Abstract
One of the readily available sources of mesenchymal stem cells (MSCs) is menstrual blood-derived stem cells (Men-SCs), which exhibit characteristics similar to other types of MSCs. This study was performed to determine the growth kinetics, plasticity, and characterization of Men-SCs in women. During spring 2014 in the southern Iranian city of Shiraz, menstrual blood (5 mL) was obtained from 10 women on their third day of menstruation in 2 age groups of 30 to 40 and 40 to 50 years old. Ficoll was used to separate the mononuclear cell fraction. After the Men-SCs were cultured, they were subcultured up to passage 4. Growth behavior and population doubling time were evaluated by seeding 5×104 cells into 12- and 24-well culture plates, and the colonies were enumerated. The expression of CD44, CD90, and CD34 was evaluated. The osteogenic potential was assessed by alizarin red staining. The Men-SCs were shown to be plastic adherent and spindle-shaped. Regarding the growth curves in the 12- and 24-well culture plates, it was demonstrated that in the women aged between 30 and 40 years, population doubling time was 55.5 and 62 hours, respectively, while these values in the women aged between 40 and 50 years were 70.4 and 72.4 hours, correspondingly. Positive expression of CD44 and CD90 and negative expression of CD34 were noted. In the osteogenic differentiation medium, the cells differentiated toward osteoblasts. As human Men-SCs are easily collectable without any invasive procedure and are a safe and rapid source of MSCs, they can be a good candidate for stem cell banking and cell transplantation in women.
Keywords: Menstrual blood, Mesenchymal stem cells, Plasticity, Reverse transcriptase polymerase chain reaction
What’s Known
The presence of stem/progenitor cells in the endometrium was shown many years ago, but the first practical evidence was reported in 2004.
The characterization of clonogenic cells in the human endometrium has opened a window in the research of endometrial stem/progenitor cells in the subsequent 10 years.
What’s New
In this study for the first time regarding the growth kinetics of human menstrual stem cells, it was shown that age — as a variant parameter — could influence the proliferation and viability of these cells, even when they were all morphologically identical and spindle-shaped.
Introduction
Mesenchymal stem cells (MSCs) are adult progenitors that can be isolated from various human and animal adult tissues.1 They are identical in their common features such as capacity for self-renewal with symmetric division, differentiation potential to many lineages, and favorable proliferative capability.2 These cells also possess some source-specific properties that have yet to be fully elucidated.3 One of the most familiar sources for harvesting MSCs is the bone marrow,2 although MSCs have also been isolated from many other tissues.4 One of the readily available sources of MSCs is menstrual blood-derived stem cells (Men-SCs), which exhibit characteristics similar to other types of MSCs.5
Men-SCs are widely available (about 12 times a year from a regular-cycle woman) and are capable of long-term proliferation; they can also be obtained via a noninvasive procedure easily.6 In recent years, Men-SCs have been deemed an easily accessible and refreshing stem cell source with no ethical considerations in the field of regenerative medicine.6 There are several reports on the differentiation of Men-SCs into osteoblasts,7 nucleus cardiomyocytes,8 glial-like cells,9 pulposus-like cells,10 hepatocyte-like cells,11 adipocytes,12 and chondrocytes.13 Men-SCs are widely regarded as a new source of MSCs with several potential therapeutic applications; nonetheless, there is a dearth of data in the existing literature on their growth kinetics. The present study was designed to isolate, culture, and determine the growth kinetics and characterization of Men-SCs in women in 2 age groups of 30 to 40 and 40 to 50 years old.
Patients and Methods
Menstrual Blood Sampling
During spring 2014, menstrual blood specimens were collected from 10 volunteer women who referred to the department of obstetrics and gynecology of hospitals affiliated to Shiraz University of Medical Sciences, Shiraz, Southern Iran (5 aged between 30 and 40 years and 5 aged between 40 and 50 years). All the women were on their third day of bleeding period, and the samples were collected with menstrual cups (Diva Cup Co., U.S.A.) inserted deeply into the vagina. All the candidates were healthy and without any history of previous genital diseases. Before the insertion of the cups, sanitary disinfectant pads were used to prevent the local bacterial flora from contaminating the samples. The collection procedure was approved only for research purposes by the institutional ethics committee, and the donors signed a written informed consent form. The sample volume obtained from each participant was 5 mL, and they were separately added to falcons containing 10% sodium citrate and 1% penicillin/streptomycin (Gibco, Germany). Subsequently, the samples were transferred to the Stem Cell Laboratory of The Stem Cell and Transgenic Technology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran, for further evaluation.
Isolation of Mononuclear Cells
Menstrual blood was gently added to an equal volume of Ficoll under laminar flow hood condition (Class II, Jal Tajhiz, Iran). Ficoll-Paque (Biowest, France) density-gradient centrifugation was performed at 1,200 rpm for 30 minutes at 4ºC, resulting in the separation of the contents in four fractions (i.e., red blood cells, Ficoll, mononuclear cells, and plasma). In brief, the transparent fraction of the mononuclear cells was separated and added to a threefold volume of Dulbecco’s Modified Eagle’s Medium-F12 (DMEM-F12, Biowest, France), supplemented with 10% fetal bovine serum (FBS) (BioIdea, Iran), 1% penicillin/streptomycin (Biowest, France), and 1% L-glutamine (BioIdea, Iran).
Culturing the Menstrual Blood-Derived Stem Cells
After centrifugation and removal of the supernatant, the cell pellet was suspended in 6 mL of fresh DMEM-F12 media, supplemented with 10% FBS, 1% penicillin/streptomycin, and 1% L-glutamine, and was then transferred into T25 flasks. The flasks were kept in a CO2 incubator (Memmert, Germany) at 37°C, 5% CO2, and saturated humidity. The media were replaced twice a week.
The primary culture (P0) was trypsinized (Trypsin, Sigma, U.S.A.) after 10 days and was transferred into new T25 culture flasks (P1, the first passage). After 3 days, the cells were evaluated for adherence, confluence, and morphology using an inverted light microscope (Nikon, Japan). When the adherent spindle-shaped cells attained 70–80% confluence, the time was considered optimum for harvesting the cells by trypsin and starting subculturing. Passaging the cells of each of the 10 samples was continued up to P4; and at the end of each passage, the number of live and dead cells was determined via the dye-exclusion method. The cell-counting and cell-staining procedures were carried out using a Neubauer chamber and trypan blue (Sigma, U.S.A.), respectively.
Growth Curve and Population Doubling Time
The passage 4 cells of both age groups were seeded into 12- and 24-well culture plates at a density of 5×104 cells per well to evaluate the growth kinetics and study the in vitro behavior of the Men-SCs. The cells were counted every 24 hours (each time 3 wells/group). This procedure was continued for 8 days, and the mean number of the cells at each time point was depicted using GraphPad Prism (version 5.01; GraphPad Software Inc., San Diego, CA, U.S.A.). The following formula was used to determine population doubling time (PDT): T×ln2/ln (Xe/Xb), while Xe, Xb, and T were defined as the final cell number, the initial cell number, and the incubation time in any unit, respectively.
Cell Viability
After the enumeration of the cells of each passage, a portion of the cells were kept frozen and their survival was thereafter checked from the beginning of the thawing process. For the freezing process, the cells were stored in CryoTubes containing a freeze medium composed of 50% FBS, 40% DMEM, and 10% dimethyl sulfoxide (DMSO) (MP Bio, U.S.A.). DMSO is toxic in room temperature; hence, the cell CryoTubes were kept in a freezer (-24°C) for 1 hour. Afterward, they were transferred into another freezer (-70°C); and 24 hours later, they were submerged into liquid nitrogen (Atocel, Austria) to be stored for long term. For the thawing process, the cell vials were removed from the liquid nitrogen and rapidly warmed in a 37°C water bath. The cell toxicity of the DMSO was prevented through the immediate addition of a DMEM medium. Next, after the centrifugation of the content at 1,200 rpm for 7 minutes, the cell precipitate was cultured using the DMEM medium. The viability of the cells was determined by adding trypan blue (0.4% trypan blue in PBS), while the number of the live cells divided by the total number of the cells was presented as a percentage.
Characterization by Reverse Transcription Polymerase Chain Reaction
The expression of mesenchymal (CD44) and hematopoietic (CD34) cell markers was determined using reverse transcription polymerase chain reaction (RT-PCR). After the extraction of the total RNA using a column RNA isolation kit (DENAzist Asia, Iran) based on the manufacturer’s instructions, the total RNA concentration was determined via spectrophotometry. The complementary DNA (cDNA) was provided from the RNA samples using an AccuPower CycleScript RT PreMix kit (Bioneer, Korea) according to the manufacturer’s instructions. Briefly, for each reaction, 15 μL of the total RNA was used and the volume reached 20 μL with diethylpyrocarbonate water. Twelve thermal cycles were done as follows: 30 seconds at 20°C for primer annealing, 4 minutes at 42°C for cDNA synthesis, 30 seconds at 55°C for melting secondary structure and cDNA synthesis, and 5 minutes at 95°C for inactivation. In the third step, 1 μL of the template (cDNA) was mixed with other reagents consisting of PCR buffer, MgCl2, H2O, dNTPs, Taq DNA polymerase, and forward and reverse primers (i.e., H45 and H73). The microtubules, containing 20 μL of the above mixture, were transferred into a thermocycler (Eppendorf Mastercycler Gradient, Eppendorf, Hamburg, Germany). Thirty amplification cycles were undertaken, consisting of 30-second denaturation at 95°C, 30-second annealing at 64 °C, and 30-second extension at 72°C with 2 minutes at 95°C for primary denaturation and 5 minutes at 72°C for final extension. The PCR products were evaluated for any considered bands using gel electrophoresis with the aid of DNA safe stain in a 1.5% agarose gel medium. The produced bands were visualized under ultraviolet radiation using a gel documentation system (UVItec, Cambridge, U.K.).
Characterization by Flow Cytometry
The phenotypes of the bone-marrow MSCs were determined using flow cytometry for the absence of CD34 and the presence of CD44 and CD90. The cells were sorted by using FITC-labeled anti-CD34 (1:20; DAKO, Carpinteria, CA, U.S.A.) and anti-CD44 and anti-CD90 (1:20; DAKO). In brief, after being stained with appropriately conjugated antibodies (Ab) and washing, the cells were studied on a BDL cytofluorimeter (BD Biosciences, Heidelberg, Germany). The area of positivity was confirmed with an isotype-matched control Ab.
Osteogenic Induction
Approximately 1×104 cells (Men-SCs) were transferred in a 35 mm culture dish (Corning, Germany). The cells were maintained in control and osteogenic media. The control medium consisted of DMEM-F12 (Biowest, France), supplemented with 10% FBS (BioIdea, Iran), 1% penicillin/streptomycin (Biowest, France), and 1% L-glutamine (BioIdea, Iran). The osteogenic medium was composed of DMEM-F12, 10% FBS, 1% penicillin-streptomycin, L-glutamine, 50 μg/mL of L-ascorbic acid-2-phosphate (Sigma, U.S.A.), 10-7 mol of dexamethasone (Sigma, U.S.A.), and 10 mmol of β-glycerophosphate (Sigma, U.S.A.). The medium was replaced every 3 days. With the use of alizarin red, the cultured cells were stained on day 21 to assess the mineralized matrix. The medium was removed, and the cell layers were washed 3 times with PBS and then fixed in cold 70% ethanol for 1 hour at 4°C. The cell layers were rinsed again with deionized water and allowed to air dry. The fixed cells were stained using 2% alizarin red S at pH 7.2 (Sigma, U.S.A.). After 1 hour at 37°C, the cell layers were washed with deionized water and investigated grossly with the light microscope.
Karyotyping
For the karyotyping process, the cells (subcultured at a 1:3 dilution, both early passages and after reaching the Hayflick limit) were subjected to a 4-hour demecolcemid (Sigma cat., no, D1925) incubation after 24 hours’ cultivation followed by trypsin-ethylenediaminetetraacetic acid (EDTA) (Sigma, U.S.A.) detachment and lysis with hypotonic KCl (Sigma, U.S.A.) and fixation in acid/alcohol. The chromosome numbers were enumerated under an oil-immersion objective. Images were captured using a digital camera and light microscopy in order to analyze the metaphysis.
Statistical Analysis
In the growth curve analysis, the normality of the data was controlled using the Kolmogorov–Smirnov test. The mean and SE of the counted cells were compared using the Mann–Whitney U test. Statistical Package for the Social Sciences (SPSS) was employed for the statistical analyses (version 11.5, SPSS Inc., Chicago, U.S.A.), and a P≤0.05 was regarded statistically significant.
Results
Morphology of the Cells
Following cell expansion, the adherent Men-SCs obtained from both groups of women revealed a spindle-shaped, fibroblast-like morphology similar to the typical appearance of MSCs in the primary culture and passage 2 (figures 1a and b).
Figure 1.
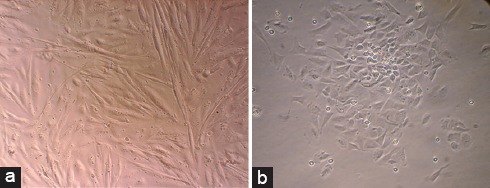
(a and b) Morphology of the menstrual blood stem cells shows a spindle shape.
Growth Kinetics of the Cells
About 3 to 5 days were needed for the cells of each passage to reach the confluence of 70 to 80%, except for the primary passage, which took 10 days. The results from the culture of the Men-SCs of the women aged between 30 and 40 years when seeding 5×104 cells into 12- and 24-well culture plates revealed PDT values of 55.5 and 62 hours, respectively (figures 2a, g, and H). However, for the women aged between 40 and 50 years, PDT values were 70.4 and 72.4 hours, correspondingly (figures 2b, e, and f). According to the cell enumeration findings in either the 12- or the 24-well plates, the growth of the Men-SCs in the women aged between 30 and 40 years was significantly more than that in the women aged between 40 and 50 years (figures 2c and 2d; P<0.05).
Figure 2.
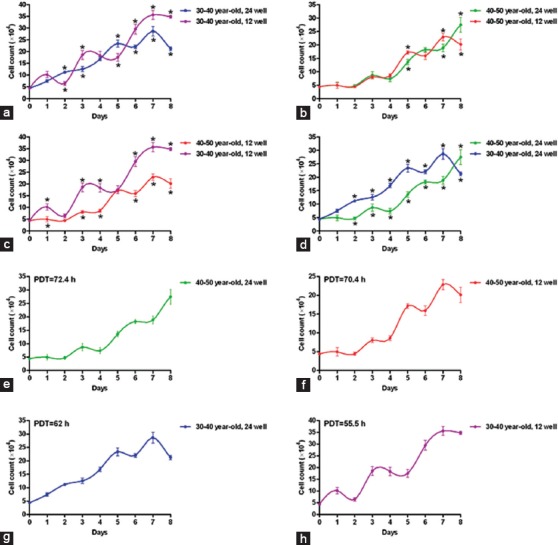
Growth kinetics of menstrual blood stem cells are compared in 12- and 24-well culture plates between women of different age groups: a) 30–40 years old when seeding 5×104 cells at the starting point in 12- and 24-well culture plates; b) 40–50 years old when seeding 5×104 cells at the starting point in 12- and 24-well culture plates; c) 30–40 and 40–50 years old when seeding 5×104 cells at the starting point in 12-well culture plates; d) 30–40 and 40–50 years old when seeding 5×104 cells at the starting point in 24-well culture plates; e) 40–50 years old when seeding 5×104 cells at the starting point in 24-well culture plates; f) 40–50 years old when seeding 5×104 cells at the starting point in 12-well culture plates; g) 30–40 and 40–50 years old when seeding 5×104 cells at the starting point in 24-well culture plates; and h) 30–40 and 40–50 years old when seeding 5×104 cells at the starting point in 12-well culture plates.
Characterization by Reverse Transcription Polymerase Chain Reaction
The RT-PCR demonstrated that the Men-SCs expressed CD44. Also, CD34 was not expressed on the Men-SCs (figure 3).
Figure 3.
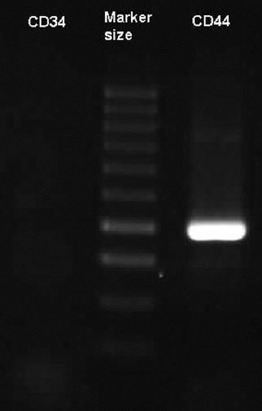
Positive expression of CD44 is compared with the negative expression of CD34 using the reverse transcription polymerase chain reaction (RT-PCR) technique.
Flow Cytometry of the Cells
The Men-SCs showed positive surface biomarker expression for CD44 and CD90. Also, the expression of CD34 was absent (figure 4).
Figure 4.
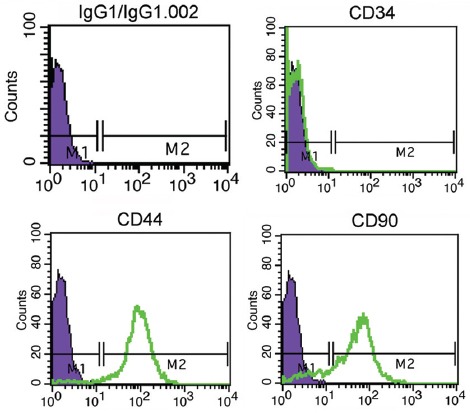
Flow cytometry characterization analyses of the menstrual blood stem cells shows that these cells are uniformly negative for CD34 and positive for CD44 and CD90.
Osteogenic Induction
Alizarin red staining showed bone-nodule formation in the presence of the osteogenic medium. The induction was positive, as is shown in figure 5a, when compared with the control in the absence of the medium (figure 5b).
Figure 5.
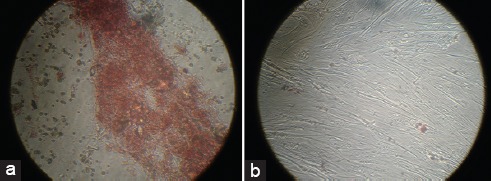
a) Positive alizarin red staining shows the osteoblast differentiation of the menstrual blood stem cells in the osteogenic medium after 21 days. b) Alizarin red staining of the menstrual blood stem cells in the control group in the absence of the osteogenic medium after 21 days does not show any differentiation to osteoblasts.
Karyotyping
There were 46 chromosomes in the Men-SCs: 44 normal autosomal and 2 normal sex chromosomes (figure 6).
Figure 6.
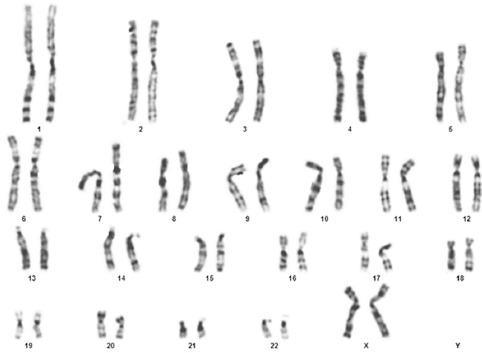
Karyotyping of the menstrual blood stem cells at passage 4 shows a normal chromosomal state.
Discussion
MSCs can be isolated from several adult tissue sources. They show progenitor cell-like features such as proliferation and differentiation capacities. One of the most historically prominent sources of MSCs has been the bone marrow, while other sources recently include the adipose tissue, bone, muscle, liver, pancreas, tooth, umbilical cord, placenta, and cord blood. The isolation of these progenitor cells requires traumatic procedures that are poorly feasible and associated with patient discomfort.2 Menstrual blood has been considered one of the most accessible sources for obtaining MSCs noninvasively.14 Recently, there has been a great deal of interests in the application of these cells in regenerative medicine due to their multilineage and highly proliferative features.14 Men-SCs have been introduced as a good source of cell transplantation for various therapeutic procedures such as the treatment of premature ovarian failure.14
In cell expansion, one of the most prominent criteria is the morphology of cultured cells.15 In our study, similar to previous findings, during subcultures, the Men-SCs displayed a spindle-shaped, fibroblast-like morphology under light microscopy, resembling the typical appearance of MSCs derived from other adult tissues.14 It has been previously shown that Men-SCs are more uniform in shape and size (10 to 100 μ) than are the cells obtained from other tissues.14 These cells, as readily available sources of MSCs, have been shown to exhibit similar morphology and characteristics like other types of MSCs, which confirms our results.5
Men-SCs have been demonstrated to have stromal cell morphology with a PDT of 24 hours.16 There is clear evidence to support the high regenerative capability of the endometrial tissue undergoing the cyclic processes of cellular proliferation and shedding.6 In the current study, a comparison of Men-SCs between the 2 groups showed that the proliferative activity of the cells obtained from the younger women (aged between 30 and 40 years) was more than that of the cells obtained from the older women (aged between 40 and 50 years), indicating the effect of ageing on the proliferation of cells even with different PDTs.16 We showed that the Men-SCs of the women aged between 30 and 40 years when seeding 5×104 cells into 12- and 24-well culture plates revealed PDT values of 55.5 and 62 hours, respectively. However, for the women aged between 40 and 50 years, the PDT values were 70.4 and 72.4 hours, respectively. These findings confirm that the proliferative activity of the cells obtained from the younger women (aged between 30 and 40 years) was more than that of the cells obtained from the older women (aged between 40 and 50 years), which chimes in with the results of some previous reports.14
In one study, the effect of ageing on the proliferation of cells was reported before revealing a reduction in the replication lifespan of somatic cells.17 Elsewhere, it was shown that Men-SCs had a proliferative capacity of more than 30 subcultures in comparison to MSCs derived from other sources such as the dental pulp and bone marrow, with limited capacity of proliferation of approximately 20 population doublings.14 Given the high proliferative rates of Men-SCs, these cells are regarded as an ideal source for autologous transplantation.14
Mesenchymal surface markers such as cluster of differentiation CD166, CD117, CD105, CD44, CD29, 9 (CD9), CD49f, CD90, major histocompatibility complex class I (MHC I), multipotent markers octamer-binding transcription factor 4 (Oct-4; the main regulator of differentiation in pluripotent cell line), stage-specific embryonic antigen 4 (SSEA-4; the marker used to differentiate between human and mouse cells and used to characterize pluripotent stem cells), and stromal-derived factor-1 (STRO-1; used as identification, isolation, and functional testing of human bone marrow stromal progenitor cells) have been identified under fluorescent microscopy or by flow cytometry.6 In our study, the presence of the mesenchymal surface markers of CD44 and CD90 was documented, while the Men-SCs were negative for the expression of CD34; these findings are concordant with those previously reported in the literature. Additionally, these findings denote the mesenchymal properties of isolated Men-SCs and candidate them as a noninvasive source for cell transplantation.
There are several reports on the differentiation of Men-SCs into osteoblasts,7 nucleus cardiomyocytes,6,8 glial-like cells,9 pulposus-like cells,10 hepatocyte-like cells,11 adipocytes,12 and chondrocytes.16 The multipotency of Men-SCs was shown by the direct differentiation of Men-SCs into adipogenic, chondrogenic, osteogenic, neurogenic, and cardiogenic cell lineages using the specific human mesenchymal stem cell differentiation bullet kit.14
Meng et al.6 (2007) showed that Men-SCs were able to differentiate into 9 lineages, namely adipocytic, osteogenic, endothelial, neurocytic, pancreatic, cardiomyocytic, respiratory epithelial, myocytic, and hepatic lineages. The authors reported that Men-SCs were able to produce matrix metalloproteinase-3 (MMP3), MMP10, granulocyte macrophage colony-stimulating factor (GM-CSF), angiopoietin-2, and platelet-derived growth factor (PDGF)-BB in quantities 10 to 10,000 times higher than those in umbilical cord blood cells.
Our findings on the differentiation of Men-SCs to osteoblasts are in agreement with previous reports, denoting the multipotency of these cells and revealing the fact that Men-SCs can be safely used in cell therapy. Accordingly, the osteogenic differentiation of Men-SCs reveals an important novel step toward safe and applied stem cell therapy of bone injuries and diseases.6 Nonetheless, donor differences, sampling day, sample source, and stem cell isolation method have been shown to affect the osteogenic differentiation capacity of Men-SCs.18
The analysis of the nuclear karyotype of MSCs has been recommended for any mutation or some other visible abnormalities at the chromosomal level.6 We also showed that the Men-SCs were able to be largely expanded in vitro to passage 4 without any mutation or some other visible abnormalities at the chromosomal level. Our results demonstrated that when the Men-SCs were utilized for cell transplantation purposes, the cells, even at passage 4, could be safely used for cell therapy.
Conclusion
The stem-cell phenotypic markers of Men-SCs were confirmed in our study, demonstrating not only the great potential of these cells for plasticity, self-renewal, and proliferation for long periods of time but also their differentiation properties. Human Men-SCs are a readily available and inexpensive source of stem cells and are collected noninvasively, rapidly, and safely; they can, therefore, be considered a good candidate for stem-cell banking and cell transplantation in women needing a cell therapy measure.
Acknowledgement
The authors wish to appreciate the financial support of Shiraz University of Medical Sciences.
Conflict of Interest: None declared.
References
- 1.Hoogduijn MJ, Betjes MG, Baan CC. Mesenchymal stromal cells for organ transplantation: different sources and unique characteristics? Curr Opin Organ Transplant. 2014;19:41–6. doi: 10.1097/MOT.0000000000000036. [DOI] [PubMed] [Google Scholar]
- 2.Ai J, Ebrahimi S, Khoshzaban A, Jafarzadeh Kashi TS, Mehrabani D. Tissue engineering using human mineralized bone xenograft and bone marrow mesenchymal stem cells allograft in healing of tibial fracture of experimental rabbit model. Iran Red Crescent Med J. 2012;14:96–103. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 3.Rossignoli F, Caselli A, Grisendi G, Piccinno S, Burns JS, Murgia A, et al. Isolation, characterization, and transduction of endometrial decidual tissue multipotent mesenchymal stromal/stem cells from menstrual blood. Biomed Res Int 2013. 2013:901821. doi: 10.1155/2013/901821. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehrabani D, Mehrabani G, Zare S, Manafi A. Adipose-derived stem cells (ADSC) and aesthetic surgery: a mini review. World J Plast Surg. 2013;2:65–70. [PMC free article] [PubMed] [Google Scholar]
- 5.Mou XZ, Lin J, Chen JY, Li YF, Wu XX, Xiang BY, et al. Menstrual blood-derived mesenchymal stem cells differentiate into functional hepatocyte-like cells. J Zhejiang Univ Sci B. 2013;14:961–72. doi: 10.1631/jzus.B1300081. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rahimi M, Zarnani AH, Mohseni-Kouchesfehani H, Soltanghoraei H, Akhondi MM, Kazemnejad S. Comparative evaluation of cardiac markers in differentiated cells from menstrual blood and bone marrow-derived stem cells in vitro. Mol Biotechnol. 2014;56:1151–62. doi: 10.1007/s12033-014-9795-4. [DOI] [PubMed] [Google Scholar]
- 7.Ai J, Davood M. The Potential of Human Endometrial Stem Cells for Os-teoblast Differentiation. Iran Red Crescent Med J 2010. 2010:585–7. [Google Scholar]
- 8.Ai J, Mehrabani D. Are Endometrial stem cells novel tools against ischemic heart failure in women? a hypothesis. Iran Red Crescent Med J. 2010;12:73–5. [Google Scholar]
- 9.Ai J, Mehrabani D. The possibility of differentiation of human endometrial stem cells into neural cells. Iran Red Crescent Med J. 2010;12:328–31. [Google Scholar]
- 10.Hu X, Zhou Y, Zheng X, Tian N, Xu C, Wu W, et al. Differentiation of menstrual blood-derived stem cells toward nucleus pulposus-like cells in a coculture system with nucleus pulposus cells. Spine. 2014;39:754–60. doi: 10.1097/BRS.0000000000000261. [DOI] [PubMed] [Google Scholar]
- 11.Mou XZ, Lin J, Chen JY, Li YF, Wu XX, Xiang BY, et al. Menstrual blood-derived mesenchymal stem cells differentiate into functional hepatocyte-like cells. J Zhejiang Univ Sci B. 2013;14:961–72. doi: 10.1631/jzus.B1300081. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khanmohammadi M, Khanjani S, Edalatkhah H, Zarnani AH, Heidari-Vala H, Soleimani M, et al. Modified protocol for improvement of differentiation potential of menstrual blood-derived stem cells into adipogenic lineage. Cell Prolif. 2014;47:615–23. doi: 10.1111/cpr.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel AN, Park E, Kuzman M, Benetti F, Silva FJ, Allickson JG. Multipotent menstrual blood stromal stem cells: isolation, characterization, and differentiation. Cell Transplant. 2008;17:303–11. doi: 10.3727/096368908784153922. [DOI] [PubMed] [Google Scholar]
- 14.Ghobadi F, Mehrabani D, Mehrabani G. Regenerative potential of endometrial stem cells: a mini review. World J Plast Surg. 2015;4:3–8. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 15.Hosseinkhani M, Mehrabani D, Karimfar MH, Bakhtiyari S, Manafi A, Shirazi R. Tissue engineered scaffolds in regenerative medicine. World J Plast Surg. 2014;3:3–7. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin J, Xiang D, Zhang JL, Allickson J, Xiang C. Plasticity of human menstrual blood stem cells derived from the endometrium. J Zhejiang Univ Sci B. 2011;12:372–80. doi: 10.1631/jzus.B1100015. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubin H. Promise and problems in relating cellular senescence in vitro to aging in vivo. Arch Gerontol Geriatr. 2002;34:275–86. doi: 10.1016/S0167-4943(01)00221-7. [DOI] [PubMed] [Google Scholar]
- 18.Darzi S, Zarnani AH, Jeddi-Tehrani M, Entezami K, Mirzadegan E, Akhondi MM, et al. Osteogenic differentiation of stem cells derived from menstrual blood versus bone marrow in the presence of human platelet releasate. Tissue Eng Part A. 2012;18:1720–8. doi: 10.1089/ten.TEA.2011.0386. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]


