Abstract
Black lipid membranes (BLMs) provide a synthetic environment that facilitates measurement of ion channel activity in diverse analytical platforms. The limited electrical, mechanical and temporal stabilities of BLMs pose a significant challenge to development of highly stable measurement platforms. Here, ethylene glycol dimethacrylate (EGDMA) and butyl methacrylate (BMA) were partitioned into BLMs and photopolymerized to create a cross-linked polymer scaffold in the bilayer lamella that dramatically improved BLM stability. The commercially available methacrylate monomers provide a simple, low cost, and broadly accessible approach for preparing highly stabilized BLMs useful for ion channel analytical platforms. When prepared on silane-modified glass microapertures, the resulting polymer scaffold-stabilized (PSS)-BLMs exhibited significantly improved lifetimes of 23 ± 9 to 40 ± 14 h and > 10-fold increase in mechanical stability, with breakdown potentials > 2000 mV attainable, depending on surface modification and polymer cross-link density. Additionally, the polymer scaffold exerted minimal perturbations to membrane electrical integrity as indicated by mean conductance measurements. When gramicidin A and α-hemolysin were reconstituted into PSS-BLMs, the ion channels retained function comparable to conventional BLMs. This approach is a key advance in the formation of stabilized BLMs and should be amenable to a wide range of receptor and ion channel functionalized platforms.
Keywords: Black Lipid Membrane, Lipid Bilayer, Polymer Scaffold, Methacrylate Polymer, Ion Channel, α-Hemolysin, Gramicidin
INTRODUCTION
Ion channels possess a number of desirable properties that make them useful for analytical applications, including ion selectivity, chemical or mechanical gating, inherent signal amplification, well-defined open and closed states and simple electrical readout.1,2,3 Suspended lipid bilayers, also known as black lipid membranes (BLMs), provide an important synthetic membrane environment to study the function and activity of ion channels and serve as key components of ion channel-functionalized analytical platforms.4,5 A major limitation of ion channel-functionalized sensor platforms is the ability to form BLMs with adequate electrical, mechanical,6 and temporal stabilities.7,8,9 BLM instability arises from the relatively weak noncovalent forces of interaction between lipid molecules in the membrane, which are insufficient to maintain the structure of BLMs under mechanical and electrical stresses.10 Additionally, the interaction forces between the lipid membrane and the underlying substrate significantly affect the temporal stability of BLMs.11,12
The development of robust BLMs has been a major research challenge. Methods have been developed to enhance the stability of BLMs including reducing aperture size,13 reducing the surface energy of aperture substrates,11 sandwiching the BLM between hydrogel layers,14 and chemical cross-linking by photopolymerization of reactive amphiphiles.15,16,17,18 Benz et al. pioneered the direct polymerization of lipid membranes as a method of stabilizing BLMs, and identified lipid compositions for developing synthetic ion channel-functionalized sensors.16 Reactive chemical functionalities can be introduced into the structure of lipid amphiphiles during synthesis to allow cross-linking at the lipid headgroup or within the lipid tail.17 The degree of cross-linking in polymeric membranes depends on the type of polymerizable lipid and method of polymerization used, and affects the fluidity and stability of the lipid membranes.10 While polymerization has significantly enhanced the stability of BLMs, rigid polymeric membranes inhibit the function of some ICs due to insufficient membrane fluidity.15 In addition, synthetic polymerizable lipids are difficult to utilize, few are commercially available, they are difficult and costly to produce, and have short shelf lives.
A number of approaches have been explored to address the challenge of membrane fluidity. Schmidt and coworkers created stable, long-lived BLM platforms for single-channel measurements by encapsulating a free-standing membrane within a polymerized gel in situ.14 Although the lifetime of the BLM was greatly enhanced, the method reduced the diffusive transport of the ion channel into the BLM by as much as 70%. BLMs have been prepared from mixtures of polymerizable and nonpolymerizable phospholipids which allowed adequate fluidity to observe normal ion channel activity.15,18 Shenoy and co-workers reported improved bilayer lifetime using a mixture of polymerizable and nonpolymerizable lipids,18 though wide fluctuations were observed in the lifetime of UV-irradiated BLMs due to variations in the amount of reactive polymerizable lipids that partitioned into the BLM. Heitz et al. demonstrated the preparation of highly stable BLMs from a mixture of polymerizable (bis-dienoyl phosphatidylcholine) and non-polymerizable 1, 2-diphytanoyl-sn-glycero-3-phosphocholine (DPhPC) lipids, a mixture that retained sufficient fluidity for reconstitution and proper function of ion channels.15 Meier et al. enhanced the electrical stability of free standing lipid membranes, in which BLMs were formed from a mixture of nonpolymerizable lipids and polymerizable styrene and divinylbenzene monomers,8 though the longevity of the BLMs, fluidity and compatibility with ion channel reconstitution were not investigated.
Here, we demonstrate a simple and cost effective method of improving the stability of BLMs from a mixture of nonpolymerizable lipids and commercially available, polymerizable methacrylate monomers that partition into the lamella region of the lipid bilayer. BLMs prepared in equimolar mixtures with nonlipid, hydrophobic methacrylate monomers were evaluated for their electrical, mechanical, and physical properties before and after UV photopolymerization. The results show dramatically enhanced BLM stability and maintenance of incorporated ion channel activity.
EXPERIMENTAL SECTION
Reagents and materials
Gramicidin A, ethylene glycol dimethacrylate (EGDMA), KCl, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) and α-hemolysin (α-HL) were purchased from Sigma-Aldrich (St. Louis, MO). Gramicidin A was diluted to 10 μg/mL in ethanol. Tridecafluoro 1, 1, 2, 2-tetrahydrodimethylchlorosilane (PFDCS) was purchased from Gelest, Inc. (Morrisville, PA). 3-cyanopropyldimethylchlorosilane (CPDCS) was purchased from TCI America, Inc. (Portland, OR). Anhydrous acetonitrile (ACN) and NaCl were purchased from EMD Chemical Inc. (Gibbstown, NJ). Ethanol was purchased from Decon Laboratories (King of Prussia, PA). Butyl methacrylate (BMA) was purchased from Alfa Aesar (Ward Hill, MA) and diethoxyacetophenone (DEAP) was purchased from Acros Organics (Pittsburgh, PA). 1, 2-diphytanoyl-sn-glycero-3-phosphocholine (DPhPC) lipid in chloroform was purchased from Avanti Polar Lipids, Inc. (Alabaster, AL). Nanopure water was obtained from a Barnstead EasyPure UV/UF purifier with resistivity of 18.3 MΩ cm.
Pipette aperture fabrication and surface modification
Borosilicate capillaries (1.5 outer diameter and 1.1 mm inner diameter) were purchased from World Precision Instruments, (Sarasota, FL) and were fabricated into pipette apertures with 25 – 30 μm diameter using a P-97 micropipette puller (Sutter Instruments, Novato, CA) and fire polished with a model MF-900 microforge (Narishige, East Meadow, NY) for BLM formation. A schematic of the pipette fabrication process is shown in Supporting Information, Figure S1. Glass pipette apertures were silane functionalized using a solution phase method reported previously.11 Briefly, glass pipettes were filled and submerged in 0.1 M HNO3 for 30 min followed by rinsing with H2O at least 3 times. The pipettes were rinsed with acetone and dried on a hot plate at 80 – 100 °C for 5 min or in an oven at 70 °C for 15 – 30 min. The pipettes were filled with then submerged in 2% v/v silane solution in either ACN (for CPDCS) or toluene (for PFDCS) for 6 – 12 h. Pipettes functionalized with CPDCS were rinsed with ACN while PFDCS-modified pipettes were rinsed with toluene. Finally, the resulting CPDCS- or PFDCS-functionalized pipettes were rinsed with ethanol and H2O. Only those silane-modified glass pipette apertures that showed a high success rate (> 80%) in the formation of BLMs, as monitored by repetitive formation and voltage-induced breakdown, were selected for use in further experiments.
Formation and characterization of BLMs
DPhPC dissolved in n-decane to a final concentration of 20 mg/mL was used to form BLMs by the painting method. Briefly, stock lipids suspended in chloroform were dried using compressed Ar followed by overnight vacuum. Conventional (unpolymerized) BLMs were prepared with this solution. Methacrylate-doped BLMs (MA-BLMs) describe BLMs prepared using a mixture of lipid, methacrylate monomers and photoinitiator in the absence of UV photopolymerization. PSS-BLMs describe MA-BLMs that were subsequently photopolymerized. For MA-BLMs and PSS-BLMs, DPhPC solutions were prepared with BMA, EGDMA and DEAP as follows. Initially, radical inhibitors were removed from BMA and EGDMA using an alumina column (Al2O3, 50–200 μm, 60 Å, Acros). The monomers were then combined in 1:1:1 ratios with DEAP to yield a solution referred to as a monomer mixture, followed by addition of one equivalent of lyophilized DPhPC to yield and overall mixture of 1:1:1:1 composition of BMA:EGDMA:DEAP:DPhPC. The lipid/monomer mixture was vortexed for 30 s prior to the addition of n-decane.
BLMs were formed by addition of 2 μL of lipid or lipid/monomer mixture solution dissolved in n-decane to the pipette tip and dried with N2 gas. Pipettes were back filled with recording buffer (1 M KCl, 5 mM HEPES, pH 7.4) and mounted on the head stage of a patch clamp amplifier (EPC-10, HEKA Electronics, Bellmore, NY). The bath chamber was filled with recording buffer and connected to the reference electrode via a salt bridge. The lipid or methacrylate-doped lipid solution was painted by gently sweeping a plastic micropipette tip across the silanized pipette aperture submerged in the recording buffer.
Formation of BLMs or MA-BLMs across silanized pipette apertures was monitored by the spontaneous increase in electrical resistance from open pipette resistance (50 – 100 KΩ) to > 2 GΩ. Further, the formation of BLM or MA-BLMs was verified by applying an increasing potential from 0 to 2000 mV in 10 mV increments of 50 ms duration. Additionally, the appearance of transient pores in BLMs under applied electrical fields was used to indicate the existence of BLMs prior to UV irradiation, although care was taken not to allow complete rupture of the BLM upon observation of transient pores. Subsequently, MA-BLMs were polymerized, forming PSS-BLMs, by UV irradiation using a pen lamp (UVP, Upland, CA, Model 90-0012-01) at a distance of 3 – 5 cm from the BLM.
The biophysical properties of conventional, MA- and PSS-BLMs were characterized by the reconstitution and measurement of gramicidin A or α-HL activity. A 0.5 μL aliquot of stock gramicidin peptide (10 μg/mL) in ethanol was added to 500 μL bath solution to a final concentration of 10 ng/mL and allowed to incubate with the BLMs. The activity of gramicidin was monitored with a potential of 70 mV applied across the BLMs. Quantized changes in current were typically observed within 2 min of adding gramicidin to the bath solution. Two μL of α-HL (0.5 mg/mL in recording buffer) was added to bath solution containing 500 μL of recording buffer and the insertion of IC measured a bias potential of + 40 mV across the BLM.
Conductance measurement
The conductance of conventional BLMs, MA-BLMs and PSS-BLMs was measured by applying a square wave of increasing potential from −100 to + 100 mV in 10 mV increments of 50 ms duration. The potential was held for 10 ms at 0 mV before and after applying each pulse. The average of the steady state current between 30 to 50 ms (following capacitive decay) was plotted versus the applied potential. The conductance is reported as the slope of the current versus potential plot and normalized for pipette aperture area, as a first approximation of BLM area. A minimum of three pipettes each were used for evaluating BLMs suspended across CPDCS or PFDCS-modified pipette apertures. For each pipette, a minimum of three BLMs was analyzed to determine the mean conductance.
Assessment of BLM stability
The stability of conventional, MA and PSS-BLMs was quantified by measuring breakdown voltage (VB), longevity and air-water transfer count (AWT). VB is the potential at which the BLM undergoes irreversible rupture, and is measured by applying an increasing potential from 0 to 2000 mV in 10 mV increments of 50 ms duration and observing the potential at which a large, non-linear increase in current occurs. The mean VB indicates the electrical stability of BLMs. AWT refers to the number of times a BLM survives transport across the air-water interface before it ruptures. To assess AWT, the aperture was removed from aqueous buffer and maintained in air for 1 s, prior to resubmersion in buffer. Each cycle of removal and return to buffer is indicated as one AWT. Longevity was measured as the average time required for the bilayer to undergo rupture under the application of a ± 5 mV 20 Hz square-wave.
Statistical Analysis
All data is presented as mean ± standard deviation. For each measurement, a minimum of three BLM replicates on at least three different pipettes were collected. For each BLM stability metric analyzed, outlying data was assessed using the Q test at the 90% confidence level. All statistical comparisons were performed using Student’s t-test at the 95% confidence interval.
Single-channel recordings with corresponding all-points histograms and mean open times were analyzed using TAC (X4.3.3) and TACfit X4.3.3 (Bruxton). The number of open/close events used to calculate mean open times for each condition is indicated in the relevant tables. The fit duration histogram for open probability and construction uses the Sigworth and Sine transformations.19
RESULTS AND DISCUSSION
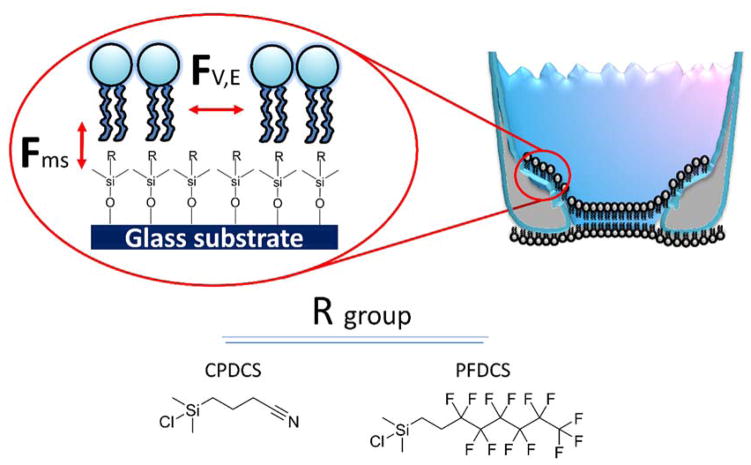
Figure 1 shows a schematic of a BLM suspended across a glass micropipette aperture functionalized with either CPDCS or PFDCS. Glass pipette aperture surfaces were first functionalized with either CPDCS or PFDCS to lower the surface energy, which is necessary to yield the tails-down lipid configuration required to form BLMs.11 The resulting membrane orientation across the modified glass pipette aperture is analogous to the folding of two lipid monolayers from opposite sides across Teflon apertures as described by Montal and Mueller.20 Decrease substrate surface energy enhances BLM stability by improving the force of interaction between the lipid membrane and the substrate (Fms).11,21,22 However, the weak van der Waals forces (FV) and electrostatic forces (FE) of interactions between adjacent lipid molecules limit the temporal, electrical, and mechanical stability of BLMs. For example, when BLMs are suspended across silane-functionalized glass apertures with decreased surface energy, the inherently weak forces of interaction between the lipid molecules in the self-assembled bilayer (Figure 1, FV,E) limit the average longevity of the BLMs formed on CPDCS- and PFDCS-modified apertures to 2 ± 1 h and 8 ± 1 h, respectively (Tables 1 and 2).11 To overcome these limitations, we investigated the fabrication of a polymer scaffold within BLMs suspended across silane functionalized glass apertures to provide additional stabilizing interactions. For these studies, BLMs were prepared on two silane modified surfaces chosen based on the prevalence of CPDCS modifications13,23 in the BLM literature and the recently demonstrated significantly enhanced BLM lifetimes provided by PFDCS.11
Figure 1. Schematic of a BLM on a modified pipette aperture.
A hybrid bilayer membrane (left) forms by the adsorption of a lipid monolayer membrane on silanized glass pipette aperture, where the R group is either CPDCS or PFDCS.11, 21 A bilayer forms across the aperture.
Table 1.
Physical and electrical properties of conventional BLMs, MA-BLMs, and PSS-BLMs on CPDCS-modified pipette apertures.
| BLM composition | UV (min) | VB (mV) | Normalized Conductance (×10−2 pS μm−2) | AWT | Longevity (h) |
|---|---|---|---|---|---|
| Conventional BLMs | 0 | 460 ± 21 | 5.5 ± 1.7 | 4 ± 3 | 2 ± 1 |
| 15 | 575 ± 124 | 6.0 ± 3.0 | 6 ± 4 | 2 ± 1 | |
| MA-BLMs | 0 | 493 ± 59 | 8.7 ± 0.5 | 14 ± 13 | 2 ± 2 |
| PSS-BLMs | 5 | 615 ± 107 | 10.7 ± 1.9 | > 50 | 23 ± 9 |
| 10 | > 2000 | 9.1 ± 0.8 | > 50 | 28 ± 16 |
Table 2.
Physical and electrical properties of conventional BLMs, MA-BLMs, and PSS-BLMs on PFDCS-modified pipette apertures
| BLM composition | UV (min) | VB (mV) | Normalized conductance (×10−2 pS μm−2) | Air – water transfer | Longevity (h) |
|---|---|---|---|---|---|
| Conventional BLMs | 0 | 984 ± 210 | 9.00 ± 2.00 | > 50 | 8 ± 1 |
| 15 | 980 ± 254 | 8.49 ± 0.91 | > 50 | 8 ± 1 | |
| MA-BLMs | 0 | 520 ± 119 | 4.95 ± 0.57 | 32 ± 11 | 6 ± 1 |
| PSS-BLMs | 5 | 1033 ± 182 | 4.81 ± 0.14 | 36 ± 13 | 26 ± 13 |
| 10 | > 2000 | 6.93 ± 1.08 | > 50 | 40 ± 14 |
To further improve the stability of BLMs for applications requiring high mechanical and temporal stability, we evaluated the integration of a polymer network into the lamella region of the BLM. We utilized a methacrylate polymer scaffold prepared from BMA and EGDMA, which has previously been shown to increase the stability of phospholipid vesicles.24–25 The BMA forms linear polymer chains that are cross-linked by the EGDMA to improve the polymer stability. While the resulting polymer does not covalently link the DPhPC monomers that form the BLM, we hypothesized that the enhanced structural stability provided by the polymer network would enhance BLM lifetime and mechanical stability. The resulting BLM architecture is referred to herein as a polymer scaffold-stabilized BLM (PSS-BLM). Figure 2 shows a schematic (not to scale) of the proposed monomer arrangement in the lamellar region of monomer doped BLMs, which is analogous to the packing of methacrylate monomers in the bilayer of lipid vesicles reported previously.24,25,26 The extent of stabilization achieved by PSS-BLMs was determined by measuring the electrical and physical properties of conventional BLMs and MA-BLMs before and after UV irradiation.
Figure 2. Schematic of methacrylate polymerization in the lamellar region of BLMs.
A. Small hydrophobic methacrylate monomers partition into the lamellar region of the lipid membrane. B. Chemical cross-linking of monomers occur when the photoinitiator (DEAP) is activated by UV-irradiation.
Physical and electrical properties of PSS-BLMs
To evaluate the physical and electrical properties of PSS-BLMs, the AWT, VB, and longevity were measured as metrics of the physical, electrical, and temporal stability, respectively. A higher value of AWT and VB indicates enhanced mechanical and electrical stability of the specific BLM composition.
The successful formation of a BLM was indicated when when VB was observed in the range of 0 – 1000 mV,11,15 prior to polymerization, as opposed to high resistance clogs which cannot be broken down in this range. The mean VB observed for conventional BLMs suspended across CPDCS-modified pipette apertures was 460 ± 21 mV, which agreed well with previous results.11 Furthermore, electrical, physical, and temporal stability were statistically similar before and after 15 min of UV irradiation of conventional BLMs, indicating no deleterious effects of UV exposure. When the monomer mixture was incorporated into the BLM in the absence of UV-irradiation to form MA-BLMs (Table 1), similar longevity and VB were observed compared to conventional BLMs. Though inclusion of the monomer mixture increases membrane conductance, the magnitude of the change is within the normal working range of BLMs on a range of aperture substrate materials. Upon cross-linking of MA-BLMs via UV irradiation for 5 min to yield PSS-BLMs, a >10 fold increase in AWT and longevity and a 30% increase in VB were observed compared to conventional BLMs. Additional improvements in electrical stability were observed upon increasing UV irradiation time to 10 min. Importantly, the membrane conductance, a key measure of membrane integrity, was statistically similar in MA-BLMs and PSS-BLMs irrespective of UV irradiation time.
Stability metrics for conventional BLMs on PFDCS-modified apertures were statistically similar before and after 15 min of UV irradiation (Table 2). Conventional BLMs formed on PFDCS-modified apertures exhibit marked stability increases compared to those formed on CPDCS apertures due to enhanced surface/lipid interactions. Unlike on CPDCS-modified apertures, MA-BLMs formed on PFDCS-modified apertures exhibited decreased electrical and mechanical stability as indicated by VB and AWT (47 % and 30% decreases, respectively) compared to conventional BLMs, though the magnitudes of these values are still comparable to BLMs formed on CPDCS-modified apertures. Thus, it is likely inclusion of the monomer mixture disrupts the lipid-surface interactions and yields BLM stabilities comparable to those formed on surfaces with weaker surface-lipid interactions.
Upon formation of PSS-BLMs via 5 min of UV irradiation, VB recovered to values equivalent to conventional BLMs; however, > 3 fold increase in longevity was observed compared to conventional BLMs. Furthermore, a 45 % reduction in membrane conductance was observed for MA-BLMs, and persisted in PSS-BLMs formed via 5 minutes of UV irradiation. When UV irradiated for 10 minutes, an increase in membrane conductance was observed, though the values were still lower than conventional BLMs prepared with this surface modification. Furthermore, VB and longevity were increased by > 2- and 5-fold, respectively, compared to conventional BLMs.
In addition to stability, we evaluated the noise characteristics of the conventional, MA- and PSS-BLMs. Table 3 presents the mean current and standard deviation for each BLM configuration in the presence and absence of gramicidin as well as for open and closed states of gramicidin (see below for more detailed description of gramicidin A reconstitution). In BLMs lacking gramicidin A baseline current values were stable and statistically similar (Figure S-5) with r.s.d. values of time-resolved current traces ranging from 9.5 – 12.5 %, with the higher r.s.d. values observed in the conventional BLMs. Similar trends were observed for reconstituted gramicidin A in both the open and closed states. Thus, the introduction of the polymer scaffold did not deleteriously effect the noise associated with the BLM current measurements supporting the potential utility for sensing using low conductance ion channels
Table 3.
Noise characteristics of conventional BLMs, MA-BLMs and PSS-BLMs on PFDCS modified pipettes.
| BLM Type | Gramicidin A | ||
|---|---|---|---|
| - | +/C | +/O | |
| DPhPC | 1.6 ± 0.20 | 1.8 ± 0.16 | 3.3 ± 0.15 |
| MA-BLM | 1.9 ± 0.18 | 1.9 ± 0.25 | 3.5 ± 0.20 |
| PSS-BLM | 1.8 ± 0.18 | 2.0 ± 0.20 | 3.6 ± 0.25 |
+ indicates addition of gramicidin A
O/C indicates open or closed state of gramicidin A
Based on the aggregate of the measurements, it appears that formation of the polymer scaffold exhibits no deleterious effects on membrane stability or membrane integrity within the BLM. In fact, PSS-BLMs formed via 5 min or 10 min of UV irradiation of MA-BLMs on CPDCS- and PFDCS-modified apertures yielded significantly improved membrane longevity and reduced membrane conductance with enhanced VB and little or no adverse effect on AWT. For membranes formed on both CPDCS and PFDCS-modified apertures, membrane conductance was unchanged when comparing MA-BLMs and PSS-BLMs formed via 5 minutes of UV irradiation. Though the conductance was increased with further irradiation time, the conductance was still lower than that obtained from a conventional BLM. The altered membrane conductance observed upon addition of monomer mixture may be due to alterations in the lipid packing or phase transition resulting from the mixed membrane composition and altered interactions between the membrane and the underlying substrate, although further study is needed to elucidate the underlying mechanisms in this complex environment.
Reconstitution of ion channels into PSS-BLMs
Robust ion channel-based biosensors and sequencing platforms necessitate high stability suspended lipid bilayers into which functional ion channels can be reconstituted. While PSS-BLMs showed significant stability improvements, the effects of UV irradiation and the presence of the polymer scaffold on ion channel function was a major concern for the application of this technology. Gramicidin A, a channel forming peptide that requires membrane fluidity to function, was reconstituted into conventional BLMs, MA-BLMs, and PSS-BLMs to probe the relationship between methacrylate cross-linking and ion channel function.
Gramicidin A forms ion-conducting channels by dimerization of peptide subunits that diffuse laterally within each monolayer leaflet of the lipid bilayer.27 Dimerization occurs via hydrogen bonding of the amino termini of each peptide subunit.28 The formation and dissociation of transmembrane dimers leads to quantized changes in transmembrane ion current, with conductance ranging from 21–24 pS.29 Since the length of a functional gramicidin pore (ca. 2.2 nm) is less than the thickness of a typical BLM (4–5 nm), the bilayer is locally deformed to facilitate the formation of conducting pores (shown schematically in Supporting Information, Figure S-2). 29,30,31,32 Kelkar et al. reported the structure and function of gramicidin in a lipid bilayer to be dependent upon the oriented dipole moments of the four C-terminal tryptophan residues of the peptide.33 Thus changes in membrane physiology upon PSS-BLM formation or UV degradation of C-terminal tryptophan residues may lead to disruption of gramicidin A function.
Gramicidin A was added to the cis side of a conventional BLM while applying a potential of 70 mV across the bilayer. Successful insertion and dimerization was indicated by quantized changes in ion current with amplitudes of ca. 1.5 pA. Ion channel conductance states were calculated by dividing the mean of each distribution in the all-points histogram (Supporting Information, Figure S-2) by the applied potential. The resulting conductance states were separated by 21 pS, characteristic of normal gramicidin activity.
Figure 3 shows the effect of UV irradiation on gramicidin A activity in conventional, MA-BLMs and PSS-BLMs. Following insertion of active gramicidin channels, BLMs were UV irradiated immediately after the verification of ion channel activity (Figure 3). On both CPDCS- and PFDCS modified apertures, a decrease in mean open time was observed in conventional BLMs after UV irradiation for 5 min, but no further change was observed when UV irradiation time was increased from 5 to 10 min (Table 4). Additionally, a decrease in mean channel conductance was observed, and the effect was much larger on PFDCS-modified apertures. The observed decrease in gramicidin conductance and mean open time after UV irradiation may be attributed to photodegradation of C-terminal tryptophan, resulting in decreased activity as reported previously.33 Ion channel activity was observed when gramicidin was reconstituted in MA-BLMs (Figure 3D); however, the mean conductance of gramicidin in MA-BLMs on CPDCS and PFDCS-modified apertures was reduced by 14% and 20%, respectively, compared to gramicidin in conventional BLMs (Table 4). Thus, the presence of the monomer mixture has a moderate but adverse effect on gramicidin A.
Figure 3. Gramicidin A activity in varying BLM configurations on CPDCS- (blue) and PFDCS- (red) modified apertures.
Conventional BLMs containing gramicidin A were UV irradiated for A. 0 min, B. 5 min, and C. 10 min. D. Gramicidin activity in MA-BLMs. E. Gramicidin activity in PSS-BLMs formed via 5 min of UV irradiation and F. PSS-BLMs formed via 10 min of UV irradiation.
Table 4.
Gramicidin A activity in conventional BLMs, MA-BLMs, and PSS-BLMs.
| BLM configuration | UV Time (min) | CPDCS
|
PFDCS
|
||
|---|---|---|---|---|---|
| Mean conductance (pS) | Mean open time (ms) | Mean conductance (pS) | Mean open time (ms) | ||
| Conventional BLMs | 0 | 21 ± 0.4 | 1067 ± 387 (91) | 21 ± 0.6 | 1091 ± 291 (83) |
| 5 | 19 ± 3 | 668 ± 170 (67) | 15 ± 2 | 625 ± 284 (64) | |
| 10 | 17 ± 3 | 653 ± 124 (45) | 14 ± 1 | 570 ± 197 (263) | |
| MA-BLMs | 0 | 19 ± 2 | 587 ± 313 (128) | 17 ± 4 | 556 ± 305 (305) |
| PSS-BLMs | 5 | 11 ± 1 | 118 ± 90 (16) | 14 ± 2 | 586 ± 162 (65) |
| 10 | NA | NA | 5 ± 1.4 | NA | |
NA = little or no ion channel activity observed.
n values are provide in parentheses for mean open time calculations
Upon 5 minutes of UV irradiation of MA-BLMs to form PSS-BLMs (Figure 3E), gramicidin conductance was reduced by 48% and 35% for CPDCS- and PFDCS-modified apertures, respectively, relative to conventional BLMs. Extending UV irradiation time to 10 min resulted in the near total loss of gramicidin activity (Figure 3F). Decreases in gramicidin conductance and mean open time upon PSS-BLM formation exceed those of UV-irradiated gramicidin in conventional BLMs, suggesting an additional mechanism of interference beyond photodegradation. Previous reports have shown that decreased bilayer fluidity decreases mean gramicidin conductance;34 thus we suspect that the observed decreases in conductance are the net result of both photodegradation and reduced bilayer fluidity upon formation of the polymer scaffold. Additionally, the presence of the monomer mixture in MA-BLMs and the polymer scaffold in PSS-BLMs may alter the physical or mechanical properties of the membrane, thus attenuating the capability of the BLM to locally deform to accommodate formation of functional gramicidin conducting pores.
In an effort to circumvent the deleterious effects of UV irradiation and/or monomer mixture on gramicidin conductance, gramicidin was reconstituted into pre-formed PSS-BLMs (Figure 4). Interestingly, when PSS-BLMs were formed via 5 min of UV irradiation, gramicidin readily reconstituted into the stabilized bilayers (Figure 4A) on both CPDCS- and PFDCS- modified apertures. Gramicidin conductance was only minimally affected under this condition (Table 5), with reductions of 14% and 3% for CPDCS and PFDCS-modified apertures, respectively, compared to conventional BLMs. While the exact nature of these observations remains to be elucidated, the observed reductions may result from interactions between gramicidin A peptides and unreacted methacrylate monomers, similar to the observations described for MA-BLMs.
Figure 4. Representative single-channel recordings of gramicidin A incubated in pre-formed PSS-BLMs suspended across CPDCS- (blue) and PFDCS-(red) modified apertures.
A. Gramicidin retains proper function when reconstituted in pre-formed PSS-BLMs polymerized via 5 min of UV irradiation. B. Incubation of gramicidin in pre-formed PSS-BLMs via 10 min of UV irradiation showed no evidence of ion channel reconstitution.
Table 5.
Gramicidin A activity when reconstituted in pre-formed PSS-BLMs
| BLM configuration | UV Time (min) | CPDCS
|
PFDCS
|
||
|---|---|---|---|---|---|
| Mean conductance (pS) | Mean open time (ms) | Mean conductance (pS) | Mean open time (ms) | ||
| PSS-BLMs | 5 | 19 ± 4 | 891 ± 147 (n = 153) | 20 ± 3 | 867 ± 167 (n = 223) |
| 10 | NA | NA | NA | NA | |
NA = no ion channel activity observed.
n values are provide in parentheses for mean open time calculations
When PSS-BLMs were formed via 10 min of UV irradiation (Figure 4B), no evidence of gramicidin reconstitution was observed. Combined, these results suggest that moderate UV irradiation times lead to partially polymerized methacrylate scaffolds that provide enhanced BLM stability and maintain bilayer fluidity adequate for peptide reconstitution and ion channel function. In contrast, excessive UV irradiation times (e.g., 10 min) result in more extensively polymerized scaffolds that decrease membrane fluidity and/or reduce the capability of the BLM to compress sufficiently to form functional gramicidin channels.
The observed number of functional ion channels provides an additional indication of the degree of fluidity and/or compressibility in PSS-BLMs. When gramicidin activity was observed in PSS-BLMs, no more than two active gramicidin channels were observed concurrently, compared to > 6 in both conventional and MA-BLMs. Thus the probability of forming a functional dimer is decreased upon increased photopolymerization. While the cause of this observation is unclear at present, it is likely that polymerization reduces the diffusion of gramicidin monomers in PSS-BLMs and that the net membrane area that is sufficiently compressible to form functional gramicidin monomers represents a small fraction of the overall membrane area.
Importantly, gramicidin reconstituted into PSS-BLMs maintained function for 7–9 h before permanent loss of peptide function was observed, possibly due to peptide denaturation (see Supporting Information, Figure S-3 for single channel recordings). Thus, with membrane lifetimes that exceed those of reconstituted ICs, PSS-BLMs may offer a route towards IC-based biosensors that are limited not by membrane stability, but by the active lifetimes of reconstituted membrane proteins.
To broaden the application of PSS-BLM ion channel platforms, we reconstituted α-HL, a pore forming channel with characteristic conductance of ca. 1 nS into the various BLM configurations.35,36 α-HL is routinely used to prepare stochastic sensors and nucleic acid sequencing platforms, thus it represents an important application for stabilized BLMs.37,35,38 Table 5 summarizes the results obtained for α-HL reconstituted into differing BLM configurations. In each case, the mean conductance values for α-HL were within the accepted experimentally measured values (see Supporting Information, Figure S-4 for single channel recordings), suggesting that UV irradiation or decreased membrane fluidity had no adverse effect on α-HL activity which agrees well with previous reports.23
Table 5.
α-HL activity in conventional BLMs, MA-BLMs, and PSS-BLMs on PFDCS-modified pipette apertures.
| BLM Configuration | UV time (min) | Mean conductance (nS) |
|---|---|---|
| Conventional BLMs | 0 | 0.80 ± 0.32 |
| 5 | 0.88 ± 0.11 | |
| 10 | 0.89 ± 0.090 | |
| MA-BLMs | 0 | 0.95 ± 0.034 |
| PSS-BLMs | 5 | 0.92 ± 0.033 |
| 10 | 0.93 ± 0.029 |
α-HL is a homoheptamer that requires insertion and assembly of the seven monomer units to form the functional channel.39 Thus, the membrane must retain sufficient fluidity to support diffusion and assembly of the channel subunits. Reconstitution of α-HL into pre-formed PSS-BLMs via 5 min of UV irradiation further confirms the existence of sufficient fluidity required for ion channel reconstitution and function, similar to that observed for gramicidin A, whereas no evidence of functional ion channel assembly was observed after extended cross-linking via 10 min of UV irradiation (Table 6). Overall, reconstitution of ion channels into pre-formed PSS-BLMs prepared via 5 min of photopolymerization show great promise for the construction of ion channel functionalized sensor technologies that may find a wide array of applications including DNA sequencing, small molecule sensing and beyond.
Table 6.
α-HL activity reconstituted in pre-formed PSS-BLMs on PFDCS-modified pipette apertures
| BLM Configuration | UV time (min) | Mean conductance (pS) |
|---|---|---|
| PSS-BLMs | 5 | 0.94 ± 0.068 |
| 10 | NA |
NA = no ion channel activity observed.
Importantly, UV-photopolymerization might also be useful to limit the number of α-HL insertions into the BLM. In typical α-HL reconstitutions, an excess of ion channel is added to the bath and immediately upon insertion of a functional channel, the bath is diluted. Thus polymerization of the PSS-BLM may provide an easily automated alternative approach for controlling ion channel insertion density, if the excessive electrical noise introduced by the UV lamp can be overcome..
Finally, it should be noted that while direct insertion of ion channels used here was readily achieved, insertion of more hydrophobic channels typically requires either surfactant dialysis or fusion of proteolysosomes. It remains to be determined if these approaches are compatible with the PSS-BLM strategy presented here.
CONCLUSION
We have demonstrated that improved stability of BLMs can be attained by chemically cross-linking methacrylate monomers within the lipid membranes to form PSS-BLMs. These stability improvements were shown to complement previously reported improvements via aperture surface chemistry modifications and with appropriate iterative optimization of the surface chemistry and polymer scaffold properties may lead to even greater enhancements. This approach is simpler, broadly applicable, less costly and more widely accessible compared to prior efforts utilizing reactive lipid monomers. PSS-BLMs can withstand potentials > 2000 mV without experiencing dielectric breakdown and show > 10 fold increase in measures of mechanical stability and >5 fold increase in BLM lifetime compared to conventional BLMs, with no deleterious effect on membrane integrity or structure. The average membrane lifetime was improved such that the lifetime of the reconstituted ion channel, gramicidin A, and not the BLM lifetime, was the fundamental limitation on sensor lifetime. PSS-BLMs offer stability advantages similar to those obtainable with polymerizable lipids but with few of the associated limitations. Thus, PSS-BLMs can offer substantial advantages for ion channel-based sensors and other BLM technologies, and may address the limitations of membrane stability on the development of these technologies.
Supplementary Material
Acknowledgments
We thank Jinyan Wang for assistance in the use of methacrylate monomers. Research reported in this publication was supported in part by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under award number R01EB007047 and the National Institute of General Medical Sciences of the National Institutes of Health under award number R01GM095763. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Footnotes
Additional description and data as indicated in the text, including additional information regarding formation of polymer scaffold stabilized BLMs, ion channel recordings and noise analysis. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Kasianowicz JJ. Introduction to Ion Channels and Disease. Chem Rev. 2012;112:6215–6217. doi: 10.1021/cr300444k. [DOI] [PubMed] [Google Scholar]
- 2.Mayer M, Semetey V, Gitlin I, Yang J, Whitesides GM. Using ion channel-forming peptides to quantify protein-ligand interactions. J Am Chem Soc. 2008;130:1453–1465. doi: 10.1021/ja077555f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moreau CJ, Dupuis JP, Revilloud J, Arumugam K, Vivaudou M. Coupling ion channels to receptors for biomolecule sensing. Nature Nanotech. 2008;3:620–625. doi: 10.1038/nnano.2008.242. [DOI] [PubMed] [Google Scholar]
- 4.Lathrop DK, Ervin EN, Barrall GA, Keehan MG, Kawano R, Krupka MA, White HS, Hibbs AH. Monitoring the escape of DNA from a nanopore using an alternating current signal. J Am Chem Soc. 2010;132:1878–1885. doi: 10.1021/ja906951g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Branton D, Deamer DW, Marziali A, Bayley H, Benner SA, Butler T, Di Ventra M, Garaj S, Hibbs A, Huang X. The potential and challenges of nanopore sequencing. Nature Biotechnology. 2008;26:1146–1153. doi: 10.1038/nbt.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oshima A, Hirano-Iwata A, Mozumi H, Ishinari Y, Kimura Y, Niwano M. Reconstitution of human ether-a-go-go-related gene channels in microfabricated silicon chips. Anal Chem. 2013;85:4363–4369. doi: 10.1021/ac303484k. [DOI] [PubMed] [Google Scholar]
- 7.Hirano-Iwata A, Oshima A, Nasu T, Taira T, Kimura Y, Niwano M. Stable lipid bilayers based on micro- and nano-fabrication. Supramolecular Chemistry. 2010;22:406–412. doi: 10.2116/analsci.28.1049. [DOI] [PubMed] [Google Scholar]
- 8.Meier W, Graff A, Diederich A, Winterhalter M. Stabilization of planar lipid membranes: A stratified layer approach. Phys Chem Chem Phys. 2000;2:4559–4562. [Google Scholar]
- 9.Hirano-Iwata A, Aoto K, Oshima A, Taira T, Yamaguchi R-t, Kimura Y, Niwano M. Free-standing lipid bilayers in silicon chips membrane stabilization based on microfabricated apertures with a nanometer-scale smoothness. Langmuir. 2010;26:1949–1952. doi: 10.1021/la902522j. [DOI] [PubMed] [Google Scholar]
- 10.Zhang H, Joubert JR, Saavedra SS. Membranes from Polymerizable Lipids. Adv Poly Sci. 2010;224:1–42. [Google Scholar]
- 11.Bright LK, Baker CA, Agasid MT, Ma L, Aspinwall CA. Decreased Aperture Surface Energy Enhances Electrical, Mechanical, and Temporal Stability of Suspended Lipid Membranes. ACS Appl Mater Interfaces. 2013;5:11918–11926. doi: 10.1021/am403605h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White RJ, Zhang B, Daniel S, Tang JM, Ervin EN, Cremer PS, White HS. Ionic conductivity of the aqueous layer separating a lipid bilayer membrane and a glass support. Langmuir. 2006;22:10777–10783. doi: 10.1021/la061457a. [DOI] [PubMed] [Google Scholar]
- 13.White RJ, Ervin EN, Yang T, Chen X, Daniel S, Cremer PS, White HS. Single ion-channel recordings using glass nanopore membranes. J Am Chem Soc. 2007;129:11766–11775. doi: 10.1021/ja073174q. [DOI] [PubMed] [Google Scholar]
- 14.Jeon TJ, Malmstadt N, Schmidt JJ. Hydrogel-encapsulated lipid membranes. J Am Chem Soc. 2007;128:42–43. doi: 10.1021/ja056901v. [DOI] [PubMed] [Google Scholar]
- 15.Heitz BA, Jones IW, Hall HK, Aspinwall CA, Saavedra SS. Fractional polymerization of a suspended planar bilayer creates a fluid, highly stable membrane for Ion channel recordings. J Am Chem Soc. 2010;132:7086–7093. doi: 10.1021/ja100245d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benz R, Elbert R, Prass W, Ringsdorf H. Polymerization in black lipid membranes. Eur Biophys J. 1986;14:83–92. [Google Scholar]
- 17.O’Brien DF, Armitage B, Benedicto A, Bennett DE, Lamparski HG, Lee YS, Srisiri W, Sisson TM. Polymerization of preformed self-organized assemblies. Acc Chem Res. 1998;31:861–868. [Google Scholar]
- 18.Daly SM, Heffernan LA, Barger WR, Shenoy DK. Photopolymerization of Mixed Monolayers and Black Lipid Membranes Containing Gramicidin A and Diacetylenic Phospholipids. Langmuir. 2005;22:1215–1222. doi: 10.1021/la052327p. [DOI] [PubMed] [Google Scholar]
- 19.Sigworth FJ, Sine SM. Data transformations for improved display and fitting of single-channel dwell time histograms. Biophy J. 1987;52:1047–1054. doi: 10.1016/S0006-3495(87)83298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montal M, Mueller P. Formation of Bimolecular Membranes from Lipid Monolayers and a Study of Their Electrical Properties. Proc Nat Acad Sci USA. 1972;69:3561–3566. doi: 10.1073/pnas.69.12.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plant AL. Supported hybrid bilayer membranes as rugged cell membrane mimics. Langmuir. 1999;15:5128–5135. [Google Scholar]
- 22.Gallagher ES, Adem SM, Bright LK, Calderon IAC, Mansfield E, Aspinwall CA. Hybrid phospholipid bilayer coatings for separations of cationic proteins in capillary zone electrophoresis. Electrophoresis. 2014;35:1099–1105. doi: 10.1002/elps.201300537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heitz BA, Xu J, Hall HK, Jr, Aspinwall CA, Saavedra SS. Enhanced long-term stability for single ion channel recordings using suspended poly (lipid) bilayers. J Am Chem Soc. 2009;131:6662–6663. doi: 10.1021/ja901442t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng Z, Aspinwall CA. Nanometre-sized molecular oxygen sensors prepared from polymer stabilized phospholipid vesicles. Analyst. 2006;131:236–243. doi: 10.1039/b511083a. [DOI] [PubMed] [Google Scholar]
- 25.Roberts DL, Ma Y, Bowles SE, Janczak CM, Pyun J, Saavedra SS, Aspinwall CA. Polymer-stabilized phospholipid vesicles with a controllable, pH-dependent disassembly mechanism. Langmuir. 2009;25:1908–1910. doi: 10.1021/la803358m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graff A, Winterhalter M, Meier W. Nanoreactors from polymer-stabilized liposomes. Langmuir. 2001;17:919–923. [Google Scholar]
- 27.Stimberg VC, Bomer JG, van Uitert I, van den Berg A, Le Gac S. High yield, reproducible and quasi-automated bilayer formation in a microfluidic format. Small. 2013;9:1076–1085. doi: 10.1002/smll.201201821. [DOI] [PubMed] [Google Scholar]
- 28.Jones D, Hayon E, Busath D. Tryptophan photolysis is responsible for gramicidin-channel inactivation by ultraviolet light. Biochim Biophys Acta (BBA) - Biomembranes. 1986;861:62–66. doi: 10.1016/0005-2736(86)90371-8. [DOI] [PubMed] [Google Scholar]
- 29.Andersen OS, Koeppe RE. Bilayer thickness and membrane protein function: an energetic perspective. Ann Rev Biophys Biomole Structure. 2007;36:107–130. doi: 10.1146/annurev.biophys.36.040306.132643. [DOI] [PubMed] [Google Scholar]
- 30.Lundbaek JA. Lipid bilayer-mediated regulation of ion channel function by amphiphilic drugs. J Gen Physiol. 2008;131:421–429. doi: 10.1085/jgp.200709948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Basu I, Chattopadhyay A, Mukhopadhyay C. Ion channel stability of Gramicidin A in lipid bilayers: Effect of hydrophobic mismatch. Biochim Biophys Acta-Biomembranes. 2014;1838:328–338. doi: 10.1016/j.bbamem.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Wu Y, He K, Ludtke SJ, Huang HW. X-ray diffraction study of lipid bilayer membranes interacting with amphiphilic helical peptides: diphytanoyl phosphatidylcholine with alamethicin at low concentrations. Biophys J. 1995;68:2361–2369. doi: 10.1016/S0006-3495(95)80418-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelkar DA, Chattopadhyay A. The gramicidin ion channel: A model membrane protein. Biochim Biophys Acta (BBA) - Biomembranes. 2007;1768:2011–2025. doi: 10.1016/j.bbamem.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 34.Besanger TR, Brennan JD. Ion Sensing and Inhibition Studies Using the Transmembrane Ion Channel Peptide Gramicidin A Entrapped in Sol Gel-Derived Silica. Anal Chem. 2003;75:1094–1101. doi: 10.1021/ac026258k. [DOI] [PubMed] [Google Scholar]
- 35.Bayley H, Cremer PS. Stochastic sensors inspired by biology. Nature. 2001;413:226–230. doi: 10.1038/35093038. [DOI] [PubMed] [Google Scholar]
- 36.Deamer DW, Branton D. Characterization of nucleic acids by nanopore analysis. Acc Chem Res. 2002;35:817–825. doi: 10.1021/ar000138m. [DOI] [PubMed] [Google Scholar]
- 37.Bayley H. Triggers and switches in a self-assembling pore-forming protein. J Cell Biochem. 1994;56:177–182. doi: 10.1002/jcb.240560210. [DOI] [PubMed] [Google Scholar]
- 38.Bayley H. Sequencing single molecules of DNA. Curr Op Chem Biol. 2006;10:628–637. doi: 10.1016/j.cbpa.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 39.Tomita T, Watanabe M, Yasuda T. Influence of membrane fluidity on the assembly of Staphylococcus aureus alpha-toxin, a channel-forming protein, in liposome membrane. J Biol Chem. 1992;267:13391–13397. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.