Abstract
Exhaustive exercise results in inflammation and oxidative stress, which can damage tissue. Previous studies have shown that vitamin D has both anti-inflammatory and antiperoxidative activity. Therefore, we aimed to test if vitamin D could reduce the damage caused by exhaustive exercise. Rats were randomized to one of four groups: control, vitamin D, exercise, and vitamin D+exercise. Exercised rats received an intravenous injection of vitamin D (1 ng/mL) or normal saline after exhaustive exercise. Blood pressure, heart rate, and blood samples were collected for biochemical testing. Histological examination and immunohistochemical (IHC) analyses were performed on lungs and kidneys after the animals were sacrificed. In comparison to the exercise group, blood markers of skeletal muscle damage, creatine kinase and lactate dehydrogenase, were significantly (P < 0.05) lower in the vitamin D+exercise group. The exercise group also had more severe tissue injury scores in the lungs (average of 2.4 ± 0.71) and kidneys (average of 3.3 ± 0.6) than the vitamin D-treated exercise group did (1.08 ± 0.57 and 1.16 ± 0.55). IHC staining showed that vitamin D reduced the oxidative product 4-Hydroxynonenal in exercised animals from 20.6% to 13.8% in the lungs and from 29.4% to 16.7% in the kidneys. In summary, postexercise intravenous injection of vitamin D can reduce the peroxidation induced by exhaustive exercise and ameliorate tissue damage, particularly in the kidneys and lungs.
Keywords: Calcitriol, 4-Hydroxynonenal, lipid peroxidation.
Introduction
Various types of exercise can be practiced. Regular, nonexhaustive physical exercise is well known to benefit human health. Many studies have proven the effectiveness of regular exercise in preventing several types of disease; for example, regular exercise has been shown to prevent cardiovascular disease, respiratory disease, hemodynamic disorder, hypertension, and obesity 1, 2. However, the beneficial effects of exercise are limited, and these effects are lost if exercise is practiced until exhaustion. Studies have reported that, in addition to energy loss, stress such as inflammatory or oxidative pressure occurs during exhaustive exercise 3, 4. This stress damages human organs such as the kidneys as well as the organs in the respiratory and cardiovascular systems 5. To address this problem, scientists have used a variant of an antioxidant agent in exercise studies 6-8.
Vitamin D is a nutrient contained in natural foods; after intake, it requires skin exposure to ultraviolet B (290-315 nm) radiation and a series of biochemical reactions to modify 7-dehydrocholesterol into a biofunctional form of vitamin D, which is called vitamin D3 or calcitriol (1,25-dihydroxycholecalciferol) 9. Vitamin D3 is crucial in calcium absorption, blood-calcium balance, and bone-density regulation; additional studies have demonstrated that vitamin D deficiency was found exercisers between 2008 and 2010 10-12. Thus, if vitamin D ameliorates damage caused by exercise, then an appropriate intake of vitamin D for exercisers offers multiple benefits: It alleviates bone-density and calcium-balance problems caused by vitamin D deficiency as well as damage caused by exercise. In 1993, Wiseman first demonstrated that vitamin D is an antioxidant vitamin, can prevent iron-dependent lipid peroxidation in the cell membrane, and acts similarly to the cancer drug Tamoxifen 13. Since then, many more studies have been conducted, and the mechanisms of vitamin D have become increasingly clear. Nevertheless, how vitamin D affects exercise has not been thoroughly discussed. Currently, we know that muscle performance significantly (P < 0.05) lowers vitamin D deficiency in exercisers 14. These results indirectly demonstrate that vitamin D potentially affects exercise performance; however, whether vitamin D reduces exercise-induced damage or the oxidative stress caused by exercise requires confirmation. Therefore, in this study, we attempted to clarify the connection between exercise-induced damage and vitamin D as well as whether vitamin D can reduce the oxidative stress caused by exercise.
Materials and Methods
Experimental Protocol
WKY rats were randomly grouped as follows: control group (NS), vitamin D group (vit D), exercise group (E), and exercise + vitamin D group (E+D). The NS and vit D groups were treated without exercise but received intravenous (IV) injections of various drugs: 1 mL of normal saline for the NS group and 1 ml 1ng/mL of vitamin D (Calcitriol, 2 mcg/mL; United Biomedical, Inc., Taiwan, ROC) for the vit D group. The E and E+D groups were treated with exercise but received IV injections of different drugs: the E+D group was injected with 1 mL of vitamin D (Calcitriol, 2 mcg/mL; United Biomedical, Inc., Taiwan, ROC ), and the E group received an IV injection of 1 mL of normal saline as a control. After the exercise was performed, femoral-artery and vein catheters were inserted; each rat was intravenously injected with vitamin D or normal saline, depending on their group. During this time, blood pressure and heart rate data were collected from the femoral-artery catheters. Blood samples for biochemical testing were also collected, and biopsies were performed after the animals were sacrificed. All experimental protocol was approved by the Tzu-Chi University Institutional Animal Care and Use Committee (Approval No. 101083).
Exercise Procedure
The WKY rats were ordered from an animal center with body weights between 260 and 280 g. Before the beginning of the 14-d experiment, the rats were trained to exercise on a treadmill (Shingshieying Instruments; Hualien, Taiwan). During this period, the rats exercised 20 min/d at a speed of 10 m/min with no incline. After 14 d, the rats were transitioned to exercising on a treadmill, and exhaustive exercise was conducted. The exhaustive exercise began at a speed of 10 m/min and increased in increments of 1 m/min every 1 min, up to a maximal running speed of 15 m/min (with no incline). Maximal running times were attained for each rat (when a rat reached exhaustion which was defined by followed the protocol in the published literature 31).
Femoral Artery and Vein Catheter Insertions
After the exhaustive exercise was performed, the rats were anesthetized through ether inhalation during a surgical procedure of approximately 15 min. During this period, polyethylene catheters (PE-50) were inserted into the right femoral artery and vein to collect blood samples and perform IV injections 15. The femoral-artery catheters were also connected to an electrophysiological amplifier (Gould Instruments, Cleveland, OH, USA) to record arterial pressure and heart rate. The operation incision was less than 0.5 cm2, and all surgical procedures were performed under sterile conditions. After the operation, the animals were placed in a metabolic cage and awakened soon thereafter. The rats were allowed free access to food and water.
Blood Biochemistry Examinations (Creatine Kinase and Lactate Dehydrogenase)
When blood sample were collected, these samples were immediately placed into heparinized tubes and centrifuged at 3,000 g for 10 min. After centrifuge, plasma was collected, and the level of creatine kinase (CK) and lactate dehydrogenase (LDH) were measured within 1 h by using an automatic biochemical analyzer (COBAS INTEGRA 800; Roche Diagnostics, Basel, Switzerland).
Histological Examination
The sacrifice was conducted 48 h after IV injections. At that time, the heart, lungs, liver, spleen, kidneys, and intestines were removed immediately. These tissue specimens were fixed overnight in 4% buffered formaldehyde, processed using standard methods, and stained with hematoxylin and eosin (HE stain). An observer blind to the group allocations performed the tissue analyses in this study and scored the organs. The severity of renal tubular injuries was scored by estimating the percentage of tubules in the cortex or the outer medulla that showed epithelial necrosis or had luminal necrotic debris, tubular dilation, and hemorrhage, as follows: 0, none; 1, < 5%; 2, 5-25%; 3, 25-75%; and 4, > 75% 16. The severity of heart injuries observed in the tissue sections was also scored from 0 (minimal or no evidence of injury) to 4 (> 75% damaged). Lung injury was scored as follows: 0, no evidence of injury; 1, mild injury; 2, moderate injury; and 3, severe injury with lung edema, interstitial inflammatory cell infiltration, and hemorrhage 15. Moreover, the severity of liver injuries observed in the tissue sections was scored as follows: 0, minimal or no evidence of injury; 1, mild injury consisting of cytoplasmic vacuolation and focal nuclear pyknosis; 2, moderate to severe injury with extensive nuclear pyknosis, cytoplasmic hypereosinophilia, and loss of intercellular borders; and 3, severe necrosis with disintegration of the hepatic cords, hemorrhage, and neutrophil infiltration 17. Furthermore, the severity of small intestine injuries was scored from 0 to 3 as follows: 0, normal with no damage; 1, mild with focal epithelial edema and necrosis; 2, moderate with diffuse swelling or necrosis of the villi; 3, severe with diffuse necrosis of the villi and evidence of neutrophil infiltration in the submucosa or hemorrhage. All evaluations were performed on five fields per section and five sections per organ.
Immunohistochemistry Stain
After the sacrifice and pathology biopsies, we used a rabbit anti-4 Hydroxynonenal (4-HNE) polyclonal antibody to detect the oxidative product (4-HNE) 18 by using a standard IHC staining method. IHC images were semiquantified using IHC Profiler automated scoring software 19.
Statistical Analysis
The PASWStatistics18 software package (SPSS Inc.), was used for statistical analysis. Unpaired Student's t test was used for assess statistical significance to comparison of preoperative and postoperative data. The level of statistical significance was set as p < 0.05.
Results
Blood Biochemistry Examinations
Blood Level of CK
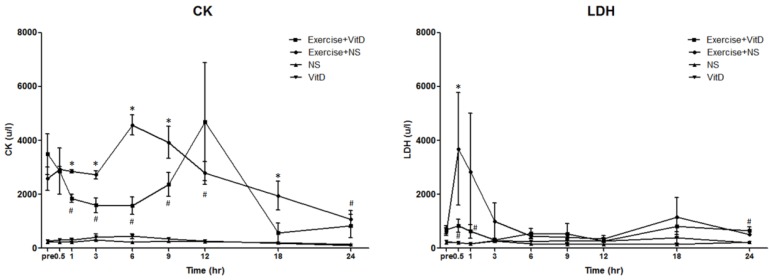
After the exercise treatment and before the IV injections, the original CK level of the no-exercise group (the NS and vit D groups) was between 200 and 280. The original CK levels of both exercise groups were between 2,000 and 3,000, with no significant (P < 0.05) difference between these groups (Fig. 1A). After the IV injections were started, in Hour 1, the E+D group showed a lower concentration of plasma CK than the E group did, which continued from Hour 1 to Hour 9. The difference of CK level between the E and E+D groups was highest at Hour 6. At Hour 12, no significant (P < 0.05) difference was found between the E and E+D groups. The no-exercise group maintained a CK level of 200 to 280 until the experiment ended at Hour 24.
Figure 1.
Biochemical markers for tissue damage. A: CK; B: LDH. *E group differed significantly from the E+D group (P < 0.05). #E+D group differed significantly from the NS and vit D groups (P < 0.05).
Blood Level of LDH
After the exercise treatment and before the IV injections, the original LDH level of the no-exercise group (the NS and vit D groups) was between 219 and 257, with no significant (P < 0.05) difference between the NS and vit D groups. The original LDH levels of both exercise groups were between 612 and 692, with no significant (P < 0.05) difference between the E and E+D groups (Fig. 1B). After 0.5 h, the LDH level of the E group was an average of 3,687 u/L, and the LDH level of the E+D group was an average of 836 u/L, significantly (P < 0.05) lower than it was in the E group, which continued until Hour 6.
Histological Examination
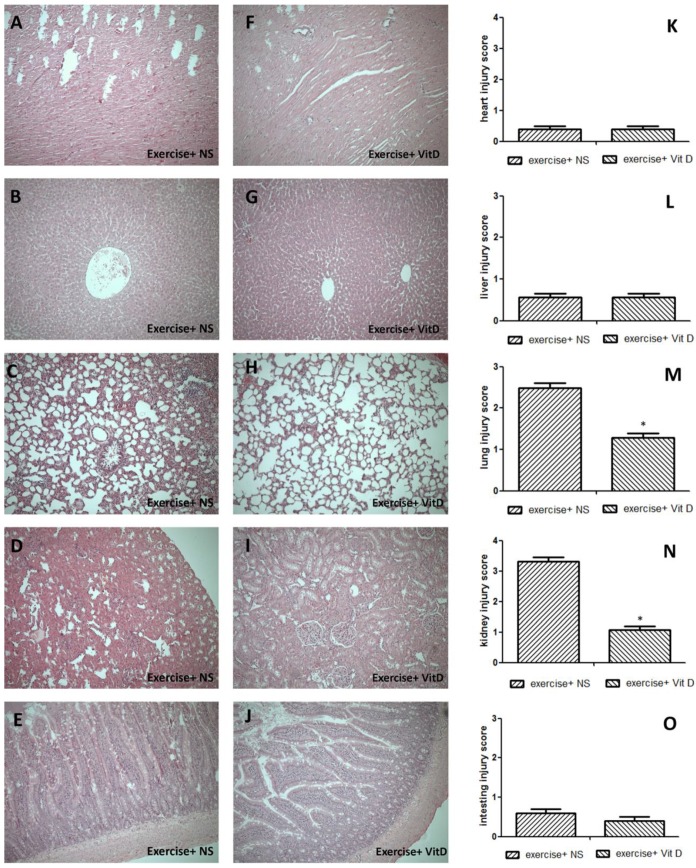
The HE staining results showed severe tissue injuries in the lungs and kidneys of the E+NS group, with average respective scores of 2.4 and 3.32; the E+D group exhibited much lower scores for the lungs (1.2) and kidneys (1.08) (Fig. 2M and N). Other tissues such as the heart, liver, and intestine were not significantly (P < 0.05) injured. All injury scores of the E and E+D groups for these three tissues were below 1 and nonsignificant (P < 0.05) (Fig. 2K, L, and O).
Figure 2.
Tissue damage caused by exercise and the rescue effect of vitamin D. A, K, F: heart; B, G, L: liver; C, H, M: lungs; D, I, N: kidneys; E, J, O: intestines. *E+D group differed significantly from the E group (P < 0.05).
IHC Staining Findings
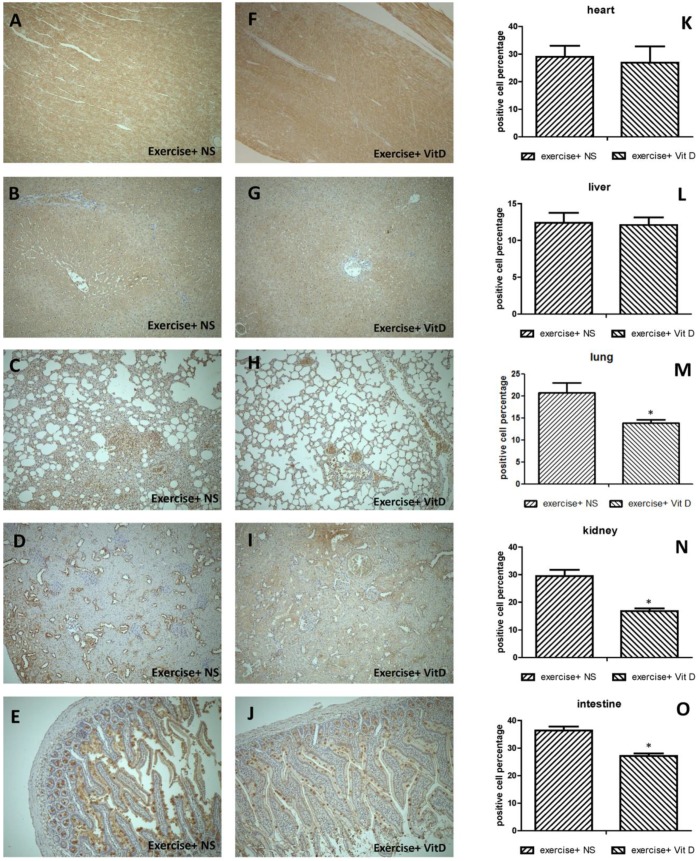
Regarding 4-HNE-positive cells in severely injured tissues, 20.6% of cells in the lungs and 29.4% of cells in the kidneys of the E group were 4-HNE positive. The levels of 4-HNE-positive cells were significantly (P < 0.05) lower in the E+D group, with 13.8% in the lungs and 16.7% in the kidneys (Fig. 3M and N). In other organs such as the heart and liver, no injured tissues occurred; no difference was found between the E and E+D groups (Fig. 3K and L). The intestines were not injured but exhibited lower levels of 4-HNE-positive cells in the E+D group. The positive cells in the intestines were distributed on mucus, not in cells (Fig. 3E and J).
Figure 3.
Distribution of oxidative stress marker 4-HNE in each organ. A, K, F: heart; B, G, L: liver; C, H, M: lungs; D, I, N: kidneys; E, J, O: intestines. *E+D group differed significantly from the E group (P < 0.05).
Discussion
In this study, exhaustive exercise induced different types of organ damage. CK, a biochemical muscle marker, was elevated after exercise; the kidney biopsies and tissue injury scores showed severe kidney damage (Fig. 2D, I, and N). After exercise, particularly strenuous muscle contractions initiate mechanical muscle damage of varying degrees. As the muscle cell degenerates, large amounts of myoglobin, CK, LDH, aspartate transaminase, and urate are released into the circulation 20, 21. Factors such as extreme temperature or strenuous exercise, which can occur during ultramarathons, for example, can result in more severe damage. When the muscle tissue strongly disintegrated, and also a higher serum CK level 22. CK are released, indicating disruption of the sarcomere architecture and surface membrane damage 23. In other recent studies, organ injuries have been discovered after exhaustive exercise through observation of human or animal biochemical parameters 24-26. These data indicate that elevated biochemical markers and exercise-induced tissue damage are often found after exhaustive exercise. Our findings in this study were similar.
Exercise is well known to cause oxidative pressure. In 1978, scientists first determined that physical exercise can lead to an increase in lipid peroxidation 27. Other studies have shown that organ damage and lipid peroxidation appear in humans after a downhill run 28. In our study, the 4-HNE lipid peroxidation marker was also observed in tissues through IHC staining (Fig. 3). In other research, the IHC results showed that 4-HNE-modified proteins accumulated in damaged muscle obtained from mice after acute running 23, which matches our findings. Our results showed that the organ damage scores were comparable with the percentages of 4-HNE-positive cells. For example, the damage scores for heart tissues were low and showed no significant (P < 0.05) differences between the E and E+D groups; the percentages of 4-HNE-positive cells also showed no significant (P < 0.05) differences in these groups (Fig. 2K and 3K). However, the organ damage scores showed that significantly (P < 0.05) damaged tissues such as the lung and kidney tissues also had higher percentages of 4-HNE-positive cells (Fig. 2M, 3M, 2N, and 3N). According to these results, tissue damage was positively correlated with lipid peroxidation. This also indicates that reactive oxygen species (ROS) play a role in exhaustive exercise-induced damage.
Our results revealed that the lungs and kidneys were damaged from exhaustive exercise; however, exercise-induced damage was reduced in the group treated with vitamin D (Fig. 2M and N). In addition, the peroxidation markers were also lower (Fig. 3M and N). According to these results, we could determine that vitamin D can reduce tissue damage by lowering peroxidation stress. A mechanism associated with muscle may also pertain to this study. Muscle accounts for approximately 40% of total body mass and can be damaged by various types of toxicity, ischemia, infection, and inflammation as well as by metabolites 29. The results of these diverse attacks may be muscle fiber dissolution, or rhabdomyolysis. As rhabdomyolysis accrues, broken-down products of damaged muscle cells are released into the bloodstream; some of these, such as protein myoglobin, are harmful to the kidneys and may cause kidney failure 30. Myoglobin can generate primary ROS and enhance the reactivity of ROS 7, indicating another possible mechanism of the exercise-induced ROS damage in this study.
Conclusion
In this study, the results show that exercise-induced tissue damage and lipid peroxidation were significantly (P < 0.05) lower by vitamin D. This provides a possible solution for two common health problems in athletes; vitamin D can remedy calcium deficiency and oxidative damage problems. Furthermore, vitamin D is a nutrient that can be provided to athletes for a long period without any life-threatening side effects. In summary, the findings in this study are useful in exercise medicine applications and contribute to the health of athletes.
Acknowledgments
This work was supported by grants from the National Science Council (NSC 102-2314-B-320-001). Also thanks to Prof. Lee R.P.'s Lab (Tzu Chi University) for technical support in conscious rat animal model.
References
- 1.Reimers CD, Knapp G, Reimers AK. Does physical activity increase life expectancy? A review of the literature. J Aging Res. 2012;2012:243958. doi: 10.1155/2012/243958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stanton T, Haluska BA, Leano R, Marwick TH, CORE Investigators. Hemodynamic benefit of rest and exercise optimization of cardiac resynchronization therapy. Echocardiography. 2014;31:980–8. doi: 10.1111/echo.12506. [DOI] [PubMed] [Google Scholar]
- 3.Deaton CM, Marlin DJ. Exercise-associated oxidative stress. Clin Tech Equine Pract. 2003;2:278–291. [Google Scholar]
- 4.Radak Z, Zhao Z, Koltai E, Ohno H, Atalay M. Oxygen consumption and usage during physical exercise: the balance between oxidative stress and ROS-dependent adaptive signaling. Antioxid Redox Signal. 2013;18:1208–46. doi: 10.1089/ars.2011.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogura S, Shimosawa T. Oxidative stress and organ damages. Curr Hypertens Rep. 2014;16:452. doi: 10.1007/s11906-014-0452-x. [DOI] [PubMed] [Google Scholar]
- 6.Aguiló A, Tauler P, Fuentespina E, Tur JA, Córdova A, Pons A. Antioxidant response to oxidative stress induced by exhaustive exercise. Physiol Behav. 2005;84:1–7. doi: 10.1016/j.physbeh.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 7.Cooper CE, Vollaard NB, Choueiri T, Wilson MT. Exercise, free radicals and oxidative stress. Biochem Soc Trans. 2002;30:280–5. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 8.Urso ML, Clarkson PM. Oxidative stress, exercise, and antioxidant supplementation. Toxicology. 2003;189:41–54. doi: 10.1016/s0300-483x(03)00151-3. [DOI] [PubMed] [Google Scholar]
- 9.Borel P, Caillaud D, Cano NJ. Vitamin D bioavailability: state of the art. Crit Rev Food Sci Nutr. 2015;55:1193–205. doi: 10.1080/10408398.2012.688897. [DOI] [PubMed] [Google Scholar]
- 10.Constantini NW, Arieli R, Chodick G, Dubnov-Raz G. High prevalence of vitamin D insufficiency in athletes and dancers. Clin J Sport Med. 2010;20:368–71. doi: 10.1097/JSM.0b013e3181f207f2. [DOI] [PubMed] [Google Scholar]
- 11.Hamilton B, Grantham J, Racinais S, Chalabi H. Vitamin D deficiency is endemic in Middle Eastern sportsmen. Public Health Nutr. 2010;13:1528–34. doi: 10.1017/S136898000999320X. [DOI] [PubMed] [Google Scholar]
- 12.Lovell G. Vitamin D status of females in an elite gymnastics program. Clin J Sport Med. 2008;18:159–61. doi: 10.1097/JSM.0b013e3181650eee. [DOI] [PubMed] [Google Scholar]
- 13.Wiseman H. Vitamin D is a membrane antioxidant Ability to inhibit iron-dependent lipid peroxidation in liposomes compared to cholesterol, ergosterol and tamoxifen and relevance to anticancer action. FEBS Lett. 1993;326:285–8. doi: 10.1016/0014-5793(93)81809-e. [DOI] [PubMed] [Google Scholar]
- 14.Zhu K, Zhang Q, Foo LH, Trube A, Ma G, Hu X. et al. Growth, bone mass, and vitamin D status of Chinese adolescent girls 3 y after withdrawal of milk supplementation. Am J Clin Nutr. 2006;83:714–21. doi: 10.1093/ajcn.83.3.714. [DOI] [PubMed] [Google Scholar]
- 15.Lee CJ, Subeq YM, Lee RP, Wu WT, Hsu BG. Low-dose propofol ameliorates haemorrhagic shock-induced organ damage in conscious rats. Clin Exp Pharmacol Physiol. 2008;35:766–74. doi: 10.1111/j.1440-1681.2007.04859.x. [DOI] [PubMed] [Google Scholar]
- 16.Chen CH, Lee RP, Wu WT, Liao KW, Hsu N, Hsu BG. Fluvastatin ameliorates endotoxin induced multiple organ failure in conscious rats. Resuscitation. 2007;74:166–74. doi: 10.1016/j.resuscitation.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Hsu BG, Lee RP, Yang FL, Harn HJ, Chen HI. Post-treatment with N-acetylcysteine ameliorates endotoxin shock-induced organ damage in conscious rats. Life Sci. 2006;79:2010–6. doi: 10.1016/j.lfs.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 18.Duong TT, Chami B, McMahon AC, Fong GM, Dennis JM, Freedman SB. et al. Pre-treatment with the synthetic antioxidant T-butyl bisphenol protects cerebral tissues from experimental ischemia reperfusion injury. J Neurochem. 2014;130:733–47. doi: 10.1111/jnc.12747. doi:10.1111/jnc.12747. [DOI] [PubMed] [Google Scholar]
- 19.Varghese F, Bukhari AB, Malhotra R, De A. IHC Profiler: an open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS ONE. 2014;9:e96801.. doi: 10.1371/journal.pone.0096801. doi:10.1371/journal.pone.0096801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huerta-Alardín AL, Varon J, Marik PE. Bench-to-bedside review: Rhabdomyolysis - an overview for clinicians. Crit Care. 2005;9:158–69. doi: 10.1186/cc2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luck RP, Verbin S. Rhabdomyolysis: a review of clinical presentation, etiology, diagnosis, and management. Pediatr Emerg Care. 2008;24:262–8. doi: 10.1097/PEC.0b013e31816bc7b7. [DOI] [PubMed] [Google Scholar]
- 22.Baird MF, Graham SM, Baker JS, Bickerstaff GF. Creatine-kinase- and exercise-related muscle damage implications for muscle performance and recovery. J Nutr Metab. 2012;2012:960363. doi: 10.1155/2012/960363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aoi W, Naito Y, Yoshikawa T. Role of oxidative stress in impaired insulin signaling associated with exercise-induced muscle damage. Free Radic Biol Med. 2013;65:1265–72. doi: 10.1016/j.freeradbiomed.2013.09.014. doi:10.1016/j.freeradbiomed.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 24.Armanfar M, Jafari A, Dehghan GR, Abdizadeh L. Effect of coenzyme Q10 supplementation on exercise-induced response of inflammatory indicators and blood lactate in male runners. Med J Islam Repub Iran. 2015;29:202. [PMC free article] [PubMed] [Google Scholar]
- 25.Bixler GM, Brown A, Way D, Ledford C, Mahan JD. Collaborative Concept Mapping and Critical Thinking in Fourth-Year Medical Students. Clin Pediatr (Phila) 2015;54:833–9. doi: 10.1177/0009922815590223. [DOI] [PubMed] [Google Scholar]
- 26.Xiao NN. Effects of Resveratrol Supplementation on Oxidative Damage and Lipid Peroxidation Induced by Strenuous Exercise in Rats. Biomol Ther (Seoul) 2015;23:374–8. doi: 10.4062/biomolther.2015.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dillard CJ, Litov RE, Savin WM, Dumelin EE, Tappel AL. Effects of exercise, vitamin E, and ozone on pulmonary function and lipid peroxidation. J Appl Physiol Respir Environ Exerc Physiol. 1978;45:927–32. doi: 10.1152/jappl.1978.45.6.927. [DOI] [PubMed] [Google Scholar]
- 28.Maughan RJ, Donnelly AE, Gleeson M, Whiting PH, Walker KA, Clough PJ. Delayed-onset muscle damage and lipid peroxidation in man after a downhill run. Muscle Nerve. 1989;12:332–6. doi: 10.1002/mus.880120412. [DOI] [PubMed] [Google Scholar]
- 29.Smith AG, Muscat GE. Skeletal muscle and nuclear hormone receptors: implications for cardiovascular and metabolic disease. Int J Biochem Cell Biol. 2005;37:2047–63. doi: 10.1016/j.biocel.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Zutt R, van der Kooi AJ, Linthorst GE, Wanders RJ, de Visser M. Rhabdomyolysis: review of the literature. Neuromuscul Disord. 2014;24:651–9. doi: 10.1016/j.nmd.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 31.Hasegawa H, Piacentini MF, Sarre S, Michotte Y, Ishiwata T, Meeusen R. Influence of brain catecholamines on the development of fatigue in exercising rats in the heat. J Physiol. 2008;586:141–9. doi: 10.1113/jphysiol.2007.142190. [DOI] [PMC free article] [PubMed] [Google Scholar]