Abstract
Cryptococcus neoformans (Cn) and Histoplasma capsulatum (Hc) co-exist in the environment and occasionally co-infect individuals, which can lead to severe disease/lethal outcomes. We investigated specific interactions between Cn-Hc to determine the impact of synchronous infection in virulence and disease. Co-infected mice had significantly higher mortality than infection with either species or acapsular Cn-Hc. Coating of Hc with cryptococcal glycans (Cn-gly) resulted in higher pulmonary fungal burden in co-infected animals relative to control. Co-cultivation or addition of Cn-gly resulted in enhanced pellicle formation with a hybrid polysaccharide matrix with higher reactivity to GXM mAbs. Transfer and incorporation of Cn polysaccharide onto Hc surface was time and temperature dependent. Cn-gly transfer altered the zeta potential of Hc and was associated with increased resistance to phagocytosis and killing by macrophages. Mice infected with Hc and subsequently injected with purified Cn-gly died significantly more rapidly than Hc alone infected, establishing the precedent that virulence factors from one fungus can enhance the virulence of unrelated species. These findings suggest a new mechanism of microbial interaction involving the transfer of virulence traits that translates into enhanced lethality during mixed fungal infections and highlights the importance of studying heterogeneous microbial populations in the setting of infection.
Cryptococcus neoformans (Cn) and Histoplasma capsulatum (Hc) are two of the major pathogenic fungi in the world, causing millions of infections annually with significant morbidity and mortality1,2. Hc is a dimorphic fungus responsible for a wide range of clinical presentations, from asymptomatic infection or a mild influenza-like illness to disseminated sepsis3 that is frequently associated with fatal infection. Epidemiological studies have estimated that ~500,000 individuals acquire Hc annually in the USA and over 80% of young adults in endemic areas have been infected by the fungus4,5. Fortunately, the majority of individuals acquiring Hc do not develop clinically significant infections, although there are still ~3,500 hospitalizations due to histoplasmosis in the USA annually, with a crude mortality rate of ~8%5. Pulmonary histoplasmosis symptoms are similar to those of pulmonary cryptococcosis, caused by the encapsulated basidomycetous yeast Cn and/or C. gattii. In addition, cryptococcosis can evolve to a life-threatening meningoencephalitis in susceptible individuals1. Globally, cryptococcal meningitis occurs in about 1 million individuals annually with a mortality rate of approximately 60%1. Individuals with histoplasmosis or cryptococcosis who are iatrogenically immunosuppresed (ie. receiving steroids or tumor necrosis factor-alpha inhibitors) or have impaired cell-mediated immunity (ie. HIV patients) are at high risk for life-threatening disease6.
Hc and Cn are widely distributed in the environment and infection by either/both fungi can be acquired after disturbance and aerosolization of soil contaminated with bird excreta1. Although co-infection rates are unknown, most adults in urban areas have serological evidence of Cn infection7 and skin testing has shown a high prevalence of Hc infection in endemic areas8. Consequently, it is possible that in Hc endemic areas there are high numbers of individuals who have been infected with both Hc and Cn, although there is no information on the timing of these infections (ie. acquisition occurring concomitantly or separately). Nevertheless, a review of the literature finds a significant number of cases of Cn-Hc co-infections9,10,11,12,13,14,15,16,17,18,19,20,21, which establishes that co-infections can and do occur, and can progress to disease with both fungi. Identification of co-infected individuals is complicated by the fact that clinical manifestations of both mycoses and the antifungal therapy administered for them are similar (typically a polyene followed by an azole). Additionally, Cn is more likely to be identified by routinely microscopy techniques and grows within 5 days on Sabouraud agar, whereas Hc is more fastidious, typically takes about 14-to-30 days for growth in culture1,8 and can also be inhibited by Cn22. Hence, it is probable that co-infection is under diagnosed and that the true incidence of concomitant infection is significantly greater than currently understood.
Many components of the cell wall of Cn are similar to those of Hc, and these surface components form the main interacting interface with their environment and cells of the host immune system. However, the outer layer of Cn consists of an additional large anti-phagocytic polysaccharide (PS) capsule, which is the fungus’ most distinctive virulence determinant. The capsule is mainly composed of glucuronoxylomannan (GXM), a high molecular mass (106–108 g/mol) α-1, 3-linked mannan backbone decorated with xylose and glucuronic acid residues23. GXM is synthesized intracellularly within the Golgi and released via vesicles to the extracellular milieu24. Eventually the GXM is incorporated into a growing capsule by complex PS-PS interactions that include GXM interaction with cell wall-derived α-glucans25, chitin-derived structures26, and other GXM molecules27. Significantly, GXM is also released into the serum and tissues during disease, frequently reaching titers >1:10,000 (or >10 μg/mL) in human disease28; hence, there is ample opportunity for the PS to interact in vivo with other microbes as well as host cells. In fact, in addition to protecting the fungus against oxidative stress23, Cn capsular PS is associated with potent detrimental effects on the immune system, such as inhibition of phagocytosis, dysregulation of immunoresponses, reduced leukocyte migration, complement depletion, interference in antigen presentation, and T-cell suppression with subsequent inhibition of inflammatory cytokines production29. Additional roles of the cryptococcal capsule in virulence have been demonstrated using congenic strain pairs that differ only by mutations or replacement of specific capsular synthesis/assembly genes, such as the well characterized CAP genes family (CAP10, CAP59, CAP60, CAP64), CAS genes (CAS1, CAS3, CAS31) and many others23,30. These mutations result in acapsular or hypocapsular phenotypes23 that were severely attenuated in murine models of infection30.
The outer layer of Hc yeast cells displays several surface molecules involved in their internalization by various phagocytes and several carbohydrate-linked structures with immunomodulatory activities are intimately linked to fungal pathogenesis and virulence31. Known Hc glycans (gly) include chitin, α-1, 3- and β-1, 3-glucans, and extracellular galactomannan32. Although the Hc surface is less well understood relative to that of Cn, and only a few gly have been partially characterized, Hc can incorporate exogenously added cryptococcal exoPS in vitro25. However, it is unclear whether PS transfer occurs in the environment or during mammalian co-infection. Moreover, the importance of this process on the outcome of Hc infection has not been explored.
In this study, we explored whether co-infection with Cn affected the virulence of Hc. Incorporation and coating of Hc yeast cells by Cn PS was detected during co-infection of mice. This incorporation of Cn PS by Hc increased pulmonary disease, as there were higher fungal burdens of encapsulated Hc in the lungs of co-infected mice compared to mice infected with Hc alone. The acquisition and incorporation of exogenous GXM on Hc yeast cell surfaces altered the cellular electrostatic potential and resulted in a reduction in phagocytosis and intracellular killing of the yeast by macrophages. The observations presented in this work raise the possibility that fungi can interchange virulence factors and that this process can modulate the immune response and lead to enhanced damage to mammalian hosts.
Results
Co-infection resulted in enhanced mouse mortality
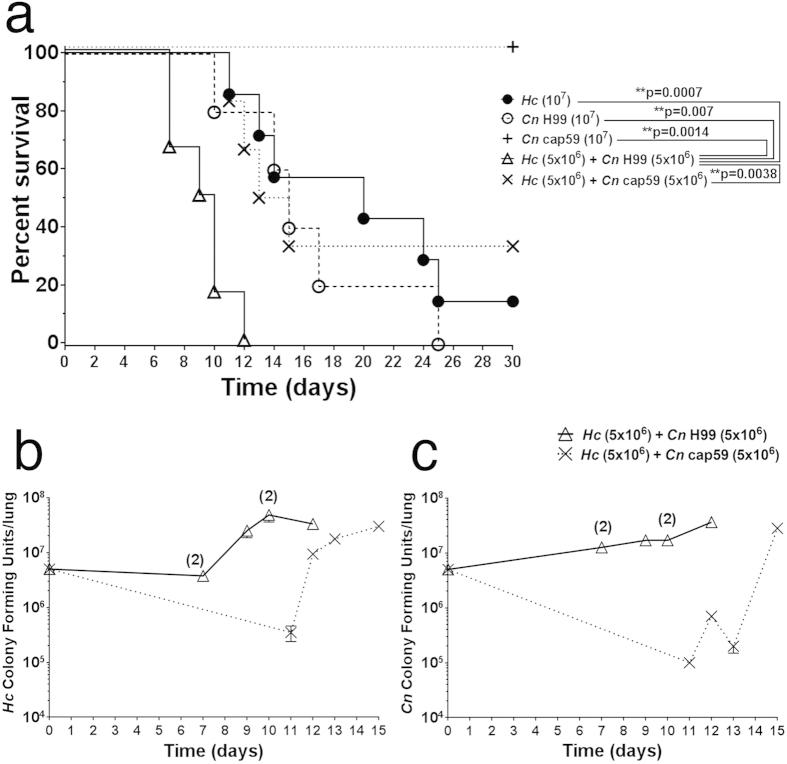
We explored the possibility that Cn and Hc co-infection could worsen disease prognosis in mice. Co-infection was assessed with an equal mixed inoculum of Hc and encapsulated Cn H99 or unencapsulated Cn cap59 (5 × 106 of each fungus) and compared with monospecies infected animals (107 yeasts). Co-infection with Hc and Cn H99 resulted in higher mortality rates, with 100% death after 12 days, relative to mice infected with Hc and acapsular Cn cap59 (p = 0.0038) or monospecies inoculum of either Hc (**p = 0.0007), Cn H99 (**p = 0.007) or Cn cap59 (**p = 0.0014; Fig. 1a).
Figure 1. Co-infection of mice with Hc and encapsulated Cn enhances virulence.
(a) Mice were infected with 107 total yeast inoculated as either a single species (107 of either Cn H99 or Hc) or a 1:1 mix of both fungi (5 × 106 Hc with 5 × 106 of either Cn H99 or Cn cap59). Co-infection with Hc and Cn H99 resulted in accelerated mortality compared to the other groups. (b) Hc and (c) Cn pulmonary CFUs from animals who expired due to infection with Hc and either Cn H99 or Cn cap59. Time 0 indicates initial inoculum of the specified fungi. Pulmonary fungal burdens of both. (b) Hc and (c) Cn were relatively higher for mice infected with Hc + Cn H99 compared to Hc co-infected with the acapsular Cn cap59. When present, digits over graph points in b and c indicate the number of deceased animals at a specific time point (cumulative death/same day), with CFUs expressed as averages.
To confirm that animals were indeed co-infected and to determine fungal burdens in the scope of Cn PS importance, the colony forming units (CFU) were determined in lungs of deceased animals in the course of the survival experiments for Hc (Fig. 1b) and Cn (Fig. 1c). Both fungal species were recovered in similar proportions, indicating they colonize the lungs with similar efficacy and that they could interact in vivo.
However, animals from the Hc + Cn H99 co-infection group had higher burdens of Hc and Cn during earlier time-points than those in the Hc + Cn cap59 group (Fig. 1b,c, respectively), which correlated with the increased lethality of the Hc co-infection with the encapsulated Cn. Hc fungal burdens from Hc + Cn H99 group varied from 2.9 × 106 to 7.0 × 107 (median 2.6 × 107), while the Hc + Cn cap59 group ranged from 3.5 × 105 to 3.0 × 107 (median 1.35 × 107). For Cn fungal burdens, group Hc + Cn H99 ranged from 1.2 × 107 to 3.6 × 107 (median 1.7 × 107), while Hc + Cn cap59 group ranged from 1.0 × 105 to 2.8 × 107 (median 4.5 × 105). These results suggest that co-infection of Hc with Cn that efficiently releases PS leads to an increase in the virulence of Hc in vivo. Additionally, the monoinfection with Hc yeast cell alone resulted in fungal burdens that were ~25% lower than the average observed for the Hc + Cn H99 co-infected animals, further suggesting that virulence of Hc is enhanced in the presence of Cn. This conclusion is further justified based on the fact that half as many Hc yeast cells (5 × 106) were introduced to the co-infected animals compared to those receiving Hc alone (107).
Hc incorporates Cn glycans in vivo
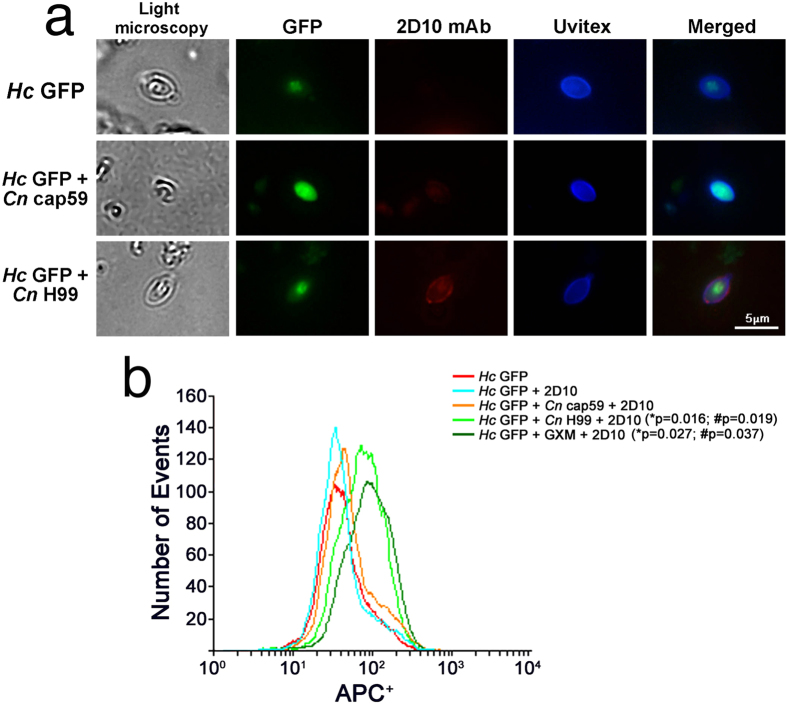
The possibility that Hc could interact in vivo with Cn-glycans was explored initially by the evaluation of the transfer of Cn PS to the surface of Hc. Recovered yeasts from co-infected lungs were incubated with 2D10 mAbs to cryptococcal GXM33 and an anti-mouse IgM Alexa 546 conjugate and evaluated by fluorescence microscopy (Fig. 2a). Hc GFP yeasts recovered from Hc + Cn H99 co-infected lungs displayed strong labelling for GXM in comparison to the absence of labelling on fungal cells obtained from mice infected with Hc + Cn cap59 or Hc GFP control alone (Fig. 2a). To obtain more quantitative information, Hc yeasts recovered from infected mice were incubated with mAb 2D10 and anti-mouse IgM allophycocyanin (APC)-conjugate and evaluated by flow cytometry (Fig. 2b). As an additional control, GXM was exogenously added to Hc samples. As expected, Hc from Hc + Cn cap59 co-infected animals displayed low levels (background) of APC+, similar to Hc incubated with 2D10 mAb (p = 0.44) or Hc alone (p = 0.52) and suggested no labelling by 2D10 mAbs. Hc isolated from lungs of Hc + Cn H99 co-infected animals had higher fluorescence labelling by mAbs to GXM than to Hc+2D10 (*p = 0.016) or Hc + Cn cap59 (#p = 0.019), indicating incorporation in vivo. Notably, the APC intensity values were similar for the Hc yeast isolated from animals co-infected with the PS-producing Cn H99 and isolated Hc spiked with GXM (p = 0.23). These results suggest that during co-infections the surface of Hc can be modified by the incorporation of cryptococcal PS material.
Figure 2. Hc incorporates Cn-glycans in vivo during co-infection.
(a) Hc binds Cn-gly during co-infection with Cn H99. Immunofluorescence punctuate surface labelling of Hc GFP recovered from lungs of Hc GFP+Cn H99 groups with GXM-binding mAb 2D10 (red) and Uvitex2B (chitin in the cell wall) after isolation from lungs of co-infected animals. In comparison, Hc recovered from lungs of Hc GFP+Cn cap59 co-infected or monospecies (Hc GFP) infected mice are not labelled by the mAb. Scale bar = 5 μm. (b) FACS demonstrates labelling of Hc cells by GXM 2D10 mAbs upon co-infection with Cn H99 or GXM added controls (in comparison to unlabelled Hc GFP (*p = 0.013 and **p = 0.0069, respectively) and Hc yeast from co-infection with Cn cap59 (#p = 0.037 and #p = 0.019).
Cn and Hc interacted during co-cultivation
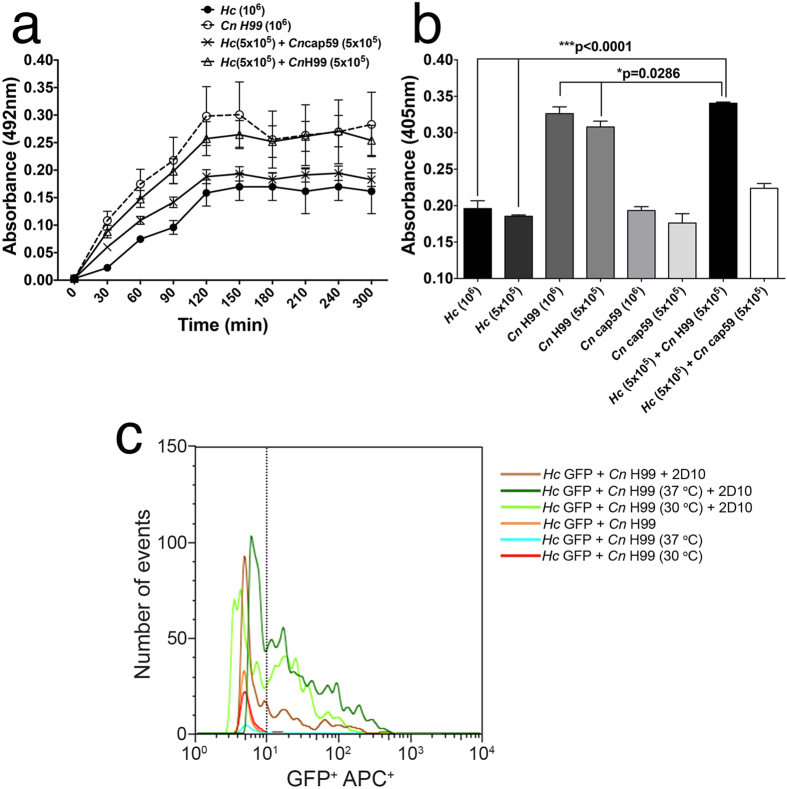
Given that both fungi can co-exist in nature and in tissues, we evaluated the interactions between Cn and Hc during in vitro cultivation. Fungal growth was examined semi-quantitatively on microtiter plates by measuring total metabolic activity of adherent cells and pellicle formation using an XTT assay (Fig. 3a)34. When cultivated separately, Cn H99 grows more robustly under biofilm conditions compared to Hc (*p = 0.016, 2 h), which is consistent with the differences in replication rate between the two fungi (approximately 2 and 6 h, respectively) and the well-described capacity of Cn to form a biofilm/PS matrix23,34,35. However, co-incubation of both fungi in a 1:1 ratio to create the same total initial inoculum, resulted in the formation of a hybrid pellicle, with similar metabolic activity relative to monospecies Cn biofilms (p = 0.46). The capacity of forming pellicles was nearly absent in Hc + Cn cap59 co-cultivation, where the metabolic activity was 27% lower relative to pellicles containing Hc and Cn H99 (*p = 0.029, 2h). 3-D image reconstruction of pellicles displayed the complex architecture formed when Hc was co-cultured in static conditions with Cn H99, in comparison with monospecies control and Hc + acapsular Cn cap59 and (Supplementary Fig. S1a–c) along with the detection of fluorescence intensity (Supplementary Fig. S1f), and correlated with the above described results.
Figure 3. Co-cultivation of Cn H99 and Hc GFP enhances pellicle formation by glycan transfer.
(a) Pellicle formation in HAM’s F-12 media was determined by measuring fungal metabolic activity using XTT colorimetric analysis. The initial inoculum for each well was 106 yeast cells, either all of one species or a 1:1 mix of 5 × 105 of Hc co-cultured with either Cn H99 or Cn cap59. Heat-killed Hc cells were used as background control and discounted from the readings. Co-cultures of Hc GFP + Cn H99 formed pellicles that were similar to biofilms produced by monospecies Cn H99. In contrast, Hc GFP + Cn cap59 and monospecies Hc GFP were extremely poor pellicle producers. (b) The reactivities of PS matrix of fungal pellicles were examined by ELISA using 2D10 IgM mAb to GXM. The pellicles from Hc GFP + Cn H99 displayed a slight, but significant increase in matrix reactivity to GXM-binding mAb compared to biofilms formed by Cn H99 alone. For both A and B, bars represent mean ± standard error of quadruplicates. (c) Cn glycan transfer to Hc surface is temperature dependent. More glycan transfer occurs at 37 °C compared to 30 °C during co-cultivation of Hc (GFP strain, FL1-H+) +Cn H99 in HAM’s F-12 medium as determined by flow cytometry using GXM-binding mAb 2D10 (IgGM) and a goat anti-mouse IgM-APC (FL4-H+), in comparison to controls or monospecies mixed Hc GFP + Cn H99 yeasts just before incubations with mAb, which displayed no PS transfer.
To examine the PS matrix of these fungal pellicles, we performed an indirect ELISA using mAb 2D10 to cryptococcal GXM. Mixed Hc + Cn H99 pellicles displayed an average reactivity increase of 10% relative to Cn biofilms (Fig. 3b, *p = 0.029). No difference on the reactivity was observed when comparing mixed pellicles of Hc + Cn cap59 with pellicles from Hc yeasts alone (p > 0.99). These results also suggest that PS material from Cn is transferred to Hc and that these fungi form a hybrid pellicle matrix with increased serological reactivity. Altogether, these findings might suggest an interplay between Cn and Hc when grown together. In fact, Cn also has been reported to produce quorum sensing molecules that affect the growth of other fungi36.
Temperature dependency of cryptococcal glycan incorporation by Hc
Hc cells were individually evaluated by FACS upon incubation with mAb 2D10 and APC-labelled conjugate anti-mouse IgM after sonication of grown Hc + Cn H99 co-cultures at 30 °C and 37 °C. The optimal growth temperature for Cn is 30 °C and Hc yeast cells grown best at 37 °C. Co-cultivation of Hc (GFP) and Cn H99 resulted in transfer and incorporation of Cn-glycan fractions on the Hc surface (Fig. 3c), related to mixed monospecies control (Hc + Cn H99), which barely had increase in Hc fluorescence in comparison to Hc control. Incubation of cells at 37 °C resulted in a 5-fold increase in average APC+ fluorescence intensity of GXM-positively labeled Hc compared to Hc grown at 37 °C, while cells co-cultured at 30 °C displayed a 3-fold increase in average fluorescence compared to Hc grown at the same temperature.
Cellular glycan cross-incorporation between Hc and Cn
Based on the incorporation of Cn PS by Hc, we investigated also whether both thermodimorphic phases of Hc would incorporate Cn-glycan, since these fungi co-exist in nature in a wide variety of temperatures. When evaluated by fluorescence microscopy, the majority of Hc yeast cells in pure culture were not labelled by mAb 18B737 although few cells displayed a discrete punctuated pattern of labelling (Supplementary Fig. S2a). The filamentous phase of Hc also displayed only few cells labelled by mAb 18B7 some concentrated at the septae (Supplementary Fig. S2d). To evaluate the Cn-glycan incorporation, as previously described for GXM and Hc25, Hc yeasts were incubated with Cn-glycan resulting in a radial labelling surrounding the Hc surface or “pseudoencapsulation” of Hc by the Cn-glycan (Supplementary Fig. S2b). The surface of filamentous forms was also able to incorporate Cn-glycan onto it’s the surface, with the most intense fluorescence surrounding micro and macroconidia (Supplementary Fig. S2e). Pre-treatment of yeast or hyphal cells with the cell wall degrading cocktail Novozyme completely abrogated the binding of Cn-glycan (Supplementary Fig. S2c,f), suggesting the requirement of cell surface molecules in this process.
Incorporation of distinct cryptococcal glycan fractions onto the Hc surface
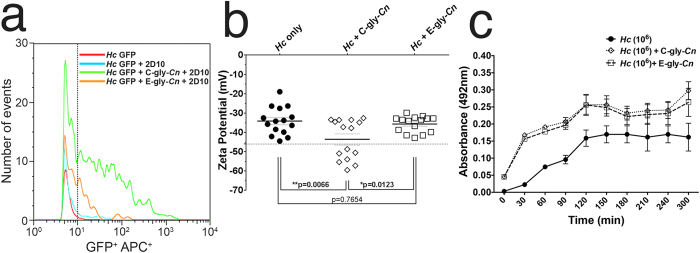
The attachment or anchoring of the Cn capsule involves PS-PS interactions between GXM and other cell wall gly (i.e. glucans and chitin)25,26. Given that the Hc surface is richly composed of glucans and N-acetylglucosamine polymers32, we examined the possibility of a transference and/or incorporation of distinct cryptococcal PS fractions onto the Hc surface. This was assessed by incubation of Hc cells with isolated capsular C-gly-Cn (DMSO extracted) and secreted E-gly-Cn (filtered supernatant) fractions, isolated from Cn. The C-gly-Cn fraction was readily incorporated by Hc yeasts based on a 7-fold increase in mAb 2D10 labelling relative to control (Fig. 4a). The E-gly-Cn fraction was less well incorporated; nevertheless, incubation with this fraction led in a 4-fold increase in antibody labelling.
Figure 4. Hc incorporates distinct Cn-gly fractions on its surface.
(a) Flow cytometry of GFP + Hc yeast (FL1-H+) following incubation with purified C-gly-Cn and E-gly-Cn and 2D10-APC conjugate reveals that C-gly-Cn is incorporated more effectively onto the cell surface by Hc compared to E-gly-Cn. (b) Cn-glycan surface incorporation changes Hc surface charge. Dashed error bars represent the standard error of average zeta potential values obtained from 10 repeated measurements. (c) Incorporation of C-gly-Cn or E-gly-Cn by Hc enabled the formation of pellicles by the fungus.
C-gly-Cn incorporation modified the charge of Hc as demonstrated by the change in the surface electrostatic potential of Hc cells. The association of C-gly-Cn with Hc cells resulted in a significant increase in the negative magnitude of the zeta potential (−46.56 ± 10.25 mV) relative to uncoated Hc yeasts (−34.10 ± 7.10 mV, *p = 0.0066) (Fig. 4b), most likely due to the addition of glucuronic acid residues, which are absent on Hc surface gly. Incubation with E-gly-Cn not significantly alter Hc surface charge (−35.63 ± 4.18 mV), consistent with the lower incorporation and the lower relative levels of glucuronic acid in this fraction compared to C-gly-Cn38. Together, these results demonstrate an interaction between both fungi involving the transfer and incorporation of Cn PS material to the Hc surface gly via PS-PS interactions, which leads to significant alterations in Hc cell surface charge.
Growth of Hc in the presence of C-gly-Cn or E-gly-Cn enabled these yeasts to more effectively form a pellicle structure (Fig. 4c) equally in the presence of either glycan fraction (p = 0.77, 2 h), relative to Hc alone (*p = 0.037 and p = 0.045, respectively). Structurally, these pellicles were characterized by dense aggregates of yeasts, which could anchor each other through interactions with Cn-gly working as a extracellular polymeric scaffold substance (Supplementary Fig. S1d,e).
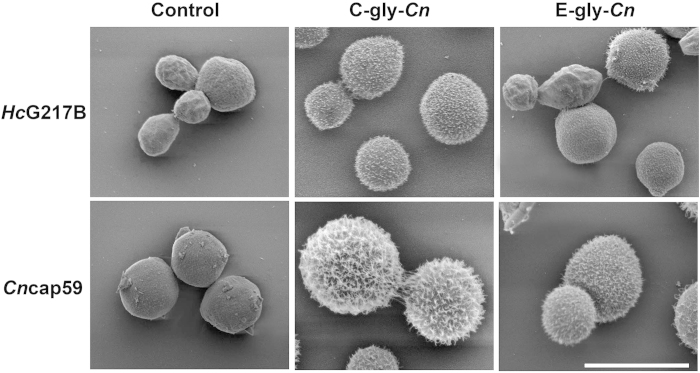
The post-incorporation ultrastructure of Hc was evaluated by SEM (Fig. 5). As a control, Cn cap59 yeasts were incubated with C-gly-Cn or E-gly-Cn and uniform attachment of capsule was observed, with C-gly-Cn producing the most robust capsules, in comparison to E-gly-Cn, and agreement with the previously described size of PS fibers from these distinct fractions38. Similarly, Hc yeasts incubated with C-gly-Cn displayed significantly larger PS fibers incorporated onto their surface compared to the smoother surface by E-gly-Cn, which had a more sparsely coated surface, but more wrinkled than Hc control. Together, these resulted were consistent with the FACS and zeta-potential determinations.
Figure 5. Incorporation of cryptococcal glycan fractions by Hc or acapsular Cn cap59 mutant produces distinct cell surface architectural features.
Scanning electron microscopy (SEM) images of Hc and acapsular cap59 Cn mutants display distinct arrangements of C-gly-Cn and E-gly-Cn on their surfaces. Both yeast species produced more complex structures through the incorporation of C-gly-Cn in comparison E-gly-Cn. Scale bar = 5 μm.
Kinetics of Cn glycan incorporation by Hc and α-glucan requirement
The requirement for α-1, 3-glucans in the incorporation of C-gly-Cn or E-gly-Cn was evaluated by comparing Hc strains expressing variable amounts of these surface glucans. Decreasing concentrations of C-gly-Cn or E-gly-Cn were incubated up to 1 h with low (G217B) or high α-1, 3-glucan content (G186A) Hc strains39. C-gly-Cn was incorporated by both Hc strains (Supplementary Fig. 3Sa,b). Despite higher incorporation of C-gly-Cn by G186A, in agreement with Reese et al.25, this process was effective only at 1 h incubation, in contrast with G217B strain, which displayed a statistically significant incorporation of C-gly-Cn after a 30 min incubation, when compared to controls. Similar behaviour was observed for both strains with E-gly-Cn incubation; however, as expected, absorbance values were lower than those obtained for C-gly-Cn, due to the lower incorporation of this fraction by Hc strains.
Cn glycans-coated Hc yeasts are more resistant to phagocytosis and antifungal activity by peritoneal macrophages
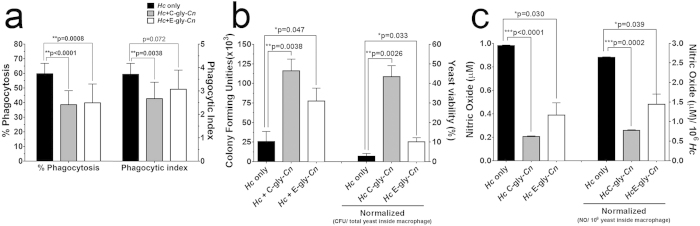
Given the antiphagocytic and immunomodulatory properties of cryptococcal PS, we examined if these virulence traits could occur with pseudoencapsulated Hc cells. Hc yeasts coated with C-gly-Cn were more resistant to phagocytosis by peritoneal macrophages compared to untreated Hc (38.6% vs 59.8% phagocytosed, ***p = 0.0001; Fig. 6a). Similar results were achieved with E-gly-Cn incorporation onto Hc (39.5%, **p = 0.0008). When the phagocytosis index was evaluated, i.e., the average number of yeast by macrophages, only C-gly-Cn reduced this number effectively (2, 67; **p = 0.0013), in comparison to E-gly-Cn (3,07; p = 0.072) and Hc control (3, 71). Moreover, resistance to killing by macrophages was also increased for Hc coated with C-gly-Cn, as the CFUs were 4.3 times higher for these cells compared to uncoated Hc (1.2 × 105 vs colonies 2.6 × 104, **p = 0.0038; Fig. 6b, left axis). E-gly-Cn coated-Hc similarly displayed a 3 times higher resistance to intracellular killing (7.8 × 104, *p = 0.047) compared to untreated Hc (2.6 × 104). Resistance to killing (CFU) was normalized by the total yeast number inside the macrophages and yeast viability under each condition evaluated (Fig. 6b, right axis). This reduced macrophage antimicrobial efficacy was in part associated with the decreased levels of nitric oxide produced by these effector phagocytic cells when infected with C-gly-Cn or E-gly-Cn coated Hc (p < 0.05; Fig. 6c, left axis). Nitric oxide levels were normalized to the number of yeast inside macrophages (p < 0.05; Fig. 6c, right axis). These results suggest that interaction between Cn and Hc can result in the generation of Hc cells with a cryptococcal-like surface and, thus, new and/or hybrid virulence properties, including ability to grow more efficiently within phagocytes and inhibition of nitric oxide production.
Figure 6. Cn-gly surface incorporation by Hc affects subsequent interactions with macrophages.
The incorporation of C-gly-Cn or E-gly-Cn onto the surface of Hc significantly increased its resistance to (a) phagocytosis and (b) killing by murine peritoneal macrophages. Bars represent mean ± standard error of quadruplicates. (c) Co-culture of macrophages with Hc cells coated with Cn-gly suppressed the production of nitric oxide by macrophages. Bars represent mean ± standard error of three independent experiments performed in triplicates.
Hc virulence is enhanced in vivo via Cn glycan transfer
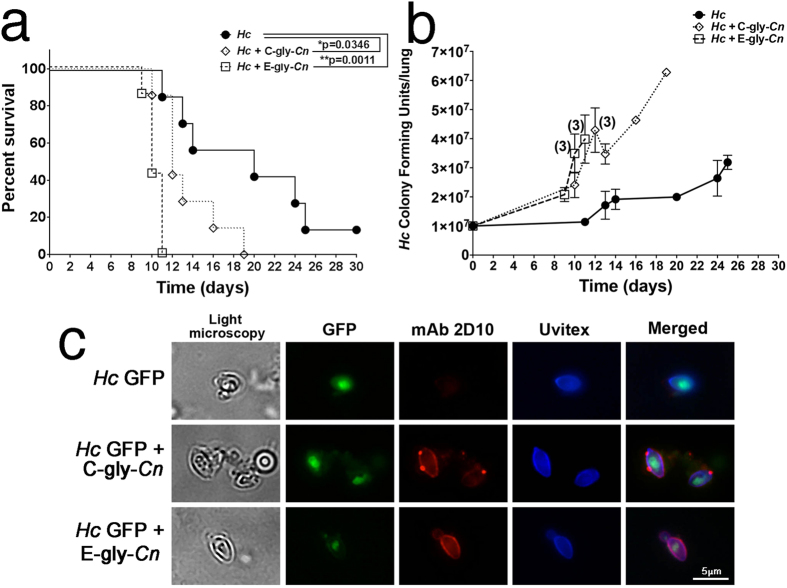
In vivo mouse models were used to determine the importance of the transfer of individual pools of Cn-gly during co-infection in vivo. After infection with Hc and administration of C-gly-Cn or E-gly-Cn intratracheally, survival rates and lung CFUs were compared. Animals challenged with Hc and treated with E-gly-Cn had the highest mortality index, with all mice dying by day 11 (**p = 0.0011 compared to Hc infection alone, Fig. 7a), followed by mice in the C-gly-Cn treatment group, with animals dying by day 19 (*p = 0.035). Notably, some animals infected with Hc that received PBS instead of PS survived until the termination of the experiment at day 30. The CFUs recovered from Hc-infected and C-gly-Cn treated animals ranged from 1.98 × 107 to 6.28 × 107 (median 4.64 × 107) and the CFUs from infected, E-gly-Cn treated mice ranged from 1.71 × 107 to 6.23 × 107 (median 2.71 × 107), both of which were significantly higher than CFUs recovered from mice infected with Hc alone (1.14 × 107 to 3.19 × 107; median 1.95 × 107; p < 0.05; Fig. 7b). Since CFU numbers were higher in animals treated with either C-gly-Cn or E-gly-Cn than control, we wanted to determine if the higher virulence was correlated with the presence of Hc-coated yeasts with the administered Cn-gly. Significantly, organ homogenates from each of the Cn-gly-treated Hc displayed Hc with intense fluorescence staining by mAb 2D10, indicating the presence of Cn-gly coated Hc yeast (Fig. 7c). Hc recovered from animals challenged with Hc alone did not react with the GXM-binding mAb.
Figure 7. Virulence is enhanced by the incorporation of cryptococcal glycan fractions onto the surface of Hc yeast in a murine infection model.
(a) Enhanced mortality occurred when mice were infected with Hc cells and subsequently injected with E-gly-Cn or C-gly-Cn in comparison with Hc infected animals. (b) Mice treated with C-gly-Cn or E-gly-Cn after infection with Hc displayed higher fungal burdens in comparison to animals infected with Hc alone. When present, digits over graph points reveal the number of deceased animals at a specific time point, with CFUs expressed as averages. Results are representative of two-independent experiments with 7 animals per group. (c) Hc binds C-gly-Cn or E-gly-Cn in vivo, displaying a punctuate surface labelling of Hc GFP recovered from lungs of mice administered with the distinct pool of Cn-gly by GXM-binding mAb 2D10 (red) and Uvitex2B. In comparison, Hc recovered from lungs of monospecies infected Hc mice are not labelled by the mAb. Scale bar = 5 μm.
We also tested the impact of PS-coating of Hc using the in vivo invertebrate model Galleria mellonella (Fig. 8); however, this approach was limited by the use of only Hc yeasts pre-incubated with purified Cn-gly. The results demonstrated a dose-dependent increase in virulence of Hc yeasts when coated with C-gly-Cn as Hc coated with 100 μg displayed higher virulence relative to untreated Hc (**p = 0.004). Treatment with 10 μg C-gly-Cn did not reach statistical significance compared to Hc alone (p = 0.062). In contrast, co-incubation with E-gly-Cn prior to infection resulted in similar mortality rates as that observed for the untreated Hc in our Galleria model. Nevertheless, our finding that coating of Hc with C-gly-Cn enhanced virulence in this second model strengthens our thesis that gly transfer between Cn and Hc during co-infection can enhance virulence and exacerbate disease.
Figure 8. Virulence is enhanced by the incorporation of cryptococcal C-glycan fractions onto the surface of Hc yeast in an invertebrate infection model.
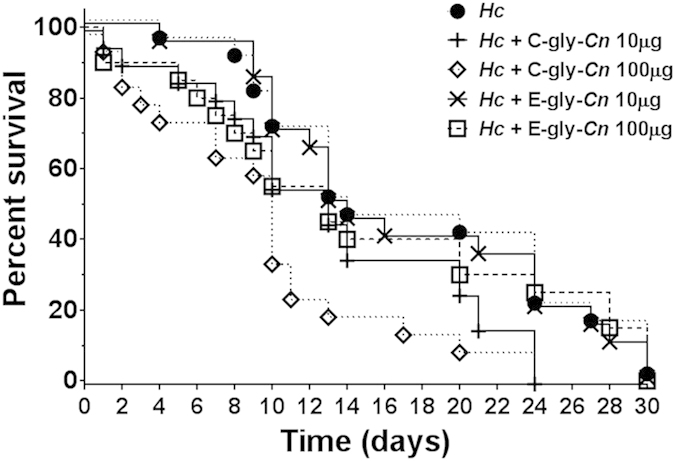
Enhanced mortality was evaluated in the invertebrate G. mellonella model. The incorporation of C-gly-Cn onto the surface of Hc increased mortality in a dose dependent manner, with the addition of 100 μg C-gly-Cn producing a statistically more rapid time to death compared to untreated Hc (100 μg,p = 0.004; 10 μg p = 0.062). There were no significant differences between Hc exposed to E-gly-Cn coated Hc and Hc alone (100 μg, p = 0.23; 10 μg, p = 0.22).
Glycan transfer occurs inside phagocytic cells
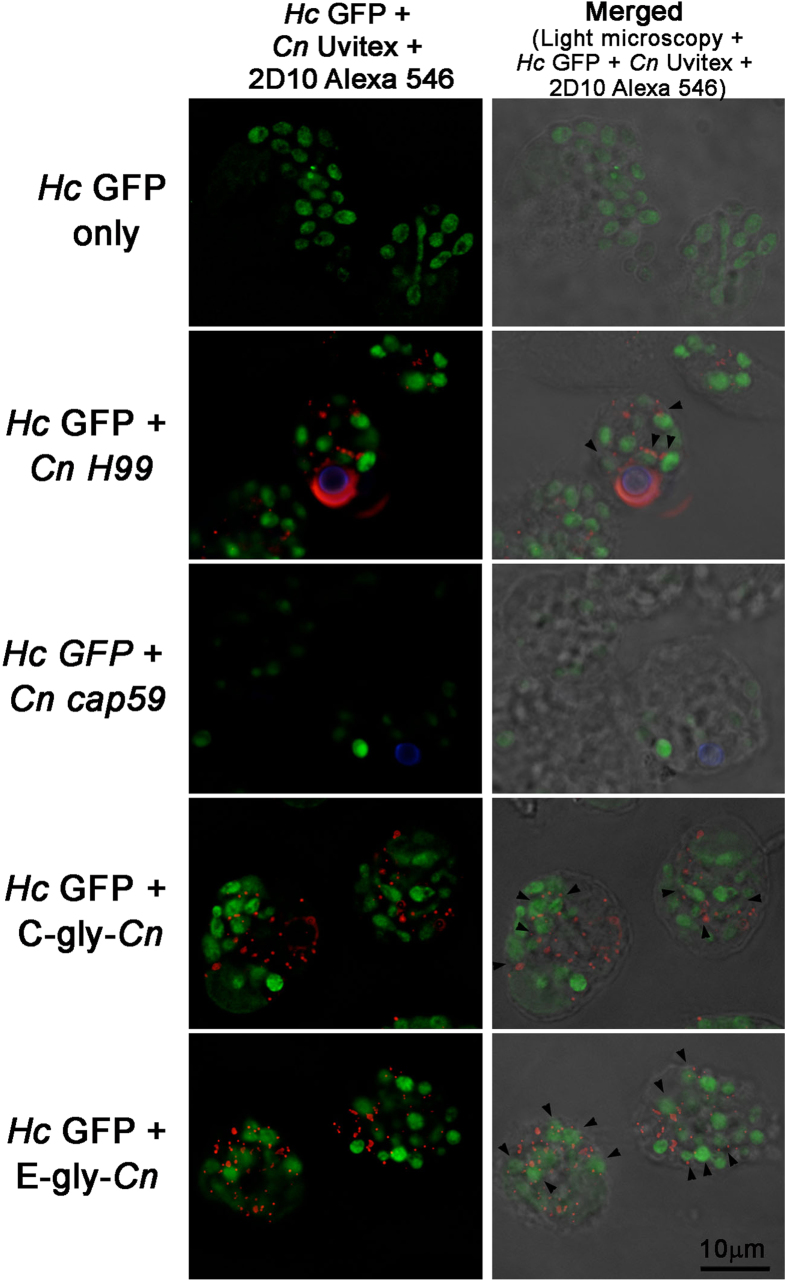
GXM can easily be ingested by macrophages through pinocytosis and phagocytosis40. Cn and Hc can also be phagocytosed by these cells and localized within phagosomes. We therefore evaluated intracellular glycan transfer within macrophage phagolysosomes. Macrophages were infected with Hc and then exposed to Cn H99, Cn cap59, C-gly-Cn or E-gly-Cn. Hc GFP was detected as a green fluorescent cells inside phogosomes (Fig. 9). Glycans reacting with mAb 2D10 were detected as punctuated patterns inside the macrophages in the presence of Cn H99, or upon incubation with C-gly-Cn or E-gly-Cn as described previosuly40. Hc GFP and Cn H99 co-localized within the same phagosome, and a punctuated labelling for GXM was observed around Hc GFP yeast (Fig. 9). When C-gly-Cn or E-gly-Cn were administered upon incubation of macrophages with Hc, higher distribution of GXM and labelling of Hc GFP yeasts by GXM antibody was observed, with a predominance of GXM staining on the surface of Hc yeasts (Fig. 9). Systems where Hc GFP was used only or where infection with Hc GFP was followed by Cn cap59 produced no labelling for GXM.
Figure 9. Hc co-localize with Cn-gly and is able to incorporate these glycans on its surface within the macrophage environment.
Macrophages were infected with Hc GFP (green) and incubated with either PBS, Cn H99 (Uvitex labeled – blue), Cn cap59 (Uvitex labeled – blue), C-gly-Cn or E-gly-Cn. Fluorescence was performed using 2D10 mAb and anti-IgM Alexa 568 conjugated (red). In the presence of Hc GFP yeasts and either Cn H99, C-gly-Cn or E-gly-Cn, Hc surface was labeled with 2D10 antibody as indicated in several instances by the black arrow heads. For Hc and PBS or Cn cap59 groups, no labelling for GXM was observed. Left column (Hc GFP-green; Cn Uvitex – blue; mAb 2D10 – red). Right column (Hc GFP-green; Cn Uvitex – blue; mAb 2D10 – red) merged with light microscopy. Scale bar = 10 μm.
Discussion
Histoplasmosis and cryptococcosis are the most prevalent pulmonary mycoses in HIV-infected patients2,3,20. Hc var. capsulatum infection has emerged as one of the most common systemic mycosis in the setting of HIV-infected patients in developing countries41, where disseminated histoplasmosis continues to cause severe morbidity and mortality. Cryptococcosis is frequently manifested in immunocompromised individuals, as meningoencephalitis particularly in the setting of HIV, with Cn var. grubii being the principal causative agent of the disease, followed by Cn var. neoformans42.
Pulmonary infections by both Hc and Cn frequently display overlapping symptoms42,43. In addition, their clinical, pathologic and imaging findings can be similar44. Both fungi can be isolated from bronchoalveolar aspirates, but Cn is able to overgrow Hc in culture and even inhibit its growth22, which may be a reflection of its simpler nutritional requirements and faster replication rate42. Besides culture, standard microscopic examination does not uniformly distinguish between these species, due to morphological similarity of these fungi in clinical samples, particularly when hypocapsular strains of Cn are involved14. Cn and Hc also share the ability to proliferate within macrophages and both species are considered to be facultative intracellular pathogens10.
A PubMed search for the words Hc, Cn and co-infection renders many hits9,10,11,12,13,14,15,16,17,18,19,20,21, including multi-center reports of several patients9,15,20, with the first co-infection observation reported by Mider et al. in 194716. The diagnoses of co-infection was made by either histological examination and/or cultures of various tissues and body fluids. The majority of the reports date from the last decade, and are frequently associated with disseminated infection by both fungi in the setting of advanced HIV disease9,12,13,15,17,18,20,21. However, as an example of co-infection in a non-HIV infected patient, Hc and Cn were found in samples of respiratory secretions in an individual on chronic steroid therapy who presented with a cavitary pulmonary lesion19. In a study to validate an ELISA for the diagnosis of histoplasmosis, 12% of the histoplasmosis patients also had positive results for the presence of Cn by detection of GXM in the cerebrospinal fluid (unpublished and45). In this context, we speculate that the total number of co-infection cases is generally underestimated, primarily due to the lack of sensitivity of the methods currently in use to diagnose histoplasmosis. Additionally, the diagnosis of non-meningeal cryptococcosis is difficult1,46. However, the advent of more sensitive molecular diagnostic techniques has increased the ability for detecting Hc in the setting of co-infections. It should be noted that other co-infections with dimorphic fungi also occur, such as the recently reported lethal human dual infection with Blastomyces and Coccidioides spp47.
We postulate that interactions of Hc with cryptococcal GXM may contribute to the pathogenesis of a significant number of histoplasmosis cases. Hc and Cn are frequently found in the same natural sites48, as they are highly associated with soils enriched with organic nitrogen sources, such as animal excrements. For instance, Cermeno et al.49 co-isolated Cn and Hc from many sites in Venezuela, reinforcing the possibility of environmental interactions and an enhanced risk of co-infection with both pathogens.
In both Hc and Cn, surface PSs are key molecules of the fungal cells since they are directly mediating interactions with the immune system. Cn GXM is recognized by Toll-like receptors 2 and 4 and/or CD14 on phagocytes, resulting in an incomplete activation of pathways necessary for TNF-α production and activation of inflammatory responses40. GXM is also recognized by CD18 resulting in the blockage of the receptor, which subsequently inhibits leukocyte infiltration into inflammatory sites23,40. In Hc, α- and β-glucans form the outer cell wall layers of both yeast cells and mycelia, playing different biological roles31. The β-1, 3-glucan, which predominates in the mycelial phase, is antigenic and modulates the host immune response31. In most Hc isolates, α-1, 3-glucan surrounds the β-1, 3-glucan layer, blocking its innate recognition by dectin-1 on host phagocytes50, and thereby suppressing the production of TNF-α31.
The interaction between Cn and Hc can result in hybrid pellicle formation. We found that pellicle formation was increased when GXM producing Cn yeast cells were co-incubated with Hc. This observation suggests that cell wall components of Hc could interact with Cn-gly to promote adhesion of matrix components resulting in effective pellicle formation. In fact, Hc can incorporate exogenous Cn GXM but the mechanism by which PSs are attached to Hc cell surfaces remains obscure. Previous reports have demonstrated that only an α-1, 3-glucan-producing Hc strain could anchor soluble GXM based on immunofluorescence staining25. However, no direct labelling control of mAbs to Hc was performed. In our system, strain G217B, which displays no α-1, 3-glucans, had a slightly lower efficacy in incorporating C-gly-Cn in comparison with the strain G186A, a well-recognized α-1, 3-glucan-producing strains39, possibly indicating that Hc α-1, 3-glucans are not specific determinants for interaction with cryptococcal gly. The PS adsorption was more efficient when C-gly-Cn were used in comparison with E-gly-Cn. Coating Hc with C-gly-Cn also resulted in an increase in the magnitude of the fungal cell’s negative charges, most likely due to the fact that this cellular fraction was better incorporated onto the cell surface and that it has higher amounts of glucuronic acid residues than the extracellular soluble fraction, E-gly-Cn38.
In addition, the incorporation of Cn-gly by Hc in both environmental and infection-related conditions, may have the potential to modify the outcome of the interaction between yeasts and phagocytes and/or environmental predators. Such an altered outcome was observed with the environmental in vivo model G. mellonela, which likely favors the survival of both microorganisms under environmental stress conditions and/or during interactions with the innate immune system. Coating of Hc with crypotoccocal PS might also inhibit the interaction with phagocytes, including macrophages, dendritic cells, neutrophils in mammalian models and haemocytes in G. mellonella invertebrate model. Within phagocytic cells, as shown with macrophages, GXM is extensively released by Cn in the phagosome51. In the case of co-infection of a single magrophage, as shown, the Cn-gly could be incorporate and associate with Hc yeast cell surface.
The results presented in this study suggest that Cn and Hc share a number of physiological steps required for gly formation and surface assembly. In addition, they also reveal a new pathogenic mechanism, resulting in increased virulence or synergism, with potential relevance for hosts co-infected with these fungi. Our in vivo observations suggest that these fungal pathogens can interact during infection, and Hc could modify its cell surfaces in a manner that alters recognition by the immune system. The explanation for the Cn-gly incorporation effect on Hc virulence may primarily be due to a subversion of the host immune recognition mechanisms of cell wall components with subsequent increase in yeast survival, which is an effect observed when comparing highly capsulated Cn strains to minimally capsulated ones30. Hence, direct PS transfer resulted in enhanced Hc virulence associated with the suppression of the antifungal functions of phagocytic cells.
Our findings also suggest that an increased understanding of the role of PS in fungal infections may lead to promising strategies for the design of new therapeutics37,52, as PSs constitute important targets for vaccines and passive immunization53. The mechanism used by fungal cells to incorporate exogenous molecules with consequent change of their surface architecture consists of a new avenue for cell biology studies and likely for the design of new therapeutic options. In summary, our findings show that Hc can co-opt GXM, the major virulence factor of Cn, during mixed infection in vivo and that this phenomenon was associated with increased virulence, both in vitro and in vivo. This observation establishes the precedent of one pathogenic microbe using a virulence factor from another to increase its virulence, suggesting that other such interactions may exist in host-microbe relationships. Although this is a new concept for synergistic dual fungal infection, the paradigm is well known in bacterial diseases and increasingly emerging in fungal-bacterial infections. For example, infection with mixed bacterial species can produce synergisms in virulence resulting in severe disease, such as Fournier gangrene. Bacterial-fungal interactions such as those described for Pseudomonas aeruginosa and Candida albicans can affect the expression of several fungal characteristics including some associated with virulence54. Our experiments extend the phenomenon of microbial synergy in virulence due to mixed infections within the fungal kingdom.
Methods
Fungal strains and growth conditions
Cn var. grubii Serotype A strain H99 (ATCC 208821), the acapsular mutant Cn cap59 (derivative of Serotype D strain B3501 ATCC 34873), Hc var. capsulatum G217B, Hc G217B GFP (kind gift from Dr. A. G. Smulian, Division of Infectious Diseases, University of Cincinnati College of Medicine, Cincinnati, Ohio, USA) and Hc G186A (ATCC 26029) were used in this study. Cn was cultured in minimal media (29.4 mM KH2PO4, 10 mM MgSO4, 13 mM Glycine, 3 μM Thiamine and 15 mM D-Glucose, pH 5.5). Hc was cultured in HAM’s F-12 (Invitrogen) medium as described55. Cn and Hc cells were grown at 30 °C and 37 °C, respectively, for 48 h with shaking at 150 rpm. For the co-cultivation of both fungi, Hc and Cn were centrifuged at 1100 × g for 10 min at room temperature (RT) and pellets were washed three times with PBS followed by centrifugation. The cells were then suspended in HAM’s F-12 and enumerated using a hemocytometer. Hc and Cn yeasts were added to a final density of 5 × 105 yeasts/mL in HAM’s F-12 and co-cultures were incubated at 30 °C and 37 °C. Monospecies controls of Hc (G217B of GFP) or Cn (H99) at 106 yeasts/mL were incubated separately in 50 mL of HAM’s F-12 at 37 °C and 30 °C.
Mouse co-infection model
To evaluate survival rates during co-infection in vivo, C57BL/6 mice (6–8 weeks old) were challenged intranasally with 5 × 106 Hc GFP, followed 2 h later by an intratracheal infection with 5 × 106 of Hc GFP (monospecies control), Cn H99 or Cn cap59. The infected mice were checked four times daily by the scientific team and daily by the veterinary staff. All animal experiments were carried out in “accordance” with the approved guidelines and protocols of the Institutes for Animal Studies at the Albert Einstein College of Medicine and the Fluminense Federal University. To determine fungal burdens, immediately after they were detected, deceased animals had their lungs removed and the organs were then weighed and homogenized in PBS using 70 μm cell strainers (BD Biosciences, NJ, USA). Organ homogenates were serially diluted and plated in duplicates on Sabouraud dextrose agar (Difco Laboratories) for Cn growth. After 2 d of incubation at 30 °C, Cn colony forming units (CFUs) were enumerated. For Hc growth determination, homogenates were simultaneously also plated on brain heart infusion (BHI) agar supplemented with 5% sheep blood and bleomycin at 10 μg/mL (to suppress Cn growth in co-infection conditions). BHI plates were incubated in the dark for 10–15 d at 37 °C and Hc CFUs were then enumerated. The plates were also observed under UV light for expression of GFP proteins by Hc GFP strain and correlated with colony morphology.
To examine cryptococcal gly incorporation by Hc during co-infection, aliquots of lung homogenates were spun down and evaluated by immunofluorescence. Homogenates were pipetted into microcentrifuge tubes and quickly spun down to remove excess liquid. For detecting bound Cn PS, Hc yeasts were incubated with 10 μg/mL of the IgM isotype GXM-binding mAb 2D10 or isotype-matched irrelevant antibody33 and a 1:100 of a goat anti-mouse IgM Alexa 546 conjugate. After three washes, fungi were stained using 0.5 mg/mL of Uvitex 2B, fixed with 4% paraformaldehyde and analysed in an AX70 fluorescence microscope. Alternatively, we used goat anti-mouse IgM APC conjugate and performed analysis of FL1+FL4+cells (GFP and APC labelled, respectively) using a FACScalibur Flow Cytometer (BD Biosciences, Franklin Lakes, NJ) and Hc fluorescence intensity was determined under each condition.
Hc pellicle formation induced by Cn or their products
Monospecies cultures of Hc and Cn yeasts were obtained as described above, collected by centrifugation, washed with PBS (3X), and suspended at 107 cells/mL in HAM F-12 media. An aliquot of Hc yeast suspension was heat-killed at 56 °C for 1 h and used as negative control. Next, 100 μL (106 total yeast) of each suspension (Hc or Cn) was added to individual wells of polystyrene 96-well plates (Fisher, MA). In co-incubations conditions, 50 μL (5 × 105) of Hc GFP and 50 μL (5 × 105) of Cn H99 or Cn cap59 were added to the same well (106 total yeast cells per well). Plates were incubated at 37 °C without shaking for 48 h. Following incubation, wells were washed (3X) with PBS 0.05% Tween 20 to remove planktonic cells. Pellicle formation, as agglutination of cells on a surface, was measured by XTT (2, 3-bis (2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino) carbonyl]-2H-tetrazolium-hydroxide) reduction assay as previously described34. Briefly, 50 μL of XTT salt solution (1 mg/mL) in PBS and 4 μL of menadione solution (1 mM in acetone) were added to each well and plates were incubated for 5 h at 37 °C. Changes in color and reduction of XTT tetrazolium salt into XTT formazan by fungal mitochondrial dehydrogenase correlate with metabolic activity and cell viability. The absorbances were measured at 492 nm using a microplatereader (SpectraMax Microplate Reader, Molecular Devices, CA, USA). The conditions were tested in quadruplicates and the results shown are the average of three independent experiments. The background activity of heat-killed Hc was discounted from all the wells as a blank control. A similar plate set-up was then used for visual documentation of the pellicle architecture by immunofluorescence of Hc-GFP yeast34.
To determine the pellicle formation and initial accumulation of PS extracellular matrix component, an ELISA with IgM mAb 2D10 was performed33. After 48 h incubation, plates were washed (3X) with TBS-T (10 mM Tris-HCl, 150 mM NaCl, 1 mM NaN3, 0.1% Tween 20, pH 7.4) and incubated with blocking solution (2% Bovine Serum Albumin in TBS-T) for 1 h at 37 °C. After washes, mAb 2D10 was diluted at 10 μg/mL in blocking solution. Fifty microliters of mAb solution was added to separate wells containing yeast cells in quadruplicate and the plates were incubated at 37 °C for 1h. An irrelevant IgM antibody 5C11 was used as a control56. Plates were washed (3X) with TBS-T and incubated with a 1:1000 dilution of a goat anti-mouse Ig (Southern Biotech) in blocking solution, for 1h at 37 °C. After washes (3X), plates were incubated with 50 μL/well of 1 mg/mL p-nitrophenyl phosphate diluted in substrate buffer (1 mM MgCl2 × 6H2O, 0.05 M Na2CO3, pH 9.8) at 25 °C for 30 min. Absorbances were measured at 405 nm on a microplate reader (BioTek Instruments, Winooski, Vermont, USA). Results shown are the average of 3 independent experiments.
Analysis of cross-incorporation of Cn polysaccharides
Co-cultivated Hc GFP and Cn yeasts at different temperatures (30 and 37 °C) were washed (3X) with PBS and incubated with 10 μg/mL of mAb 2D10 or irrelevant isotype-matched antibody, diluted in blocking solution for 1 h at RT. As a control, Hc and Cn were mixed right before incubation with mAb. The yeasts were washed and suspended in 100 μL of a goat anti-mouse IgM APC-conjugate (Southern Biotech) diluted 1:100 in blocking solution. The suspension was incubated for 1h at RT and washed with PBS. Cells were sonicated with 1 min cycles to disrupt any possible aggregates or biofilm formed during growth/incubations, fixed for 20 min using formalin buffer (Fisher Scientifics) and washed with excess of PBS. Analysis of FL1+FL4+cells (GFP and APC double-labelled) was performed in a FACScan Flow Cytometer (BD Biosciences, Franklin Lakes, NJ) and fluorescence intensity was determined for each condition.
Alternatively, Hc grown in the filamentous phase on microslides at RT were incubated with 100 μg/mL of total PS obtained from Cn culture supernatants25,38 for 1h at RT. Similarly, Hc yeast were adhered to poly-L-lysine coated slides and incubated with Cn PS. Slides were then washed and incubated with 10 μg/mL of mAb 18B737 to GXM or isotype-matched antibody and a 1:100 of a goat anti-mouse FITC-conjugated Ab. As a control for glycan incorporation through the requirement of cell surface carbohydrates or proteins, cells were treated with Novozyme 234 (Novoenzyme, Windsor, UK), a multi-enzyme preparation containing carbohydrate and peptide hydrolases57. After three washes, fungi were stained using 0.5 mg/mL of Uvitex 2B and fixed with 4% paraformaldehyde. Glycan incorporation was examined with an immunofluorescence Olympus AX70 fluorescence microscope, with a magnification of 40X.
Isolation of fungal glycans
Two-day old 1 L cultures of Cn H99 yeasts were centrifuged for 10 min at 1100 × g. Both cells and culture supernatants were collected for the extraction of cellular attached gly (C-gly) and isolation of secreted extracellular gly (E-gly), respectively. C-gly extraction was performed with DMSO as described38. E-gly were obtained by ultrafiltration of the supernatant using nitrocellulose membranes with a nominal molecular weight limit (NMWL) of 10 kDa (Millipore, MA, USA) as described38. Concentrated E-gly and C-gly were dialyzed against water for 24 h (with at least 8 water exchanges) and then lyophilized. The Cn-gly were quantitated by inhibition ELISA as described58.
Incorporation of cryptococcal cellular and extracellular glycan fractions by Hc
C-gly-Cn and E-gly-Cn (100 μg) were incubated with 107 GFP Hc yeasts for 1 h at 37 °C in PBS. Hc yeasts incubated in PBS alone were used as a control. Following incubation, cells were washed (3X) with PBS to remove unbound gly and enumerated using a haemocytometer. Gly incorporation by Hc GFP yeast was determined by FACS analysis using mAb 2D10 as described above.
Hc were also suspended at 107 cells/mL in HAM F-12 medium. Next, 50 μL (106 total yeast) was added to individual wells of polystyrene 96-well plates (Fisher, MA) and incubated with 10μg of C-gly-Cn or E-gly-Cn in 50 μL of HAM F-12 media). Plates were incubated at 37 °C without shaking for 48 h. Pellicle formation was assessed as described previously.
To compare the relative incorporation of Hc G217B with the high α-1, 3-glucan strain Hc G186A, yeasts were incubated with the Cn gly fractions for different time intervals and incorporation was detected by indirect ELISA as described58.
Zeta potential measurements
Hc zeta potential was examined before and after incubation with C-gly-Cn or E-gly-Cn, and included untreated Hc yeast as a control. Analysis was done using 106 cells/mL in pure distilled LPS free water (Thermo Scientific HyClone). Zeta potential (ζ) and mobility values of intact cells were measured in a Zeta potential analyser (ZetaPlus, Brookhaven Instruments Corp., Holtsville, NY) as described58.
Scanning electron microscopy
Acapsular Cn cap59 mutant, Hc or the Cn-gly-coated yeasts were washed three times in PBS and fixed with 0.1 M sodium cacodylate buffer containing 2.5% glutaraldehyde for 1 h. Yeast were washed with 0.1 M sodium cacodylate, 0.2 M sucrose and 2 mM MgCl2 and fixed on coverslips coated with poly-L-lysine for 20 min. Preparations were then gradually dehydrated in alcohol (30%, 50%, 70% and 100% for 5 min and 95% and twice in 100% for 10 min), and submitted to critical point drying and metallization. The cells were observed in a Quanta-FEI scanning electron microscope (FEI,USA).
Phagocytosis
Four-to-six weeks-old female BALB/c mice were used for the isolation of peritoneal macrophages59. Macrophages were plated onto a culture chamber at 2 × 105 cells/well. Hc yeasts were labelled with 40 μg/mL of NHS Rhodamine (Thermo Scientific, Rockford, lL, USA) for 30 min at 25 °C and washed (3X) with excess of PBS. Cells were incubated with the distinct Cn-gly or PBS as described above. Following incubation, cells were washed, suspended in DMEM, enumerated, and added to the macrophages in a 5:1 (yeast:macrophage) ratio. Plates were incubated for 1 h in 5% CO2 atmosphere. After three washes with PBS, yeasts were stained using 0.5 mg/mL of Uvitex 2B to distinguish internalized versus extracellular yeasts. Wells were washed (3X) with PBS and fixed with a 4% formaldehyde solution in PBS. The number of macrophages and yeasts were recorded for each field by microscopic enumeration and at least 200 macrophages were counted. The percentage of phagocytosis was determined as the ratio of macrophages with internalized yeast cells divided by total macrophages, and the phagocytic index as the average number of yeast inside macrophages55.
Yeast killing assay
Cn-gly-coated Hc yeast cells were suspended in DMEM and added in a 5:1 (yeast:macrophage) ratio to 96-well culture plates containing 105 macrophages/well. Plates were incubated overnight at 37 °C under 5% CO2. The wells were washed with cold PBS and macrophages lysed by adding sterile water. Aliquots were plated onto BHI-blood agar plates (10 g/L glucose, 0.1 g/L cysteine, 1% Pen-Strep and 5% v/v sheep red blood cells) and incubated at 37 °C for 10–15 days. The numbers of CFUs were enumerated and compared among groups.
Nitric oxide synthase activity
Nitric oxide production by peritoneal macrophages following incubation with control or gly-coated Hc yeast cells was determined from culture supernatant using the Griess reagent (Promega, Madison, WI, USA) according to manufacturer’s instructions. A nitrite standard reference curve was prepared for accurate quantization of NO2 levels in experimental samples. Experimental conditions were performed in quadruplicates. Plates were read in a spectrophotometer at 540 nm.
Survival in mammalian and invertebrate host models against Cn-PS coated Hc
Mice were intranasally infected with 107 Hc GFP yeast followed 2h later by intratracheal injection with 10 μg (in 50 μL) of C-gly-Cn, E-gly-Cn or PBS. Mice were checked four times daily by the scientific team and daily by the veterinary staff. Evaluations of Hc virulence were performed by survival and CFU quantification as described previously.
To further assess the effects of the incorporation of distinct gly pools by Hc in pathogenesis, survival experiments were also conducted in Galleria mellonella according to our established methods60. Prior to infection, Hc yeast cells were treated with cellular C-gly-Cn, extracellular E-gly-Cn or PBS (control) as described above. Infections were performed by injecting the hemocoel of each caterpillar via the last left proleg with 10μL aliquot containing 106 yeast using a 10-μl Hamilton syringe. Groups consisted of 10 larvae per group and experiments was repeated 3 times with similar results achieved.
Model of glycan transfer during infection of macrophages
Peritoneal macrophages (2 × 105 in 200 μL) were plated on 8-chambers culture slides (Falcon) and cultivated overnight at 37 °C under 5% CO2. Hc GFP yeasts were washed and added to macrophages at a 2:1 ratio, and infection performed for 2 hours. Chambers were washed three times with DMEM to remove extracellular Hc GFP yeasts. Cn yeasts were incubated with Uvitex 2B as described above, and either Cn H99 or Cn cap59 were added to macrophages in a 5:1 ratio. For C-gly-Cn or E-gly-Cn, glycans were diluted at 10 μg/mL in 200 μL of DMEM and added to individual wells. Chambers were incubated overnight at at 37 °C under 5% CO2. After washing with PBS, chambers were fixed as described and immunofluorescence conducted as described above.
Statistical analysis
All analyses were performed using GraphPad Prism version 6.00 for Windows (GraphPad Software, San Diego California USA). One-way ANOVA statistics using a Kruskall-Wallis non-parametric test was used to compare the differences among groups with a 95% confidence interval in all experiments. Individual comparison between groups was performed using Bonferoni post-test. Survival results were analyzed by Kaplan-Meyer to determine the difference among groups.
Additional Information
How to cite this article: Cordero, R. J. B. et al. Enhanced virulence of Histoplasma capsulatum through transfer and surface incorporation of glycans from Cryptococcus neoformans during co-infection. Sci. Rep. 6, 21765; doi: 10.1038/srep21765 (2016).
Supplementary Material
Acknowledgments
AJG, SF, LN, MLR and JMP are supported by grants from the Brazilian agencies Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). JDN was supported by an Irma T. Hirschl/Monique Weill-Caulier Trust Research Award and in part by NIH AI52733. RJBC was supported in part by the Training Program in Cellular and Molecular Biology and Genetics Grant T32 GM007491. AC is supported by NIH awards 5R01HL059842, 5R01AI033774, 5R37AI033142, and 5R01AI052733. JDN and RJBC are special visiting-researcher fellows of the Science Without Borders Program, CNPq-Brazil.
Footnotes
Author Contributions R.J.B.C., S.C.L. and A.J.G. performed all the experiments. R.J.B.C., S.C.L., G.S.A., S.F. and A.J.G. performed the microscopy techniques. R.J.B.C., S.C.L., L.R.M. and A.J.G. performed the in vivo experiments. R.J.B.C., S.C.L., L.R.M., L.N., J.M.P., A.C., M.L.R., J.D.N. and A.J.G. designed all the experiments and wrote the manuscript. R.J.B.C., S.C.L. and A.J.G. prepared the figures. All authors reviewed the manuscript.
References
- Chayakulkeeree M. & Perfect J. R. Cryptococcosis. Infect Dis Clin North Am 20, 507–544 v–vi, 10.1016/j.idc.2006.07.001 (2006). [DOI] [PubMed] [Google Scholar]
- Pfaller M. A. & Diekema D. J. Epidemiology of invasive mycoses in North America. Crit Rev Microbiol 36, 1–53 10.3109/10408410903241444 (2010). [DOI] [PubMed] [Google Scholar]
- Wheat J. Histoplasmosis: recognition and treatment. Clin. Infect. Dis. 19 Suppl 1, S19–27 10.1093/clinids/19.Supplement_1.S19 (1994). [DOI] [PubMed] [Google Scholar]
- Deepe G. S. Jr. Immune response to early and late Histoplasma capsulatum infections. Curr Opin Microbiol 3, 359–362 10.1016/S1369-5274(00)00104-1 (2000). [DOI] [PubMed] [Google Scholar]
- Chu J. H., Feudtner C., Heydon K., Walsh T. J. & Zaoutis T. E. Hospitalizations for endemic mycoses: a population-based national study. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 42, 822–825 10.1086/500405 (2006). [DOI] [PubMed] [Google Scholar]
- Hsu L. Y., Ng E. S. & Koh L. P. Common and emerging fungal pulmonary infections. Infect Dis Clin North Am 24, 557–577 10.1016/j.idc.2010.04.003 (2010). [DOI] [PubMed] [Google Scholar]
- Goldman D. L. et al. Serologic evidence for Cryptococcus neoformans infection in early childhood. Pediatrics 107, E66, 10.1542/peds.107.5.e66 (2001). [DOI] [PubMed] [Google Scholar]
- Guimaraes A. J., Nosanchuk J. D. & Zancope-Oliveira R. M. Diagnosis of Histoplasmosis. Braz J Microbiol 37, 1–13 10.1590/S1517-83822006000100001 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronis M. L., dos Santos R. P. & Goldani L. Z. Disseminated Histoplasma capsulatum and Cryptococcus neoformans co-infection in patients with AIDS. Mycopathologia 172, 233–236 10.1007/s11046-011-9422-x (2011). [DOI] [PubMed] [Google Scholar]
- Chipungu G. A., Christians S. J. & Oliver S. P. Cutaneous cryptococcosis erroneously diagnosed as Histoplasma capsulatum infection. S Afr Med J 98, 85–86 10.7196/SAMJ.871 (2008). [DOI] [PubMed] [Google Scholar]
- Dantas K. C. et al. Importance of the association of molecular and immunological diagnosis in immunocompetent patient with Histoplasma capsulatum and Cryptoccocus neoformans infection: a case report. The journal of venomous animals and toxins including tropical diseases 20, 36, 10.1186/1678-9199-20-36 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry T. et al. Disseminated cryptococcosis and histoplasmosis co-infection in a HIV-infected woman in France. J Infect 51, e173–176 10.1016/j.jinf.2004.12.017 (2005). [DOI] [PubMed] [Google Scholar]
- Ghosh A., Tilak R., Bhushan R., Dhameja N. & Chakravarty J. Lymphnodal Co-infection of Cryptococcus and Histoplasma in a HIV-Infected Patient and Review of Published Reports. Mycopathologia 180, 105–110 10.1007/s11046-015-9882-5 (2015). [DOI] [PubMed] [Google Scholar]
- Guarner J. & Brandt M. E. Histopathologic diagnosis of fungal infections in the 21st century. Clinical microbiology reviews 24, 247–280 10.1128/CMR.00053-10 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marukutira T. et al. Clinical characteristics and outcomes in 303 HIV-infected patients with invasive fungal infections: data from the Prospective Antifungal Therapy Alliance registry, a multicenter, observational study. Hiv/Aids 6, 39–47 10.2147/HIV.S53910 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mider G. B., Smith F. D. & Bray W. E. Jr. Systemic infection with Cryptococcus neoformans (Torula histolytica) and Histoplasma capsulatum in the same patient. Arch Pathol (Chic) 43, 102–110 (1947). [PubMed] [Google Scholar]
- Myers S. A. & Kamino H. Cutaneous cryptococcosis and histoplasmosis coinfection in a patient with AIDS. J Am Acad Dermatol 34, 898–900 10.1016/S0190-9622(96)90075-4 (1996). [DOI] [PubMed] [Google Scholar]
- Rali P., Soni H., Naing W. & Gandhi V. Cryptococcal Pneumonia and Disseminated Histoplasmosis Coinfection in Newly Diagnosed HIV Patient. Chest Infections 145, 102A, 10.1378/chest.1822027 (2014). [DOI] [Google Scholar]
- Ramirez-Ortiz R., Rodriguez J., Soto Z., Rivas M. & Rodriguez-Cintron W. Synchronous pulmonary cryptococcosis and histoplasmosis. Southern medical journal 90, 729–732 (1997). [DOI] [PubMed] [Google Scholar]
- Ribeiro L. C., Hahn R. C., Favalessa O. C., Tadano T. & Fontes C. J. [Systemic mycosis: factors associated with death among patients infected with the human immunodeficiency virus, Cuiaba, State of Mato Grosso, Brazil, 2005-2008]. Rev Soc Bras Med Trop 42, 698–705 10.1590/S0037-86822009000600017 (2009). [DOI] [PubMed] [Google Scholar]
- Supparatpinyo K., Kwangsuksatith C., Hirunsri P., Uthammachai C. & Sirisanthana T. J. Systemic mycosis caused by Cryptococcus neoformans, Penicillium marneffei and Histoplasma capsulatum in a patient with Acquired Immunodefficiency Syndrome. Infect Dis Antimicrob Agents 9, 77–79 (1992). [Google Scholar]
- Kapica L., Shaw C. E. & Bartlett G. W. Inhibition of Histoplasma capsulatum by Candida albicans and other yeasts on Sabouraud’s agar media. Journal of bacteriology 95, 2171–2176 (1968). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaragoza O. et al. The capsule of the fungal pathogen Cryptococcus neoformans. Adv Appl Microbiol 68, 133–216 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues M. L. et al. Vesicular polysaccharide export in Cryptococcus neoformans is a eukaryotic solution to the problem of fungal trans-cell wall transport. Eukaryot Cell 6, 48–59 10.1128/EC.00318-06 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese A. J. & Doering T. L. Cell wall alpha-1, 3-glucan is required to anchor the Cryptococcus neoformans capsule. Mol Microbiol 50, 1401–1409 10.1046/j.1365-2958.2003.03780.x (2003). [DOI] [PubMed] [Google Scholar]
- Ramos C. L. et al. Chitin-like molecules associate with Cryptococcus neoformans glucuronoxylomannan to form a glycan complex with previously unknown properties. Eukaryot Cell 11, 1086–1094 10.1128/EC.00001-12 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimrichter L. et al. Self-aggregation of Cryptococcus neoformans capsular glucuronoxylomannan is dependent on divalent cations. Eukaryot Cell 6, 1400–1410 10.1128/EC.00122-07 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis J. N. et al. Evaluation of a novel point-of-care cryptococcal antigen test on serum, plasma, and urine from patients with HIV-associated cryptococcal meningitis. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 53, 1019–1023 10.1093/cid/cir613 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yauch L. E., Lam J. S. & Levitz S. M. Direct inhibition of T-cell responses by the Cryptococcus capsular polysaccharide glucuronoxylomannan. PLoS Pathog 2, e120, 10.1371/journal.ppat.0020120 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromtling R. A., Shadomy H. J. & Jacobson E. S. Decreased virulence in stable, acapsular mutants of cryptococcus neoformans. Mycopathologia 79, 23–29 10.1007/BF00636177 (1982). [DOI] [PubMed] [Google Scholar]
- Guimaraes A. J., de Cerqueira M. D. & Nosanchuk J. D. Surface architecture of histoplasma capsulatum. Front Microbiol 2, 225, 10.3389/fmicb.2011.00225 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanetsuna F., Carbonell L. M., Gil F. & Azuma I. Chemical and ultrastructural studies on the cell walls of the yeastlike and mycelial forms of Histoplasma capsulatum. Mycopathol Mycol Appl 54, 1–13 10.1007/BF02055967 (1974). [DOI] [PubMed] [Google Scholar]
- Mukherjee J., Casadevall A. & Scharff M. D. Molecular characterization of the humoral responses to Cryptococcus neoformans infection and glucuronoxylomannan-tetanus toxoid conjugate immunization. The Journal of experimental medicine 177, 1105–1116 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez L. R. & Casadevall A. Cryptococcus neoformans biofilm formation depends on surface support and carbon source and reduces fungal cell susceptibility to heat, cold, and UV light. Appl Environ Microbiol 73, 4592–4601 10.1128/AEM.02506-06 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresca B. & Kobayashi G. S. Dimorphism in Histoplasma capsulatum: a model for the study of cell differentiation in pathogenic fungi. Microbiol Rev 53, 186–209 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque P. et al. Quorum sensing-mediated, cell density-dependent regulation of growth and virulence in Cryptococcus neoformans. mBio 5, e00986–00913 10.1128/mBio.00986-13 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadevall A. et al. Characterization of a murine monoclonal antibody to Cryptococcus neoformans polysaccharide that is a candidate for human therapeutic studies. Antimicrob Agents Chemother 42, 1437–1446 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frases S., Nimrichter L., Viana N. B., Nakouzi A. & Casadevall A. Cryptococcus neoformans capsular polysaccharide and exopolysaccharide fractions manifest physical, chemical, and antigenic differences. Eukaryot Cell 7, 319–327 10.1128/EC.00378-07 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook E. D. & Rappleye C. A. Histoplasma capsulatum pathogenesis: making a lifestyle switch. Curr Opin Microbiol 11, 318–324 10.1016/j.mib.2008.05.010 (2008). [DOI] [PubMed] [Google Scholar]
- Chang Z. L., Netski D., Thorkildson P. & Kozel T. R. Binding and internalization of glucuronoxylomannan, the major capsular polysaccharide of Cryptococcus neoformans, by murine peritoneal macrophages. Infect Immun 74, 144–151 10.1128/IAI.74.1.144-151.2006 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couppie P., Aznar C., Carme B. & Nacher M. American histoplasmosis in developing countries with a special focus on patients with HIV: diagnosis, treatment, and prognosis. Curr Opin Infect Dis 19, 443–449 10.1097/01.qco.0000244049.15888.b9 (2006). [DOI] [PubMed] [Google Scholar]
- Brizendine K. D., Baddley J. W. & Pappas P. G. Pulmonary cryptococcosis. Seminars in respiratory and critical care medicine 32, 727–734 10.1055/s-0031-1295720 (2011). [DOI] [PubMed] [Google Scholar]
- McKinsey D. S. & McKinsey J. P. Pulmonary histoplasmosis. Seminars in respiratory and critical care medicine 32, 735–744 10.1055/s-0031-1295721 (2011). [DOI] [PubMed] [Google Scholar]
- Hidalgo A. [Radiology of invasive fungal infections of the respiratory tract]. Rev Iberoam Micol 24, 14–18 (2007). [DOI] [PubMed] [Google Scholar]
- Guimaraes A. J. et al. Evaluation of an enzyme-linked immunosorbent assay using purified, deglycosylated histoplasmin for different clinical manifestations of histoplasmosis. Microbiol Res (Pavia) 2, 10.4081/mr.2009.e1 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester S. J., Malik R., Bartlett K. H. & Duncan C. G. Cryptococcosis: update and emergence of Cryptococcus gattii. Vet Clin Pathol 40, 4–17 10.1111/j.1939-165X.2010.00281.x (2011). [DOI] [PubMed] [Google Scholar]
- Jehangir W., Tadepalli G. S., Sen S., Regevik N. & Sen P. Coccidioidomycosis and Blastomycosis: Endemic Mycotic Co-Infections in the HIV Patient. Journal of clinical medicine research 7, 196–198 10.14740/jocmr2036w (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton J. F. & DiSalvo A. F. The isolation of Histoplasma capsulatum, Cryptococcus neoformans and Blastomyces dermatitidis from the same natural site. Sabouraudia 17, 193–195 10.1080/00362177985380281 (1979). [DOI] [PubMed] [Google Scholar]
- Cermeno J. R. et al. Cryptococcus neoformans and Histoplasma capsulatum in dove’s (Columbia livia) excreta in Bolivar state, Venezuela. Rev Latinoam Microbiol 48, 6–9 (2006). [PubMed] [Google Scholar]
- Rappleye C. A., Eissenberg L. G. & Goldman W. E. Histoplasma capsulatum alpha-(1, 3)-glucan blocks innate immune recognition by the beta-glucan receptor. Proc Natl Acad Sci USA 104, 1366–1370 10.1073/pnas.0609848104 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker S. C. & Casadevall A. Replication of Cryptococcus neoformans in macrophages is accompanied by phagosomal permeabilization and accumulation of vesicles containing polysaccharide in the cytoplasm. Proc Natl Acad Sci USA 99, 3165–3170 10.1073/pnas.052702799 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadevall A. & Pirofski L. A. Polysaccharide-containing conjugate vaccines for fungal diseases. Trends in molecular medicine 12, 6–9 10.1016/j.molmed.2005.11.003 (2006). [DOI] [PubMed] [Google Scholar]
- Saylor C., Dadachova E. & Casadevall A. Monoclonal antibody-based therapies for microbial diseases. Vaccine 27 Suppl 6, G38–46 10.1016/j.vaccine.2009.09.105 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wargo M. J. & Hogan D. A. Fungal–bacterial interactions: a mixed bag of mingling microbes. Curr Opin Microbiol 9, 359–364 10.1016/j.mib.2006.06.001 (2006). [DOI] [PubMed] [Google Scholar]
- Nosanchuk J. D., Steenbergen J. N., Shi L., Deepe G. S. J. & Casadevall A. Antibodies to a cell surface histone-like protein protect against Histoplasma capsulatum. J. Clin. Invest. 112, 1164–1175 10.1172/JCI200319361 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navoa J. A. et al. Specificity and diversity of antibodies to Mycobacterium tuberculosis arabinomannan. Clinical and diagnostic laboratory immunology 10, 88–94 10.1128/CDLI.10.1.88–94. 2003 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma A. & Kwon-Chung K. J. Rapid method to extract DNA from Cryptococcus neoformans. J Clin Microbiol 29, 810–812 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimaraes A. J. et al. Cryptococcus neoformans responds to mannitol by increasing capsule size in vitro and in vivo. Cell Microbiol 12, 740–753 10.1111/j.1462-5822.2010.01430.x (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Goncalves R. & Mosser D. M. The isolation and characterization of murine macrophages. Curr Protoc Immunol Chapter 14, Unit 14 11, 10.1002/0471142735 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomaz L. et al. Galleria mellonella as a model host to study Paracoccidioides lutzii and Histoplasma capsulatum. Virulence 4, 139–146 10.4161/viru.23047 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.