Abstract
Background:
We compared the wave amplitude of visually evoked potential (VEP) between patients with esotropic and anisometropic amblyopic eyes and a normal group.
Methods:
The wave amplitude of VEP was documented in 2 groups of persons with amblyopia (15 with esotropia and 28 with anisometropia) and 1 group of individuals with normal visual acuity (n, 15). The amplitude of P100 was recorded monocularly with different spatial frequencies.
Results:
Our statistical analysis revealed that the wave amplitude in the 2 groups with amblyopia was significantly decreased compared to that in the normal group (P<0.001). There was a significant difference regarding the amplitude in high spatial frequencies in both high- and low-contrast conditions between the groups with esotropia and anisometropia and the normal group (P<0.001). There were also significant differences in large check-size stimuli and low-contrast condition between the amblyopic groups with esotropia and anisometropia and the normal group (P=0.013 and P=0.044, respectively). In large check-size stimuli and high-contrast condition, a significant difference was indicated only in the comparison between the esotropic amblyopic eyes and the normal eyes (P=0.036).
Conclusion:
The wave amplitude parameter of VEP was influenced by both types of amblyopia, but it seems that this parameter was more sensitive to esotropic amblyopia than anisometropic amblyopia. This outcome may reflect a non-parallel pattern of cortical responses in the comparison of the 2 types of amblyopia with each other and with the control group, which may be beneficial for the diagnosis and treatment of amblyopia.
Keywords: Amblyopia, Anisometropia, Esotropia, Visual evoked potentials
What’s Known
Amblyopia is not merely a simple reduction in visual acuity, but it is rather a complex mechanism of brain information processing.
The visually evoked potential (VEP) test records the activity of the primary visual cortex in response to visual stimuli and is one of the current techniques used to understand the complicated mechanism of amblyopia.
What’s New
In the present study, the wave amplitude parameter of VEP was influenced by both types of amblyopia (esotropic amblyopia and anisometropic amblyopia).
This parameter was more sensitive to esotropic amblyopia than anisometropic amblyopia.
Introduction
Amblyopia is one of the major causes of visual loss in the pediatric group, with an incidence of approximately 3% of the population, and has been the subject of numerous studies.1 During the visual evolution, any reduction in vision resulting from a blurred retinal image, including anisometropia, strabismus, and deprivation amblyopia (such as congenital cataract), is considered amblyopia.2 A number of studies have indicated amblyopia during the neural plasticity period early in life.3 Amblyopia is not just a simple reduction in visual acuity but rather a complex mechanism of brain information-processing deficit accompanied by irreversible risks to the life of the indivisual.4,5 In addition, the clinical prevalence of esotropia and anisometropia is of significance.6
The mechanism of vision loss in the amblyopic eye is related to unequal competing data reaching the primary visual cortex.7 While understanding the neural nature of amblyopia has been the subject of various investigations over many years, given the amblyopic relationship with the spatial properties of the neurons in the primary visual cortex, neurophysiological researchers believe that such damage can be observed in higher visual pathway centers.1 Neurological research evaluating glucose metabolism and cerebral blood flow has also yielded evidence of malfunction in the visual cortex of individuals with amblyopia.8,9
The visually evoked potential (VEP) test records the activity of the primary visual cortex (V1) in response to visual stimuli and is one of the current techniques used to understand the complicated mechanism of amblyopia.7,10 Central visual pathways transmit the visual input in a parallel manner via the 2 main magnocellular and parvocellular pathways.11
Parvocellular pathways are sensitive to high-spatial-frequency stimuli and low temporal frequencies, while magnocellular pathways are sensitive to low-spatial-frequency stimuli and high temporal frequencies.12 It seems that it is possible to investigate the effect of amblyopia on the function of these 2 pathways using the change in the temporal and spatial-frequency properties of the stimuli.4
Amblyopia, as one of the controversial challenges in vision studies, has been investigated by many researchers drawing upon the differences in the sensitivity of the neural vision pathways in the amblyopia syndrome. The findings of such studies have been both consistent13-18 and inconsistent.19-22 Despite extensive studies involving the neurological investigation of human and animal models, however, no one right answer is available to the question of where and how changes in visual communication lead to amblyopia.23 These results are controversial and, in some cases, inconsistent, thereby indicating that further studies are needed to understand the complex mechanism of amblyopia in various populations. We aimed to compare the wave amplitude of VEP between patients with esotropic and anisometropic amblyopic eyes and a normal group.
Patients and Methods
In the present study, 58 participants with ages ranging from 4 to 14 years were selected by convenience sampling. The study comprised 43 patients with unilateral amblyopia (28 with anisometropia and 15 with esotropia) and 15 persons with normal visual acuity (via random sampling). The participants were recruited from governmental ophthalmological centers (Poostchi Eye Clinic and Motahari) and nongovernmental centers (Maaliabad Optometry and Vision-Therapy) in Shiraz. The procedure was explained to all the participants, and informed consent was obtained from their parents. All the participants were examined under refraction conditions with and without eye drops (cyclopentolate 0.5%). In strabismus cases, suppression was checked with the Worth 4-dot test, and anomalous corresponding was tested with the Bagolini test.
The participants were examined for the following inclusion criteria: clear media, normal fundus, at least a 2-line difference with the best optical correction in the visual acuity test between the eyes, and age between 4 and 14 years. Additionally, the participants had more than 1.00 diopter difference in anisometropia between the eyes with amblyopic vision reduction, visual acuity of 0.3–0.7 LogMAR in the amblyopic eye after best optical correction, and vision better than 0.1 LogMAR in the non-amblyopic eye as tested by the standard Snellen distance chart (Abtahi Medicine) and the Yang acuity chart. All the cases were either diagnosed as esotropia without optical correction or diagnosed as esotropia, esophoria, microtropia, or eccentric fixation; they all received proper optical corrections. The esotropic group had vision loss in the deviated eye. None of the participants had taken part in previous studies. All the patients had refractive errors <0.52±0.24. All the individuals in the control group had full vision. The exclusion criteria consisted of pathological complications as determined by ophthalmological evaluations, presence of a history of neurological diseases, previous eye surgery, abnormal retinal correspondence with the Bagolini test, and vertical deviations secondary to surgery. The participants’ fixation was carefully evaluated by direct ophthalmoscopy. All the subjects were matched in terms of age, gender, refractive error, and visual acuity.
Pattern reversal VEPs were recorded using the Roland RETI system with spatial frequencies of 15 and 60 minutes of arc and contrasts of 30 and 100% in 1 eye for each participant. Temporal frequency was considered as 1.5 Hz for all the tests. Electrodes were placed according to the instruction of the International Society of Electrophysiological Vision (ISCEV). The tested eyes were optically corrected, and refractive correction was used during recording. The participants were instructed to maintain fixation at the center of the stimulus located at a distance of 100 centimeters on a 20×30 centimeter black-and-white video display monitor. The stimulus was displayed with a pattern reversal rate of 1.5 times per second. The VEP recording was repeated 3 times in cases where participant cooperation was poor. The fixation stability of the eyes was monitored closely by an experienced technician. The same conditions were observed for all the VEP recordings. The collected data were analyzed using a connected computer. P100 wave amplitude was measured for each check-size stimulus in 2 different contrasts for each eye, and the pattern VEP was recorded. In each recording, 200 sweeps were averaged. All the VEP tests were performed at the Electrophysiology Laboratory of Poostchi Ophthalmology Research Center, an eye research center in Shiraz, Iran. Finally, the data were analyzed using Statistical Package for the Social Sciences (SPSS), version 15. A P value =0.05 was considered statistically significant. The statistical analyses were performed using the one-way analysis of variance (ANOVA) for the comparison of the 3 groups and the independent t-test for between-group comparison.
Results
The study population was comprised of 58 participants: 43 with amblyopia and 15 with normal vision. The following tables and figures show the means and SDs of the wave amplitude of P100 in the 2 amblyopic groups of esotropia and anisometropia and the control group as well as the mean percentage of the difference between the 2 amblyopic groups and the normal group (mean % difference). According to the one-way ANOVA, the P value of the mean of the amplitude between all the cases was significant. These values in high spatial frequency with high- and low-contrast conditions were 0.002 and 0.000, respectively, while they were 0.012 and 0.035, correspondingly, in low spatial frequency with high- and low-contrast conditions (table 1).
Table 1.
Comparison of the mean±SD of P100 amplitude (µV) between the group with anisometropic amblyopic eyes, group with esotropic amblyopic eyes, and normal group
| Mean P100 amplitude (µV) | P value | |||
|---|---|---|---|---|
| Mean±SD | ||||
| Anisometropic amblyopia | Esotropic amblyopia | Normal eyes | ||
| Checksize stimuli of 15 min – 100% contrast | 20.5393±7.89338 | 16.7533±8.81894 | 28.9360±11.76226 | 0.002 |
| Checksize stimuli of 15 min – 30% contrast | 11.8332±6.07355 | 11.8360±4.86075 | 20.8327±7.98315 | 0.000 |
| Checksize stimuli of 60 min – 100% contrast | 26.7964±8.89334 | 20.3527±12.42690 | 32.8867±13.19269 | 0.012 |
| Checksize stimuli of 60 min – 30% contrast | 16.3025±4.55289 | 14.7420±6.90307 | 19.9820±5.86053 | 0.035 |
Comparison of P100 Amplitude between the Group with Anisometropic Amblyopic Eyes and the Normal Group
The P100 amplitude of the amblyopic eyes in the group with anisometropia in small check-size stimuli was considerably shorter than that in the control group (P<0.001) (table 2, figures 1 and 2). The percentage of the mean difference of P100 amplitude (mean % difference) in the group with anisometropia, as compared with the normal amplitude, was mainly reduced (table 3). While the amplitude recorded in the anisometropic eyes with large check-size stimuli in both contrast conditions was significantly lower than normal, it was statistically significant only in low-contrast condition (P=0.028) (table 2, figure 3) and it was not significant in high-contrast condition (P=0.124) (table 2, figure 4).
Table 2.
Comparison of the mean±SD of P100 amplitude (µV) between the group with anisometropic amblyopic eyes and the normal group
| Mean P100 amplitude (µV) | P value | ||
|---|---|---|---|
| Mean±SD | |||
| Group with anisometropia | Control group | ||
| Checksize stimuli of 15 min – 100% contrast | 20.5393±7.89338 | 28.9360±11.76226 | 0.008 |
| Checksize stimuli of 15 min – 30% contrast | 11.8332±6.07355 | 20.8327±7.98315 | <0.001 |
| Checksize stimuli of 60 min – 100% contrast | 26.7964±8.89334 | 32.8867±13.19269 | 0.124 |
| Checksize stimuli of 60 min – 30% contrast | 16.3025±4.55289 | 19.9820±5.86053 | 0.028 |
Figure 1.
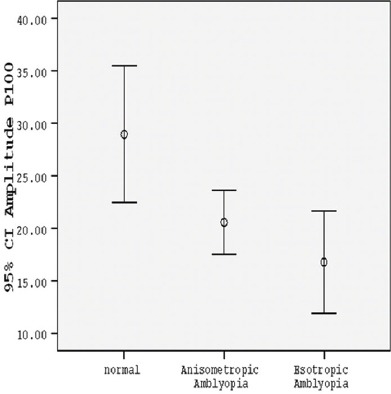
Comparison between the normal group, group with anisometropic amblyopia, and group with esotropic amblyopia according to mean P100 amplitude with small check-size stimuli in 100% contrast.
Figure 2.
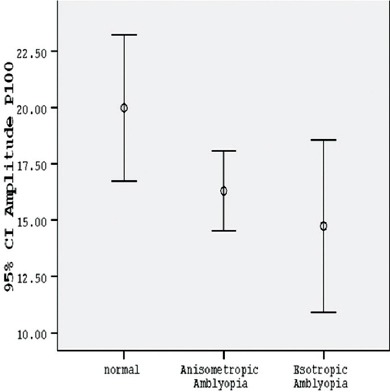
Comparison between the normal group, group with anisometropic amblyopia, and group with esotropic amblyopia according to mean P100 amplitude with small check-size stimuli in 30% contrast.
Table 3.
Mean difference from normal of the P100 amplitude (µV) of the anisometropic and esotropic amblyopic eyes
| P100 amplitude (µV) | ||||||
|---|---|---|---|---|---|---|
| Amplitude 15 minutes – 100% contrast | Amplitude 15 minutes – 30% contrast | |||||
| ANOVA analysis | ANOVA analysis | |||||
| Mean % difference from normal | t | P value | Mean % difference from normal | t | P value | |
| Anisometropic amblyopia | −8.39671 | 2.793 | 0.008 | −8.99945 | 4.145 | 0.001> |
| Esotropic amblyopia | −12.18267 | 3.209 | 0.003 | − 8.99667 | 3.728 | 0.001 |
| Amplitude 60 minutes – 100% contrast | Amplitude 60 minutes – 30% contrast | |||||
|---|---|---|---|---|---|---|
| Mean % difference from normal | t | P value | Mean % difference from normal | t | P value | |
| Anisometropic amblyopia | −6.09024 | 1.603 | 0.124 | −3.67950 | 2.283 | 0.028 |
| Esotropic amblyopia | −12.53400 | 2.678 | 0.012 | −5.2400 | 2.241 | 0.033 |
Figure 3.
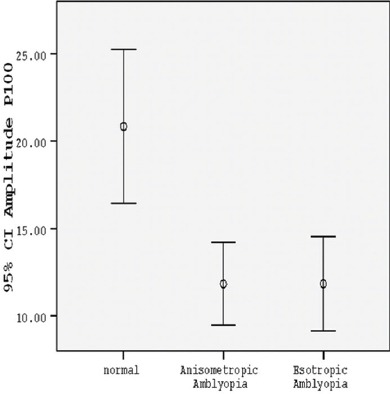
Comparison between the normal group, group with anisometropic amblyopia, and group with esotropic amblyopia according to mean P100 amplitude with large check-size stimuli in 30% contrast.
Figure 4.
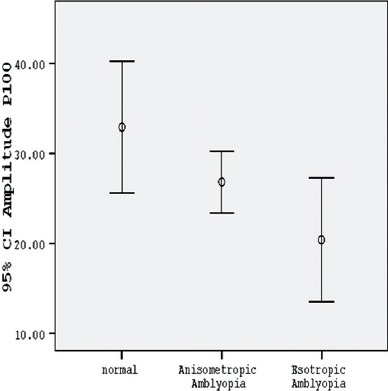
Comparison between the normal group, group with anisometropic amblyopia, and group with esotropic amblyopia according to mean P100 amplitude with large check-size stimuli in 100% contrast.
Comparison of P100 Amplitude between the Group with Esotropic Amblyopic Eyes and the Normal Group
The P100 amplitude of the esotropic amblyopic eyes, when compared with that in the normal group, with small check-size stimuli was mainly less than normal (P<0.001) (table 4, figures 1 and 2). Additionally, in larger check-size stimuli, these changes were statistically significant in high-and low-contrast conditions (P=0.012 and P=0.033, respectively) (table 4, figures 3 and 4). As is depicted in table 3, with the use of larger check-size stimuli, the percentage of the mean difference of the amplitude was associated with a greater reduction in using high-contrast condition than low-contrast condition (P=0.012). Nonetheless, in low-contrast condition, the amplitude was also statistically significant (P=0.033) (table 3, figures 3 and 4). In fact, the P100 amplitude reduction of the amblyopic eyes in the esotropic group, as compared with the normal group, in both spatial frequencies and contrasts was recorded as statistically significant.
Table 4.
Mean±SD of P100 amplitude (µV) between the group with esotropic amblyopic eyes and the normal group
| Mean P100 amplitude (µV) | P value | ||
|---|---|---|---|
| Mean±SD | |||
| Group with esotropia | Control group | ||
| Checksize stimuli of 15 min – 100% contrast | 16.7533±8.81894 | 28.9360±11.76226 | 0.003 |
| Checksize stimuli of 15 min – 30% contrast | 11.8360±4.86075 | 20.8327±7.98315 | 0.001 |
| Checksize stimuli of 60 min – 100% contrast | 20.3527±12.42690 | 32.8867±13.19269 | 0.012 |
| Checksize stimuli of 60 min – 30% contrast | 14.7420±6.90307 | 19.9820±5.86053 | 0.033 |
Comparison of P100 Amplitude between the Anisometropic and Esotropic Amblyopic Eyes
According to table 5, in small check-size stimuli and low-contrast condition, the response of both amblyopic groups was very similar. In the other stimuli, although the mean of the amplitude of the esotropic group was smaller than that of the anisometropic group, no statistical difference was shown (table 5).
Table 5.
Comparison of the mean±SD of P100 amplitude (µV) between the group with esotropic eyes and the group with anisometropic eyes
| Mean P100 amplitude (µV) | P value | ||
|---|---|---|---|
| Mean±SD | |||
| Group with anisometropia | Group with esotropia | ||
| Checksize stimuli of 15 min – 100% contrast | 20.5393±7.89338 | 16.7533±8.81894 | 0.158 |
| Checksize stimuli of 15 min – 30% contrast | 11.8332±6.07355 | 11.8360±4.86075 | 0.999 |
| Checksize stimuli of 60 min – 100% contrast | 26.7964±8.89334 | 20.3527±12.42690 | 0. 089 |
| Checksize stimuli of 60 min – 30% contrast | 16.3025±4.55289 | 14.7420±6.90307 | 0.378 |
Discussion
Amblyopia is considered a functional cortical disorder7 and has been studied over many years by a variety of techniques such as electrophysiology, functional magnetic resonance imaging, and positron emissiontomography.8,9,15,20-27
In the current study, a comparison of the mean of P100 amplitude between all the participants indicated a significant decrease in P100 amplitude (table 1), which is consistent with similar results reported in previous studies.17,24,28,29 Most studies have mainly focused on latency parameters, and the amplitude of VEP in patients with amblyopia has rarely been described. However, according to our findings, the sensitivity of the amplitude parameter in the evaluation of amblyopia can be considerable.
As is shown in tables 1 to 4, the amplitude of VEP using all stimuli showed a significant reduction in the amblyopic eye in the patients with esotropia as compared to the group with normal visual acuity. Nevertheless, previous studies have mentioned a lack of sensitivity in the changes in the amplitude in patients with esotropia and suggested that the amplitude is affected only in patients with anisometropic amblyopia.20 Other researchers have suggested that the amplitude of the pattern reversal VEP can be decreased only with a small pattern stimulation.29
The effect of stimuli on the central visual pathways has been extensively studied. Further stimulation of neurons with shorter axons and lower speed transmission is performed using high-spatial-frequency stimuli, which seems to be attributed to the parvocellular system. By contrast, the stimulation of neurons with long axons that transmit messages too quickly occurs through stimuli with low spatial frequency, which is probably called magnocellular.27
Our results suggested that the amplitude in the patients with esotropic eyes, compared to the normal group, was significantly reduced. In former studies of esotropic amblyopia, abnormal amplitude values have been recorded only by presenting small check sizes or high-spatial-frequency stimuli, implying parvocellular defects.19-28
More recent studies, however, have mentioned greater sensitivity to the P100 latency of the VEP test in amblyopia.16-20,27 According to these findings, it seems that amplitude sensitivity in amblyopia is also significant. Other studies have also reported the reduced amplitude in esotropic eyes.30
In comparing the patients with anisometropic amblyopia and the normal group, both the stimuli of the parvocellular pathway (100% contrast and high spatial frequency as the features of the parvocellular system) and the magnocellular pathway (30% contrast and low spatial frequency as the features of the magnocellular system) showed a significant reduction in the amplitude of the wave. This difference in the cell function has also been reported in other studies on patients with anisometropia.13 Studies by Shan et al.,21 however, mentioned only defects of the parvocellular system. Differences in the cortical function of patients with anisometropia have been revealed using functional magnetic resonance imaging.15
Conversely, as is shown in table 5, the mean P100 amplitude of the patients with esotropia, when compared to that in the group with anisometropia, exhibited a significant difference in response to high-contrast and large check-size stimuli compared to the other stimuli. Although these differences were not statistically significant, they indicated non-parallel information processing of the 2 groups compared with each other. These results revealed that these 2 groups of amblyopia did not follow the same neural patterns in their appearance and function. The absence of statistically significant differences in P100 amplitude between the 2 amblyopic groups may be due to our small sample size. Accordingly, it seems that high-contrast and large-sized stimuli may have induced different responses compared with the other stimuli in these participants. This finding could help practitioners select the proper stimuli to investigate the visual function in these 2 kinds of amblyopia groups.
Our research findings apropos the patients with anisometropia chime in with the results of the previous investigations conducted via electrophysiology techniques and positron emission tomography insofar as they indicated apparent damage to the parvocellular and magnocellular pathways. Nonetheless, there are inconsistencies with respect to esotropic eyes in the results of the previous studies.19,20,24,28
Although it is not possible to separate the parvocellular and magnocellular pathways completely, in light of the previous studies it can be suggested that defects in the entire frequency range imply the existence of both parvocellular and magnocellular pathway defects, while defects only in high spatial frequencies represent damage to the parvocellular pathway only.16
The small sample size and lack of multi-centrality can be deemed the salient limitations in the current study. Be that as it may, the strengths of our study lie in its meticulously rigorous inclusion and exclusion criteria and inclusion of reasonably statistically acceptable number of cases, most of whom usually tend to be uncooperative. We would suggest that further studies recruiting adequate numbers of subjects in multi-central locations be conducted to analyze the latency of VEP.
Conclusion
Differences in the electrophysiological responses of the patients with esotropic and anisometropic amblyopic eyes, compared with those of the control group, reflected some changes in the function of the cells in the visual system and the number of neurons transferring visual processing to the visual cortex of these participants. While these 2 types of amblyopia were similar in response to some stimuli, they showed differences in others, which is of great significance in terms of diagnosis and treatment.
Acknowledgement
This work was supported by a grant from Shiraz University of Medical Sciences (Grant # 91-01-01-5205). The authors wish to thank the Research Vice-Chancellorship of Shiraz University of Medical Sciences, Shiraz, Iran, for its financial support. The authors also would like to thank Dr. Nasrin Shokrpour at the Center for Development of Clinical Research of Namazee Hospital for editorial assistance.
Conflict of Interest: None declared.
References
- 1.Gharebaghi AH, Heidary F, Gharebaghi R, Heidary R. Mehdi-ODM;a modified digital monitoring of the occlusion therapy for amblyopia. Graefes Arch Clin Exp Ophthalmol. 2011;249:945–6. doi: 10.1007/s00417-010-1491-x. [DOI] [PubMed] [Google Scholar]
- 2.Chung W, Hong S, Lee JB, Han SH. Pattern visual evoked potential as a predictor of occlusion therapy for amblyopia. Korean J Ophthalmol. 2008;22:251–4. doi: 10.3341/kjo.2008.22.4.251. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tognini P, Manno I, Bonaccorsi J, Cenni MC, Sale A, Maffei L. Environmental enrichment promotes plasticity and visual acuity recovery in adult monocular amblyopic rats. PLoS One. 2012;7:e34815. doi: 10.1371/journal.pone.0034815. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sloper J. Amblyopia beyond acuity. J AAPOS. 2008;12:3–4. doi: 10.1016/j.jaapos.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 5.Holmes JM, Clarke MP. Amblyopia. Lancet. 2006;367:1343–51. doi: 10.1016/S0140-6736(06)68581-4. [DOI] [PubMed] [Google Scholar]
- 6.Simmers AJ, Ledgeway T, Hess RF, McGraw PV. Deficits to global motion processing in human amblyopia. Vision Res. 2003;43:729–38. doi: 10.1016/S0042-6989(02)00684-3. [DOI] [PubMed] [Google Scholar]
- 7.Halfeld Furtado de Mendonca R, Abbruzzese S, Bagolini B, Nofroni I, Ferreira EL, Odom JV. Visual evoked potential importance in the complex mechanism of amblyopia. Int Ophthalmol. 2013;33:515–9. doi: 10.1007/s10792-013-9734-6. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi MY, Lee DS, Hwang JM, Choi DG, Lee KM, Park KH, et al. Characteristics of glucose metabolism in the visual cortex of amblyopes using positron-emission tomography and statistical parametric mapping. J Pediatr Ophthalmol Strabismus. 2002;39:11–9. doi: 10.3928/0191-3913-20020101-05. [DOI] [PubMed] [Google Scholar]
- 9.Wong AM. New concepts concerning the neural mechanisms of amblyopia and their clinical implications. Can J Ophthalmol. 2012;47:399–409. doi: 10.1016/j.jcjo.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Tello C, De Moraes CG, Prata TS, Derr P, Patel J, Siegfried J, et al. Repeatability of short-duration transient visual evoked potentials in normal subjects. Doc Ophthalmol. 2010;120:219–28. doi: 10.1007/s10633-010-9216-3. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis AR, Sloper JJ, Neveu MM, Hogg CR, Morgan MJ, Holder GE. Differential changes of magnocellular and parvocellular visual function in early- and late-onset strabismic amblyopia. Invest Ophthalmol Vis Sci. 2006;47:4836–41. doi: 10.1167/iovs.06-0382. [DOI] [PubMed] [Google Scholar]
- 12.Lalor EC, Foxe JJ. Visual evoked spread spectrum analysis (VESPA) responses to stimuli biased towards magnocellular and parvocellular pathways. Vision Res. 2009;49:127–33. doi: 10.1016/j.visres.2008.09.032. [DOI] [PubMed] [Google Scholar]
- 13.Zele AJ, Pokorny J, Lee DY, Ireland D. Anisometropic amblyopia: spatial contrast sensitivity deficits in inferred magnocellular and parvocellular vision. Invest Ophthalmol Vis Sci. 2007;48:3622–31. doi: 10.1167/iovs.06-1207. [DOI] [PubMed] [Google Scholar]
- 14.Feng LX, Zhao KX. Study on anisometropic amblyopia by simultaneously recording multifocal VEP and multifocal ERG. Zhonghua Yan Ke Za Zhi. 2005;41:41–6. [PubMed] [Google Scholar]
- 15.Bonhomme GR, Liu GT, Miki A, Francis E, Dobre MC, Modestino EJ, et al. Decreased cortical activation in response to a motion stimulus in anisometropic amblyopic eyes using functional magnetic resonance imaging. J AAPOS. 2006;10:540–6. doi: 10.1016/j.jaapos.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Davis AR, Sloper JJ, Neveu MM, Hogg CR, Morgan MJ, Holder GE. Differential changes in color and motion-onset visual evoked potentials from both eyes in early- and late-onset strabismic amblyopia. Invest Ophthalmol Vis Sci. 2008;49:4418–26. doi: 10.1167/iovs.07-1437. [DOI] [PubMed] [Google Scholar]
- 17.Davis AR, Sloper JJ, Neveu MM, Hogg CR, Morgan MJ, Holder GE. Electrophysiological and psychophysical differences between early- and late-onset strabismic amblyopia. Invest Ophthalmol Vis Sci. 2003;44:610–7. doi: 10.1167/iovs.02-0240. [DOI] [PubMed] [Google Scholar]
- 18.Moschos MM, Margetis I, Tsapakis S, Panagakis G, Chatzistephanou IK, Iliakis E. Multifocal visual evoked potentials in amblyopia due to anisometropia. Clin Ophthalmol. 2010;4:849–53. doi: 10.2147/OPTH.S11762. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Demirci H, Gezer A, Sezen F, Ovali T, Demiralp T, Isoglu-Alkoc U. Evaluation of the functions of the parvocellular and magnocellular pathways in strabismic amblyopia. J Pediatr Ophthalmol Strabismus. 2002;39:215–21. doi: 10.3928/0191-3913-20020701-09. [DOI] [PubMed] [Google Scholar]
- 20.Heravian J, Daneshvar R, Dashti F, Azimi A, Ostadi Moghaddam H, Yekta AA, et al. Simultaneous pattern visual evoked potential and pattern electroretinogram in strabismic and anisometropic amblyopia. Iran Red Crescent Med J. 2011;13:21–6. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 21.Shan Y, Moster ML, Roemer RA, Siegfried JB. Abnormal function of the parvocellular visual system in anisometropic amblyopia. J Pediatr Ophthalmol Strabismus. 2000;37:73–8. doi: 10.3928/0191-3913-20000301-05. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Cui D, Zheng L, Yang X, Yang H, Zeng J. Combination of blood oxygen level-dependent functional magnetic resonance imaging and visual evoked potential recordings for abnormal visual cortex in two types of amblyopia. Mol Vis. 2012;18:909–19. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 23.Hess RF, Thompson B, Baker DH. Binocular vision in amblyopia: structure, suppression and plasticity. Ophthalmic Physiol Opt. 2014;34:146–62. doi: 10.1111/opo.12123. [DOI] [PubMed] [Google Scholar]
- 24.Oner A, Coskun M, Evereklioglu C, Dogan H. Pattern VEP is a useful technique in monitoring the effectiveness of occlusion therapy in amblyopic eyes under occlusion therapy. Doc Ophthalmol. 2004;109:223–7. doi: 10.1007/s10633-004-7098-y. [DOI] [PubMed] [Google Scholar]
- 25.Choi MY, Lee KM, Hwang JM, Choi DG, Lee DS, Park KH, et al. Comparison between anisometropic and strabismic amblyopia using functional magnetic resonance imaging. Br J Ophthalmol. 2001;85:1052–6. doi: 10.1136/bjo.85.9.1052. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li C, Cheng L, Yu Q, Xie B, Wang J. Relationship of visual cortex function and visual acuity in anisometropic amblyopic children. Int J Med Sci. 2012;9:115–20. doi: 10.7150/ijms.9.115. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parisi V, Scarale ME, Balducci N, Fresina M, Campos EC. Electrophysiological detection of delayed postretinal neural conduction in human amblyopia. Invest Ophthalmol Vis Sci. 2010;51:5041–8. doi: 10.1167/iovs.10-5412. [DOI] [PubMed] [Google Scholar]
- 28.Watts PO, Neveu MM, Holder GE, Sloper JJ. Visual evoked potentials in successfully treated strabismic amblyopes and normal subjects. J AAPOS. 2002;6:389–92. doi: 10.1067/mps.2002.129046. [DOI] [PubMed] [Google Scholar]
- 29.Krzystkowa KM, Kubatko-Zielinska A, Wojcik E, Strek W, Lebiedz J. Changes observed in electrophysiological investigations in amblyopia and strabismus. Klin Oczna. 1998;100:229–34. [PubMed] [Google Scholar]
- 30.Zhang W, Zhao K. Multifocal VEP difference between early- and late-onset strabismus amblyopia. Doc Ophthalmol. 2005;110:173–80. doi: 10.1007/s10633-005-4312-5. [DOI] [PubMed] [Google Scholar]


