Abstract
Importance
The role of aspiration associated extra-esophageal reflux disease (AERD) in patients with chronic respiratory symptoms is not well defined. Identifying the frequency of AERD in these patients may provide us with further guidance in treatment and management of these patients.
Objective
The purpose of this study is to determine the prevalence of AERD in patients with chronic respiratory symptoms compared to controls and, secondly, to assess the utility of pepsin as a new marker for AERD.
Design
Case-control study performed from 2008-2012.
Setting
Tertiary referral center.
Participants
Patients (4.5 months-24 years) with chronic pulmonary disease, both with and without tracheostomy, were compared to controls with no prior history of pulmonary disease undergoing elective surgery.
Interventions: Lavage fluid specimen was obtained from each participant.
Main Outcome Measures
Western blot analysis for pepsin and oil red O staining for lipid-laden macrophages (LLM) was performed on lavage fluid specimens to assess for AERD.
Results
Seventy-six total patients were enrolled: 65 study patients, of which, 34 patients underwent bronchoscopy, 31 patients had tracheostomy for sampling, and 11 controls. Pepsin positive lavage fluid specimens were identified in 25 (74%) bronchoscopy patients and 22 (71%) tracheostomy patients. All control specimens were negative for pepsin. Presence of LLM was identified in 91% of bronchoscopy group, 52% of tracheostomy patients, and 64% of controls, with a similar distribution of the quantity of LLM in each lavage fluid specimen amongst the groups.
Conclusions and Relevance
Patients with chronic pulmonary disease have a high prevalence of AERD, which may have important treatment implications. The presence of pepsin was a better predictor of AERD in patients with respiratory symptoms compared to controls than LLM. Detection of pepsin in BAL can serve as a biomarker for AERD and is potentially superior to the current method of measuring LLM. While there is a significant association among patients with AERD and those patients with chronic respiratory symptoms, this study does not verify causation. Additional study investigating the mechanism of pepsin on the respiratory epithelium may provide further understanding of the pathophysiology of this association and provide additional management options for these patients.
Introduction
Direct aspiration of ingested material and reflux aspiration have both been implicated in the development and/or progression of pulmonary disease.1,2 Distinguishing between these two types of aspiration is important in guiding treatment recommendations. However, the diagnosis of aspiration associated extra-esophageal reflux disease (AERD) continues to be difficult, as well as defining its role in patients with chronic pulmonary symptoms. Clinical tests currently used to assess presence of AERD are limited. Modified barium swallow studies often have a poor negative predictive value, frequently missing episodes of reflux and intermittent aspiration.3 Twenty-four hour pH probe monitoring was considered the gold standard for diagnosing gastroesophageal reflux disease (GERD) however this method may miss episodes of non-acidic reflux. Multichannel intraluminal impedance monitoring was introduced to help capture weakly acidic episodes of reflux.2,4 Prior methods also looked at measuring glucose in tracheal secretions as a measure of aspiration without effective results.5 Measurement of lipid laden alveolar macrophages (LLM) from bronchoalveolar lavage (BAL) is the most widely used test to identify AERD. This test is based on the hypothesis that refluxate will be phagocytosed by alveolar macrophages, and that staining for these in the BAL would verify AERD.6 Prior studies demonstrated conflicting results. Higher levels of LLM in BAL samples were found in patients with lung disease and gastroesophageal reflux (GER).4 However, the LLM were also found in patients without GER and in control patients, thus not great predictors of aspirators.4,7 Different methods to measure the LLM were investigated, including the lipid laden macrophage index or classifying the amount of lipid in each cell. However, the diagnostic utility of these methods is limited and variable among studies.3,8,9
Pepsin, an exogenous protein, is proposed as a good biomarker of aspiration in animal studies.10 Pepsin was shown to potentially have a role in acute exacerbations of idiopathic pulmonary fibrosis11 and was detected in patients requiring mechanical ventilation at risk for aspiration.5 Stovold et al. used pepsin as a biomarker of gastric aspiration and reported elevated levels of pepsin in BAL of lung allografts, the highest levels found in patients with acute rejection.1 Fisichella et al. also used pepsin as a biomarker for aspiration and reported that laparoscopic anti-reflux surgery is an effective means to present aspiration as defined by the presence of pepsin in the BAL.12While the literature continues to have more studies demonstrate the effective use of pepsin as a biomarker of aspiration, this technique has not been fully translated to the clinical setting and often pathology labs are not fully equipped to perform this testing.
The purpose of this study was to determine the prevalence of AERD in our cohort of patients with chronic respiratory symptoms and in patients with tracheostomies by detecting the presence or absence of pepsin in BAL specimens. Additionally, the effectiveness of pepsin as a biomarker for AERD was investigated by comparing the results of pepsin detection in the BAL specimens with the data measuring LLM obtained from the same tracheal aspirate. The findings from these study objectives may highlight the importance of more routine testing of pepsin in BAL specimens of specific patient populations.
Methods
Patient selection and study design
The Institutional Review Board at Children's Hospital of Wisconsin (CHW, IRB protocol# 122706) approved this study. Written informed consent was obtained from each enrolled patient. A total of 11 control patients, 34 bronchoscopy patients, and 31 chronic tracheostomy patients were enrolled in the study during a four-year period (2008-2012). Those patients included in the study were 4.5 months to 24 years old and fulfilling one of the following criteria: patient without pulmonary disease undergoing an elective procedure, a patient undergoing a diagnostic bronchoscopy, or a patient with a tracheostomy. Patients without history of respiratory symptoms or GER undergoing an elective procedure were recruited to the control group. Patients with history of chronic cough, wheezing, recurrent pneumonias, abnormal lung exam, or increased work of breathing that warranted a diagnostic bronchoscopy were recruited to the bronchoscopy group. Additionally patients with a previously diagnosed chronic lung disease with worsening symptoms requiring a bronchoscopy were also enrolled in the bronchoscopy group. Patients with tracheostomy dependency requiring an airway evaluation with included in the tracheostomy group. Additionally, there were two patients used as a control for the pepsin test that had laryngotracheal separation and tracheal gastric separation. Patient demographic data and clinical characteristics were collected during a medical record review. Those patients excluded from the study did not have an adequate fluid specimen for complete analysis, or the presence of pulmonary disease in a patient undergoing elective surgery.
BAL specimens were obtained by flexible bronchoscopy under general anesthesia from each subject patients. Lavage fluid specimens were also obtained from control patients during an unrelated surgical procedure. After intubation, one milliliter of normal saline was infused through the endotracheal tube and immediately suctioned. For study patients undergoing a diagnostic bronchoscopy, a portion of the aspirated fluid during the procedure was used. Each specimen was assigned a code to correlate with patient's clinical data. Immediately after obtaining the specimen, it was placed on ice and transported to the research laboratory. Each specimen was mixed and divided into two samples. One sample was sent to pathologist for further LLM analysis and the other sample was snap-frozen on dry ice and stored at -80°C for Western blot analysis.
Western blot analysis for pepsin
Twenty to thirty microliters of lavage fluid was separated on a 10% sodium dodecyl sulfate polyacrylamide gel by electrophoresis (SDS-PAGE). Purified human pepsin 3b (isolated from human gastric juice by ion exchange chromatography; MCW IRB Protocol # PRO00004759)13 and human pepsinogen I (Sigma, St. Louis, MO) were run alongside clinical samples as positive and negative controls, respectively. Protein was then transferred to a polyvinylidene difluoride (PVDF) membrane (GE Healthcare, Piscataway, NJ). Blots were incubated with rabbit anti-human pepsin HU3 peptide antibody (1:350 dilution)14 and goat anti-rabbit secondary antibody conjugated to horseradish peroxidase diluted 1:5,000 (Dako, Copenhagen, Denmark). All antibodies were diluted in 5% non-fat dried milk in phosphate buffered saline with 0.1%Tween-20. Blots were exposed to enhanced chemiluminescence reagents (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) followed by radiographic exposure and development. The presence or absence of a pepsin band was recorded. The person performing the western blot for pepsin was not aware of the clinical findings including the LLM number.
Analysis of diagnostic bronchoscopy specimen
Specimens obtained during diagnostic bronchoscopy were transported to the clinical laboratory at CHW. The pathologist determined presence of LLM in each specimen. The specimens were centrifuged and cell suspensions from a portion of the bronchoscopy sample were prepared. These were stained with Oil red O stain. Under light microscopy, one pathologist counted the number of LLM was counted for each specimen. The LLM for each patient sampling were further quantified on a five-point scale as described by Corwin et al.15 The lipid laden macrophage index was also determined by combining the scores for 100 consecutive macrophages. The pathologist performing this analysis was not aware of the clinical findings of the patient or results of the pepsin analysis.
Results
A total of 76 patients met the inclusion criteria and were enrolled in the study at CHW. Demographic data of enrolled patients is summarized in Table 1. Sixty-one percent were male and 39% were female with age of subjects ranging from 4.5 months to 24 years old, with the average age at 6.5 years old. There were two patients older than 18 years of age in the tracheostomy group included in the study as these patients had congenital disease processes that contributed to the long-term tracheostomy dependency and development of chronic lung disease. Of the 76 patients, 34 patients underwent bronchoscopy, 31 patients had a tracheostomy and there were 11 total control patients. In regards to age, there was no statistical difference between the control group and the two study groups (p=0.989). Gender was not perfectly balanced between the bronchoscopy group and the tracheostomy group however this was not statistically significant (p=0.336). Nine patients in the control group underwent elective procedures including liver biopsy, osteotomy, toe amputation, implantation of osseointegrated bone anchored hearing device, neck mass excision, incision and drainage of preauricular cyst, and palatal reconstruction. Additionally, there was one patient that had a laryngotracheal separation and one patient that had a history of a tracheoesophageal fistula with esophageal atresia status post closure of fistula. These patients were also used as controls as they did not have a communication between the trachea and stomach to allow reflux of gastric contents.
Table 1. Patient Demographics.
| Total (n=76) | Control (n=11) | Bronchoscopy group (n=34) | Tracheostomy group (n=31) | ||
|---|---|---|---|---|---|
| Gender | Male | 46 (61%) | 6 (55%) | 18 (53%) | 22 (71%) |
| Female | 30 (39%) | 5 (45%) | 16 (47%) | 9 (29%) | |
| Average age of patients (years) | 6.5 | 6 | 6.5 | 6.6 | |
| Average BMI percentile | 63% | 63% | 54% | 75% | |
Abbreviations: BMI-body mass index
Of the patients in the bronchoscopy group, 14 patients had chronic respiratory symptoms, including recurrent wheezing, chronic cough, shortness of breath and underwent a bronchoscopy for an airway evaluation, 10 patients had recurrent pneumonias, and the remaining four patients for other indications. One patient did not have a reason recorded. Other indications included two patients with history of bilateral lung transplant and the remaining patients had an abnormal lung exam andhemoptysis. Of the patients in the tracheostomy group, 17 patients had a tracheostomy performed for an anatomic abnormality including bilateral vocal cord paralysis, upper airway obstruction, subglottic stenosis, and facial trauma. Nine patients had tracheostomy for neuromuscular disease. Seven patients had tracheostomy performed for other reasons including chronic lung disease, central hypoventilation syndrome, hypoxic ischemic encephalopathy, apnea and pulmonary hemorrhage.
Additional clinical features for these patients were also investigated and obtained during retrospective chart review, as shown in Table 2. Half of the patients in the bronchoscopy group were being treated for gastroesophageal reflux with five (15%) on H2 blockers (two of which were pepsin positive in BAL sample), 12 (35%) on proton pump inhibitors (PPIs) (10 of which were pepsin positive in BAL sample), and three (9%) on metoclopramide (all of which were pepsin positive in BAL sample). More than half of the patients in the tracheostomy group were on anti-reflux medications, with five (17%) on H2 blockers, three of which had pepsin detected in BAL, and 14 (45%) on PPIs, 12 of which had pepsin detected in BAL. Few patients had a documented swallow study performed, five in the bronchoscopy group and 15 in the tracheostomy group. Of these swallow studies performed, only one in the bronchoscopy group and three in the tracheostomy group demonstrated aspiration. The majority of patients in both groups were on bronchodilators for control of respiratory symptoms. One patient in the bronchoscopy group had a Nissen fundoplication performed, and this sample was pepsin positive. Five of eight patients in the tracheostomy group had a Nissen fundoplication performed were pepsin positive. Administration of steroids was also evaluated in the bronchoscopy group of patients. Twenty-five out of 29 (86%) patients (five were not recorded) were on steroids, mostly on inhaled steroids. Two patients were on both inhaled and oral steroids, and both of these patients had pepsin detected in BAL specimens. There was one patient on only oral steroids, which was also a patient with a pepsin positive BAL.
Table 2. Clinical characteristics in each group.
| Bronchoscopy group (n=34) | Tracheostomy group (n=31) | |
|---|---|---|
| Number on PPI | 12 (35%) | 14 (45%) |
| Number on H2 blocker | 5 (15%) | 5 (17%) |
| Number on metoclopramide | 3 (9%) | 0 (0%) |
| Number on bronchodilators | 26 (76%) | 26 (84%) |
| Swallow studies performed | 5 (15%) | 18 (58%) |
| If swallow study, number positive for aspiration | 1 | 3 |
Abbreviations: PPI-Proton pump inhibitor
All patients had a western blot analysis of a BAL performed for pepsin. Of the control patients, all samples were negative for pepsin. Of the bronchoscopy group, 25 patients had positive pepsin samples. Twenty-two patients in the tracheostomy group had pepsin positive samples. LLM data was also collected. Seven control patients had LLM noted in BAL samples, 31 patients in the bronchoscopy group had LLM detected and 16 patients in the tracheostomy group. Results of western blot and LLM data are displayed in Table 3.
Table 3. Pepsin and LLM results from lavage fluid specimen for each group.
| Total (n=76) | Control (n=11) | Bronchoscopy group (n=34) | Tracheostomy group (n=31) | |
|---|---|---|---|---|
| Pepsin positive samples | 47 (62%) | 0 (0%) | 25 (74%) | 22 (71%) |
| LLM positive samples | 54 (71%) | 7 (64%) | 31 (91%) | 16 (52%) |
Abbreviations: LLM-Lipid laden macrophages
ROC analysis predicting patient group (control versus bronchoscopy group or tracheostomy group) from LLM data was performed. When initially looking at the presence of any LLM or pepsin in the specimens, there is a moderate agreement between LLM and pepsin (kappa=0.27, p < 0.05), and there is no apparent agreement among controls (kappa = -0.2, p>0.05). When looking at their ability to predict control versus at-risk patients, both LLM and pepsin have reasonable AUC (area under the ROC curve), 0.53 and 0.86, respectively, which are not significantly different from each other. However when quantifying LLM as the number of LLM among all macrophages in the specimen, there was no agreement between LLM and pepsin and LLM are not predictive of patients undergoing bronchoscopy or with tracheostomy. Additionally, LLM data was not predictive of pepsin status when this quantification was performed. (Figure 1)
Figure 1. Predictive power of pepsin vs. LLM.
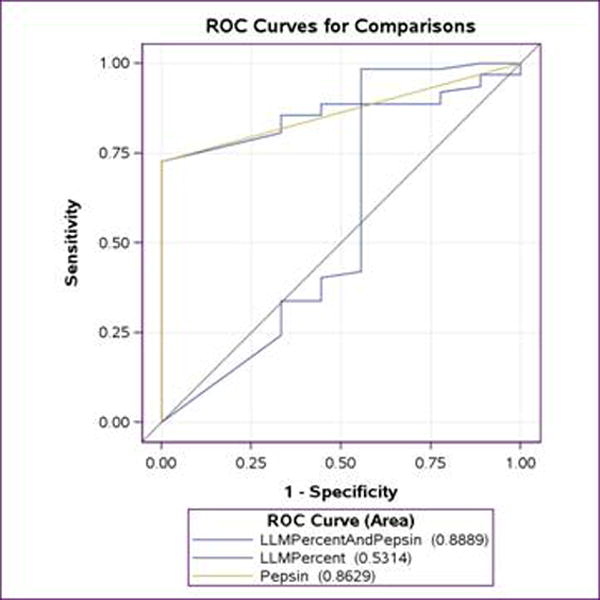
ROC analysis was performed to evaluate the ability of pepsin and LLM (as quantified as percentage of LLM among all macrophages) to predict the at-risk group compared to control group.
Discussion
AERD may have implications on our understanding and approach to patients with chronic respiratory symptoms and those with chronic lung disease. Using pepsin as a marker for AERD demonstrated a high prevalence of AERD in our cohort of patients with chronic respiratory symptoms and tracheostomy, with more than 70% of the patients in the two groups having pepsin detected in the BAL specimen. Our data would suggest that the frequency of AERD is likely underestimated in patients with disease patterns similar to our study cohort, and it may have a stronger role in chronic pulmonary disease than previously recognized. Prior studies have begun to investigate the role of silent aspiration in some patient groups. Gopalareddy et al.16 identified a high rate of silent aspiration in critically ill pediatric patients requiring mechanical ventilation in the intensive care unit. Additionally, Krishnan et al.17 demonstrated a high correlation of patients with respiratory disease and GER to have pepsin in tracheal secretions. However, using conventional methods, detection of “silent” aspiration has been difficulty and warrants the development of new techniques.
Previously, quantification of LLM in a BAL specimen has been a proposed measurement tool to detect aspiration in patients. Varying and conflicting data regarding the reliability of this test has been reported in the literature.3,4,7,9,18 The LLM are not necessarily exogenous and may be a measurement of phospholipid degradation from pulmonary inflammation or infection. Additionally a previous study demonstrated the presence of LLM in control patients, further emphasizing the nonspecific nature of this marker.19 Although further study is needed to confirm, our findings would suggest analysis of LLM has substantial potential for false positive tests. Our data would implicate that analysis of LLM misses true positive patients with AERD. As such, the positive predictive value (true positive/(true positive + false positive)) of the LLM test would appear to be quite poor.
Conversely, pepsin is shown to be a superior test; it is purely exogenous unlike LLM. In this study, all controls were negative for this test, which is consistent with our knowledge of pepsin in physiologic conditions. Pepsin is only produced in the stomach by gastric chief cells and thus its' presence in BAL fluid indicates extra-esophageal reflux/aspiration. Our anti-human pepsin can be used to discriminate between pepsin and pepsinogen, allowing pepsin to be used reliably to detect extra-esophageal reflux/aspiration. Fisichella and colleagues also noted similar results in a prior study while evaluating the effectiveness of laparoscopic antireflux surgery in lung transplant patients by measuring the presence of pepsin in the BAL fluid specimens of these patients. These results were compared with BAL specimens from 11 healthy controls patients; all specimens from the control patients were negative for the presence of pepsin.12 Prior studies have also supported its' use as a measurement of aspiration.5,6,17
Farrell et al.6 looked at a group of patients with proven macroscopic aspiration event with significantly higher level of pepsin compared to negative controls. Also a significantly higher level of patients with proximal GER had pepsin positive samples and cough related symptoms. Thus supporting the negative impact of refluxate after exposure to the respiratory epithelium leading to disease.
The suggested high prevalence of AERD in this study cohort has the potential for important management decisions for these patients. Prior investigations of the effects of reflux on hypopharyngeal and laryngeal structures have shown that pepsin may be a damaging agent to the laryngeal epithelium. Pepsin, from laryngopharyngeal reflux, was shown to have a negative impact on the defense mechanisms of laryngeal epithelium including decreased levels of laryngeal carbonic anhydrase III and Sep 70 protective proteins.20 There may be additional damage occurring in the respiratory epithelium as well. Animal studies have also shown an impact of pepsin on cytokine expression and airway remodeling.21
The frequency of silent aspiration in our patients with chronic respiratory symptoms may have further implications on diagnosis and treatment options. While looking at the clinical data, only half of the patients were on a form of anti-reflux medical therapy. And those that may have been on medications, continued to have pepsin detected in the BAL specimen. Pepsin at neutral pH was shown to retain its' original activity and ability to be reactivated with drop in pH during repeat reflux event or when taken up into acidic intracellular environment.20 Silent aspiration in these patients may contribute to worsening pulmonary function and understanding its impact has the potential to change both medical and surgical interventions for these often medically fragile patients.
Additionally, it was interesting to look at the pepsin results of our subpopulation of patients that previously had a Nissen fundoplication performed. It was surprising that six out of nine of these patients had pepsin detected in the lavage fluid specimens despite having a Nissen fundoplication. When further reviewing the records of these six patients, four of them had return of reflux symptoms after the fundoplication and were continued on a proton pump inhibitor. However, this finding is supported by several studies in the literature reporting high failure rates (60-70%).22 Vakil et al. reported reflux symptoms in 67% patients after surgery.23 In a large controlled study, Spechler et al. found 62% of adults were taking proton pump inhibitor medicationsfor reflux symptoms at a seven year follow up after anti-reflux surgery.24 Postoperative outcome measurements are also not always objective and consistent, therefore determining the success of surgery sometimes difficult.25
While our results demonstrate a high prevalence of pepsin positive samples, we were unable to identify any specific clinical factors that may predict those patients that are more likely to have silent aspiration events. In the absence of any specific factors, pepsin testing of BAL samples may be a feasible test, which appears to have a very high positive predictive value, to further identify those patients.
There are several limitations noted in our study. First, when evaluating the technique to determine presence of pepsin in the BAL specimens, the amount of pepsin identified was not further quantified. Therefore the severity of the aspiration-associated reflux in patients is unable to be further defined by this test. The amount of reflux that would cause lung pathology is not understood. Additionally, the time frame of pepsin to be detectable from the BAL is not known, and there may be more false negative tests in patients with intermittent AERD.
Conclusion
There is a high prevalence of AERD in pediatric patients with chronic respiratory symptoms and tracheostomy. Clinically, the impact of this finding is likely underestimated. Pepsin is a reliable biomarker to detect AERD and practical to perform in the clinical setting. Using this test may allow improved recognition of AERD in patients and lead to more focused management.
Acknowledgments
We thank Aniko Szabo, PhD, for biostatistics consultation and Tina L. Samuels, MS, for assistance in data collection and analysis.
Funding/Support: Children's Hospital and Health System Foundation provided funding for the collection, management, and analysis of the data. The statistical support and interpretation of the data is provided, in part, by grant 1UL1RR031973 from the clinical and translational Science Award program of the national center for research resources, National Institutes of Health.
Footnotes
Presented at the American Society of Pediatric Otolaryngology annual meeting in Arlington, VA on April 26, 2013.
Disclosures: Dr. Nikki Johnston is a consultant for Koufman Diagnostics LLC.
References
- 1.Trinick R, Johnston N, Dalzell AM, McNamara PS. Reflux aspiration in children with neurodisability--a significant problem, but can we measure it? J Pediatr Surg. 2012;47(2):291–298. doi: 10.1016/j.jpedsurg.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 2.Pacheco-Galván A, Hart SP, Morice AH. Relationship between gastro-oesophageal reflux and airway diseases: the airway reflux paradigm. Arch Bronconeumol. 2011;47(4):195–203. doi: 10.1016/j.arbres.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Reilly BK, Katz ES, Misono AS, et al. Utilization of lipid-laden macrophage index in evaluation of aerodigestive disorders. Laryngoscope. 2011;121(5):1055–1059. doi: 10.1002/lary.21467. [DOI] [PubMed] [Google Scholar]
- 4.Ahrens P, Noll C, Kitz R, et al. Lipid-laden alveolar macrophages (LLAM): a useful marker of silent aspiration in children. Pediatr Pulmonol. 1999;28(2):83–88. doi: 10.1002/(sici)1099-0496(199908)28:2<83::aid-ppul2>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 5.Meert KL, Daphtary KM, Metheny NA. Detection of pepsin and glucose in tracheal secretions as indicators of aspiration in mechanically ventilated children. Pediatr Crit Care Med. 2002;3(1):19–22. doi: 10.1097/00130478-200201000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Farrell S, McMaster C, Gibson D, Shields MD, McCallion WA. Pepsin in bronchoalveolar lavage fluid: a specific and sensitive method of diagnosing gastro-oesophageal reflux-related pulmonary aspiration. J Pediatr Surg. 2006;41(2):289–293. doi: 10.1016/j.jpedsurg.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Kitz R, Boehles HJ, Rosewich M, Rose MA. Lipid-Laden Alveolar Macrophages and pH Monitoring in Gastroesophageal Reflux-Related Respiratory Symptoms. Pulm Med. 2012;2012:673637. doi: 10.1155/2012/673637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boesch RP, Daines C, Willging JP, et al. Advances in the diagnosis and management of chronic pulmonary aspiration in children. Eur Respir J. 2006;28(4):847–861. doi: 10.1183/09031936.06.00138305. [DOI] [PubMed] [Google Scholar]
- 9.Furuya MEY, Moreno-Córdova V, Ramírez-Figueroa JL, et al. Cutoff value of lipid-laden alveolar macrophages for diagnosing aspiration in infants and children. Pediatr Pulmonol. 2007;42(5):452–457. doi: 10.1002/ppul.20593. [DOI] [PubMed] [Google Scholar]
- 10.Badellino MM, Buckman RF, Malaspina PJ, et al. Detection of pulmonary aspiration of gastric contents in an animal model by assay of peptic activity in bronchoalveolar fluid. Crit Care Med. 1996;24(11):1881–1885. doi: 10.1097/00003246-199611000-00019. [DOI] [PubMed] [Google Scholar]
- 11.Lee JS, Song JW, Wolters PJ, et al. Bronchoalveolar lavage pepsin in acute exacerbation of idiopathic pulmonary fibrosis. Eur Respir J. 2012;39(2):352–358. doi: 10.1183/09031936.00050911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisichella PM, Davis CS, Lundberg PW, et al. The protective role of laparoscopic antireflux surgery against aspiration of pepsin after lung transplantation. Surgery. 2011;150(4):598–606. doi: 10.1016/j.surg.2011.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crapko M, Kerschner JE, Syring M, Johnston N. Role of extra-esophageal reflux in chronic otitis media with effusion. Laryngoscope. 2007;117(8):1419–1423. doi: 10.1097/MLG.0b013e318064f177. [DOI] [PubMed] [Google Scholar]
- 14.Johnston N, Knight J, Dettmar PW, Lively MO, Koufman J. Pepsin and carbonic anhydrase isoenzyme III as diagnostic markers for laryngopharyngeal reflux disease. Laryngoscope. 2004;114(12):2129–2134. doi: 10.1097/01.mlg.0000149445.07146.03. [DOI] [PubMed] [Google Scholar]
- 15.Corwin RW, Irwin RS. The lipid-laden alveolar macrophage as a marker of aspiration in parenchymal lung disease. Am Rev Respir Dis. 1985;132(3):576–581. doi: 10.1164/arrd.1985.132.3.576. [DOI] [PubMed] [Google Scholar]
- 16.Gopalareddy V, He Z, Soundar S, et al. Assessment of the prevalence of microaspiration by gastric pepsin in the airway of ventilated children. Acta Paediatr. 2008;97(1):55–60. doi: 10.1111/j.1651-2227.2007.00578.x. [DOI] [PubMed] [Google Scholar]
- 17.Krishnan U, Mitchell JD, Messina I, Day AS, Bohane TD. Assay of tracheal pepsin as a marker of reflux aspiration. J Pediatr Gastroenterol Nutr. 2002;35(3):303–308. doi: 10.1097/00005176-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Reid-Nicholson M, Kulkarni R, Adeagbo B, Looney S, Crosby J. Interobserver and intraobserver variability in the calculation of the lipid-laden macrophage index: implications for its use in the evaluation of aspiration in children. Diagn Cytopathol. 2010;38(12):861–865. doi: 10.1002/dc.21298. [DOI] [PubMed] [Google Scholar]
- 19.Knauer-Fischer S, Ratjen F. Lipid-laden macrophages in bronchoalveolar lavage fluid as a marker for pulmonary aspiration. Pediatr Pulmonol. 1999;27(6):419–422. doi: 10.1002/(sici)1099-0496(199906)27:6<419::aid-ppul9>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 20.Johnston N, Dettmar PW, Bishwokarma B, Lively MO, Koufman JA. Activity/stability of human pepsin: implications for reflux attributed laryngeal disease. Laryngoscope. 2007;117(6):1036–1039. doi: 10.1097/MLG.0b013e31804154c3. [DOI] [PubMed] [Google Scholar]
- 21.Chiu HY, Chen CW, Lin HT, et al. Study of gastric fluid induced cytokine and chemokine expression in airway smooth muscle cells and airway remodeling. Cytokine. 2011;56(3):726–731. doi: 10.1016/j.cyto.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 22.Hassall E. Outcomes of fundoplication: causes for concern, newer options. Arch Dis Child. 2005;90(10):1047–1052. doi: 10.1136/adc.2004.069674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vakil N, Shaw M, Kirby R. Clinical effectiveness of laparoscopic fundoplication in a U.S. community. Am J Med. 2003;114(1):1–5. doi: 10.1016/s0002-9343(02)01390-6. [DOI] [PubMed] [Google Scholar]
- 24.Spechler SJ, Lee E, Ahnen D, et al. Long-term outcome of medical and surgical therapies for gastroesophageal reflux disease: follow-up of a randomized controlled trial. JAMA. 2001;285(18):2331–2338. doi: 10.1001/jama.285.18.2331. [DOI] [PubMed] [Google Scholar]
- 25.Contini S, Scarpignato C. Evaluation of clinical outcome after laparoscopic antireflux surgery in clinical practice: still a controversial issue. Minim Invasive Surg. 2011;2011:725472. doi: 10.1155/2011/725472. [DOI] [PMC free article] [PubMed] [Google Scholar]


