) are shown.