Abstract
Objective
Non-episodic irritability is a common and impairing problem, leading to the development of the diagnoses severe mood dysregulation (SMD) and disruptive mood dysregulation disorder (DMDD). No psychosocial therapies have been formally evaluated for either, with medication being the most common treatment. This study examined the feasibility and efficacy of a joint parent–child intervention for SMD.
Method
Sixty-eight particpants ages 7–12 with attention-deficit/hyperactivity disorder (ADHD) and SMD were randomly assigned to the 11-week therapy or community-based psychosocial treatment. All participants were first stabilized on psychostimulant medication by study physicians. Fifty-six still manifested impairing SMD symptoms and entered the therapy phase. Masked evaluators assessed participants at baseline, midpoint, and endpoint, with therapy participants reassessed 6 weeks later.
Results
All but two therapy participants attended the majority of sessions (n=29), with families reporting high levels of satisfaction. The primary outcome of change in mood symptoms using the Mood Severity Index (MSI) did not reach significance except in the subset attending the majority of sessions (effect size [ES]=0.53). Therapy was associated with significantly greater improvement in parent-rated irritability (ES=0.63). Treatment effects for irritability but not MSI diminished after therapy stopped. Little impact on ADHD symptoms was seen. Results may not be generalizable to youth with SMD and different comorbidities than seen in this sample of children with ADHD and are limited by the lack of a gold standard for measuring change in SMD symptoms.
Conclusion
While failing to significantly improve mood symptoms versus community treatment, the integrative therapy was found to be a feasible and efficacious treatment for irritability in participants with SMD and ADHD.
Clinical trial registration information
Group-Based Behavioral Therapy Combined With Stimulant Medication for Treating Children With Attention Deficit Hyperactivity Disorder and Impaired Mood; http://clinicaltrials.gov/; NCT00632619.
Keywords: Severe mood dysregulation, disruptive mood dysregulation disorder, ADHD, psychosocial treatment, group therapy
INTRODUCTION
There has been increasing recognition that many children with attention-deficit/hyperactivity disorder (ADHD) exhibit non-episodic irritability and deficits in emotion regulation.1,2,3 While found not to be at increased risk for bipolar disorder (BD),4 children with non-episodic irritability have elevated rates of other internalizing disorders.4,5 For instance, they have a seven-fold greater risk for major depressive disorder (MDD).6 These children also exhibit high rates of aggression and are more impaired compared to children with ADHD and oppositional defiant disorder (ODD). 7–10 These impairments progress to a wide range of adverse outcomes in adulthood.11 The National Institute of Mental Health (NIMH) created the construct of “severe mood dysregulation” (SMD) to study youth with persistent irritability, excessive reactivity to stimuli, and hyperarousal. The SMD criteria were slightly modified for the DSM-512 to create the diagnosis of disruptive mood dysregulation disorder (DMDD), eliminating the hyperarousal criteria and sadness as a qualifying abnormal interval mood.
Little is known about the treatment of SMD.2,13 The only controlled medication trial found no benefit of lithium over placebo.14 The lack of evidenced-based interventions for non-episodic irritability has been linked to the increased prescribing of antipsychotics to youth with ADHD.15,16 It has been suggested that increased use of psychosocial interventions may decrease polypharmacy. Psychosocial treatments may at least serve as an adjunct that enhances outcomes at lower medication doses, as seen in schizophrenia.17,18 Not surprisingly, there has been a call for psychosocial treatments tailored to address the most impairing symptoms seen in SMD: temper outbursts and persistent irritablity.13
Behavioral parent training (BPT) is efficacious for improving ADHD, oppositional behaviors, and aggression.19 However, BPT and psychostimulants have not proven sufficient to normalize functioning in youth with SMD and ADHD.8,20 One reason for the limited efficacy may be that BPT does not address deficits in emotion regulation.3 The addition of an emotion regulation component may be particularly essential for treatment of SMD, as the presence of high levels of externalizing symptoms predicts reduced efforts by parents to engage in emotion regulation coaching with their child.21 Moreover, concerns have been raised that BPT programs addressing volitional temper outbursts may need to be modified for youth with prominent emotional lability in recognition that these children experience sustained negative mood states that are difficult to voluntarily supress.22
Abnormal responses to frustration may drive the anger outbursts and persistent irritability seen in SMD. Once frustrated, youth with SMD experience excessive arousal23,24 and state-dependent impairments in attentional flexibility.25 They exhibit differential patterns of central nervous system (CNS) activation in response to negative feedback13 and experience prolonged recovery from frustration that can be particularly impairing for peer relationships.3,9 Because of these deficits, problem-solving efforts after the onset of prominent irritability may prove quite challenging. Therefore, it may be advisable to focus parental efforts on antecedent management and soothing of negative affect and delay engaging the child in problem-solving efforts until the negative affective state has diminished. At the same time, parents must be conscientious to not inadvertently reward defiance.
Youth with SMD also have difficulty identifying negative emotions26 and experience greater fear when viewing neutral faces.27 These impairments in emotion processing have been theorized to cause the elevated rates of reactive aggression seen with SMD.28 Social cognitive programs for reducing aggressive behaviors may be efficacious for SMD, as they emphasize affect monitoring as well as coping skills for managing anger. They also promote assessment of antecedents and potential consequences prior to action and address hostile attribution biases and other distortions that may promote aggression.29,30 These techniques may be effective for targeting deficits in response reversal seen in SMD.2,9 However, cognitive interventions for social skills deficits in youth with ADHD have not proven routinely efficacious, possibly because of executive functioning impairments.3,31 Therefore, modifications to existing therapies may be necessary to optimize efficacy.
Parents play a critical role in the development of emotion regulation skills, and persistent negative family cycles are theorized to lead to deficits in emotion regulation.3,21 Families of children with ADHD are particularly likely to engage in aversive and conflictual parenting response.32 As parental emotion regulation skills impact the efficacy of parenting interventions in mood-labile youth, 21 inclusion of a parenting component promoting coaching of emotion regulation skills may be advisable for the treatment of SMD.
In creating a treatment tailored to the needs of children with ADHD and SMD, we integrated and modified established techniques from cognitive-behavioral therapy (CBT) for mood disorders, social cognitive programs for aggression, and BPT programs for oppositional behaviors to target the core SMD symptoms of temper outbursts and irritability.33–35 As children with SMD exhibit a wide range of internalizing symptoms,2,6,9 the treatment’s impact on other mood symptoms was examined. In an open pilot trial of the therapy entitled AIM (ADHD plus Impairments in Mood), reductions in mood and behavioral symptoms were observed in seven children with SMD.36 Based on these promising results, a randomized trial comparing AIM plus ADHD medication versus community psychosocial treatment plus ADHD medication was conducted. As stimulant medications are a first-line treatment for ADHD that improves irritability,20,37 both groups were stabilized on stimulant medication before baseline.
METHOD
The study was conducted in two separate cohorts due to the research center relocating in the middle of the study. Between cohorts, identical procedures and many of the same staff were used at each site. The study was approved by governing institutional review boards (IRBs) at both sites.
Participants
Eligible participants were ages 7 –12 and had the combined subtype of ADHD and SMD.2 Both disorders were required, as it is youth with ADHD and behavioral dyscontrol that are being increasingly prescribed antipsychotic medications, 15,16 and most children with SMD drawn from clinical samples will have ADHD.9,14,25 Exclusionary criteria included an IQ below 80, prominent traits of autism spectrum disorder, use of any non-stimulant psychotropic, bipolar I/II, or psychoses. Children with suicidal ideation needing emergent treatment were excluded. Otherwise, mood/anxiety disorders or suicidal ideation were not exclusionary.
Procedures
Families were recruited through advertisement and referrals from local providers. Written consent was obtained from the parents. Participants gave assent. ADHD, ODD, and conduct disorder (CD) were assessed using the Disruptive Behavior Disorders Structured Parent Interview (DBD-I), which also queries about the frequency and severity of temper outbursts at home, school, and with peers.38 It was completed by masters-level clinicians with two plus years of experience in assessing ADHD. ADHD was confirmed by teacher report using the DBD Rating Scale (DBD-RS), which assesses all DSM-IV symptoms of ADHD, ODD, and CD on a 0–3 scale.39 The SMD symptoms of excessive reactivity to negative stimuli, persistent angry/sad mood and hyperarousal, plus symptoms of other mood disorders were assessed using the mood modules from the Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS; Table 1). The WASH-U-KSADS asks about developmentally appropriate symptoms of mood disorders using probes designed to disentangle mood from ADHD symptoms.40,41 These modules specifically query participants and parents about the frequency, severity, and duration of angry/sad moods and temper outbursts. Parent interviews were completed by MD/PhD-staff and participant assessments by experienced graduate students. Integration of both reports along with relevant information gathered from the DBD-I was used to achieve a final composite score, with greater weight given to the reporter deemed most reliable on an item-by-item basis. All raters completed a systematic training course consisting of video reviews and live assessment (Kappa > .9 at the diagnosis level). Participants were required to meet the additional SMD onset, impairment, and duration criteria.2
Table 1.
Diagnostic Assessment Methods
| SMD Criteria | Questions | Scale | Informant |
|---|---|---|---|
|
| |||
| (1) Markedly increased reactivity to negative emotional stimuli manifesting verbally or behaviorally on average at least three times weekly |
|
DBD Parent Interview | Parent only |
|
| |||
|
WASH-U-KSADS Irritability item | Parent and child | |
|
| |||
| (2) Abnormal mood (anger or sadness), present at least half of the day most days |
|
DBD Parent Interview | Parent only |
|
| |||
|
WASH-U-KSADS Depressed mood, irritability, reactivity of mood, rejection sensitivity items | Parent and child | |
|
| |||
| (3) Hyperarousal (≥3 of insomnia, agitation, distractibility, racing thoughts or flight of ideas, pressured speech, intrusiveness) |
|
DBD Parent Interview | Parent only |
|
| |||
|
WASH-U-KSADS | Parent and child | |
|
| |||
| (4) Symptoms cause severe impairment in at least one setting and at least mild in a second | “How much of a problem does this cause?” “How impairing is the problem?” | WASH-U-KSADS and DBD Interview | Parent and child |
| (5) SMD symptom onset must be before age 12 and must be currently present for at least 12 months without symptom-free periods greater than 2 months | “At what age did this start? How long does it last? Has it been present for most of the year? Have you been completely free of… for more than two consecutive months during any 12-month period?” | WASH-U-KSADS and DBD Interview | Parent and child |
| (6) Absence of cardinal manic symptoms, psychoses, or PTSD. | WASH-U-KSADS and KSADS-PL SCQ WISC-IV |
Parent and child Parent Child only |
|
| (7) Absence of autism spectrum disorder | |||
| (8) IQ>70 | |||
Note: DBD Parent Interview= Disruptive Behavior Disorders Parent Interview; KSADS-PL=Kiddie-Schedule for Affective Disorders and Schizophrenia-Present and Lifetime Version; PTSD= posttraumatic stress disorder; SMD= severe mood dysregulation; WASH-U-KSADS=Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia; SCQ= Social Communication Questionnaire; WISC-IV=Wechsler Intelligence Scale for Children–Fourth Edition.
The KSADS-PL was used to assess other comorbidities.42 The Social Communications Questionnaire (SCQ) was used to screen for autism spectrum disorder, using a cutoff score of 15 or more.43 MD/PhD staff confirmed all comorbid diagnoses using best-estimate procedures prior to enrollment. Maternal depression predicts increasing irritability over middle childhood, 44 and parental ADHD predicts reduced response to ADHD treatments.45 Therefore, parents reported on their own level of depressive symptoms (Beck Depression Inventory II [BDI])46 and ADHD symptoms (Adult ADHD Self-Report Scale [ASRS]).47 The cutoffs were 14+ on the BDI and endorsement of 6+ symptoms from one subtype on the ASRS.
Given the debate as to whether DMDD is distinct from ODD,48 we required that participants have elevated levels of hyperarousal and other mood symptoms beyond what is typically seen in youth with ADHD and ODD. Participants had to score ≥ 27 on the Children’s Depressive Rating Scale-Revised (CDRS-R, which ranges from 17–117)49 or ≥12 on the Young Mania Rating Scale (YMRS, which ranges from 0–64).50 These values are the remission cutoffs, and children with only disruptive behavioral disorders typically do not score above them.8,51 They were administered separately to parents and participants by MD/PhD staff to obtain a final composite rating. Raters had to complete a systematic training course consisting of scoring videotaped assessments (Kappa > .8 for the final score).
Prior to the therapy phase, study physicians optimized the psychostimulant dose for all participants to ensure that under-treated ADHD symptoms were not the source of mood dysregulation.52 Many participants had substantial past medication trials, with 24 (37%) being deemed to be on their optimal regimen based on consensus opinion from study physicians. The other 41(63%) had their medication adjusted by study physicians using weekly parent and teacher ratings. It took an average of three visits to optimize dose, with a minimum of two and a maximum of six. All but two were treated with extended-release stimulants, with 39% treated with amphetamine products and the rest with methylphenidate (MPH). The mean dose was .90mg/kg/day in MPH equivalents. Only those continuing to exhibit impairing SMD symptoms plus elevated scores on the CDRS or YMRS progressed to the therapy phase. Baseline measures were completed after medication optimization. Stimulant dose was held stable for the rest of the trial and prescribed by study doctors through endpoint.
Treatment Conditions
Participants were randomized to ADHD medication plus the experimental therapy (AIM group) or medication plus community psychosocial care (CC group) using a computer-generated permutated blocking procedure. Similar to other stepped treatment trials in pediatric mental health, randomization occurred before the medication phase so parents would be aware of therapy status prior to making decisions about medication.53 Families assigned to community care were encouraged to engage with local psychosocial providers. Referrals were provided, but the CC group did not receive any psychosocial treatment from study staff. Families assigned to AIM were asked to suspend non-study psychosocial services for the trial’s duration. Psychotropic medication was prescribed only by study doctors for both groups.
AIM consisted of eleven 105-minute parent and child group sessions. The child program was designed to address SMD’s primary theorized deficits: 1) impairment in identifying negative affective states; 2) over-reactivity to minor stressors; 3) deficits in self-soothing negative affective states; 4) impaired problem solving when emotionally aroused.2 With authors’ permission, evidenced-based programs employing relevant techniques (Fristad’s Multifamily Psychoeducation Groups [MFPG], Cunningham’s Community Parent Education Program [COPE] and Lochman’s Coping Power)33–35 were used as models to guide the development of the integrative SMD treatment. Parent and child sessions were run in parallel to ensure that parents and children were learning complementary content, which has been found to enhance outcomes.54,55 For example, during Session 4, children learned how to apply coping skills to soothe hyperaroused states while parents learned how to coach them in effective application of these skills.
The primary focus of AIM is the connection between mood and behavior with a goal to teach participants that it is best to make choices when calm. Sessions 1–3 focused on emotion recognition in self and others, including what calm feels like; sessions 4–6 on connections between emotions and cognitions including the challenge of problem solving when upset, how to stay calm in the face of frustration, and the development of contextually appropriate coping tools with an emphasis on behavioral over cognitive techniques, as the latter may be challenging for youth with ADHD to reliably employ;3 sessions 7–9 on application of coping tools and problem-solving skills at school and home with an emphasis on self-soothing prior to problem solving. The last sessions focused on integration and practice of learned skills. PhD or graduate-level clinicians experienced with pediatric ADHD led the sessions. A contingency management system was implemented, modeled after the social skills program from the Summer Treatment Program.56 Therapy participants earned points for participation, good behavior, and application of therapy skills, which could be exchanged for gift cards at midpoint and endpoint. The last twenty minutes of each session was a structured recess to provide opportunities to employ learned skills under staff supervision so that participants experienced immediate benefits of effectively handling frustration. For a more detailed description, see Waxmonsky et al., 2013.36
Initial parent sessions provided psychoeducation on how minor irritants can lead to large outbursts. It also focused on behavior modification principles, emphasizing antecedent versus consequence management given the proclivity of youth with SMD for extended and impairing negative mood states following frustration that could impair processing of events. Middle sessions focused on connections between mood and behavior, including identifying triggers for negative mood states, how parental mood and behavior serve as an antecedent for the child’s behavior, and identifying and interrupting negative family cycles that reinforce outbursts. These sessions focused on teaching parents how to be effective emotion regulation coaches. Later sessions emphasized problem solving techniques with an emphasis on addressing negative mood states before engaging in problem solving and managing the balancing act between assisting with emotion regulation while appropriately responding to oppositional behaviors. Final sessions discussed the links between SMD and depression and working cooperatively with the school and other adults in the child’s life. Parents were not compensated for attendance but could earn up to $100 for completing assessments. Parents and participants missing group sessions were offered individual make-up sessions.
Lead therapists met weekly with all therapy aides to review content and program implementation. All sessions were videotaped. One randomly selected weekly session per cohort was scored for treatment integrity and fidelity (range 0–10) using structured checklists to rate adherence to manualized content and techniques by staff trained in AIM who did not participate in that session.
Outcome Assessments
Ratings were completed at baseline (week 0), midpoint (week 6), and endpoint (week 11) with the AIM group reassessed at week 17. All clinician-rated assessments were completed by MD/PhD-level staff masked to therapy status. Participants were interviewed by the same rater whenever possible.
The CDRS-R49 and YMRS50 were used to assess changes in hyperarousal that are a component of SMD and other mood symptoms that commonly occur with SMD.2 While not designed to assess SMD, these measures have been used in other studies of irritable/aggressive youth to examine treatment effects.8,14,60 As participants were recruited for mood dysregulation versus mania or MDD, a Mood Severity Index (MSI=[(CDRS-R score-17)*(11/17)]+ YMRS) was calculated as used by Fristad in her controlled trial of children with a range of mood disorders and served as the primary outcome measure.61 The formula adjusts for the differences in items and minimum severity score between the scales. Unlike Fristad, we did not halve the irritability score, as irritability is a core component of SMD. Lower scores indicate milder symptoms.
Parents and teachers completed the DBD-RS to measure change in the DSM-IV symptoms of ADHD, ODD, and CD.39 The sum score of the three irritability items from the ODD subscale of this measure served as the measure of irritability (range 0–9). These items (“often angry or resentful,” “often touchy/easily annoyed by others,” and “often loses temper”) comprise the angry/irritable DSM-5 cluster of ODD symptoms. They have been found to be a distinct dimension from other oppositional behaviors,12,62,63 predict similar outcomes to an SMD diagnosis that is not seen with the other ODD symptoms, 4 have been used as a proxy for SMD across several studies, 57,58,59 and used to detect treatment effects of psychosocial and pharmacological interventions within ADHD studies. 20
The Social Skills (higher scores equal better functioning) and Problem Behavior (high scores equal more severe functioning) subscales of the Social Skills Rating System (SSRS)64 were completed by parents at baseline and endpoint, as these subscales measure a wide range of internalizing and externalizing symptoms often seen in SMD.2,9 A Satisfaction scale employing a 5-item Likert scale (1=strongly agree, 5=strongly disagree) was completed by parents and participants. Parents also rated the demands of therapy on a 1–7 Likert scale (1 =very unacceptable, 7= very acceptable). The internal consistency was high (Cronbach’s alpha =.86) and consistent with that reported in other samples with ADHD.
Data Analysis
The primary hypothesis was that AIM would lead to a greater reduction in clinician-rated mood symptoms as measured by the Mood Severity Index (MSI). Changes in parent and teacher ratings of irritability and other externalizing symptoms (DBD-RS, SSRS) as well as satisfaction and feasibility were secondary outcomes. Groups were compared using mixed models (PROC MIXED in SAS 9.3) to account for the correlations of repeated observations within participants. Treatment (CC, AIM), time, and the interactions between treatment and time were included as the primary predictors. Gender, racial/ethnic minority status (yes, no), IQ, SES, elevated parental ADHD symptoms (yes/no) and parental mood symptoms (yes/no) and other mental health service status (yes, no) were identified in advance as relevant covariates. Preliminary analyses also controlled for the effect of cohort, but it was never significant and therefore dropped. All other covariates were retained in all analyses as the specific ones that were significant varied across measures. Time was modeled continuously for DBD, CDRS-R, YMRS, and MSI because they were measured at three time points (baseline, midpoint, and endpoint). Time was modeled categorically for the SSRS because it was measured only at baseline and endpoint. Rates of session attendance and homework completion have been found to predict treatment effects, with consistent attendance associated with the best outcomes.65,66 Therefore, similar to the controlled trial of MFPG,61 a secondary analysis of the same outcomes was completed in only those therapy participants attending at least six sessions (n=29). For completers, six is the smallest number of sessions to achieve any consistency in attendance. Models were evaluated using Kenward-Rogers adjustment for degrees of freedom due to the sample size, and robustness of models was evaluated by re-estimating them after dropping influential participants as identified by Cook’s D values. Influential participants did not meaningfully change the findings, and thus results are presented with all participants included. Significant interactions were decomposed using simple slope tests, simple effects tests, and pairwise comparisons. Treatment effects were only interpreted if they interacted with time because treatment main effects average across all stages of treatment (including pretreatment) and therefore have little obvious meaning. Simple t-test analyses were used to examine maintenance of significant effects from therapy endpoint to follow-up. Significant time effects were still reported when the interaction was significant, as little is known about the symptom trajectory of SMD. The mixed model used to analyze the data incorporates fixed and random effects, impacting calculation of the standard deviation. Therefore, Cohen’s d is not available for our mixed model analysis. Instead, an adjusted effect size (ES) that accounts for both fixed and random effects and covariates was used to estimate the size of the treatment effect.67 This effect size was computed by multiplying the parameter estimate of the time x treatment interaction (from the mixed model) by the study duration (11 weeks). This adjusted effect size can be interpreted as a standardized measure of how much the groups differed in their change over treatment.
RESULTS
Study Flow and Demographics
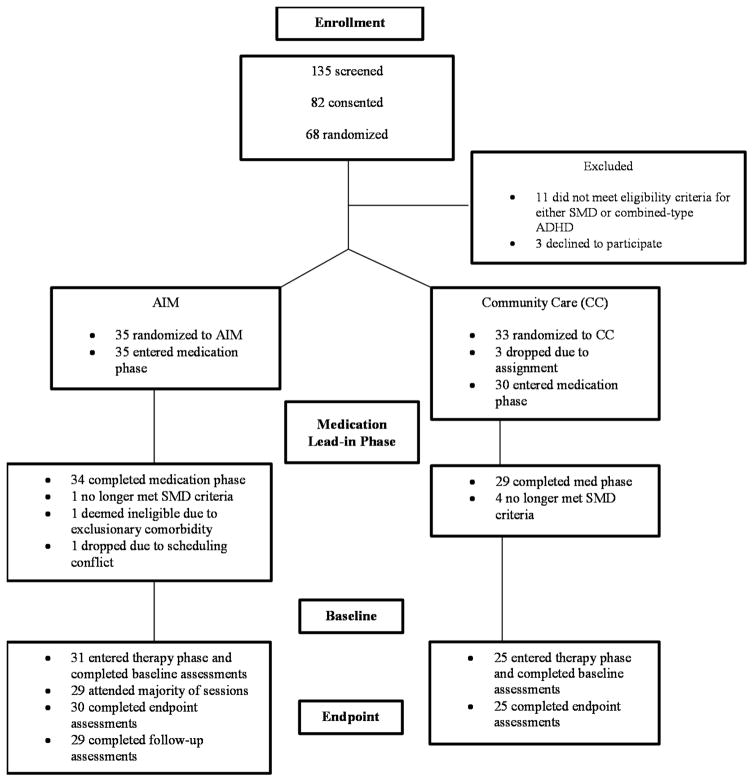
Three pairs of siblings were randomized as one unit each since the parent would be unlikely to apply learned skills to just one participant. There were 68 participants randomized, with 65 entering the medication trial.52 Two discontinued during the medication phase (Figure 1). Five participants no longer exhibited impairing SMD symptoms after optimizing medication. One was excluded for detection of an exclusionary comorbidity and an additional participant assigned to the therapy group could not attend sessions and so dropped before baseline, leaving 56 participants entering the therapy phase. There were 29 AIM participants completing follow-up assessments. Due to differential rates of dropout during the medication phase, the cells became slightly unbalanced, although there were no differences in baseline demographics (Table 2). Most participants had previously used ADHD medications (68%) and had parents with elevated symptoms of MDD or ADHD (59%). The majority of families were middle class. In the community care (CC) group, 15 (60%) participants received outside mental health services during the study. Two (8%) received only school-based counseling, and 13 (52%) received individual sessions with community providers for a mixture of behavior problems, anger management, and social skills issues.
Figure 1.
Consort diagram. Note: ADHD = attention-deficit/hyperactivity disorder; AIM = Novel therapy for ADHD plus Impairments in Mood; CC = community care comparison group; SMD = severe mood dysregulation.
Table 2.
Entry Characteristics
| Variable | AIM (n=31) | Community Care (n=25) |
|---|---|---|
| Age, mean (SD) | 9.3(1.6) | 9.4(1.5) |
| Male (%) | 20(65%) | 19(76%) |
| Racial/ethnic minority (%) | 12(39%) | 9(36%) |
| IQ, mean (SD) | 100.6(15.4) | 100.7(10.6) |
| Entry Stimulant dose mg/kg/daya (SD) | .90(.40) | .90(.43) |
| Using outside counseling services (%) | 18(58%) | 15(60%) |
| Conduct disorder (%) | 4(13%) | 1(4%) |
| Oppositional defiant disorder (%) | 29(94%) | 24(96%) |
| Anxiety Disorders/Subthreshold Anxietyb (%) | 9(29%) | 11(44%) |
| Elevated parental ADHD symptoms (ASRS) (%) | 17(55%) | 7(28%) |
| Elevated parental depressive symptoms (BDI)(%) | 9(29%) | 7(28%) |
| Socioeconomic Index, c mean (SD) | 42.3(15.2) | 42.03(12.8) |
Note: ADHD = attention-deficit/hyperactivity disorder; AIM = Novel therapy for ADHD plus Impairments in Mood; ASRS = Adult ADHD Self-Report Scale; BDI = Beck Depression Inventory
In methylphenidate equivalents on a mg/kg/day basis.
Anxiety symptoms assessed with the Schedule for Affective Disorders and Schizophrenia for School-Age Children: Present and Lifetime version (K-SADS-PL).
Based on Nakao and Treas (1994).
Fidelity and Satisfaction
High fidelity was observed, with 89% of individualized components delivered as prescribed. Families attended a mean of 9.7 out of 11group sessions. All but two attended at least half of the group or make-up sessions (one quit after four sessions and a second intermittently attended five sessions, including the final one). Parents in AIM were highly satisfied, with 97% saying they would recommend it to others. In addition, 80% reported that the program helped them to manage their child’s oppositional behaviors, and 90% said it helped them manage their child’s emotional reactivity. Nearly all parents (90%) rated the demands of therapy as acceptable or very acceptable. Therapy participants reported high satisfaction with the groups, with 83% reporting they liked the program and 77% that it improved their behavior.
Mood and Hyperarousal
The groups did not significantly differ at baseline (CC:M=22.26[95% CI=19.23,25.26]; AIM:M=22.09[95% CI= 19.62,24.57]) or endpoint (CC:M=17.18[95% CI=14.05,20.31]; AIM:M=14.14[95% CI=11.55,16.74]). MSI significantly decreased over time (Table 3). Moderately larger reductions were seen with AIM. The time x treatment interaction (ES=0.53) only reached significance amongst therapy participants attending the majority of sessions (n=29; F[1, 45]=4.88, p=.0323). There was a nonsignificant decrease in MSI scores within the AIM group during the 6-week follow-up period (follow-up Mean=13.9[8.1]). On the CDRS-R, 53 (95%) had an elevated score (≥28). There was a significant time effect with a time x treatment interaction (ES=0.51) that only reached significance in participants attending the majority of therapy sessions (AIM; F[1, 45]=4.48, p=.040). The largest improvements were in the social withdrawal, tearfulness, self-esteem, irritability, sleep, and depressed feelings items. On the YMRS, 33 (59%) participants had an elevated score (≥12). Only time was significant.
Table 3.
Study Outcomes
| Variable | Community Care | Community Care | AIM | AIM | Time | Time x Treat | ||
|---|---|---|---|---|---|---|---|---|
| Prea (sd) | Post a (sd) | Pre a (sd) | Post a (sd) | f | p | f | P | |
| Parent DBD irritability | 4.11 (2.79) | 3.72 (2.69) | 4.96 (2.57) | 3.22 (2.46) | 13.81 | .0005 | 5.59 | .0234 |
| Parent DBD ADHD | 29.49(12.00) | 27.93(11.56) | 28.61(11.06) | 25.76(10.57) | 5.59 | 0.0222 | 0.47 | NS |
| Parent DBD ODD | 11.66(6.52) | 11.26(6.56) | 12.37(6.02) | 10.36(6.02) | 5.23 | 0.0268 | 2.34 | NSb |
| Parent DBD CD | 3.13(3.72) | 3.01(3.72) | 3.72(3.40) | 2.95(3.40) | 2.21 | NS | 1.15 | NS |
| Mood Severity Index | 22.26(7.30) | 17.18(7.59) | 22.09(6.74) | 14.14(7.07) | 80.08 | <.0001 | 3.89 | 0.055c |
| CDRS-R | 32.30(6.27) | 28.67(6.17) | 31.16(5.81) | 25.19(5.70) | 60.35 | <.0001 | 3.56 | 0.065 c |
| YMRS | 12.30 (5.34) | 9.59(5.73) | 12.92(4.93) | 8.89(5.31) | 40.04 | <.0001 | 1.53 | NS |
| SSRS social skills | 79.38(14.84) | 80.57 (17.05) | 72.12(14.02) | 76.45 (16.27) | 6.22 | 0.0163 | 2.01 | NS |
| SSRS problem behaviors | 117.08(20.28) | 119.48 (15.43) | 122.59(19.88) | 116.79 (14.79) | 0.51 | NS | 296 | .0920 |
| Teacher DBD irritability | 2.44 (3.09) | 2.68 (3.04) | 2.52 (2.90) | 2.29(2.90) | 0.01 | NS | 0.54 | NS |
| Teacher DBD ADHD | 23.45(15.04) | 19.26(14.60) | 19.31(14.13) | 17.87(13.97) | 3.96 | 0.0526 | 0.94 | NS |
| Teacher DBD ODD | 5.99(6.66) | 5.70(6.91) | 5.84(6.24) | 5.34(6.68) | 0.31 | NS | 0.02 | NS |
| Teacher DBD CD | 0.97(2.69) | 0.66(2.45) | 1.44(2.52) | 0.91(2.36) | 2.27 | NS | 0.15 | NS |
Note: ADHD = attention-deficit/hyperactivity disorder; AIM = experimental group therapy; CD = conduct disorder; CDRS-R = Children’s Depression Rating Scale Revised; DBD= Disruptive Behavior Disorder Rating Scale; Irritability = sum of items “often angry or resentful”, “often touchy/easily annoyed by others,” and “often loses temper” from the DBD; NS = nonsignificant; ODD = oppositional defiant disorder; SSRS= Social Skills Rating Scale; YMRS = Young Mania Rating Scale.
Values presented are least square means adjusted for covariates.
When AIM group restricted to those attending the majority of sessions (n=29), p=.079.
Reached significance (p<.05) when AIM group restricted to those attending the majority of sessions (n=29).
Irritability
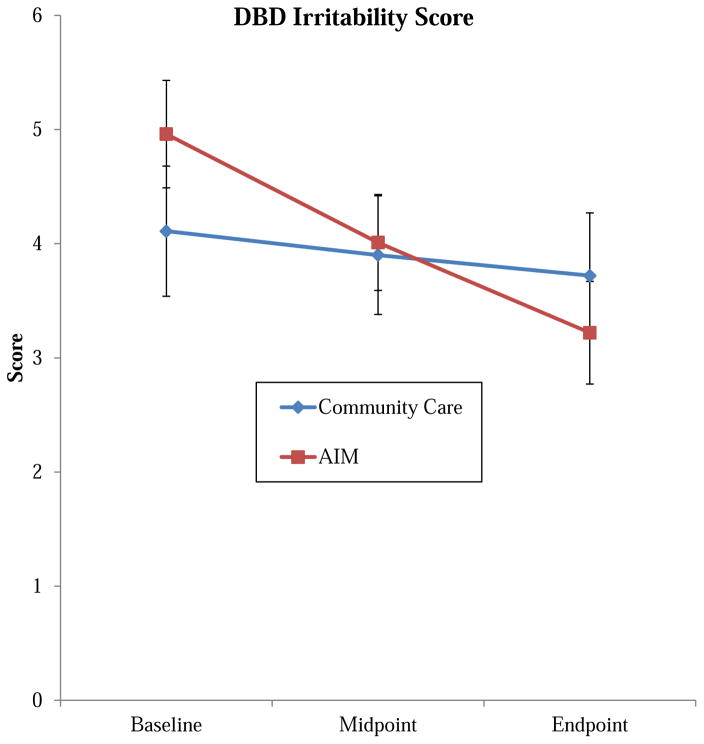
The groups did not significantly differ at baseline (CC: M=4.11[95%CI =2.96, 5.26]; AIM: M=4.96[95% CI =4.01, 5.92]) or endpoint (CC: M=3.72[95% CI =2.61,4.83]; AIM: M=3.22[95% CI= 2.31, 4.13]). There was a significant time effect and time x treatment interaction with larger changes in the therapy group (ES=0.63) (Figure 2). The largest change was seen within the “often angry” item (ES=.73), with moderate effects seen for “often loses temper” (ES=0.47) and small effects for “easily annoyed by others” (ES=0.27). Results were not different when restricted to those attending the majority of sessions. During follow-up, the irritability score significantly increased (Follow-up mean=3.89[2.3], p=.0436), primarily due to an increase in the “loses temper” rating.
Figure 2.
Change in parent-rated irritability. Note: AIM = Novel therapy for ADHD plus Impairments in Mood; DBD irritability score = sum of items “often angry or resentful,” “often touchy/easily annoyed by others,” and “often loses temper” from Disruptive Behavior Disorders Parent Rating Scale (range 0–9).
Other Parent Ratings of Behavior
ADHD symptoms (DBD-RS) significantly declined over time but not differentially by group. Greater improvements in total ODD symptoms (ES=0.42) were seen with AIM, but the time x treatment interaction was not significant. Similar results were seen for the headstrong and hurtful ODD symptom clusters. No significant effects were seen for conduct disorder symptoms (DBD-RS), although larger reductions were seen with AIM (ES=0.29). While not significant at the p<.05 level, larger improvements with AIM were seen in both the social skills (ES= −0.40) and problem behaviors subscales of the SSRS (ES=0.47).
Teacher Ratings
No significant effects were seen for the irritability ratings. There were no significant time effects or time x treatment interactions. Results were not different when limited to those who attended regularly.
Tolerability
No participant met criteria for a manic episode. There were two incidences of suicidal ideation (one per group), with one leading to hospitalization. Neither incident involved a self-harm attempt. A nonsignificant but larger decline in suicidal ideation (CDRS-R, Item 13) was seen with AIM (F[1,45]=3.35, p=.0740; ES=0.57). There was one episode of aggression at school leading to hospitalization. This therapy participant was discharged without any change in their psychotropic medication. Two therapy participants were briefly hospitalized for medical issues (appendicitis and asthma exacerbation) unrelated to study participation.
DISCUSSION
This study is the first randomized trial of a psychosocial therapy (AIM) designed for youth with SMD. All participants were stabilized on psychostimulant medication to ensure that impairing SMD symptoms persisted after ADHD treatment was optimized. AIM led to significantly greater reductions in parent-rated irritability. For those attending a majority of sessions, significant reductions in hyperarousal and other mood symptoms were also seen.
AIM was found to be feasible, with most parents and participants reporting high satisfaction with their treatment experience. Parents identified the joint parent/child sessions as a particular strength. Even though treatment with psychostimulants has been found to reduce attendance rates for behavioral therapies,68 only two (6%) families failed to attend the majority of sessions.
Significantly greater improvement in the irritability cluster of ODD symptoms was seen in therapy participants with their mean endpoint rating declining from the moderate to mild range. Reductions were seen for the phasic (temper outbursts) and tonic components (often angry/easily annoyed). Improvements were larger than those observed during the first 3 months of the MTA for either of the arms with behavioral treatment. These gains occurred after significant reductions in irritability were achieved with optimization of the CNS stimulant dose during the medication lead-in phase of this study.52 Therapy plus medication significantly outperformed medication alone, which was not observed in the MTA, even though AIM was less intensive than the MTA treatment package and applied to participants with more severe parent-rated irritability.20 Results suggest that interventions specifically targeting irritability may be preferable for youth with SMD and ADHD over those with a more general focus on externalizing behaviors. The frequency of temper outbursts significantly increased during follow-up, suggesting that booster sessions may be needed to maintain gains. While all but two AIM participants completed at least partial follow-up ratings, 8 (28%) were missing parent ratings of irritability, which may have impacted results.
Significant group differences were not observed for the other ODD symptoms or for ADHD. However, there were nonsignificant but not insubstantial group differences favoring AIM for the whole spectrum of oppositional behaviors on the DBD (ES=0.41) and SSRS (ES=0.47). The latter asks about a mix of internalizing and externalizing symptoms, consistent with current conceptualizations of DMDD.2,9 While the preferential impact on the irritability cluster of ODD symptoms is encouraging given the focus of AIM, it suggests that reductions in irritability may not translate to meaningful improvements in headstrong or hurtful behaviors, consistent with a multidimensional model of ODD.63 It may also be that youth with SMD are less responsive to behavioral interventions for headstrong/hurtful behaviors than those with just ODD. Few psychosocial intervention trials employ a formal medication phase as was done here,19 so the design may have impacted results. Behavioral therapies produce smaller reductions in externalizing symptoms in medicated versus unmedicated youth.56 Adding behavioral therapy to optimized medication did not significantly reduce ODD or ADHD symptoms versus medication alone in the MTA.37 Likewise, ADHD medication trials evaluating adjunctive supplementation of CNS stimulants find smaller effects than monotherapy trials.69
It is important to note that the change in mood symptoms, which was the primary outcome, did not reach significance. As SMD does not require the presence of prominent mood symptoms other than irritability, the failure to detect significant effects may have been in part due to many of the participants having relatively low baseline scores on the MSI compared to trials of youth with MDD or BP.61 However, amongst those attending at least half of the sessions (93% of AIM participants), significantly greater reductions in hyperarousal and other mood symptoms were seen with therapy as rated by blinded clinicians using established measures of treatment change (CDRS-R, YMRS) that integrated input from participants and parents. Larger effects in completers than in intent-to-treat (ITT) samples have been observed in other psychosocial trials for pediatric mood disorders.61 These combined results emphasize the need for therapies to address potential barriers to attendance. Sustained improvements were seen across a range of depressive symptoms versus just those overlapping with ADHD. While observed effects sizes were comparable to those reported in trials for pediatric mood disorders,61,70 the milder baseline scores and smaller raw changes question the acute clinical significance of the findings. However, SMD predicts depression,4,6,11 so the observed reductions suggest that AIM could reduce the risk for future depressive episodes.
Initial treatments for SMD/DMDD are broad as symptoms span the internalizing and externalizing spectrums, yet there is little evidence to guide clinicians. In children, those with ADHD and conduct problems are most likely to be prescribed antipsychotics, and they are likely to be maintained on them for an extended time.15,16 Although guidelines for aggression recommend multimodal treatment with prioritization of psychosocial modalities,71 the great majority of youth prescribed adjunctive antipsychotics are not engaged in psychosocial treatment.16 Increasing uptake of psychosocial interventions has been forwarded as means to reduce antipsychotic usage.17 Using comparable measures, significant but small reductions for irritability (ES=0.19) and ODD (ES=0.27) were observed with risperidone added to CNS stimulants and parent training in youth with ADHD with recurrent aggression. No differences in parent-rated ADHD or CD symptoms were seen.59
The primary limitation of the study is the novelty of SMD and DMDD, as it remains controversial whether they are unique disorders.48 However, participants had ODD, ADHD, prominent irritability, and other mood symptoms that have been associated with a host of future psychopathology and functional impairments.6,11 Baseline mood scores in our sample were comparable to other SMD samples, except for lower rates of anxiety disorders that were closer to those seen in trials of aggressive youth with ADHD.14,27,72 Future trials should examine the impact of anxiety and other comorbidities on treatment response. Secondly, there is no gold standard for detecting treatment effects on irritability. The DSM-5 ODD irritability items have been used to examine the prevalence of irritability,6,10 and the impact of treatment on irritability in youth with ADHD. 20,59 However, they focus on frequency over duration or severity and do not assess hyperarousal or other mood symptoms often seen in SMD.2,9 Therefore, we selected the MSI as the primary outcome, as it incorporates the rich interview data from the CDRS-R and YMRS to create a composite rating of affective symptomatology that includes all SMD symptoms. Others have used the MSI to detect treatment effects in children with a mix of affective symptoms.61 Moreover, little is known about the course of hyperarousal and other mood symptoms within SMD or their response to treatment.20 With the creation of DMDD for DSM-5 that focuses on irritability while eliminating hyperarousal and sadness as criteria, it may be most prudent to employ a primary outcome that emphasizes irritability in future treatment trials. Third, we used community treatment as the comparison group, with families aware of treatment assignment. Participants in both arms received systematic pharmacological treatment, and the primary outcomes were completed by masked clinicians, minimizing the impact of this limitation. Therapy recipients had substantially more contact with study staff. As this was the first controlled trial of any psychosocial therapy for SMD or DMDD, use of a community care comparator was considered reasonable. The majority of control families received community-based psychosocial treatment. Additional limitations include the sample size, the unbalanced arms in the therapy trial due to the multistage design, lack of follow-up data for the CC group, and the use of study staff as fidelity raters. This study included supports such as financial incentives, dinner, and daycare that are not available in community settings. Therefore, the effectiveness of the intervention remains to be established, as does its efficacy in children without ADHD. Similar to pharmacological trials for irritability, little effects were seen by teacher report, possibly due to milder baseline severity versus parent ratings and the limited utility of teacher ratings for assessing mood symptoms.20,59
The integrative psychosocial treatment for SMD was associated with significant improvement in irritability. Moderate reductions in hyperarousal and other mood symptoms that reached significance in those regularly attending sessions were also observed. The program was well received and applied with high fidelity. Results support the use of psychosocial treatments before advancing to polypharmacy for youth with ADHD and non-episodic irritability that persists after treatment with psychostimulants. Further testing in larger samples, against an active comparator and over an extended timeframe, is merited.
CLINICAL GUIDANCE.
A sizable percentage of children with ADHD exhibit impairing non-episodic irritability, conceptualized as severe mood dysregulation (SMD) by NIMH and disruptive mood dysregulation disorder (DMDD) by DSM-5. There has been little evaluation of psychosocial interventions for either disorder, as medication has been the most commonly employed treatment.
An eleven-session, group therapy program for parents and children that integrates components from cognitive-behavioral therapy for mood disorders, parent training for externalizing disorders, and social-cognitive programs for aggression was designed for children with ADHD and SMD.
The therapy program was well attended, with families reporting high levels of treatment satisfaction.
The experimental therapy produced significant reductions in irritability versus community psychosocial care in children already stabilized on CNS stimulants. Results suggest that tailored psychosocial treatments may be a viable adjunct to CNS stimulants in children with ADHD and SMD that could be implemented prior to prescribing antipsychotic medication.
Acknowledgments
This study was fully funded by a grant from the National Institute of Mental Health (MH080791; Principal Investigator: Waxmonsky).
The authors would like to thank Mary Fristad, PhD, of Ohio State University, and Charles Cunningham, PhD, of McMaster University, for consulting on this project and allowing them to use their programs as guides for the development of the program, as well as John Lochman, PhD, of the University of Alabama, for allowing the authors to use his program as a guide for the development of AIM.
Footnotes
Disclosure: Dr. Waxmonsky has received research funding from the National Institutes of Health (NIH), Noven Pharmaceuticals, and Shire Inc., served on the advisory board for Noven and Iron Shore, and provided CME talks funded by Quintiles Inc., in the past three years. Dr. Waschbusch has received funding from NIH. Dr. Fabiano has received funding from the Institute of Education Sciences (IES), the Administration for Children and Families (ACF), and NIH, and royalties from Guilford Press. Dr. Pettit has received research funding from the American Psychological Foundation and NIH, and book royalties from the American Psychological Association and New Harbinger Publications. Dr. Pelham has received funding from NIH, the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Drug Abuse, IES, and the Society of Clinical Child and Adolescent Psychology. Drs. Belin, Li, Babocsai, Humphrey, Pariseau, Babinski, Hoffman, Haak, Mazzant, and Ms. Fallahazad report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stringaris A. Irritability in children and adolescents: a challenge for DSM-5. Eur Child Adolesc Psychiatry. 2011;20:61–66. doi: 10.1007/s00787-010-0150-4. [DOI] [PubMed] [Google Scholar]
- 2.Leibenluft E. Severe mood dysregulation, irritability, and the diagnostic boundaries of bipolar disorder in youths. Am J Psychiatry. 2011;168:129–142. doi: 10.1176/appi.ajp.2010.10050766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bunford N, Evans SW, Wymbs F. ADHD and emotion dysregulation among children and adolescents. Clin Child Fam Psychol Rev. 2015;18:185–217. doi: 10.1007/s10567-015-0187-5. [DOI] [PubMed] [Google Scholar]
- 4.Stringaris A, Cohen P, Pine DS, Leibenluft E. Adult outcomes of youth irritability: a 20-year prospective community-based study. Am J Psychiatry. 2009;166:1048–1054. doi: 10.1176/appi.ajp.2009.08121849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whelan YM, Stringaris A, Maughan B, Barker ED. Developmental continuity of oppositional defiant disorder subdimensions at ages 8, 10, and 13 years and their distinct psychiatric outcomes at age 16 years. J Am Acad Child Adolesc Psychiatry. 2013;52:961–969. doi: 10.1016/j.jaac.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brotman MA, Schmajuk M, Rich BA, et al. Prevalence, clinical correlates, and longitudinal course of severe mood dysregulation in children. Biol Psychiatry. 2006;60:991–997. doi: 10.1016/j.biopsych.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 7.Anastopoulos AD, Smith TF, Garrett ME, et al. Self-Regulation of Emotion, Functional Impairment, and Comorbidity Among ChildrenWith AD/HD. J Atten Disord. 2011;15:583–592. doi: 10.1177/1087054710370567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waxmonsky J, Pelham WE, Gnagy E, et al. The efficacy and tolerability of methylphenidate and behavior modification in children with attention-deficit/hyperactivity disorder and severe mood dysregulation. J Child Adolesc Psychopharmacol. 2008;18:573–588. doi: 10.1089/cap.2008.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roy AK, Lopes V, Klein RG. Disruptive mood dysregulation disorder: a new diagnostic approach to chronic irritability in youth. Am J Psychiatry. 2014;171:918–924. doi: 10.1176/appi.ajp.2014.13101301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Copeland WE, Angold A, Costello EJ, Egger H. Prevalence, comorbidity, and correlates of DSM-5 proposed disruptive mood dysregulation disorder. Am J Psychiatry. 2013;170:173–179. doi: 10.1176/appi.ajp.2012.12010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Copeland WE, Shanahan L, Egger H, Angold A, Costello EJ. Adult diagnostic and functional outcomes of DSM-5 disruptive mood dysregulation disorder. Am J Psychiatry. 2014;171:668–674. doi: 10.1176/appi.ajp.2014.13091213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5. Washington, DC: American Psychiatric Publishing; 2013. [Google Scholar]
- 13.Leibenluft E, Stoddard J. The developmental psychopathology of irritability. Dev Psychopathol. 2013;25:1473–87. doi: 10.1017/S0954579413000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dickstein DP, Towbin KE, Van Der Veen JW, et al. Randomized double-blind placebo-controlled trial of lithium in youths with severe mood dysregulation. J Child Adolesc Psychopharmacol. 2009;19:61–73. doi: 10.1089/cap.2008.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kreider AR, Matone M, Bellonci C, et al. Growth in the concurrent use of antipsychotics with other psychotropic medications in Medicaid-enrolled children. J Am Acad Child Adolesc Psychiatry. 2014;53:960–970. e962. doi: 10.1016/j.jaac.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Olfson M, King M, Schoenbaum M. Treatment of young people with antipsychotic medications in the United States. JAMA Psychiatry. 2015;72:867–874. doi: 10.1001/jamapsychiatry.2015.0500. [DOI] [PubMed] [Google Scholar]
- 17.Correll CU, Blader JC. Antipsychotic use in youth without psychosis: A double-edged sword. JAMA Psychiatry. 2015;72:859–860. doi: 10.1001/jamapsychiatry.2015.0632. [DOI] [PubMed] [Google Scholar]
- 18.Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE early treatment program [Epub ahead of print September 4, 2015] Am J Psychiatry. doi: 10.1176/appi.ajp.2015.15050632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fabiano GA, Pelham WE, Jr, Coles EK, Gnagy EM, Chronis-Tuscano A, O’Connor BC. A meta-analysis of behavioral treatments for attention-deficit/hyperactivity disorder. Clin Psychol Rev. 2009;29:129–140. doi: 10.1016/j.cpr.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez de la Cruz L, Simonoff E, McGough JJ, Halperin JM, Arnold LE, Stringaris A. Treatment of children with attention-deficit/hyperactivity disorder (ADHD) and irritability: results from the multimodal treatment study of children with ADHD (MTA) J Am Acad Child Adolesc Psychiatry. 2015;54:62–70. e63. doi: 10.1016/j.jaac.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dunsmore JC, Booker JA, Ollendick TH, Greene RW. Emotion socialization in the context of risk and psychopathology: Maternal emotion coaching predicts better treatment outcomes for emotionally labile children with Oppositional Defiant Disorder [Epub ahead of print February 5, 2015] Social Development. doi: 10.1111/sode.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mackinaw-Koons B, Fristad MA, University OS. Children with bipolar disorder: how to break down barriers and work effectively together. Professional Psychol: Res Practice. 2004;35:481–4. [Google Scholar]
- 23.Rich BA, Carver FW, Holroyd T, et al. Different neural pathways to negative affect in youth with pediatric bipolar disorder and severe mood dysregulation. J Psychiatr Res. 2011;45:1283–1294. doi: 10.1016/j.jpsychires.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roy AK, Klein RG, Angelosante A, et al. Clinical features of young children referred for impairing temper outbursts. J Child Adolesc Psychopharmacol. 2013;23:588–596. doi: 10.1089/cap.2013.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deveney CM, Connolly ME, Haring CT, et al. Neural mechanisms of frustration in chronically irritable children. Am J Psychiatry. 2013;170:1186–1194. doi: 10.1176/appi.ajp.2013.12070917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guyer AE, McClure EB, Adler AD, et al. Specificity of facial expression labeling deficits in childhood psychopathology. J Child Psychol Psychiatry. 2007;48:863–871. doi: 10.1111/j.1469-7610.2007.01758.x. [DOI] [PubMed] [Google Scholar]
- 27.Brotman MA, Rich BA, Guyer AE, et al. Amygdala activation during emotion processing of neutral faces in children with severe mood dysregulation versus ADHD or bipolar disorder. Am J Psychiatry. 2010;167:61–9. doi: 10.1176/appi.ajp.2009.09010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deveney CM, Brotman MA, Decker AM, Pine DS, Leibenluft E. Affective prosody labeling in youths with bipolar disorder or severe mood dysregulation. J Child Psychol Psychiatry. 2012;53:262–70. doi: 10.1111/j.1469-7610.2011.02482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lochman JE, Wells KC. The coping power program for preadolescent aggressive boys and their parents: outcome effects at the 1-year follow-up. J Consult Clin Psychol. 2004;72:571–578. doi: 10.1037/0022-006X.72.4.571. [DOI] [PubMed] [Google Scholar]
- 30.Feindler E, Marriott S, Iwata M. Group anger control training for junior high school delinquents. Cognitive Therapy ad Research. 1984;8:299–311. [Google Scholar]
- 31.Pfiffner LJ, McBurnett K. Social skills training with parent generalization: treatment effects for children with attention deficit disorder. J Consult Clin Psychol. 1997;65:749–757. doi: 10.1037//0022-006x.65.5.749. [DOI] [PubMed] [Google Scholar]
- 32.Johnston C, Mash EJ. Families of children with attention-deficit/hyperactivity disorder: review and recommendations for future research. Clin Child Fam Psychol Rev. 2001;4:183–207. doi: 10.1023/a:1017592030434. [DOI] [PubMed] [Google Scholar]
- 33.Lochman JE, Wells KC, Lenhart LA. Coping Power: Child group facilitator’s guide. New York: Oxford University Press; 2008. [Google Scholar]
- 34.Fristad MA, Gavazzi SM, Soldano KW. Multi-family psychoeducation groups for childhood mood disorders: a program description and preliminary efficacy data. Contemporary Family Therapy. 1998;20:385–402. [Google Scholar]
- 35.Cunningham CE, Brenner R, Secord-Gilbert M. The community parent education program (COPE): A school based family systems oriented course for parents of children with disruptive behavior disorders. Hamilton, ON: Chedoke-McMaster Hospitals and McMaster University; 1998. [Google Scholar]
- 36.Waxmonsky JG, Wymbs FA, Pariseau ME, et al. A novel group therapy for children with ADHD and severe mood dysregulation. J Atten Disord. 2013;17:527–541. doi: 10.1177/1087054711433423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jensen PS, Arnold LE, Swanson JM, et al. 3-year follow-up of the NIMH MTA study. J Am Acad Child Adolesc Psychiatry. 2007;46:989–1002. doi: 10.1097/CHI.0b013e3180686d48. [DOI] [PubMed] [Google Scholar]
- 38.Hartung CM, McCarthy DM, Martin CA. Parent adolescent agreement on ADHD symptoms: a multi-trait, multi-methods model. J Psychopathol Behavioral Assessment. 2005;27:159–168. [Google Scholar]
- 39.Pelham WE, Jr, Gnagy EM, Greenslade KE, Milich R. Teacher ratings of DSM-III-R symptoms for the disruptive behavior disorders. J Am Acad Child Adolesc Psychiatry. 1992;31:210–8. doi: 10.1097/00004583-199203000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Geller BWM, Zimmerman B, Frazier J. Washington University of St. Louis Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) St Louis: Washington University; 1996. [DOI] [PubMed] [Google Scholar]
- 41.Geller B, Zimerman B, Williams M, et al. Reliability of the Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) mania and rapid cycling sections. J Am Acad Child Adolesc Psychiatry. 2001;40:450–5. doi: 10.1097/00004583-200104000-00014. [DOI] [PubMed] [Google Scholar]
- 42.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–8. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 43.Chandler S, Charman T, Baird G, et al. Validation of the social communication questionnaire in a population cohort of children with autism spectrum disorders. J Am Acad Child Adolesc Psychiatry. 2007;46:1324–32. doi: 10.1097/chi.0b013e31812f7d8d. [DOI] [PubMed] [Google Scholar]
- 44.Wiggins JL, Mitchell C, Stringaris A, Leibenluft E. Developmental trajectories of irritability and bidirectional associations with maternal depression. J Am Acad Child Adolesc Psychiatry. 2014;53:1191–1205. doi: 10.1016/j.jaac.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chronis-Tuscano A, Stein MA. Pharmacotherapy for parents with attention-deficit hyperactivity disorder (ADHD): impact on maternal ADHD and parenting. CNS Drugs. 2012;26:725–32. doi: 10.2165/11633910-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 46.Steer RA, Ball R, Ranieri WF, Beck AT. Dimensions of the Beck Depression Inventory-II in clinically depressed outpatients. J Clin Psychol. 1999;55:117–28. doi: 10.1002/(sici)1097-4679(199901)55:1<117::aid-jclp12>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 47.Adler LA, Spencer T, Faraone SV, et al. Validity of pilot Adult ADHD Self- Report Scale (ASRS) to Rate Adult ADHD symptoms. Ann Clin Psychiatry. 2006;18:145–8. doi: 10.1080/10401230600801077. [DOI] [PubMed] [Google Scholar]
- 48.Axelson DA, Birmaher B, Findling RL, et al. Concerns regarding the inclusion of temper dysregulation disorder with dysphoria in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. J Clin Psychiatry. 2011;72:1257–62. doi: 10.4088/JCP.10com06220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poznanski EOMH. Children’s Depression Rating Scale-Revised (CDRS-R) Los Angeles: Western Psychological Services; 1996. [Google Scholar]
- 50.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–35. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 51.Fristad MA, Weller EB, Weller RA. The Mania Rating Scale: can it be used in children? A preliminary report. J Am Acad Child Adolesc Psychiatry. 1992;31:252–7. doi: 10.1097/00004583-199203000-00011. [DOI] [PubMed] [Google Scholar]
- 52.Baweja R, Belin P, Humphrey H, et al. The efficacy and tolerability of CNS stimulants in school aged children with Attention Deficit/hyperactivity disorder and Disruptive Mood Dysregulation Disorder across Home and School. J Child Adolesc Psychopharmacol. 2015 doi: 10.1089/cap.2015.0053. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Farmer CA, Arnold LE, Bukstein OG, et al. The treatment of severe child aggression (TOSCA) study: Design challenges. Child Adolesc Psychiatry Ment Health. 2011;5:36. doi: 10.1186/1753-2000-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Curtis DF, Chapman S, Dempsey J, Mire S. Classroom changes in ADHD symptoms following clinic-based behavior therapy. J Clin Psychol Med Settings. 2013;20:114–122. doi: 10.1007/s10880-012-9307-2. [DOI] [PubMed] [Google Scholar]
- 55.Piacentini J, Bergman RL, Chang S, et al. Controlled comparison of family cognitive behavioral therapy and psychoeducation/relaxation training for child obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2011;50:1149–61. doi: 10.1016/j.jaac.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pelham WE, Burrows-MacLean L, Gnagy EM, et al. A dose-ranging study of behavioral and pharmacological treatment in social settings for children with ADHD. J Abnorm Child Psychol. 2014;42:1019–31. doi: 10.1007/s10802-013-9843-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Copeland WE, Angold A, Costello EJ, Egger H. Prevalence, comorbidity, and correlates of DSM-5 proposed disruptive mood dysregulation disorder. Am J Psychiatry. 2013;170:173–179. doi: 10.1176/appi.ajp.2012.12010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stringaris A, Goodman R. Longitudinal outcome of youth oppositionality: irritable, headstrong, and hurtful behaviors have distinctive predictions. J Am Acad Child Adolesc Psychiatry. 2009;48:404–12. doi: 10.1097/CHI.0b013e3181984f30. [DOI] [PubMed] [Google Scholar]
- 59.Gadow KD, Arnold LE, Molina BS, et al. Risperidone added to parent training and stimulant medication: effects on attention-deficit/hyperactivity disorder, oppositional defiant disorder, conduct disorder, and peer aggression. J Am Acad Child Adolesc Psychiatry. 2014;53:948–59. doi: 10.1016/j.jaac.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blader JC, Pliszka SR, Jensen PS, Schooler NR, Kafantaris V. Stimulant-responsive and stimulant-refractory aggressive behavior among children with ADHD. Pediatrics. 2010;126:e796–806. doi: 10.1542/peds.2010-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fristad MA, Verducci JS, Walters K, Young ME. Impact of multifamily psychoeducational psychotherapy in treating children aged 8 to 12 years with mood disorders. Arch Gen Psychiatry. 2009;66:1013–21. doi: 10.1001/archgenpsychiatry.2009.112. [DOI] [PubMed] [Google Scholar]
- 62.Burke JD, Boylan K, Rowe R, et al. Identifying the irritability dimension of ODD: Application of a modified bifactor model across five large community samples of children. J Abnorm Psychol. 2014;123:841–51. doi: 10.1037/a0037898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krieger FV, Polanczyk VG, Goodman R, et al. Dimensions of oppositionality in a Brazilian community sample: testing the DSM-5 proposal and etiological links. J Am Acad Child Adolesc Psychiatry. 2013;52:389–400. doi: 10.1016/j.jaac.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gresham F, Elliott S. Social Skills Rating Scales (SSRS) Circle Pines, MN: American Guidance Systems; 1990. [Google Scholar]
- 65.Kazantzis N, Deane FP, Ronan KR. Assessing compliance with homework assignments: review and recommendations for clinical practice. J Clin Psychol. 2004;60:627–41. doi: 10.1002/jclp.10239. [DOI] [PubMed] [Google Scholar]
- 66.Nock MK, Ferriter C. Parent management of attendance and adherence in child and adolescent therapy: a conceptual and empirical review. Clin Child Fam Psychol Rev. 2005;8:149–66. doi: 10.1007/s10567-005-4753-0. [DOI] [PubMed] [Google Scholar]
- 67.Olejnik S, Algina J. Generalized eta and omega squared statistics: measures of effect size for some common research designs. Psychol Methods. 2003;8:434–47. doi: 10.1037/1082-989X.8.4.434. [DOI] [PubMed] [Google Scholar]
- 68.Pelham W, Fabiano G, Waxmonsky J, et al. Treatment Sequencing for Childhood ADHD: A Multiple-Randomization study of adaptive medication and behavioral interventions. J Clin Child Adolesc Psychol. 2015 doi: 10.1080/15374416.2015.1105138. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hirota T, Schwartz S, Correll CU. Alpha-2 agonists for attention-deficit/hyperactivity disorder in youth: a systematic review and meta-analysis of monotherapy and add-on trials to stimulant therapy. J Am Acad Child Adolesc Psychiatry. 2014;53:153–73. doi: 10.1016/j.jaac.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 70.Compton SN, March JS, Brent D, Albano AMt, Weersing R, Curry J. Cognitive-behavioral psychotherapy for anxiety and depressive disorders in children and adolescents: an evidence-based medicine review. J Am Acad Child Adolesc Psychiatry. 2004;43:930–959. doi: 10.1097/01.chi.0000127589.57468.bf. [DOI] [PubMed] [Google Scholar]
- 71.Pappadopulos E, Macintyre JC, Crismon ML, et al. Treatment recommendations for the use of antipsychotics for aggressive youth (TRAAY). Part II. J Am Acad Child Adolesc Psychiatry. 2003;42:145–61. doi: 10.1097/00004583-200302000-00008. [DOI] [PubMed] [Google Scholar]
- 72.Blader JC, Schooler NR, Jensen PS, Pliszka SR, Kafantaris V. Adjunctive divalproex versus placebo for children with ADHD and aggression refractory to stimulant monotherapy. Am J Psychiatry. 2009;166:1392–1401. doi: 10.1176/appi.ajp.2009.09020233. [DOI] [PMC free article] [PubMed] [Google Scholar]