Abstract
Whale carcasses create remarkable habitats in the deep-sea by producing concentrated sources of organic matter for a food-deprived biota as well as places of evolutionary novelty and biodiversity. Although many of the faunal patterns on whale falls have already been described, the biogeography of these communities is still poorly known especially from basins other than the NE Pacific Ocean. The present work describes the community composition of the deepest natural whale carcass described to date found at 4204 m depth on Southwest Atlantic Ocean with manned submersible Shinkai 6500. This is the first record of a natural whale fall in the deep Atlantic Ocean. The skeleton belonged to an Antarctic Minke whale composed of only nine caudal vertebrae, whose degradation state suggests it was on the bottom for 5–10 years. The fauna consisted mainly of galatheid crabs, a new species of the snail Rubyspira and polychaete worms, including a new Osedax species. Most of the 41 species found in the carcass are new to science, with several genera shared with NE Pacific whale falls and vent and seep ecosystems. This similarity suggests the whale-fall fauna is widespread and has dispersed in a stepping stone fashion, deeply influencing its evolutionary history.
Whale carcasses are considered the largest organic inputs reaching the deep ocean floor in a single event. Carcasses attract a suite of opportunistic and specialist organisms (see1 for a review) that feast on the flesh and lipid-rich bones. Specialized organisms have been evolving in these habitats for millions of years since the appearance of large ocean-going whales and other vertebrates before them1,2,3,4,5,6,7. Whale falls can thus be considered as sources of evolutionary novelty and biodiversity in the deep-sea, since they form isles of organic enrichment and biodiversity in an extremely food-limited environment1,8.
The degradation process of a whale carcass can pass through several overlapping successional stages1,9,10,11,12. During the first stages, necrophages/scavengers remove soft tissues while high densities of opportunists colonize both bones and surrounding sediments. The “sulfophilic stage” occurs when anaerobic microbial degradation of organic-enriched sediments and the lipid-rich skeleton create high fluxes of reduced compounds, which allow the development of a chemosynthesis-based community11,12,13,14,15. This stage shows faunal overlap with other deep-sea chemosynthetic communities, such as hydrothermal vents, cold seeps and wood falls1,10,12,16,17,18,19,20.
Based on this faunal overlap, Smith et al.16 theorized that whale falls may act as stepping-stones for faunal dispersal among different chemosynthetic communities, and could contribute to the colonization of new habitats separated by hundreds of kilometers (e.g. hydrothermal vents). In addition, this theory has also deep evolutionary implications. For instance, some of the most abundant symbiont-bearing invertebrates, such as mytilid mussels, evolved from shallow waters probably using organic-fall islands as dispersal stepping stones1,21,22,23,24,25,26,27.
Despite the importance of evolutionary and ecological relationships among biological communities at different deep-sea chemosynthetic habitats, the biodiversity and biogeography of hydrothermal vents and cold seeps has been by far much more studied. Whale falls are likely to occur worldwide along whale migratory routes as well as in whale breeding and feeding areas16,20. However, only 7 natural whale carcasses have been studied in detail in the deep-sea since 198916,20,28,29,30,31 (although many more have been observed or remotely sampled1). In consequence, the advancement in the understanding of these poorly known communities has been mainly due to time-series studies of artificially implanted whale carcasses on the seafloor1,20,32,33,34,35,36.
Most natural and implanted deep-sea whale-fall community studies are from the deep Northeast Pacific Ocean, specifically from the California slope and Monterey Canyon20,29,30. Consequently, the paucity of studies on whale falls as well as the scarce data available beyond the Northeast Pacific make biogeographic and evolutionary syntheses of both whale-fall fauna, and other related chemosynthetic communities, challenging1.
Here we describe the community composition of the first whale carcass found in the deep Atlantic Ocean (off the S-SE Brazilian continental margin). We show that, although separated by thousands of kilometers, this abyssal Southwest Atlantic whale fall is inhabited by many lineages previously only found in the Pacific chemosynthesis-based communities. In addition, many other chemosynthetically-related genera have their bathymetric and latitudinal ranges expanded. The findings reported here have deep implications for the poorly known biogeography of deep-sea whale-fall communities and suggest a worldwide distribution for some whale-fall specialists.
Results
Physico-chemical characteristics of the study site and whale carcass description
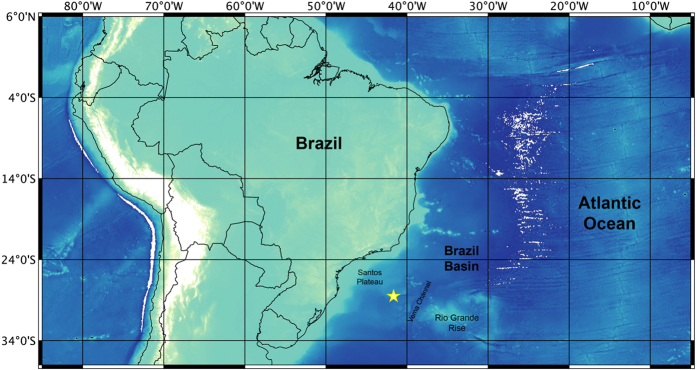
The whale fall was located ca. 700 km from the Brazilian coast at the base of the São Paulo Ridge (SPR; 28° 31.1191′ S, 41° 39.4097′ W) at a depth of 4204 m (Fig. 1). The surrounding area was characterized by a thin layer (<20 cm) of sediments overlying basaltic rocks. During our study, the area was under the influence of the Antarctic Bottom Water (AABW)37 with a temperature of 0.4 °C and salinity 34.7.
Figure 1. Location of the whale carcass found at the base of São Paulo Ridge at 4204 m depth.
The map was created using the QGIS software, bathymetric data from CleanTOPO2 (http://www.shadedrelief.com/cleantopo2/index.html) and Word borders from Thematic Mapping (http://thematicmapping.org/downloads/world_borders.php). QGIS Development Team, 2015. QGIS Geographic Information System. Open Source Geospatial Foundation Project. http://qgis.osgeo.org. The World Borders Dataset and the data obtained from the QGIS Open Source Geospatial Foundation Project is licensed under the Attribution-Share-Alike 3.0 Unported license. The license terms can be found on the following link: http://creativecommons.org/licenses/by-sa/3.0/.
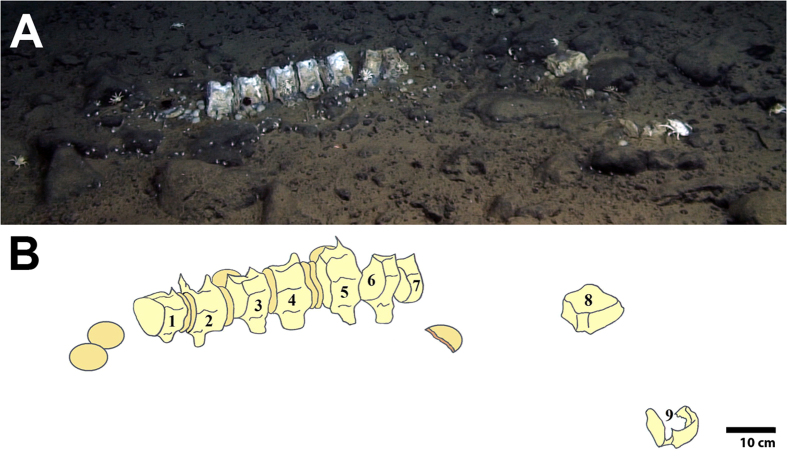
Mitochondrial COI analysis revealed that the carcass belonged to an Antarctic Minke whale (Balaenoptera bonaerensis) (99% identity). The sequence was deposited in the DNA Databank of Japan (DDBJ) under the accession number LC106302. This partial carcass was composed of nine small vertebrae, seven of which were standing side by side. Among those, five vertebrae were loosely joined by intervertebral discs (vertebrae 1–5) (Fig. 2). Additionally, five intervertebral discs were scattered around the skeleton. No soft tissues were present on the bones, which were all exposed to the surrounding water (i.e. not covered by sediment). All vertebrae were similar in shape and dimensions (ca. 11.5 cm in diameter) and their anatomical characteristics suggest they belong to the caudal portion of the animal. The sediment underneath bones and discs was dark in color suggesting anoxia.
Figure 2. Partial Antarctic Minke whale skeleton (Balaenoptera bonaerensis) found at 4204 m in the Southwest Atlantic Ocean using the manned submersible Shinkai 6500.
(A) Caudal vertebrae lying on a thin layer of fine sediment over basaltic rocks; (B) Schematic view of the whale skeleton reconstructed from Shinkai 6500 videos. The nine vertebrae are numbered and shown in pale yellow color, while the round intervertebral discs are darker. Vertebrae were numbered from the posterior end of the animal towards the head.
Qualitative and quantitative analysis of the macrofauna assemblage and species distributions
Only epifaunal organisms larger than ca. 5 mm could be identified and counted in videos. Five phyla were recovered from the study area comprising at least 41 species (Table 1). Nematoda occurred in large numbers both inside bones and in the surrounding sediments and may be represented by more than one species. Nematodes are currently being quantified and will be treated in detail in a later publication.
Table 1. Species collected at the SW Atlantic whale fall site at 4204 m depth.
| Phylum | Class | Order | Family | Species or tag name | Number of Individuals* | Location |
|---|---|---|---|---|---|---|
| Cnidaria | Anthozoa | Actiniaria | n.d. | Cnidaria sp. | n.d. | Rocks |
| Annelida | Polychaeta | Aciculata | Dorvilleidae | Ophryotrocha spp.** | n.d. | Bone/Sediment |
| Annelida | Polychaeta | Aciculata | Nereididae | Neanthes sp. nov.† | n.d. | Sediment |
| Annelida | Polychaeta | Canalipalpata | Ampharetidae | cf. Grassleia sp. | 40 | Sediment |
| Annelida | Polychaeta | Canalipalpata | Chaetopteridae | Spiochaetopterus sp. | n.d. | Sediment |
| Annelida | Polychaeta | Canalipalpata | Cirratulidae | Raphidrilus sp. | n.d. | Bone |
| Annelida | Polychaeta | Canalipalpata | Cirratulidae | Tharyx sp. | n.d. | Sediment |
| Annelida | Polychaeta | Canalipalpata | Spionidae | Lindaspio sp. nov. | n.d. | Bone |
| Annelida | Polychaeta | Canalipalpata | Spionidae | Prionospio sp. | n.d. | Sediment |
| Annelida | Polychaeta | Capitellida | Capitellidae | Capitella iatapiuna‡ | n.d. | Bone/Sediment |
| Annelida | Polychaeta | Phyllodocida | Hesionidae | Hesiocaeca sp. nov.∆ | n.d. | Bone/Sediment |
| Annelida | Polychaeta | Phyllodocida | Hesionidae | Microphthalmus sp. nov. ∆ | n.d. | Bone/Sediment |
| Annelida | Polychaeta | Phyllodocida | Hesionidae | Pleijelius sp. nov.1∆ | n.d. | Bone |
| Annelida | Polychaeta | Phyllodocida | Hesionidae | Pleijelius sp. nov.2∆ | n.d. | Bone |
| Annelida | Polychaeta | Phyllodocida | Hesionidae | Vrijenhoekia sp. nov. ∆ | n.d. | Bone/Sediment |
| Annelida | Polychaeta | Phyllodocida | Polynoidae | Polynoidae | 18*** | Bone/sediment |
| Annelida | Polychaeta | Phyllodocida | Polynoidae | Polynoidae sp. | n.d. | Bone/sediment |
| Annelida | Polychaeta | Phyllodocida | Polynoidae | Bathykurila cf. guaymasensis | n.d. | Bone/sediment |
| Annelida | Polychaeta | Phyllodocida | Polynoidae | Bathyfauvelia sp. | n.d. | Bone/sediment |
| Annelida | Polychaeta | Phyllodocida | Sigalionidae | Sigalionidae | n.d. | Sediment |
| Annelida | Polychaeta | Phyllodocida | Sphaerodoridae | Sphaerodoropsis sp. nov.☆ | n.d. | Sediment |
| Annelida | Polychaeta | Phyllodocida | Chrysopetalidae | Vigtorniella sp. | n.d. | Bone |
| Annelida | Polychaeta | Sabellida | Siboglinidae | Osedax sp. nov.⨂ | 98 | Bone |
| Arthropoda | Malacostraca | Amphipoda | Uristidae | Stephonyx sp. | 17 | Bone/sediment |
| Arthropoda | Malacostraca | Isopoda | n.d. | Epicaridea sp. | n.d. | Sediment |
| Arthropoda | Malacostraca | Decapoda | Munidopsidae | Munidopsis spp. | 295 | Bone/sediment |
| Arthropoda | Maxillopoda | n.d. | n.d. | Copepoda sp.1 | n.d. | Parasitic on Osedax |
| Arthropoda | Maxillopoda | Harpacticoida | n.d. | Copepoda sp.2 | n.d. | Bone |
| Arthropoda | Maxillopoda | Cyclopoida | n.d. | Copepoda sp.3 | n.d. | Bone |
| Nematoda | Chromadorea | Monhysterida | Xyalidae | Theristus sp. | n.d. | Bone |
| Mollusca | Bivalvia | Nuculanoida | Malletiidae | Malletia sp. | n.d. | Sediment |
| Mollusca | Gastropoda | unassigned | unassigned | Rubyspira sp. nov.▼ | 52 | Sediment |
| Mollusca | Gastropoda | Neogastropoda | Raphitomidae | Gastropoda sp. | 20 | Bone epifaunal |
| Echinodermata | Echinoidea | indet. | indet. | Echinoidea sp. | 2 | Bone epifaunal |
| Echinodermata | Ophiuroidea | indet. | indet. | Ophiuroidea sp. | n.d. | Sediment |
Each species is assigned to a location within the habitat. *Only for organisms that could be counted in videos. **Includes eight different species. ***Includes all three polynoid species collected. n.d. = not determined.
†Shimabukuro et al., in prep.;
‡Silva et al.38.
∆Shimabukuro et al., in prep.;
☆Shimabukuro et al., in prep.;
⨂Fujiwara et al., in prep.;
▾Fujiwara et al., in prep.
Polychaetes were the most speciose taxon on both whale bones and soft sediments, with at least 28 species (≈68%), most of which are probably new to science. Among these was a new species of the bone-eating worm Osedax (Fig. 3C,D). We found at least eight morphotypes of the dorvilleid Ophryotrocha and the new species Capitella iatapiuna38 boring into the bones, with the latter also found inhabiting the surrounding sediment sampled with a slurp gun. Three species of polynoid polychaetes, indistinguishable in video analyses, occurred on the surface of bones and sediments (Figs 3E and 4H), with a higher abundance on the former. Interestingly, antagonistic behavior could be observed in videos, where two polynoids were fighting, possibly for space or food resources (see supplemental video material). Five species of Hesionidae (Hesiocaeca sp. nov., Microphthalmus sp. nov., Pleijelius sp. nov. 1 and 2 and Vrijenhoekia sp. nov.) (Fig. 4I) and two species of cirratulids (Raphidrilus and Tharyx) were also present in both sediment and bone, except for both species of Pleijelius which were found only on bones. Another important species occurring in bones was the chrysopetalid Vigtorniella.
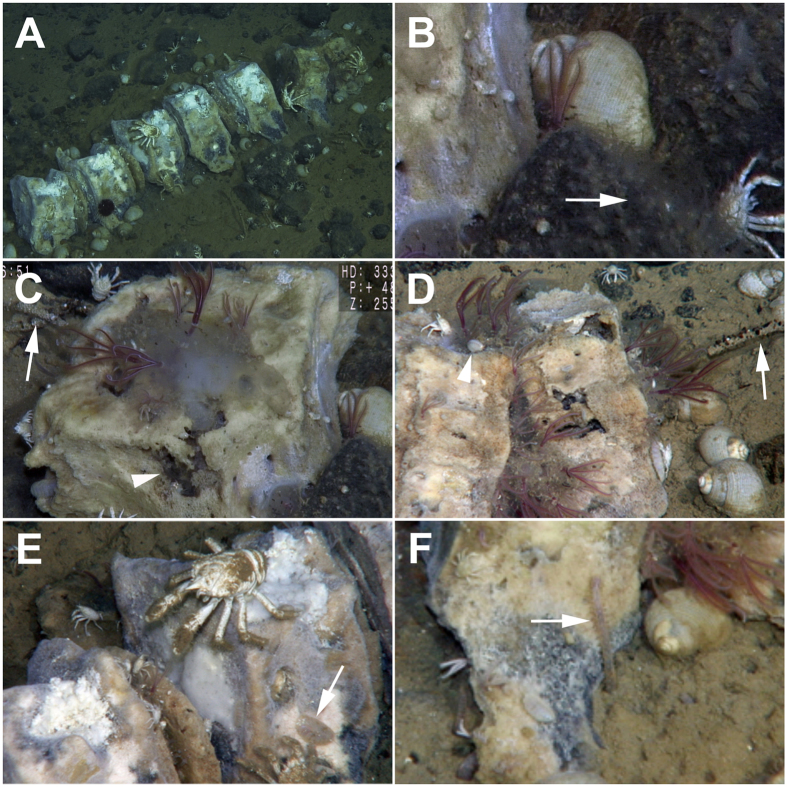
Figure 3. Distribution of epifauna on the whale fall and surrounding sediments and rocks.
A) General view of the SW Atlantic whale carcass vertebrae 1–7. Note the abundant fauna and the bacterial mats on vertebrae 1–5. A dark echinoid (Echinoidea sp. 1) can be seen on top of vertebra 2; (B) Black basaltic rocks around the whale fall were heavily colonized by dense carpets of anemones (arrow) (up to 10 ind. cm−2). The large gastropod Rubyspira sp. nov. lies behind the red palps of Osedax sp. nov. Note also the small unidentified gastropods attached to the bone; (C) Red palps and gelatinous tubes of several Osedax sp. nov. in vertebra 8. Note the ampharetid polychaete tubes (arrow) and the bone degraded area (arrowhead); (D) Clusters of Osedax sp. nov. in vertebrae 6 and 7. On the surrounding sediment, Rubyspira sp. nov. and a tube of an ampharetid polychaete (arrow). The small lysianassoid amphipod Stephonix sp. lies on the top of the bone (arrowhead); (E) Dense bacterial mats covering vertebrae 2 and 3. Here we can see the small and the large Munidopsis and a polynoid polychaete (arrow); (F) The eyeless nereid polychaete Neanthes sp. nov. climbing the surface of vertebra 6 (arrow).
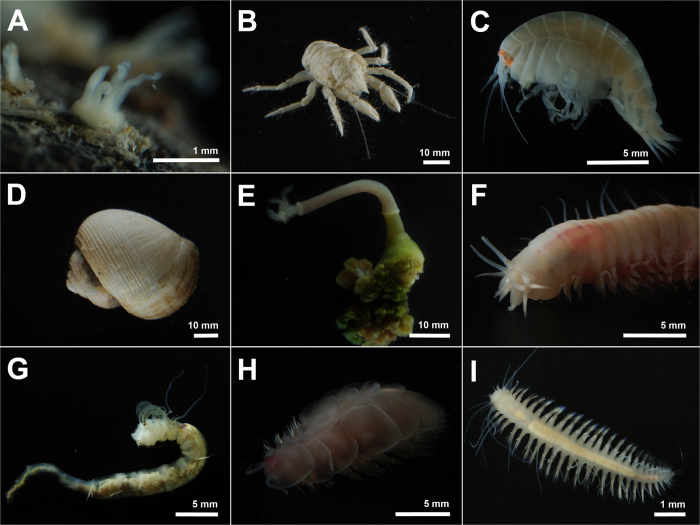
Figure 4. Some of the most abundant organisms collected at the 4204 m depth whale fall in the São Paulo Ridge, Southwest Atlantic Ocean.
(A) Unidentified sea anemone inhabiting the rocks surrounding the whale skeleton; (B) Large Munidopsis sp.; (C) The amphipod Stephonix sp.; (D) Rubyspira sp. nov.; (E) Osedax sp. nov.; (F) Neanthes sp. nov.; (G) cf. Grassleia sp.; (H) Bathykurila cf. guaymasensis; (I) Vrijenhoekia sp. nov.
Some polychaetes were found exclusively in sediments surrounding the bones. Among them, one species of Ampharetidae resembling the genus Grassleia (Figs 3C,D and 4G) and a new species of eyeless nereid from the genus Neanthes were abundant (Figs 3F and 4F). Ampharetids dwelt in tubes that were widespread in sediments close to the bones and were only less abundant in videos than Osedax sp. nov. (Table 1) (Fig. 3C,D). Neanthes sp. nov. could not be counted in videos, however it was observed in videos in the anoxic sediment under bones and intervertebral discs coming out the sediment and climbing the bones, without totally leaving its gallery of burrows (Fig. 3F) (supplemental video material).
Among mollusks, a new species of the abyssochrysoid gastropod Rubyspira was present in large numbers (Figs 3B,D,F and 4D) and individuals were quite large in size, attaining up to 3–4 cm in length. The other gastropod was a small species found on the surface of bones (Fig. 3B). Preliminary molecular data place this small gastropod in the family Raphitomidae (Conoidea). Around the skeleton we also found many large empty shells of Rubyspira. No empty shells of the small unidentified gastropod were registered.
Seven species of crustaceans occurred on bones and surrounding sediments (Table 1), including a species of copepod parasitic on Osedax. Munidopsis spp. were found in large numbers being widely distributed up to 1 m away from the carcass (Figs 2, 3E, and 4B). We found two morphotypes of Munidopsis, one large and one small, which probably represent different species. A total of 295 individuals of both species were counted in videos (Table 1) and observations suggest these organisms feed on bacterial mats (supplemental video material). However, some of the galatheid crabs were also seen processing sediments in their mouthparts. One ovigerous female of the large morphotype was collected, which suggests that at least one of the galatheid species is reproducing on site. The amphipod Stephonix sp. (Figs 3D and 4C) occurred mainly on bones, frequently coming out of the bones or entering into cracks and holes in degraded areas of bones probably produced by Osedax activity (supplemental video material).
A small species of anemone (polyps ca. 1–2 mm in size) was observed forming extensive carpets of thousands of polyps on rocks around the carcass (Figs 3B and 4A). It was probably the most abundant epifaunal organism, with photographs suggesting a density of ca. 10 ind. cm−2. However, these anemones could not be counted since they could not be resolved in video analyses due to their small size. This anemone was not observed on rocks far from bones.
Discussion
We find a close affinity between the SW Atlantic whale fall fauna with that of the NE Pacific, especially with genera found in the Monterey Canyon and off southern California9,12,36. We also found a large generic overlap with other chemosynthetic ecosystems. These findings have deep implications for the almost unknown biogeography of whale-fall communities and contrast/conform with patterns proposed for other chemosynthetic communities, such as vents and seeps.
Vent fields can be ephemeral and separated by large distances, occurring mainly along active mid-oceanic ridges and back arc spreading centers (reviewed in39). They show great endemicity and different biogeographic provinces fit well with different ocean basins and their history of geological events (reviewed in39,40). On the other hand, cold seeps may be longer lasting and widespread along all continental margins (e.g.41). These environments, however, do not present such endemicity and other factors such as depth rather than geography may better explain their faunal distributions (e.g.42,43).
Here we show for the first time an inter-basin distribution for many whale-fall specialists and other genera previously only known to occur in other chemosynthetic-based ecosystems (i.e., vents, seeps and wood parcels). Strikingly, some of the fauna found in the present study appears to be related to that of the NE Pacific. Five genera or 12% of all genera reported in this study were previously found exclusively in the NE Pacific (see Table 2). For instance, specialists such as the gastropod Rubyspira sp. nov., the polynoids Bathyfauvelia sp. and Bathykurila cf. guaymasensis, the ampharetid Grassleia sp. and the hesionid Vrijenhoekia sp. nov. comprise genera with distribution hitherto restricted to the Pacific44,45,46,47. In fact, for Rubyspira and Vrijenhoekia this is the first record anywhere outside Monterey Canyon and expands their bathymetric ranges by more than 1300 m depth (see44,47).
Table 2. Genera reported in the present study and their previous geographic records.
| Genus | Previously known from | Habitat | Reference |
|---|---|---|---|
| cf. Grassleia | NE Pacific | Hydrothermal vents and cold seeps | 45 |
| Vrijenhoekia | NE Pacific | Whale falls | 47 |
| Bathykurila | NE Pacific | Hydrothermal vents and whale falls | 9,46,60 |
| Bathyfauvelia | NE Pacific | Abyssal plain | 61 |
| Rubyspira | NE Pacific | Whale falls | 44 |
| Hesiocaeca | NE Pacific/NE Atlantic | Cold seeps (NE Atlantic) and whale falls (NE Pacific) | 48 |
| Pleijelius | NE Atlantic | Wood falls | 49 |
| Vigtorniella | N Pacific/NE Atlantic | Whale falls | 62,63 |
| Lindaspio | NE Pacific/SW Atlantic | Hydrothermal vents (NE Pacific) and oil seeps (SW Atlantic) | 50 |
| Osedax | All Pacific/NE Atlantic/Southern Ocean | Whale falls | 1 |
Most genera are shared between the NE Pacific Ocean whale falls and hydrothermal vents and cold seeps.
There was also a substantial overlapping with vent and seep fauna, such as Grassleia, a NE Pacific vents and seep inhabitant45, and Bathykurila cf. guaymasensis, that occurs in NE Pacific vents and whale falls46. The polynoid polychaete genus Bathyfauvelia is also registered for the first time on a chemosynthethic-related habitat. Other genera present in our study area were also found in cognate communities of the Atlantic Ocean. This is the case of the new hesionid polychaetes Hesiocaeca sp. nov. (sensu48) and Pleijelius sp. nov. 1 and 2, and the spionid Lindaspio sp. nov., previously registered in NW Atlantic methane hydrates48, NW Atlantic wood-fall experiments49 and SE Atlantic oil fields50, respectively (Table 2).
These findings support the stepping-stone hypothesis of Smith et al.16 and suggest that dispersal rather than vicariance is a major driver for diversification in whale fall ecosystems (see1,51). In fact, whale falls are likely to occur worldwide although heterogeneously distributed11. Some large baleen whales, such as humpbacks, migrate from high-latitude high-productivity feeding areas to low-latitude low-productivity breeding areas along continental margins in all oceans and to some specific oceanic islands (e.g. Hawaii) (reviewed in52). In addition, other species such as sperm whales, exhibit cosmopolitan distribution and can potentially sink everywhere in the ocean, especially supplying the deep ocean at equatorial latitudes1. In some areas carcasses may be relatively close to each other, e.g., Smith et al.11 estimated average nearest neighbor distances for whale falls from gray whales to occur every 5–16 km in the NE Pacific Ocean.
Some genera of the whale fall specialists appear to have a worldwide distribution, such as the bone-eating genus Osedax. Osedax rubiplumus illustrates well this idea having been reported in both sides of the Pacific Ocean and even in the Southern Ocean, which suggests a large inter-basin connectivity1. Furthermore, the present Osedax phylogeny does not seem to fit any specific geographical or bathymetrical pattern suggesting that dispersion is an important factor for the evolution of Osedax species51,53. Mitochondrial COI data (DDBJ accession number LC106303) from the new Osedax found in the present work place it near the NE Pacific species O. frankpressi (species description currently in prep.). It is the deepest Osedax species found to date, extending the genus depth range by more than 1300 m28,54 and it is the first found in the deep Atlantic Ocean. Similarly, mtCOI studies (DDBJ accession number LC106304) cluster Rubyspira sp. nov. with the two previously described species (R. osteovora and R. goffrediae44), both of them from the Monterey Canyon (NE Pacific). The occurrence of other Pacific genera in our study, such as Vrijenhoekia and Bathykurila cf. guaymasensis, also supports the idea that many whale-fall specialist lineages may be distributed worldwide (Table 2).
Thus, it is feasible to imagine a “worldwide whale-fall corridor” along continental margins, somewhat similar to the distribution of cold-seeps, but also along equatorial areas. Both “corridors” would allow faunal dispersion by a stepping-stone dispersal mechanism. This mechanism may be important for some chemosynthetic-generalist invertebrates with worldwide distributions, such as vesicomyid clams16,55, and also for whale-fall specialists.
Methods
A whale carcass was serendipitously found at 4204 m depth in the Southwest Atlantic Ocean during a Shinkai 6500 dive in April 24, 2013. This finding is a result of the Iatá-Piúna Research Consortium, a collaborative scientific partnership between Brazil and Japan. The Iatá-Piúna research cruise comprised two legs of the around-the-world Project Quelle 2013 (Quest for the Limit of Life) of the Japan Agency for Marine-Earth Science and Technology (JAMSTEC) using R/V Yokosuka.
Video surveys and sampling were carried out during two dives of the deep-sea manned submersible Shinkai 6500 (Dives 1334 and 1336). On each dive, a detailed video survey was made, including whole community surveys and close-ups of the fauna. Owing to the small habitat size, epifaunal organisms larger that ca. 5 mm were identified to the lowest taxonomic rank possible and quantified in videos. Videos were also used to verify faunal distribution patterns along the skeleton. Images were processed using the computer program Image J56.
Whalebones were collected using the submersible manipulators and the fauna surrounding the area was retrieved using a slurp gun and maintained in local cold seawater during submersible ascent. Upon arrival on deck, bones were immediately transferred to a cold room at a constant temperature of 1 °C. Bone and sediment epifauna and infauna were sorted manually and under stereomicroscope. Samples were taken for morphological identification and molecular (deep-frozen at −80 °C and 99.5% non-denatured ethanol). In addition, samples were fixed in glutaraldehyde for SEM and TEM analyses.
Sediments were collected using a slurp gun and push corers and were used in the present work only for qualitative analysis. Sediment was fixed with 4% formalin (final concentration) in filtered seawater buffered with sodium tetraborate and stained with 0.05 gL−1. Rose Bengal dye was used to distinguish meiofauna from sediment particles. Sediment samples for metazoan meiofaunal analysis were treated according to the procedure described by57. The samples were washed over 63-μm mesh sieves. The sediment that remained on the 63-μm mesh sieve was resuspended and centrifuged for 10 min at 800 g with colloidal silica (Ludox HS40; Sigma-Aldrich, St Louis, MO, USA) to separate meiofauna and other lighter particles from mineral particles. The supernatants were transferred to flat-bottomed Petri dishes. Rose Bengal-stained organisms were then collected using an Irwin loop58, sorted into higher taxa under a binocular.
A piece of a deep-frozen vertebra was used for DNA sequencing to verify the identity of the whale skeleton. DNA was directly extracted from the bone. The vertebra sample was thoroughly washed in autoclaved and filtered seawater to eliminate surface contaminants. DNA extraction was conducted using the DNeasy Tissue Kit (Qiagen Japan, Tokyo, Japan).
The cytochrome c oxidase subunit I (COI) gene was amplified by PCR using the Ex Taq PCR Kit (Takara, Kyoto, Japan). Two oligonucleotide primers (1 μM each) and <1 μg of DNA template were added to the reaction mixtures. Thermal cycling was as follows: denatured at 96 °C for 20 s; annealed at 55 °C for 45 s; and extended at 72 °C for 2 min for a total of 35 cycles. The oligonucleotide primer sequences used for this amplification were LCO1490 and HCO219859. The molecular size of the PCR products was checked with 1.2% Agarose S (Nippon Gene, Toyama, Japan) gel electrophoresis.
DNA sequencing of the amplified COI genes was performed using the BigDye Terminator Cycling Sequencing Ready Reaction Kit (PE Applied Biosystems, Foster City, CA, USA). The LCO1490 and HCO2198 primers were used in sequencing reactions. Sequencing was performed using an ABI PRISM 3100 genetic analyzer (PE Applied Biosystems).
Additional Information
How to cite this article: Sumida, P. Y. G. et al. Deep-sea whale fall fauna from the Atlantic resembles that of the Pacific Ocean. Sci. Rep. 6, 22139; doi: 10.1038/srep22139 (2016).
Supplementary Material
Acknowledgments
We are deeply indebted to the various representatives of the Japan Agency for Marine-Earth Science and Technology (JAMSTEC), the Japanese Consulate at São Paulo, the Brazilian Ministry of Science and Technology and Innovation, Brazilian Ministry of Foreign Affairs and the Brazilian Navy for helping establishing the Brazil-Japan Marine Science Agreement. We also wish to thank the Master and crews of RV Yokosuka and DSRV Shinkai 6500 for invaluable help at sea. PYGS benefitted from a BIOTA-FAPESP Grant 2011/50185-1 and a CNPq research productivity fellowship 302526/2012-9 to whom he is grateful. We also wish to thank Craig R. Smith (University of Hawaii) for insightful comments that greatly improved the manuscript.
Footnotes
Author Contributions Collected and processed data: Y.F., P.Y.G.S., K.A., H.K., J.A.A.P., A.S.G., T.T. and A.O.S.L. Performed laboratory analyses and species identification: J.M.A.L., M.S., P.Y.G.S. and Y.F. Wrote paper: P.Y.G.S., J.M.A.L., M.S. and Y.F.
References
- Smith C. R., Glover A. G., Treude T., Higgs N. D. & Amon D. J. Whale-Fall ecosystems: Recent insights into ecology, paleoecology, and evolution. Ann Rev Mar Sci 7, 571–596 (2015). [DOI] [PubMed] [Google Scholar]
- Kiel S., Goedert J. L., Kahl W. & Rouse G. W. Fossil traces of the bone-eating worm Osedax in early Oligocene whale bones. P Natl Acad Sci 107, 8656–8659 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowen M. R., Gatesy J. & Wildman D. E. Molecular evolution tracks macroevolutionary transitions in Cetacea. Trends Ecol Evol 29, 336–346 (2014). [DOI] [PubMed] [Google Scholar]
- Kaim A., Kobayashi Y., Echizenya H., Jenkins R. G. & Tanabe K. Chemosynthesis-based associations on Cretaceous plesiosaurid carcasses. Acta Palaeontol Pol 53, 97–104 (2008). [Google Scholar]
- Kiel S. Fossil evidence for micro- and macrofaunal utilization of large nekton falls: Examples from early Cenozoic deep-water sediments in Washington State, USA. Palaeogeogr Palaeocl Palaeoecol 267, 161–174 (2008). [Google Scholar]
- Kiel S., Kahl W. & Goedert J. L. Osedax borings in fossil marine bird bones. Naturwissenschaften 98, 51–55 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danise S. & Higgs N. D. Bone-eating Osedax worms lived on Mesozoic marine reptile deadfalls. Biol Lett 11, 20150072 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baco A. R. & Smith C. R. High species richness in deep-sea chemoautotrophic whale skeleton communities. Mar Ecol Progr Ser 260, 109–114 (2003). [Google Scholar]
- Bennett B. A., Smith C. R., Glaser B. & Maybaum H. L. Faunal community structure of a chemoautotrophic assemblage on whale bones in the deep northeast Pacific Ocean. Mar Ecol Progr Ser 108, 205–223 (1994). [Google Scholar]
- Smith C. R., Baco A. R. & Glover A. Faunal succession on replicate deep-sea whale falls: time scales and vent-seep affinities. Cah Biol Mar 43, 293–297 (2002). [Google Scholar]
- Smith C. R. & Baco A. R. Ecology of whale falls at the deep-sea floor. Oceanogr Mar Biol Annu Rev 41, 311–354 (2003). [Google Scholar]
- Smith C. R., Bernardino A. F., Baco A., Hannides A. & Altamira I. Seven-year enrichment: macrofaunal succession in deep-sea sediments around a 30 tonne whale fall in the Northeast Pacific. Mar Ecol Prog Ser 515, 133–149 (2014). [Google Scholar]
- Deming J. W., Reysenbach A. L., Mako S. A. & Smith C. R. Evidence for the microbial basis of a chemoautotrophic invertebrate community at a whale fall on the deep seafloor: Bone-colonizing bacteria and invertebrate endosymbionts. Microsc Res Techniq 37, 162–170 (1997). [DOI] [PubMed] [Google Scholar]
- Goffredi S. K., Wilpiszenski R., Lee R. & Orphan V. J. Temporal evolution of methane cycling and phylogenetic diversity of Archaea in sediments from a deep-sea whale-fall in Monterey Canyon, California. ISME J 2, 204–220 (2008). [DOI] [PubMed] [Google Scholar]
- Treude T. et al. Biogeochemistry of a deep-sea whale fall: Sulfate reduction, sulfide efflux and methanogenesis. Mar Ecol Prog Ser 382, 1–21 (2009). [Google Scholar]
- Smith C. R., Kukert H., Wheatcroft R. A., Jumars P. A. & Deming J. W. Vent fauna on whale remains. Nature 34, 127–128 (1989). [Google Scholar]
- Smith C. R. & Baco A. R. Phylogenetic and functional affinities between whale-fall, seep and vent chemoautotrophic communities. Cah Biol Mar 39, 345–346 (1998). [Google Scholar]
- Feldman R. A. et al. Vestimentiferan on a whale fall. Biol Bull 194, 116–119 (1998). [DOI] [PubMed] [Google Scholar]
- Baco A. R., Smith C. R., Peek A. S., Roderick G. K. & Vrijenhoek R. C. The phylogenetic relationships of whale-fall vesicomyid clams based on mitochondrial COI DNA sequences. Mar Ecol Prog Ser 182, 137–147 (1999). [Google Scholar]
- Smith C. R. & Baco A. R. Ecology of whale falls at the deep-sea floor. Oceanogr Mar Biol Annu Rev 41, 311–354 (2003). [Google Scholar]
- Distel D. L. et al. Do mussels take wooden steps to deep-sea vents? Nature 403, 725–726 (2000). [DOI] [PubMed] [Google Scholar]
- Jones W. J. et al. Evolution of habitat use by deep-sea mussels. Mar Biol 148, 841–851 (2006). [Google Scholar]
- Fujiwara Y. et al. Extracellular and mixotrophic symbiosis in the whale-fall mussel Adipicola pacifica: A trend in evolution from extra- to intracellular symbiosis. PLoS ONE 5, e11808. doi: 10.1371/journal.pone.0011808 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki J.-I., Martins L. O., Fujita Y., Matsumoto H. & Fujiwara Y. Evolutionary process of deep-sea Bathymodiolus mussels. PLoS ONE 5, e10363. doi: 10.1371/journal.pone.0010363 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorion J. et al. Evolutionary history of Idas sp. Med (Bivalvia: mytilidae), a cold seep mussel bearing multiple symbionts. Cah. Biol. Mar. 53, 77–87 (2012). [Google Scholar]
- Lorion J. et al. Adaptive radiation of chemosymbiotic deep-sea mussels. Proc. R. Soc. B 280, 2013 1243 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thubaut J., Puillandre N., Faure B., Cruaud C. & Samadi S. The contrasted evolutionary fates of deep-sea chemosynthetic mussels (Bivalvia, Bathymodiolinae). Ecol. Evol. 3, 4748–4766 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka K., Wada H. & Okano H. Torishima whale deep-sea animal community assemblage- new findings by “Shinkai 6500”. J Geogr 102, 507–517 (1993). [Google Scholar]
- Goffredi S. K., Paull C. K., Fulton-Bennet K., Hurtado L. A. & Vrijenhoek R. C. Unusual benthic fauna associated with a whale fall in Monterey Canyon, California. Deep-Sea Res I 51, 1295–1306 (2004). [Google Scholar]
- Lundsten L., Paull C. K., Schlining K. L., McGann M. & Ussler W. III Biological characterization of a whale-fall near Vancouver Island, British Columbia, Canada. Deep-Sea Res I 57, 918–922 (2010). [Google Scholar]
- Amon D. J. et al. The discovery of a natural whale fall in the Antarctic deep sea. Deep-Sea Res II 92, 87–96 (2013). [Google Scholar]
- Dahlgren T. G. et al. A shallow-water experiment in the north Atlantic. Cah Biol Mar 47, 385–389 (2006). [Google Scholar]
- Braby C. E., Rouse G. W., Johnson S. B., Jones W. J. & Vrijenhoek R. C. Bathymetric and temporal variation among Osedax boneworms and associated megafauna on whale-falls in Monterey bay, California. Deep-sea Res I 54, 1773–1791 (2007). [Google Scholar]
- Fujiwara Y. et al. Three-year investigations into sperm whale-fall ecosystems in Japan. Mar Ecol SI 28, 1219–1232 (2007). [Google Scholar]
- Glover A. G. et al. A live video observatory reveals temporal processes at a shelf-depth whale-fall. Cah Biol Mar 51, 375–381 (2010). [Google Scholar]
- Lundsten L. et al. Time-series analysis of six whale-fall communities in Monterey Canyon, California, USA. Deep-Sea Res I 57, 1573–1584 (2010). [Google Scholar]
- Speer K. G. & Zenk W. The flow of Antarctic Bottom Water into the Brazil Basin. J Phys Oceanogr 23, 2667–2682 (1993). [Google Scholar]
- Silva C. F., Shimabukuro M., Alfaro-Lucas J. M., Fujiwara Y., Sumida P. Y. G. & Amaral A. C. Z. A new Capitella polychaete worm (Annelida: Capitellidae) living inside whale bones in the abyssal South Atlantic. Deep-Sea Research I, doi: 10.1016/j.dsr.2015.12.004 (in press). [DOI] [Google Scholar]
- Van Dover C. L., German C. R., Speer K. G., Parson L. M. & Vrijenhoek R. C. Evolution and biogeography of deep-sea vent and seep invertebrates. Science 295, 1253–1257 (2002). [DOI] [PubMed] [Google Scholar]
- Moalic Y. et al. Biogeography revisited with network theory: Retracing the history of hydrothermal vent communities. Syst Biol 61, 127–137 (2012). [DOI] [PubMed] [Google Scholar]
- Levin L.A. Ecology of cold seep sediments: Interactions of fauna with flow, chemistry and microbes. Oceanogr Mar Biol Annu Rev 43, 1–46 (2005). [Google Scholar]
- Olu K., Cordes E. E., Fisher C. R., Brooks J. M. & Sibuet M. Biogeography and potential exchanges among the Atlantic Equatorial Belt cold-seep faunas. PLoS ONE 5, e11967. doi: 10.1371/journal.pone.0011967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira S. et al. High connectivity across the fragmented chemosynthetic ecosystems of the deep Atlantic Equatorial Belt: Efficient dispersal mechanisms or questionable endemism? Mol Ecol 22, 4663–4680 (2013). [DOI] [PubMed] [Google Scholar]
- Johnson S. B. et al. Rubyspira, new genus and two new species of bone-eating deep-sea snails with ancient habits. Biol Bull 219, 166–177 (2010). [DOI] [PubMed] [Google Scholar]
- Reuscher M., Fiege D. & Wehe T. Terebellomorph polychaetes from hydrothermal vents and cold seeps with the description of two new species of Terebellidae (Annelida: Polychaeta) representing the first records of the family from deep-sea vents. J. Mar. Biol. Ass. U.K. 92, 997–1012 (2011). [Google Scholar]
- Glover A. G., Goetze E., Dahlgren T. G. & Smith C. R. Morphology, reproductive biology and genetic structure of the whale-fall and hydrothermal vent specialist, Bathykurila guaymasensis Pettibone, 1989 (Annelida: Polynoidae). Mar Ecol 26, 223–234 (2005). [Google Scholar]
- Pleijel F., Rouse G. W., Ruta C., Wiklund H. & Nygren A. Vrijenhoekia balaenophila, a new hesionid polychaete from a whale fall off California. Zool J Linn Soc 152, 625–634 (2008). [Google Scholar]
- Desbruyères D. & Toulmond A. A new species of hesionid worm, Hesiocaeca methanicola sp. nov. (Polychaeta: Hesionidae), living in ice-like methane hydrates in the deep Gulf of Mexico. Cah Biol Mar 39, 93–98 (1998). [Google Scholar]
- Salazar-Vallejo S. I. & Orensanz J. M. Pleijelius longae n. gen., n. sp., a remarkable deep water polychaete from the Northwestern Atlantic (Polychaeta: Hesionidae). Scient Mar 70, 157–166 (2006). [Google Scholar]
- Bellan G., Dauvin J. C. & Laubier L. The genus Lindaspio (Annelida: Polychaeta: Spionidae), and a new species from an oil field off Congo, western Africa. J Nat Hist 37, 2413–2424 (2003). [Google Scholar]
- Glover A. G., Kallstrom B., Smith C. R. & Dahlgren T. G. World-wide whale worms? A new species of Osedax from the shallow north Atlantic. Proc Royal Soc B 272, 2587–2592 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman J. et al. Whales as marine ecosystem engineers. Front Ecol Environ 12, 377–385 (2014). [Google Scholar]
- Glover A. G. et al. Bone-eating worms from the Antarctic: the contrasting fate of whale and wood remains on the Southern Ocean seafloor. Proc Royal Soc B 280, 20131390 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse G. W., Goffredi S. K. & Vrijenhoek R. C. Osedax: bone-eating marine worms with dwarf males. Science 305, 668–671 (2004). [DOI] [PubMed] [Google Scholar]
- Tunnicliffe V. & Juniper S. K. Cosmopolitan underwater fauna. Nature 344, 300 (1990). [Google Scholar]
- Schneider C. A., Rasband W. S. & Eliceiri K. W. NIH Image to ImageJ: 25 years of image analysis”. Nature Met 9, 671–675 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danovaro R. et al. Major viral impact on the functioning of benthic deep-sea ecosystems. Nature 454, 1084–1088 (2008). [DOI] [PubMed] [Google Scholar]
- Westheide W. & Purschke G. Organism processing in Introduction to the Study of Meiofauna (eds Higgins R.P. & Thiel H.) 146–160 (Smithsonian Institute Press, 1988).
- Folmer O., Black M., Hoeh W., Lutz R. & Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotech 3, 294–299 (1994). [PubMed] [Google Scholar]
- Pettibone M. H. Polynoidae and Sigalionidae (Polychaeta) from Guaymas Basin, with descriptions of 2 new species, and additional records from hydrothermal vents of Galapagos Rift, 21 N, and seep site in the Gulf of Mexico (Florida and Louisiana). P Biol Soc Wash 102, 154–168 (1989). [Google Scholar]
- Pettibone M. H. Revision of the genus Macellicephala McIntosh and the subfamily Macellicephalinae Hartmann-Schröder (Polychaeta: Polynoidae). Smithson Contrib Zool 229, 71 p. (1976). [Google Scholar]
- Dahlgren T. G., Glover A. G., Baco A. & Smith C. R. Fauna of whale falls: systematics and ecology of a new polychaete (Annelida: Chrysopetalidae) from the deep Pacific Ocean. Deep-Sea Res I 51, 1873–1887 (2004). [Google Scholar]
- Wiklund H., Glover A. G., Johannessen P. J. & Dahlgren T. G. Cryptic speciation at organic-rich marine habitats: a new bacteriovore annelid from whale-fall and fish farms in the North-East Atlantic. Zool J Linn Soc 155, 774–785 (2009). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.