Abstract
Objectives
It is not known whether current use of the medication primidone affects brain gamma-aminobutyric acid (GABA) concentrations. This is an important potential confound in studies of the pathophysiology of essential tremor (ET), one of the most common neurological diseases. We compared GABA concentrations in the dentate nucleus in 6 ET patients taking primidone vs. 26 ET patients not taking primidone.
Methods
1H magnetic resonance spectroscopy (MRS) was performed using a 3.0 Tesla Siemens Tim Trio scanner. The MEGA-PRESS J-editing sequence was used for GABA detection in two cerebellar volumes of interest (left and right) that included the dentate nucleus.
Results
The right dentate GABA concentration was similar in the two groups (2.21 ± 0.46 [on primidone] vs. 1.93 ± 0.39 [not on primidone], p=0.15), as was the left dentate GABA concentration (1.61 ± 0.35 [on primidone] vs. 1.67 ± 0.34 [not on primidone], p=0.72). The daily primidone dose was not associated with either right or left dentate GABA concentrations (respective p = 0.89 and 0.76).
Conclusions
We did not find a difference in dentate GABA concentrations between 6 ET cases taking daily primidone and 26 ET cases not taking primidone. Furthermore, there was no association between daily primidone dose and dentate GABA concentration. These data suggest that it is not necessary to exclude ET patients on primidone from MRS studies of dentate GABA concentration and, if assessment of these concentrations were to be developed as a biomarker for ET, primidone usage would not confound interpretation of the results.
Keywords: Primidone, medication, gamma-aminobutyric acid, concentration, essential tremor
Introduction
Despite its high prevalence, essential tremor (ET) is among the most poorly understood neurological diseases. On the most basic biological level, little is known about its underlying pathologic-anatomy and pathophysiology.1 Several recent postmortem studies report a 30 - 40% reduction in Purkinje cells (PCs) in ET, suggesting that on a mechanistic level, this common neurological disease could be neurodegenerative.2–6 However, the presence and extent of PC loss in ET is the subject of considerable controversy,7 and therefore, the focus of current scrutiny.
PCs are a major storehouse of brain gamma-aminobutyric acid (GABA), releasing GABA into the synaptic cleft at the level of the cerebellar dentate nucleus.8, 9 Thus, the cerebellar dentate GABA level could be a convenient in vivo marker of PC number. At present, we are conducting a longitudinal study that uses in-vivo magnetic resonance spectroscopy (MRS) to quantify GABA concentrations in the cerebellar dentate nucleus. We expect that the proposed research, by testing the hypothesis that these concentrations will be low in ET patients compared to age-matched controls, will elucidate a critically important question about the underlying pathophysiology of ET. Aside from its scientific value, demonstrating low MRS-assessed GABA concentrations in ET could have an important clinical implication - MRS-assessed GABA concentration could serve as an imaging biomarker for ET. No such biomarkers currently exist.
Yet there is a complication. A potential contraindication for enrollment in this and other such studies is current exposure to medications that could influence brain GABA-ergic neurotransmission. By far, the most commonly used of these agents in ET is primidone, which is one of only two front-line medications used to treat ET.10, 11 Studies estimate that approximately 50% of all ET patients have tried primidone.12 Primidone is metabolized to phenobarbital, which binds to the GABAA receptor, potentiating GABA-ergic neurotransmission.13 The question, which we now address, is whether current use of primidone affects dentate GABA concentrations. To our knowledge, this is unknown. The issue is an important one – if one were to exclude such treated ET patients, the potential loss of patients would be as much as 50%. In this cross-sectional study, we compared GABA concentrations in two cerebellar volumes of interest (left and right), which included the dentate nucleus, comparing 6 ET patients taking daily primidone to 26 ET patients who were not taking primidone.
Materials and Methods
Subjects and clinical evaluation
ET cases were recruited from several sources, including a clinical-epidemiological study of ET,14 one of the author’s (E.D.L.) neurological practices, and study advertisements. Inclusion criteria were: (1) a prior diagnosis of ET assigned by a treating neurologist, (2) willingness to undergo a magnetic resonance imaging (MRI) scan as part of the current baseline study as well as in a longitudinal study, and (3) living within two hours of the recruiting site. Exclusion criteria were: (1) heavy exposure to ethanol (as defined previously),15 (2) history of a neurodegenerative disease (Parkinson’s disease, Alzheimer’s disease), (3) prior deep brain stimulation or other neurosurgery (e.g., gamma knife, thalamotomy) for ET, (4) a reason to be excluded from MRI scanning (e.g., metal in their bodies). Furthermore, we excluded most cases who were taking medications that bind to the GABAA receptor or that enhance GABA tone (e.g., clonazepam, diazepam, gabapentin, phenobarbital, primidone, progabide, propofol, tigabine, valproate, vigabatrin); however, a small number of cases who were taking primidone were enrolled specifically for the purposes of the present analyses. In total, 106 ET cases were excluded for one of the aforementioned reasons.
Once enrolled, a trained research assistant conducted an in-person evaluation, administering demographic (e.g., age, gender, race, education) and medical history questionnaires (e.g., hand-dominance, smoking history, tremor duration, number of drinks of ethanol per month). The Montreal Cognitive Assessment (MoCA) was performed as a brief assessment of cognitive function.16 During the in-person assessment, a videotaped neurological examination was also performed. This included one test for postural tremor and five for kinetic tremor (12 tests total). A senior neurologist specializing in movement disorders (E.D.L.) used a reliable and valid clinical rating scale, the Washington Heights-Inwood Genetic Study of ET (WHIGET) tremor rating scale, to rate postural and kinetic tremor during each test: 0 (none), 1 (mild), 2 (moderate), 3 (severe), resulting in a total tremor score (range = 0 – 36).17 Diagnoses of ET were re-confirmed by E.D.L. using the videotaped neurological examination and WHIGET diagnostic criteria (moderate or greater amplitude kinetic tremor [tremor rating ≥ 2] during three or more tests or a head tremor, in the absence of Parkinson’s disease, dystonia or another cause).18, 19
The study protocol was reviewed and approved by the Human Subjects Institutional Review Board at Yale University, Purdue University and at Weill Cornell Medical College. Written informed consent was obtained from each subject prior to participation in the study.
In vivo MRI/MRS measurements
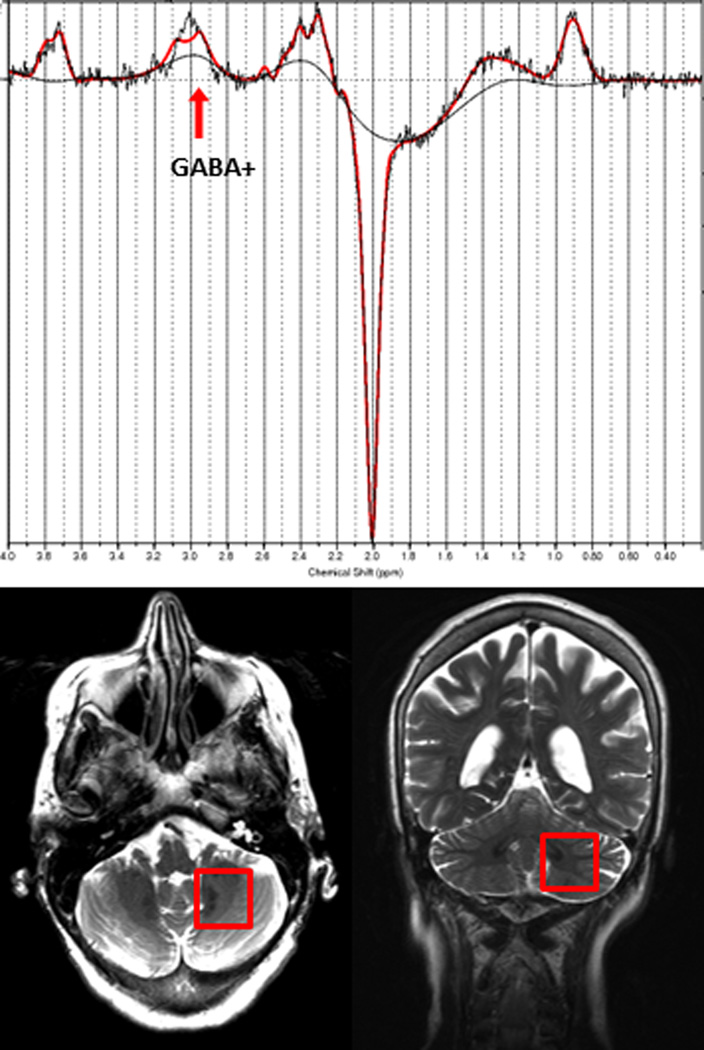
MRI and 1H MRS exams were performed on a 3.0 Tesla Siemens Tim Trio scanner (Siemens Healthcare, Erlangen, Germany), equipped with a 32-channel head coil. All scans were performed at Weill Cornell Medical College. Fast T2-weighted images were acquired in all three orientations to ensure exact localization of the MRS volumes of interest (VOIs). GABA-edited MRS data was acquired from two VOIs containing the left and right cerebellar dentate nucleus, respectively (both 25 mm × 25 mm × 25 mm, 128 averages) (Figure 1). Both dentate nuclei were clearly identified on the T2-weighted images on both axial and coronal planes. Each GABA VOI was placed on the axial plane such that the entire dentate nucleus was included, given the fact that this is the level at which the Purkinje cells release their GABA into the synaptic cleft,8 while minimizing contributions from vascular and CSF compartments. The VOI was then confirmed to be completely within the cerebellum on the coronal plane. The MEGA-PRESS J-editing sequence was used for GABA detection (TR/TE = 1500/68 ms).20
Figure 1.
Top: Representative GABA-edited spectrum from the dentate VOI showing the raw data and the LCModel fit. Bottom: Placement of the GABA VOI, containing the left cerebellar dentate.
196 averages were acquired with the spectrally selective editing pulse centered at 1.9 ppm (edit-on) and 196 averages with the pulse centered at 7.5 ppm (edit-off) in an interleaved fashion. The resulting difference spectrum contains a GABA peak at 3.0 ppm, which also includes contributions from co-edited macromolecules and homocarnosine, a dipeptide consisting of GABA and histidine. Therefore, the signal will be referred to as GABA+. For both VOIs, a reference spectrum was acquired without water suppression. These reference spectra were then used for phase and frequency correction of the corresponding water-suppressed spectra. FASTESTMAP shimming (IPR#577; Siemens Healthcare) was performed before each voxel measurement to achieve water line widths of < 20 Hz.21 In order to determine voxel tissue composition, high-resolution MPRAGE images were acquired (TR/TE/TI = 2300/2.91/900 ms, flip angle = 9°, bandwidth: 240 Hz/pixel, voxel size: 1.0 mm × 1.0 mm × 1.2 mm, GRAPPA = 2). Every effort was made to ensure the subjects were as comfortable as possible in the scanner.
Data processing and analysis
MRS data processing and quantification were performed with LCModel 6.3-0L,22 fitting each spectrum as a weighted linear combination of basis spectra from individual metabolites. For fitting the MEGA-PRESS spectra, basis sets were generated from density matrix simulations of the sequence using published values for chemical shifts and J-couplings from Kaiser et al.,23 with an exact treatment of metabolite evolution during the two frequency-selective MEGA inversion pulses. LCModel fitting %SD values were lower than 20% for GABA+ in all spectra. GABA+ concentrations were derived from raw GABA+ output values from LCModel, multiplied with a water calibration factor provided by LCModel (FCALIB factor). Due to scaling uncertainties, GABA+ concentration values are given in institutional units, but are proportional to and in the range of the true GABA+ concentrations in mM.
To determine and correct for the tissue composition of the MRS voxels, MPRAGE images were segmented into grey matter (GM), white matter (WM) and cerebrospinal fluid (CSF) using an in-house MATLAB 2013a (MathWorks Inc., Natick, MA, USA) code, incorporated with statistical parametric mapping (SPM8, Wellcome Department of Imaging Neuroscience, London, UK). GABA levels corrected for CSF were obtained using the method described by Chowdhury et al.24
Sample size calculation
In order to calculate sample size, we used our won pilot MRS data on dentate GABA concentration in five ET cases who were not taking primidone. We determined that with 6 treated and 26 untreated cases, we would have 80% power to detect as little as a 25% difference in dentate GABA concentration between groups (assuming a two-sided Student’s t test and alpha = 0.5). Given published data showing 50 – 70% increases in MRS-assessed brain GABA concentrations in animals and humans treated with GABA-ergic medications,25–28 this sample size was considered adequate.
Statistical analyses
All analyses were performed in SPSS (version 21.0). We used Student’s t tests, Fisher’s exact tests, and Mann Whitney tests (for variables that were not normally distributed) to compare the demographic and clinical features of ET cases on primidone vs. ET cases off primidone (Table 1). We also assessed the correlations between dentate GABA concentration and demographic and clinical features using Student’s t tests as well as correlation coefficients (Pearson’s for normally distributed variables and Spearman’s for variables that were not normally distributed) (Table 2). In linear regression models, we adjusted for potential confounds (i.e., variables that were associated, even to a marginal degree, with primidone status or with dentate GABA concentration) in the association between primidone status (treated vs. untreated) and dentate GABA concentrations (dependent variable).
Table 1.
Demographic and clinical characteristics of ET cases who were taking primidone vs. ET cases who were not taking primidone
| ET cases on primidone (n = 6) |
ET cases not on primidone (n = 26) |
Significance | |
|---|---|---|---|
| Age (years) | 78.7 ± 4.4 | 75.4 ± 6.6 | p = 0.26 a |
| Female gender | 2 (33.3) | 11 (42.3) | p = 1.00 b |
| White race | 6 (100) | 25 (96.2) | p = 1.00 b |
| Education (years) | 17.3 ± 4.8 | 17.7 ± 2.3 | p = 0.79 a |
| Current smoker | 0 (0.0) | 0 (0.0) | p = 1.00 b |
| Total tremor score | 19.3 ± 5.5 | 21.8 ± 4.6 | p = 0.27 a |
| Tremor duration (years) | 42.3 ± 16.3 | 33.1 ± 21.9 | p = 0.35 a |
| Right hand dominant | 4 (75.0) | 25 (96.2) | p = 0.17 b |
| MoCA score | 27.0 ± 2.6 | 27.1 ± 2.3 | p = 0.97 a |
| Median number of drinks of ethanol per month | 21.5 | 3.2 | p = 0.097 c |
Values represent numbers (percentages) or mean ± standard deviation.
Student’s t test.
Fisher’s exact test.
Mann-Whitney test.
MoCA = Montreal Cognitive Assessment
Table 2.
Correlation between dentate GABA concentration and demographic and clinical characteristics of ET cases
| Right dentate GABA concentration |
Left dentate GABA concentration |
|
|---|---|---|
| Age (years) | rp = −0.006 p = 0.98 |
rp = 0.10 p = 0.61 |
| Gender Male Female |
1.91 ± 0.46 2.08 ± 0.33 t = 1.12, p = 0.27 |
1.66 ± 0.36 1.65 ± 0.32 t = 0.05, p = 0.96 |
| Education (years) | rp = −0.16 p = 0.43 |
rp = −0.002 p = 0.99 |
| Total tremor score | rp = −0.17 p = 0.36 |
rp = 0.11 p = 0.60 |
| Tremor duration (years) | rp = −0.19 p = 0.34 |
rp = 0.39 p = 0.06 |
| MoCA score | rp = −0.42 p = 0.03 |
rp = 0.16 p = 0.45 |
| Median number of drinks of ethanol per month | rs = 0.03 p = 0.86 |
rs = 0.16 p = 0.43 |
Values represent correlation coefficients (rp = Pearson’s and rs = Spearman’s) or mean ± standard deviation.
MoCA = Montreal Cognitive Assessment
Results
The two groups were similar to one another in terms of age, gender, race, education, smoking status, hand dominance, total tremor score, and MoCA score (Table 1). The mean tremor duration was nearly 10 years longer for the primidone group, although this difference did not reach statistical significance (Table 1). The median number of drinks of ethanol was greater for the primidone group (21.5 vs. 3.2), but this difference did not reach statistical significance either (Table 1).
In our 32 cases, dentate GABA concentration was not correlated with age, gender, years of education, total tremor score or median number of drinks of ethanol per month (Table 2). Higher right, but not left, dentate GABA concentration was weakly associated with lower MoCA score (r = -0.42, p = 0.03, Table 2). Higher left, but not right, dentate GABA concentration was marginally associated with longer tremor duration (r = 0.39, p = 0.06, Table 2).
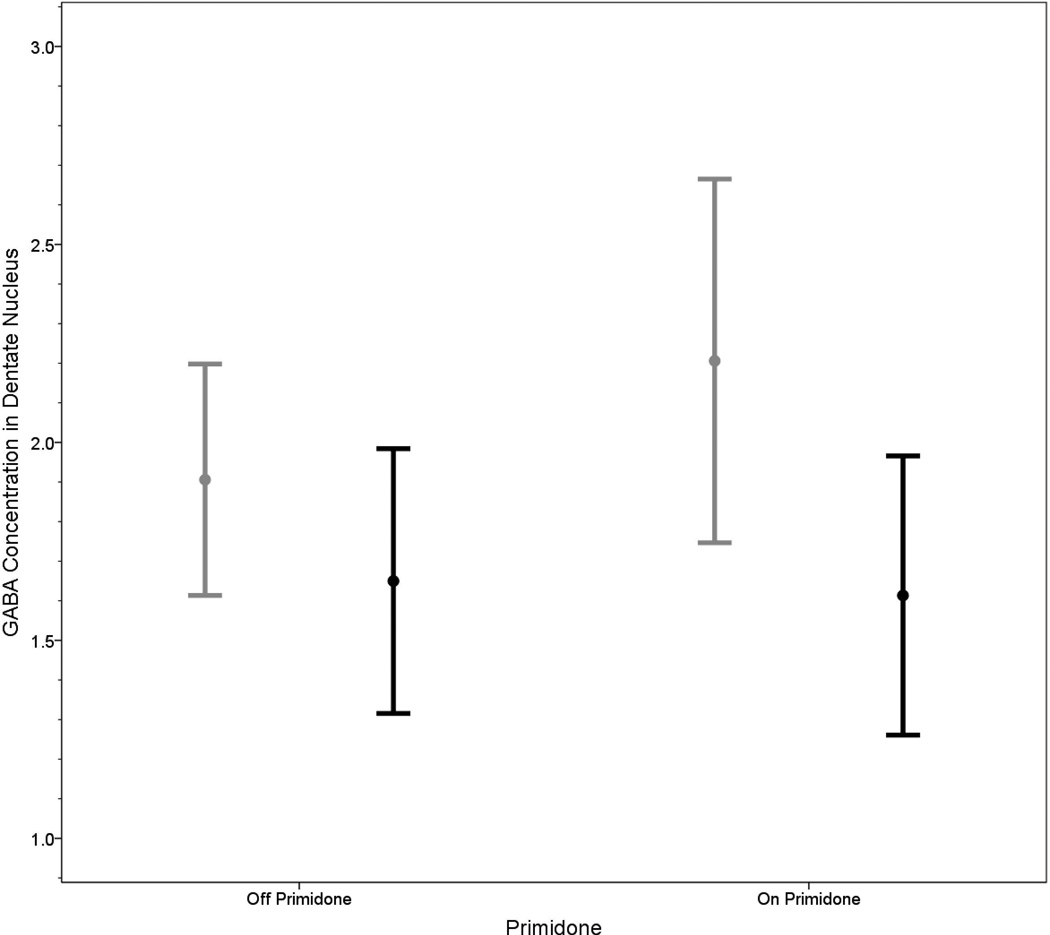
Both, the right and left dentate GABA concentrations were similar in the two groups (right: 2.21 ± 0.46 [on primidone] vs. 1.93 ± 0.39 [not on primidone], t = 1.49, p = 0.15; left: 1.61 ± 0.35 [on primidone] vs. 1.67 ± 0.34 [not on primidone], t = 0.36, p = 0.72) (Figure 2).
Figure 2.
GABA concentrations in the right dentate nuclei in ET patients on vs. off primidone (gray bars) and GABA concentrations in the left dentate nucleus in ET patients on vs. off primidone (black bars). Bars represent means and one standard deviation.
In the six treated cases, the daily primidone dose ranged from 50 to 750 mg (mean = 312.5 mg, standard deviation = 309.2 mg); there was no association between this dose and either right dentate GABA concentration (Pearson’s r = 0.11, p = 0.89) or left dentate GABA concentration (Pearson’s r = -0.24, p = 0.76).
In a linear regression model that adjusted for potential confounds (number of drinks of ethanol per month, tremor duration, MoCA score), taking primidone was not associated with the right dentate GABA concentration (beta = 0.27, p = 0.17). Similarly, adjusting for the same potential confounds, taking primidone was not associated with the left dentate GABA concentration (beta = -0.24, p = 0.22).
Discussion
To our knowledge, this is the first study to examine the effects of current, daily administration of primidone on MRS-assessed dentate GABA concentration. The current data address a question about a major potential confound in studies of GABA in ET. Importantly, we did not find a difference in dentate GABA concentrations in 6 ET cases taking daily primidone compared to 26 ET cases who were not taking primidone. Furthermore, there was no association between daily primidone dose and dentate GABA concentration.
The published literature has not directly addressed the issue we focus on in this study. Indeed, the small number of related published studies differ from ours in numerous respects and their results are mixed. These data will be reviewed in the paragraphs below, beginning with the human studies and then moving to the animal studies.
In an MRS study, 17 healthy adults were randomly assigned to receive one of the following medications with GABA-ergic properties: topiramate, gabapentin or lamotrigine. GABA was measured using a 4.1-T magnet from a 13.5-mL volume over the occipital region 3 and 6 hours following administration of an acute single dose and 2 and 4 weeks after dose titration. During the acute phase, GABA concentrations rose 70% (topiramate) and 48% (gabapentin), but not with lamotrigine. With long term dosing, significant elevations in GABA were observed compared to baseline for all three drugs (topiramate 46%, gabapentin 25%, and lamotrigine 25%).25 The study differed from ours in the sense that the patients were not ET patients, the medication of interest in our study (primidone) was not studied, and GABA concentrations in a VOI containing the dentate nucleus were not assessed.
In another study, the authors performed a 900 mg gabapentin single-dose challenge in healthy human subjects in order to explore its effects on GABA concentrations measured with ¹H-MRS in the visual cortex. Gabapentin administration was associated with an average increase in GABA concentration of 55.7% (range = 6.9 -91.0%).26 That study differed from ours in multiple respects, including the specific medication used, the study population (ET vs. controls), the chronicity of drug usage (single-dose vs. chronic use) and the location of the MRS voxel.
In another study, the effects of gabapentin and vigabatrin were assessed on GABA concentrations in human (n = 14) and rat (n = 6) neocortical slice preparations. Neocortical slices were incubated with gabapentin, vigabatrin or no drugs for 3 hours. Proton MRS of perchloric acid (PCA) extracts was used to measure GABA concentrations. Vigabatrin increased cellular GABA concentrations in both human and rat neocortical slices by 62% (p <0.001) and 88% (p <0.03), respectively. Gabapentin significantly increased GABA concentrations by 13% (p <0.02) in human neocortical slices but not in rat neocortical slices.27
In another study, the effects of gabapentin, pregabalin and vigabatrin on brain MRS GABA concentrations were studied in Long Evans rats. Two hours after intraperitoneal injection of 100mg/kg gabapentin no differences were observed in GABA concentrations in rats treated with gabapentin. For pregabalin, no effect was observed. Vigabatrin produced a 50% increase in GABA. The authors concluded that although gabapentin and pregabalin are anticonvulsants designed to mimic GABA, these drugs do not raise GABA levels acutely.29
In an earlier study 28 of albino mice, the authors administered primidone and measured brain GABA levels in the cerebral hemispheres. This is the only study we are aware of that has focused on this medication (primidone), as did we. They found that primidone (50 mg/kg intra-peritoneally) did not raise the brain GABA concentrations in these animals.
Overall, one can see that the results are mixed with respect to animal vs. human data, with respect to the effects of specific GABA-ergic agents relative to one another, and with respect to the acute vs. chronic effects of the same agents. The one study28 that assessed primidone; however, found that intra-peritoneally-delivered primidone did not raise the brain GABA concentrations.
This study had a number of limitations. First, our VOI was large compared to the size of the dentate nucleus. This was necessary in order to obtain a GABA-edited spectrum with sufficient signal to noise ratio for adequate quantification. Thus, it is conceivable that our sensitivity to detect very small changes in dentate GABA may have been insufficient, especially if the GABA concentration of the surrounding cerebellar tissue, which was co-measured, did not change. Second, we did not precisely standardize when the six treated study subjects took their usual morning primidone dose. However, all six of them took their dose on the morning of the scan, and the latency between dose and scan ranged from 1 – 6 hours. As noted above, in a study 28 of albino mice, the authors noted that primidone administered intra-peritoneally did not acutely raise brain GABA concentrations. Third, the number of study subjects taking primidone was small (n = 6). Despite this, we were powered to detect as little as a 25% difference between study groups. Given the available published data, which show 50 – 70% increases in MRS-assessed brain GABA concentrations in animals and humans treated with GABA-ergic medications,25–28 this sample size was considered more than adequate.
In summary, we did not find a difference in dentate GABA concentrations in 6 ET cases taking daily primidone compared to 26 ET cases who were not taking primidone. Furthermore, there was no association between daily primidone dose and dentate GABA concentration. These data suggest that it is not necessary to exclude ET patients on primidone from MRS studies of dentate GABA concentration and, if such concentrations were eventually to be developed as a biomarker for ET, that use of primidone would not confound the interpretation of the results.
Acknowledgments
Sources of Funding
This work was supported by NINDS R01 NS085136 from the National Institutes of Health.
Footnotes
Conflicts of Interest
None of the authors has any conflicts of interest.
References
- 1.Louis ED. Essential tremor: from bedside to bench and back to bedside. Curr Opin Neurol. 2014;27:461–467. doi: 10.1097/WCO.0000000000000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louis ED, Faust PL, Vonsattel JP, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain. 2007;130:3297–3307. doi: 10.1093/brain/awm266. [DOI] [PubMed] [Google Scholar]
- 3.Louis ED, Babij R, Lee M, et al. Quantification of cerebellar hemispheric purkinje cell linear density: 32 ET cases versus 16 controls. Mov Disord. 2013;28:1854–1859. doi: 10.1002/mds.25629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grimaldi G, Manto M. Is essential tremor a Purkinjopathy? The role of the cerebellar cortex in its pathogenesis. Mov Disord. 2013;28:1759–1761. doi: 10.1002/mds.25645. [DOI] [PubMed] [Google Scholar]
- 5.Bonuccelli U. Essential tremor is a neurodegenerative disease. J Neural Transm. 2012;119:1383–1387. doi: 10.1007/s00702-012-0878-8. [DOI] [PubMed] [Google Scholar]
- 6.Benito-Leon J. Essential tremor: a neurodegenerative disease? Tremor Other Hyperkinet Mov (N Y) 2014;4:252. doi: 10.7916/D8765CG0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Louis ED, Faust PL, Vonsattel JP. Purkinje cell loss is a characteristic of essential tremor: Towards a more mature understanding of pathogenesis. Parkinsonism Relat Disord. 2012;18:1003–1004. doi: 10.1016/j.parkreldis.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 8.Gambarana C, Loria CJ, Siegel RE. GABAA receptor messenger RNA expression in the deep cerebellar nuclei of Purkinje cell degeneration mutants is maintained following the loss of innervating Purkinje neurons. Neuroscience. 1993;52:63–71. doi: 10.1016/0306-4522(93)90182-f. [DOI] [PubMed] [Google Scholar]
- 9.Linnemann C, Sultan F, Pedroarena CM, et al. Lurcher mice exhibit potentiation of GABAA-receptor-mediated conductance in cerebellar nuclei neurons in close temporal relationship to Purkinje cell death. J Neurophysiol. 2004;91:1102–1107. doi: 10.1152/jn.00163.2003. [DOI] [PubMed] [Google Scholar]
- 10.Zesiewicz TA, Elble RJ, Louis ED, et al. Evidence-based guideline update: treatment of essential tremor: report of the Quality Standards subcommittee of the American Academy of Neurology. Neurology. 77:1752–1755. doi: 10.1212/WNL.0b013e318236f0fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benito-Leon J, Louis ED. Clinical update: diagnosis and treatment of essential tremor. Lancet. 2007;369:1152–1154. doi: 10.1016/S0140-6736(07)60544-3. [DOI] [PubMed] [Google Scholar]
- 12.Louis ED, Rios E, Henchcliffe C. How are we doing with the treatment of essential tremor (ET)?: Persistence of patients with ET on medication: data from 528 patients in three settings. Eur J Neurol. 2010;17:882–884. doi: 10.1111/j.1468-1331.2009.02926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sasso E, Perucca E, Calzetti S. Double-blind comparison of primidone and phenobarbital in essential tremor. Neurology. 1988;38:808–810. doi: 10.1212/wnl.38.5.808. [DOI] [PubMed] [Google Scholar]
- 14.Michalec M, Hernandez N, Clark LN, et al. The spiral axis as a clinical tool to distinguish essential tremor from dystonia cases. Parkinsonism Relat Disord. 2014;20:541–544. doi: 10.1016/j.parkreldis.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harasymiw JW, Bean P. Identification of heavy drinkers by using the early detection of alcohol consumption score. Alcohol Clin Exp Res. 2001;25:228–235. [PubMed] [Google Scholar]
- 16.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 17.Louis ED. Utility of the hand-drawn spiral as a tool in clinical-epidemiological research on essential tremor: data from four essential tremor cohorts. Neuroepidemiology. 2015;44:45–50. doi: 10.1159/000371850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Louis ED, Ottman R, Ford B, et al. The Washington Heights-Inwood Genetic Study of Essential Tremor: methodologic issues in essential-tremor research. Neuroepidemiology. 1997;16:124–133. doi: 10.1159/000109681. [DOI] [PubMed] [Google Scholar]
- 19.Louis ED, Ford B, Lee H, et al. Diagnostic criteria for essential tremor: a population perspective. Arch Neurol. 1998;55:823–828. doi: 10.1001/archneur.55.6.823. [DOI] [PubMed] [Google Scholar]
- 20.Mullins PG, McGonigle DJ, O'Gorman RL, et al. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. Neuroimage. 2014;86:43–52. doi: 10.1016/j.neuroimage.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gruetter R, Tkac I. Field mapping without reference scan using asymmetric echo-planar techniques. Magn Reson Med. 2000;43:319–323. doi: 10.1002/(sici)1522-2594(200002)43:2<319::aid-mrm22>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 22.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 23.Kaiser LG, Young K, Meyerhoff DJ, et al. A detailed analysis of localized J-difference GABA editing: theoretical and experimental study at 4 T. NMR Biomed. 2008;21:22–32. doi: 10.1002/nbm.1150. [DOI] [PubMed] [Google Scholar]
- 24.Chowdhury FA, O'Gorman RL, Nashef L, et al. Investigation of Glutamine and GABA levels in patients with idiopathic generalized epilepsy using MEGAPRESS. J Magn Reson Imag. 2015;41:697–699. doi: 10.1002/jmri.24611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuzniecky R, Ho S, Pan J, et al. Modulation of cerebral GABA by topiramate, lamotrigine, and gabapentin in healthy adults. Neurology. 2002;58:368–372. doi: 10.1212/wnl.58.3.368. [DOI] [PubMed] [Google Scholar]
- 26.Cai K, Nanga RP, Lamprou L, et al. The impact of gabapentin administration on brain GABA and glutamate concentrations: a 7T 1H-MRS study. Neuropsychopharmacology. 2012;37:2764–2771. doi: 10.1038/npp.2012.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Errante LD, Williamson A, Spencer DD, et al. Gabapentin and vigabatrin increase GABA in the human neocortical slice. Epilepsy Res. 2002;49:203–210. doi: 10.1016/s0920-1211(02)00034-7. [DOI] [PubMed] [Google Scholar]
- 28.Matin MA, Kar PP. Effect of barbiturates and isoniazid on cerebral hemisphere gamma-aminobutyric acid content in pp' DDT treated mice. Pharmacol Res Commun. 1974;6:357–362. doi: 10.1016/s0031-6989(74)80035-4. [DOI] [PubMed] [Google Scholar]
- 29.Errante LD, Petroff OA. Acute effects of gabapentin and pregabalin on rat forebrain cellular GABA, glutamate, and glutamine concentrations. Seizure. 2003;12:300–306. doi: 10.1016/s1059-1311(02)00295-9. [DOI] [PubMed] [Google Scholar]