Abstract
Heritable ectopic mineralization disorders represent a phenotypically diverse group of conditions characterized by deposition of calcium phosphate complexes in soft connective tissues. The prototype of such conditions is pseudoxanthoma elasticum (PXE), and related conditions with overlapping clinical features include generalized arterial calcification of infancy (GACI) and arterial calcification due to CD73 deficiency (ACDC). Molecular genetic investigations have revealed mutations in the genes physiologically involved in generation of inorganic pyrophosphate (PPi) and phosphate (Pi), and the findings suggest a unifying pathomechanism relating to reduced PPi/Pi ratio. This hypothesis is based on the notion that PPi serves as a powerful inhibitor of mineralization while Pi is a pro-mineralization factor, and an appropriate PPi/Pi ratio is critical for prevention of ectopic mineralization under homeostatic conditions.
PXE International, the premiere patient support organization, advocating on behalf of patients and families with PXE, sponsors regular research meetings evaluating the progress in this and related ectopic mineralization disorders. The latest meetings were held in September 2014 in Bethesda, MD and in September 2015 in Budapest, Hungary. This report summarizes the latest progress in research on PXE and related ectopic mineralization disorders, based on presentations and discussions in these meetings, with pharmacologic implications for currently intractable disorders.
Introduction
Ectopic mineralization of soft connective tissues, particularly the cardiovascular system, is globally a major cause of morbidity and early mortality (Budoff et al., 2007). The pathomechanisms leading to deposition of mineral complexes in these tissues are exceedingly complex, but significant insights have been gained from observations in a group of heritable ectopic mineralization disorders with defined gene defects (Li and Uitto, 2013). The prototype of such conditions is pseudoxanthoma elasticum (PXE), a multi-system disorder characterized by deposition of calcium hydroxyapatite in various connective tissues, with protean manifestations (Neldner, 1988; Uitto et al., 2013). The classic form of PXE is of late-onset and slowly progressing, and the major clinical problems relate to loss of eyesight and development of vascular complications (Neldner, 1988).
Another, characteristically more severe ectopic mineralization disorder is the generalized arterial calcification of infancy (GACI), frequently diagnosed by prenatal ultrasound revealing vascular calcium deposits (Nitschke and Rutsch, 2012a; Rutsch et al., 2011). The newborns manifest with cardiovascular complications and many of these patients die within the first year of life, particularly if not receiving bisphosphonate treatment (see below). Finally, a more recently described condition, arterial calcification due to CD73 deficiency (ACDC), manifests with calcification of arterial blood vessels particularly in the lower extremities and in periarticular ligaments and tendons, typically encountered in older individuals (Markello et al., 2011; St Hilaire et al., 2011). Thus, these clinical entities all manifest with varying degrees of mineralization of the arterial blood vessels, with evidence of phenotypic overlap (Le Boulanger et al., 2010; Li et al., 2012c).
Genetic Bases and Phenotypic Modulation
Significant progress has recently been made in understanding the genetic basis of these heritable ectopic mineralization disorders (Table 1). Specifically, the classic form of PXE is now known to be caused by loss-of-function mutations in the ABCC6 gene encoding ATP-binding cassette subfamily C, member 6 (ABCC6), a putative transmembrane efflux transporter protein expressed primarily in the baso-lateral plasma membrane of hepatocytes and in the proximal tubules of the kidney (Belinsky and Kruh, 1999; Pomozi et al., 2014; Scheffer et al., 2002; Uitto et al., 2013). The molecules transported physiologically by ABCC6 from the intracellular milieu to extracellular space have not been identified as yet, but recent studies have demonstrated that ABCC6 is required for the release of ATP from the hepatocytes raising the question whether ATP, is the physiologic target molecule to be transported by ABCC6 (Jansen et al., 2014; Jansen et al., 2013).
Table 1.
Heritable human diseases with ectopic mineralization phenotypes and the corresponding mutated genes
| Disease* | Predominant organs affected | Clinicopathological features | Mutated genes |
|---|---|---|---|
| PXE | Skin, eyes and cardiovascular system | Late-onset, slowly progressive mineralization; loss of visual acuity and cardiovascular complications | ABCC6+, ENPP1 |
| PXE-like cutaneous findings | Skin | Mineral deposits in mid-dermis; loose and sagging skin | GGCX |
| GACI | Arterial blood vessels | Prenatal or early postnatal mineralization; demise <1 year of age | ENPP1+, ABCC6 |
| ACDC | Arterial blood vessels and joints | Calcification of lower extremity arteries, hand and feet joint capsules | NT5E |
Abbreviations: PXE, pseudoxanthoma elasticum; GACI, generalized arterial calcification of infancy; ACDC, arterial calcification due to CD73 deficiency.
These genes harbor mutations in the majority of cases with this disease.
PXE is characterized by considerable both intra- and inter-familial heterogeneity, suggesting a role for genetic and environmental modifying factors. Researchers are now taking candidate gene and whole exome sequencing approaches to further dissect out the effects of putative modifier genes on clinical presentations in PXE (Dabisch-Ruthe et al., 2014; Hendig et al., 2007; Zarbock et al., 2009). In addition, there are reports that alterations in lipid metabolism and soft tissue calcification are closely related pathologies, and in this context, the role of ABCC6 deficiency has been studied in lipoprotein and cholesterol homeostasis and in the development of atherosclerosis (Kuzaj et al., 2014; Le Saux et al., 2012; Trip et al., 2002).
Several studies performed on PXE fibroblasts have demonstrated that mesenchymal cells, whether locally producing pro- and anti-calcifying factors or being involved in extracellular matrix synthesis and degradation, are involved in the mineralization of soft connective tissues (Boraldi et al., 2014; Ronchetti et al., 2013). However, it is unclear at the present if all mesenchymal cells in PXE lesions behave similarly, or whether their tissue-specific differentiation contributes to different susceptibility of connective tissues to mineralization.
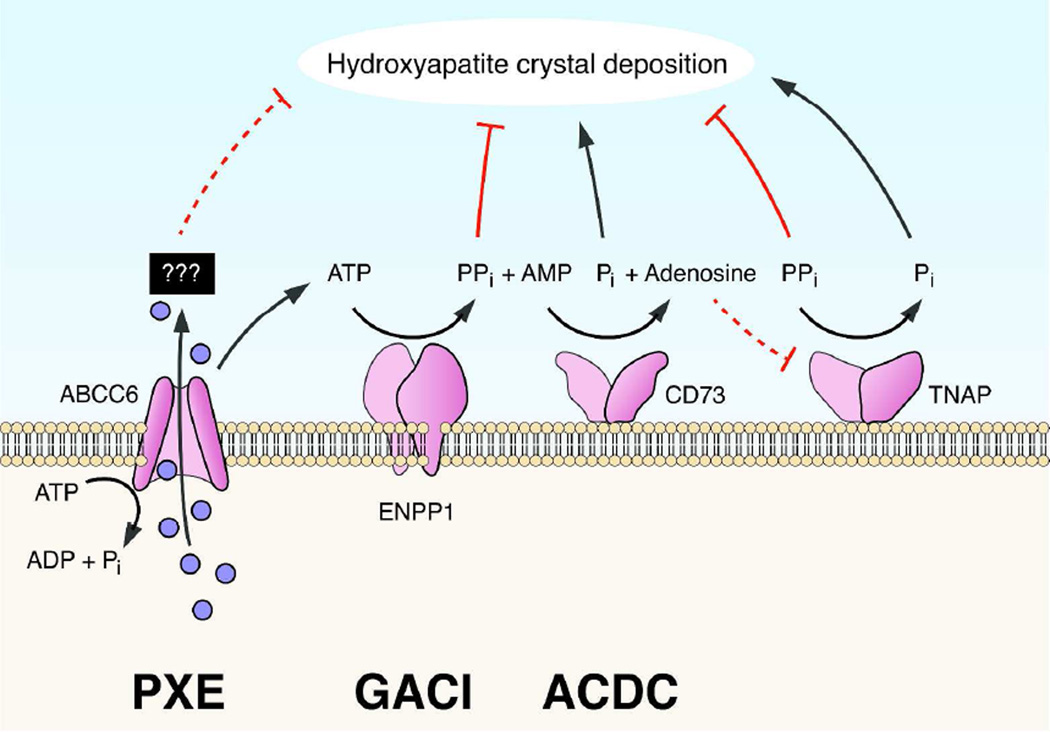
The classic form of GACI is caused by mutations in the ENPP1 gene which encodes ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1), an enzyme facilitating hydrolysis of ATP to AMP and inorganic pyrophosphate (PPi) (Fig. 1). PPi is a powerful anti-mineralization factor, while inorganic phosphate (Pi) serves as a pro-mineralization factor, and consequently, an appropriate ratio of PPi/Pi is required to prevent spontaneous calcium phosphate precipitation under normal homeostasis. In the absence of ENPP1, this ratio is reduced and ectopic mineralization ensues. Interestingly, recent studies have demonstrated that some patients with PXE-like clinical features harbor mutations in the ENPP1 gene while some patients clinically diagnosed as GACI have mutations in the ABCC6 gene (Li et al., 2012b; Nitschke et al., 2012). Therefore, these genotypic findings can explain the overlapping phenotypic features in some patients with ectopic mineralization disorders, pointing to shared pathomechanistic pathways leading to ectopic mineralization.
Figure 1.
Genetic complexity of the pro-mineralization/anti-mineralization network. Mutations in specific genes (also see Table 1) can result in deposition of hydroxyapatite crystals in tissues in heritable ectopic mineralization disorders: PXE, pseudoxanthoma elasticum; GACI, generalized arterial calcification of infancy; ACDC, arterial calcification due to CD73 deficiency. The solid blue circles represent currently unidentified molecules postulated to be transported under physiologic homeostasis by ABCC6 from the intracellular (IC) space to extracellular (EC) milieu. Note that release of ATP from the hepatocytes has been shown to depend on active ABCC6. (Modified from Li and Uitto, 2013, with permission).
Patients with ACDC with vascular calcification have been shown to harbor mutations in the NT5E gene which encodes CD73, a membrane-bound ecto-5’-nucleotidase that breaks down AMP to adenosine and Pi (Markello et al., 2011; St Hilaire et al., 2011). CD73 deficiency also increases the activity of tissue non-specific alkaline phosphatase (TNAP), accelerating conversion of PPi to Pi. Adenosine is a physiologic inhibitor of TNAP, and reduced adenosine concentration results in TNAP activation further facilitating the conversion of PPi to Pi (Fig. 1).
Molecular Diagnostics
In patients with PXE, the mutation database consists of ~300 distinct loss-of-function mutations in the ABCC6 gene, including recurrent p.R1141X and g.del23–29 mutations, which account for up to ~45% of all reported pathogenic alleles (Terry and Hefferson, 2013). It should be noted, however, that most of the findings have been reported in Caucasian patients from the United States and Europe, and for example, only recently, mutations in PXE patients of Asian ancestry have appeared in press. Specifically, a recent study on Chinese patients with PXE revealed mostly previously unreported mutations, and there is an apparent paucity of the recurrent p.R1141X and g.del23–29 mutations (Jin et al., 2015). This observation emphasizes the importance of studying patients of various ethnic backgrounds and different ancestries to allow development of allele-specific therapies for PXE.
Animal Models for Heritable Ectopic Mineralization Disorders
Significant progress in understanding of the pathomechanistic details leading to ectopic mineralization in these disorders has been made by examination of animal models, particularly mice (Nitschke and Rutsch, 2012b). Specifically, histopathologic examination of Abcc6 knock-out mice (Abcc6tm1JfK and Abcc6tm1Aabb) demonstrated the presence of mineral deposits in a number of tissues, including skin, eyes, and the cardiovascular system, similar to that in patients with PXE (Gorgels et al., 2005; Klement et al., 2005). In addition to genetically engineered knock-out mice, a number of inbred mouse strains, such as KK/HlJ, have been demonstrated to harbor a specific mutation in the Abcc6 gene which predisposes these animals to ectopic mineralization similar to the corresponding targeted knock-out mice (Berndt et al., 2013; Li et al., 2012a). It should be noted that the degree of mineralization in these different mouse strains is highly variable even under standardized laboratory conditions, attesting to the presence of putative modifier genes (Li et al., 2013a). In addition to the mouse models, a rat model through inactivation of Abcc6 gene using zinc finger nuclease technology has recently been developed. Preliminary data demonstrated mineralization of the dermal sheath of vibrissae of the homozygous mutant rat, similar to findings in Abcc6 knock-out mice (Li et al., unpublished).
Another animal model for PXE consists of a zebrafish which has an orthologous abcc6a gene. Knock-down of the abcc6a gene in 1–4 cell stage embryos by a morpholino, a stable antisense nucleotide eliciting suppression of expression of the targeted gene, results in profound phenotype consisting of pericardiac edema, stunted and curved tail (Li et al., 2010). This phenotype can be readily rescued by concomitant injection of human or mouse Abcc6 mRNA together with the morpholino, attesting to the specificity of the abcc6a phenotype. This model has provided a system to assess the pathogenicity of missense mutations in the human ABCC6 gene by testing whether such mutant mRNAs do or do not rescue the phenotype (Jin et al., 2015; Pomozi et al., 2014). In the absence of rescue, the missense mutation is deemed to be pathogenic, especially when combined with bioinformatics analysis.
In addition to the morpholino-mediated abcc6a knock-down system, a zebrafish grt mutant was recently identified in a forward genetic screen, with a p.L1429R mutation in the abcc6a gene (Mackay et al., 2015). In contrast to morpholino-injected embryos with early lethality, the grt mutant fish survived for at least one year. Both heterozygous and homozygous mutant fish show signs of excessive mineralization in the craniofacial region and axial skeleton, and infrequently also in the skin. In addition, abcc6a was found to be strongly expressed at the sites of mineralization rather than in the liver, as it is in human and mice. Therefore, the grt phenotype does not fully recapitulate that of humans with PXE due to bi-allelic mutations in the ABCC6 gene.
A number of animal models, particularly targeted and spontaneous mutant mice, have also been identified and characterized to recapitulate the features of GACI caused by ENPP1 mutations. ‘Tip toe walking’ (Enpp1ttw/Enpp1ttw) was first described as a spontaneous mutant mouse harboring a p.G568X nonsense mutation in the Enpp1 gene (Okawa et al., 1998). These mice exhibit accelerated bone formation, ossification of the spinal ligaments and ectopic mineralization of the aortic medial layer at the level of the internal elastic lamina. Enpp1tm1Gdg knock-out mice exhibit abnormalities similar to those in “tip toe walking” mice (Sali et al., 1999). Another Enpp1 mutant mouse (Enpp1m1Amgn), with a p.C397S missense mutation, has been characterized by low bone mineral density, crystal-related arthropathy and vascular calcification (Babij et al., 2009). A spontaneous mutant mouse with similar phenotype (Enpp1ttw-Ham) was recently identified in a colony in Japan. These mice were shown to harbor a splice-site mutation c.259+1G>T in Enpp1 gene (Takabayashi et al., 2014).
A mutant mouse with a missense mutation (p.V246D) in the Enpp1 gene was recently identified at The Jackson Laboratory as a result of ENU treatment (Li et al., 2013b). These mice demonstrated stiff posture, abnormalities in the front legs and stiffening of the joints; hence, this mutation was named ‘ages with stiffened joints’ (asj). The lack of ENPP1 enzymatic activity in asj homozygous mice results in reduced PPi levels in the plasma, accompanied by extensive mineralization of a number of tissues, including arterial blood vessels and the dermal sheath of vibrissae. Another mutant mouse, asj-2J, was also identified at The Jackson Laboratory with a phenotypic gait due to periarticular mineral deposits. This mouse was noted by histology to have extensive mineralization of the dermal sheath of vibrissae as well as arterial blood vessels. The asj-2J mice were shown to be allelic to asj mice by complementation studies, and these mice harbor of a large 40,035 bp deletion spanning from intron 1 to the 3’-untranslated region of the Enpp1 gene, coupled with a 74 bp insertion, thus completely eliminating functional gene in these mice (Li et al., 2014a). Thus, these targeted and spontaneous mutant mice display features of GACI, and can serve as a platform to explore treatment of this ectopic mineralization disorder.
Finally, a mouse model has been recently characterized with features of ACDC. This mouse (Nt5etm1JgSC), developed by ablation of the Nt5e gene (Castrop et al., 2004), showed stiffening of the joints, and micro CT revealed distinct changes in the thoracic skeletal structure with profound mineralization of the costochondral junctions (Li et al., 2014b). Mineralization was also noted in juxta-articular joint-capsules as well as ligaments adjacent to the bony structures, features in patients with ACDC. No evidence of vascular mineralization was noted, indicating that the Nt5e knock-out mice recapitulate some, but not all, features of ACDC. There was a markedly reduced PPi/Pi ratio in the plasma, attesting to the presence of a complex promineralization/ anti-mineralization network, characteristic to ectopic mineralization disorders, particularly in this case in ACDC.
The Unifying Concept of Pathomechanistic Pathways
The metabolic hypothesis concerning PXE postulates that the absence of functional ABCC6 activity, primarily in the liver, results in deficiency of circulating factor(s) that are physiologically required to prevent aberrant mineralization under normal calcium and phosphate homeostatic conditions (Jiang and Uitto, 2006). However, the physiological role of ABCC6 protein is currently unknown, and in particular, the identity of the factor(s) being transported in vivo has long remained a mystery. Recently, using cultured cells, ABCC6 was found to mediate the cellular release of ATP which is rapidly converted extracellularly into AMP and PPi (Jansen et al., 2014; Jansen et al., 2013). Outside hepatocytes, but still within the liver vasculature, released ATP is converted into AMP and PPi, revealing an unanticipated role of the liver in systemic PPi homeostasis. These findings raised the question whether ATP is the physiologic substrate of ABCC6, however, transport of ATP outside of the cells has not been demonstrated so far.
The genes mutated in PXE, GAC1, and ACDC, viz., ABCC6, ENPP1, and NT5E, respectively, are all part of the pathway that involves generation of PPi and Pi from ATP (Fig. 1). The unifying feature in these patients is reduction in the plasma PPi concentration resulting in reduced PPi/Pi ratio. Since PPi is a powerful anti-mineralization factor and the primary deficiency in ENPP1 or CD73 activity results in depletion of the PPi pool, it appears that these two conditions are pathomechanistically related. Furthermore, in the absence of functional ABCC6, the release of ATP to the extracellular milieu is reduced, potentially lowering the extracellular pool of ATP. In such situation, less ATP is available as a substrate for ENPP1, again resulting in reduced PPi plasma concentration (Fig. 1). In support of this hypothesis are recent demonstrations that PPi levels in the plasma of patients with PXE are reduced leading to lower PPi/Pi ratio (Jansen et al., 2014; Jansen et al., 2013). Thus, all three ectopic mineralization disorders, PXE, GACI and ACDC, with overlapping phenotypic features, share the deficiency in the same metabolic pathway. The phenotypic differences in these conditions may reflect the specific additional roles of the mutated genes when combined with modifying genetic factors and the environmental influences. Nevertheless, understanding the pathomechanistic alterations in these diseases may provide targets for pharmacologic modulation of the disease.
Development of Novel Treatment Modalities
No specific treatment modality is currently available for prevention of systemic ectopic mineralization in PXE or GACI. Remarkable success has been achieved, however, in the treatment of ocular complications in PXE using vascular endothelial growth factor antagonists (Lucentis and Avastin) which prevent neovascularization and preserve the vision (Myung et al., 2010; Verbraak, 2010). In fact, this approach has markedly reduced the incidence of the most severe complication of PXE, i.e., loss of vision, and has changed the focus of the disease to be largely on the skin and arterial vasculature.
The first attempts to develop treatment for systemic mineralization in PXE focused on modifications of the mineral content of the diet. Preclinical animal studies, using Abcc6 knock-out mice as a platform, demonstrated that a diet with elevated phosphorus and reduced magnesium content (so-called “acceleration diet”) enhanced the ectopic mineralization in this model, while conversely, increasing the magnesium content in the mouse diet by 5-fold completely abolished ectopic mineralization (Jiang and Uitto, 2012; LaRusso et al., 2009). Furthermore, recent studies have demonstrated that feeding of pregnant mice with the “acceleration diet” increases the mineralization of the newborn pups, with apparent relevance to GACI characterized by ectopic mineralization of blood vessels during fetal development (Li et al., 2015b). Based on the observations of the critical role of magnesium in modifying the degree of mineralization, a double-blinded placebo-controlled clinical trial with diet fortified with magnesium has been initiated in patients with PXE (https://clinicaltrials.gov/ct2/show/NCT01525875). This study has recently ended and data analysis has begun.
Considering the evidence in support of the notion that reduced PPi concentration is a critical factor allowing ectopic mineralization to ensue, a suggestion has been made that introduction of PPi would be helpful in prevention of the mineralization processes (O'Neill et al., 2011). Unfortunately, inorganic pyrophosphate is extremely labile and has a short half-life due to hydrolysis of the molecule. This has raised the possibility to use stable, non-hydrolysable pyrophosphate analogs, bisphosphonates, for treatment of ectopic mineralization disorders. In fact, bisphosphonates have been used for either prenatal or perinatal treatment of GACI patients, but careful examination of the literature reveals varying results, some studies showing apparent improvement, while in others very little, if any, effect is found (Edouard et al., 2011; Galletti et al., 2011; Rutsch et al., 2008). Furthermore, bisphosphonate administration has resulted in severe toxicity after protracted therapy (Otero et al., 2013). To explore the efficacy of bisphosphonates for treatment of ectopic mineralization disorders, recent studies have used Abcc6 knock-out and asj mice as a model of PXE and GACI, respectively (Li et al., 2015a; Li et al., 2015c).
In principle, bisphosphonates have two properties on mineralization: (i) anti-mineralization activity which is derived from incorporation of the bisphosphonate into calcium phosphate crystals preventing their growth, and (ii) anti-osteoclastic activity which prevents bone resorption. The latter activity is the basis for extensive use of bisphosphonates for treatment of osteoporosis and other disorders affecting bone, including Paget’s disease, bone metastases, osteogenesis imperfecta and multiple myeloma (Uludag, 2002). Bisphosphonates, etidronate or alendronate, demonstrated that they were able to prevent ectopic mineralization in Abcc6−/− or asj mice when administered either by mouth at relatively high concentrations or by subcutaneous administration in 100-times lower concentration (Li et al., 2015a; Li et al., 2015c). Interestingly, the microarchitecture of the bones, which is severely perturbed in the asj mice, was corrected with etidronate. Thus, there is a dual beneficial effect, i.e., inhibition of soft tissue mineralization and improvement in bone microarchitecture, which was achieved by bisphosphonate administration. It should be noted, that these beneficial effects were achieved with relatively high concentrations of bisphosphonates, and further long-term toxicity studies are required before this approach should be tested in clinical trials in humans, particularly concerning potential side effects.
The potential treatment of PXE has also recently been explored by a number of molecular and cell-based strategies. For example, the potential correction of nonsense mutations in the ABCC6 gene by read-through mechanism facilitated by PTC124, a non-aminoglycoside nonsense mutation suppressor, has been tested (Zhou et al., 2013). Preliminary studies demonstrated that PTC124 enhances read-through of stop codon mutations in this gene in cell culture systems. With respect to missense mutations with preserved transport activity but with improper plasma membrane localization of the protein, a chemical chaperone, 4-phenylbutyrate (4-PBA), a drug approved for clinical use for other indications, has been tested in PXE mice. The results revealed restoration of the plasma membrane localization of ABCC6 in mouse hepatocytes in a limited number of specific mutations, suggesting that allele-specific therapy may be useful for selected patients with PXE and GACI, i.e., those carrying missense mutations in their ABCC6 gene (Le Saux et al., 2011; Pomozi et al., 2014). Furthermore, transplantation of cells with hepatoblastic lineage differentiation, either representing a hepatoblast cell line or differentiated from induced pluripotent stem cells (iPSCs), has demonstrated homing of these cells to the liver and has resulted in ABCC6 protein expression in Abcc6 knock-out mice (Jiang et al., 2012).
Conclusions
Significant progress has been made in understanding the molecular basis and pathomechanistic pathways involved in heritable ectopic mineralization disorders, as exemplified by PXE, GACI and ACDC. This progress has now identified pathways that can potentially serve as pharmacologic targets towards treatment of these currently intractable disorders. While these heritable disorders are rare, study of these conditions has allowed significant insights into complex, much more common disorders. For example, utilization of mouse models of PXE, the level of hepatic expression of ABCC6 has been shown to determine the severity of calcification and the infarct size after cardiac injury, contribute to arterial calcification, and participate in development of chronic kidney disease (Brampton et al., 2014; Lau et al., 2014; Mungrue et al., 2011; Prunier et al., 2013). In that sense, the ABCC6 transporter system can be considered as a paradigm for extending the knowledge from rare heritable diseases to complex disorders (De Vilder et al., 2015). Finally, development of novel pharmacologic interventions for rare heritable ectopic mineralization disorders, such as PXE, may be applicable to disorders, such as arteriosclerosis, a much more common condition in general populations, with a major burden to the global healthcare systems.
Acknowledgements
Carol Kelly assisted in manuscript preparation. These meetings were supported by PXE International. Dr. Li is the recipient of a K01 AR064766 award from NIH/NIAMS.
Abbreviations
- PXE
pseudoxanthoma elasticum
- GACI
generalized arterial calcification of infancy
- ACDC
arterial calcification due to CD73 deficiency
- MGP
matrix Gla protein
Footnotes
Conflict of Interest
The authors state no conflict of interest.
References
- Babij P, Roudier M, Graves T, et al. New variants in the Enpp1 and Ptpn6 genes cause low BMD, crystal-related arthropathy, and vascular calcification. J Bone Min Res. 2009;24:1552–1564. doi: 10.1359/jbmr.090417. [DOI] [PubMed] [Google Scholar]
- Belinsky MG, Kruh GD. MOAT-E (ARA) is a full-length MRP/cMOAT subfamily transporter expressed in kidney and liver. Br J Cancer. 1999;80:1342–1349. doi: 10.1038/sj.bjc.6690527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt A, Li Q, Potter CS, et al. A single-nucleotide polymorphism in the Abcc6 gene associates with connective tissue mineralization in mice similar to targeted models for pseudoxanthoma elasticum. J Invest Dermatol. 2013;133:833–836. doi: 10.1038/jid.2012.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boraldi F, Annovi G, Bartolomeo A, et al. Fibroblasts from patients affected by Pseudoxanthoma elasticum exhibit an altered PPi metabolism and are more responsive to pro-calcifying stimuli. J Dermatol Sci. 2014;74:72–80. doi: 10.1016/j.jdermsci.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Brampton C, Aherrahrou Z, Chen LH, et al. The level of hepatic ABCC6 expression determines the severity of calcification after cardiac injury. Am J Pathol. 2014;184:159–170. doi: 10.1016/j.ajpath.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budoff MJ, Shaw LJ, Liu ST, et al. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007;49:1860–1870. doi: 10.1016/j.jacc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- Castrop H, Huang Y, Hashimoto S, et al. Impairment of tubuloglomerular feedback regulation of GFR in ecto-5'-nucleotidase/CD73-deficient mice. J Clin Invest. 2004;114:634–642. doi: 10.1172/JCI21851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabisch-Ruthe M, Brock A, Kuzaj P, et al. Variants in genes encoding pyrophosphate metabolizing enzymes are associated with Pseudoxanthoma elasticum. Clin Biochem. 2014;47:60–67. doi: 10.1016/j.clinbiochem.2014.07.003. [DOI] [PubMed] [Google Scholar]
- De Vilder EYG, Hosen MJ, Vanakker OM. The ABCC6 transporter as a paradigm for networking from an orphan disease to complex disorders. Biomed Res Int. 2015;2015 doi: 10.1155/2015/648569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edouard T, Chabot G, Miro J, et al. Efficacy and safety of 2-year etidronate treatment in a child with generalized arterial calcification of infancy. Eur J Pediatr. 2011;170:1585–1590. doi: 10.1007/s00431-011-1572-9. [DOI] [PubMed] [Google Scholar]
- Galletti S, Nitschke Y, Malavolti AM, et al. Generalized arterial calcification of infancy: Fatal clinical course associated with a novel mutation in ENPP1. JIMD Rep. 2011;1:23–27. doi: 10.1007/8904_2011_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgels TG, Hu X, Scheffer GL, et al. Disruption of Abcc6 in the mouse: novel insight in the pathogenesis of pseudoxanthoma elasticum. Hum Mol Genet. 2005;14:1763–1773. doi: 10.1093/hmg/ddi183. [DOI] [PubMed] [Google Scholar]
- Hendig D, Arndt M, Szliska C, et al. SPP1 promoter polymorphisms: identification of the first modifier gene for pseudoxanthoma elasticum. Clin Chem. 2007;53:829–836. doi: 10.1373/clinchem.2006.083675. [DOI] [PubMed] [Google Scholar]
- Jansen RS, Duijst S, Mahakena S, et al. ABCC6-mediated ATP secretion by the liver is the main source of the mineralization inhibitor inorganic pyrophosphate in the systemic circulation-brief report. Arterioscler Thromb Vasc Biol. 2014;34:1985–1989. doi: 10.1161/ATVBAHA.114.304017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen RS, Kucukosmanoglu A, de Haas M, et al. ABCC6 prevents ectopic mineralization seen in pseudoxanthoma elasticum by inducing cellular nucleotide release. Proc Nat Acad Sci USA. 2013;110:20206–20211. doi: 10.1073/pnas.1319582110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, Takahagi S, Uitto J. Administration of bone marrow derived mesenchymal stem cells into the liver: Potential to rescue pseudoxanthoma elasticum in a mouse model (Abcc6−/−) J Biomed Biotech. 2012;2012:818937. doi: 10.1155/2012/818937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, Uitto J. Pseudoxanthoma elasticum: a metabolic disease? J Invest Dermatol. 2006;126:1440–1441. doi: 10.1038/sj.jid.5700267. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Uitto J. Restricting dietary magnesium accelerates ectopic connective tissue mineralization in a mouse model of pseudoxanthoma elasticum (Abcc6−/−) Exp Dermatol. 2012;21:694–699. doi: 10.1111/j.1600-0625.2012.01553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Jiang Q, Wu Z, et al. Genetic heterogeneity of pseudoxanthoma elasticum: The Chinese signature profile of ABCC6 and ENPP1 mutations. J Invest Dermatol. 2015;135:1294–1302. doi: 10.1038/jid.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klement JF, Matsuzaki Y, Jiang QJ, et al. Targeted ablation of the Abcc6 gene results in ectopic mineralization of connective tissues. Mol Cell Biol. 2005;25:8299–8310. doi: 10.1128/MCB.25.18.8299-8310.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzaj P, Kuhn J, Dabisch-Ruthe M, et al. ABCC6- a new player in cellular cholesterol and lipoprotein metabolism? Lipids Health Dis. 2014;13:118. doi: 10.1186/1476-511X-13-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRusso J, Li Q, Jiang Q, et al. Elevated dietary magnesium prevents connective tissue mineralization in a mouse model of pseudoxanthoma elasticum (Abcc6−/−) J Invest Dermatol. 2009;129:1388–13894. doi: 10.1038/jid.2008.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau WL, Liu S, Vaziri ND. Chronic kidney disease results in deficiency of ABCC6, the novel inhibitor of vascular calcification. Am J Nephrol. 2014;40:51–55. doi: 10.1159/000365014. [DOI] [PubMed] [Google Scholar]
- Le Boulanger G, Labreze C, Croue A, et al. An unusual severe vascular case of pseudoxanthoma elasticum presenting as generalized arterial calcification of infancy. Am J Med Genet A. 2010;152A:118–123. doi: 10.1002/ajmg.a.33162. [DOI] [PubMed] [Google Scholar]
- Le Saux O, Fülop K, Yamaguchi Y, et al. Expression and in vivo rescue of human ABCC6 disease-causing mutants in mouse liver. PLoS One. 2011;6:e24738. doi: 10.1371/journal.pone.0024738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Saux O, Martin L, Aherrahrou Z, et al. The molecular and physiological roles of ABCC6: more than meets the eye. Front Genet. 2012;3:289. doi: 10.3389/fgene.2012.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Berndt A, Guo H, et al. A novel animal model for pseudoxanthoma elasticum - the KK/HlJ mouse. Am J Pathol. 2012a;181:1190–1196. doi: 10.1016/j.ajpath.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Brodsky JL, Conlin L, et al. Mutations in the ABCC6 gene can cause generalized arterial calcification of infancy in addition to pseudoxanthoma elasticum. J Invest Dermatol. 2012b;132:S91. doi: 10.1038/jid.2013.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Guo H, Chou DW, et al. Mouse models for pseudoxanthoma elasticum: Genetic and dietary modulation of the ectopic mineralization phenotypes. PLoS One. 2013a;9:e89268. doi: 10.1371/journal.pone.0089268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Guo H, Chou DW, et al. Mutant Enpp1asj mouse as a model for generalized arterial calcification of infancy. Dis Model Mech. 2013b;6:1227–1235. doi: 10.1242/dmm.012765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Kingman J, Sundberg JP, et al. Dual effects of bisphosphonates on ectopic skin and vascular soft tissue mineralization versus bone microarchitecture in a mouse model of generalized arterial calcification of infancy. J Invest Dermatol. 2015a doi: 10.1038/JID.2015.377. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Kingman J, Uitto J. Mineral content of the maternal diet influences ectopic mineralization in offspring of Abcc6 mice. Cell Cycle. 2015b doi: 10.1080/15384101.2015.1068473. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Pratt CH, Dionne LA, et al. Spontaneous asj-2J mutant mouse as a model for generalized arterial calcification of infancy: A large deletion/insertion mutation in the Enpp1 gene. PLoS One. 2014a;9:e113542. doi: 10.1371/journal.pone.0113542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Price TP, Sundberg JP, et al. Juxta-articular joint-capsule mineralization in CD73 deficient mice: Similarities to patients with NT5E mutations. Cell Cycle. 2014b;13:2609–2615. doi: 10.4161/15384101.2014.943567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Sadowski S, Frank M, et al. The abcc6a gene expression is required for normal zebrafish development. J Invest Dermatol. 2010;130:2561–2568. doi: 10.1038/jid.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Schumacher W, Siegel D, et al. Cutaneous features of pseudoxanthoma elasticum in a patient with generalized arterial calcification of infancy due to a homozygous missense mutation in the ENPP1 gene. Br J Dermatol. 2012c;166:1107–1111. doi: 10.1111/j.1365-2133.2012.10811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Sundberg JP, Levine MA, et al. The effects of bisphosphonates on ectopic soft tissue mineralization caused by mutations in the ABCC6 gene. Cell Cycle. 2015c;14:1082–1089. doi: 10.1080/15384101.2015.1007809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Uitto J. Mineralization/anti-mineralization networks in the skin and vascular connective tissues. Am J Pathol. 2013;183:10–18. doi: 10.1016/j.ajpath.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay EW, Apschner A, Schulte-Merker S. Vitamin K reduces hypermineralisation in zebrafish models of PXE and GACI. Development. 2015;142:1095–1101. doi: 10.1242/dev.113811. [DOI] [PubMed] [Google Scholar]
- Markello TC, Pak LK, St Hilaire C, et al. Vascular pathology of medial arterial calcifications in NT5E deficiency: Implications for the role of adenosine in pseudoxanthoma elasticum. Mol Genet Metab. 2011;103:44–50. doi: 10.1016/j.ymgme.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungrue IN, Zhao P, Yao Y, et al. Abcc6 deficiency causes increased infarct size and apoptosis in a mouse cardiac ischemia-reperfusion model. Arterioscler Thromb Vasc Biol. 2011;31:2806–2812. doi: 10.1161/ATVBAHA.111.237420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myung JS, Bhatnagar P, Spaide RF, et al. Long-term outcomes of intravitreal antivascular endothelial growth factor therapy for the management of choroidal neovascularization in pseudoxanthoma elasticum. Retina. 2010;30:748–755. doi: 10.1097/IAE.0b013e3181c596b1. [DOI] [PubMed] [Google Scholar]
- Neldner KH. Pseudoxanthoma elasticum. Clin Dermatol. 1988;6:1–159. doi: 10.1016/0738-081x(88)90003-x. [DOI] [PubMed] [Google Scholar]
- Nitschke Y, Baujat G, Botschen U, et al. Generalized arterial calcification of infancy and pseudoxanthoma elasticum can be caused by mutations in either ENPP1 or ABCC6 . Am J Hum Genet. 2012;90:25–39. doi: 10.1016/j.ajhg.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschke Y, Rutsch F. Generalized arterial calcification of infancy and pseudoxanthoma elasticum: two sides of the same coin. Front Genet. 2012a;3:302. doi: 10.3389/fgene.2012.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschke Y, Rutsch F. Genetics in arterial calcification: lessons learned from rare diseases. Trends Cardiovasc Med. 2012b;22:145–149. doi: 10.1016/j.tcm.2012.07.011. [DOI] [PubMed] [Google Scholar]
- O'Neill WC, Lomashvili KA, Malluche HH, et al. Treatment with pyrophosphate inhibits uremic vascular calcification. Kidney Int. 2011;79:512–517. doi: 10.1038/ki.2010.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okawa A, Nakamura I, Goto S, et al. Mutation in Npps in a mouse model of ossification of the posterior longitudinal ligament of the spine. Nature Genet. 1998;19:271–273. doi: 10.1038/956. [DOI] [PubMed] [Google Scholar]
- Otero JE, Gottesman GS, McAlister WH, et al. Severe skeletal toxicity from protracted etidronate therapy for generalized arterial calcification of infancy. J Bone Miner Res. 2013;28:419–430. doi: 10.1002/jbmr.1752. [DOI] [PubMed] [Google Scholar]
- Pomozi V, Brampton C, Fulop K, et al. Analysis of pseudoxanthoma elasticum-causing missense mutants of ABCC6 in vivo; pharmacological correction of the mislocalized proteins. J Invest Dermatol. 2014;134:946–953. doi: 10.1038/jid.2013.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prunier F, Terrien G, Le Corre Y, et al. Pseudoxanthoma elasticum: cardiac findings in patients and Abcc6-deficient mouse model. PLoS One. 2013;8:e68700. doi: 10.1371/journal.pone.0068700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronchetti I, Boraldi F, Annovi G, et al. Fibroblast involvement in soft connective tissue calcification. Front Genet. 2013;4:22. doi: 10.3389/fgene.2013.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutsch F, Boyer P, Nitschke Y, et al. Hypophosphatemia, hyperphosphaturia, and bisphosphonate treatment are associated with survival beyond infancy in generalized arterial calcification of infancy. Circ Cardiovasc Genet. 2008;1:133–140. doi: 10.1161/CIRCGENETICS.108.797704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutsch F, Nitschke Y, Terkeltaub R. Genetics in arterial calcification: pieces of a puzzle and cogs in a wheel. Circ Res. 2011;109:578–592. doi: 10.1161/CIRCRESAHA.111.247965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sali A, Favaloro JM, Terkeltaub R, et al. Germline deletion of the nucleoside triphosphate pyrophosphohydrolase (NTPPPH) plasma cell membrane glycoprotein-1 (PC-1) produces abnormal calcification of periarticular tissues. In: Vanduffel LLR, editor. Ecto-ATPases and related ectoenzymes. Maastricht, The Netherlands: Shaker Publishing; 1999. pp. 267–282. [Google Scholar]
- Scheffer GL, Hu X, Pijnenborg AC, et al. MRP6 (ABCC6) detection in normal human tissues and tumors. Lab Invest. 2002;82:515–518. doi: 10.1038/labinvest.3780444. [DOI] [PubMed] [Google Scholar]
- St Hilaire C, Ziegler SG, Markello TC, et al. NT5E mutations and arterial calcifications. N Engl J Med. 2011;364:432–442. doi: 10.1056/NEJMoa0912923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takabayashi S, Seto S, Katoh H. A new Enpp1 allele, Enpp1(ttw-Ham), identified in an ICR closed colony. Exp Anim. 2014;63:193–204. doi: 10.1538/expanim.63.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry S, Hefferson T. LOVD Gene Homepage. [Accessed 10/22/2015];2013 < http://www.ncbi.nlm.nih.gov/lovd/home.php?select_db=ABCC6>.
- Trip MD, Smulders YM, Wegman JJ, et al. Frequent mutation in the ABCC6 gene (R1141X) is associated with a strong increase in the prevalence of coronary artery disease. Circulation. 2002;106:773–775. doi: 10.1161/01.cir.0000028420.27813.c0. [DOI] [PubMed] [Google Scholar]
- Uitto J, Varadi A, Bercovitch L, et al. Pseudoxanthoma elasticum: progress in research toward treatment: summary of the 2012 PXE International Research Meeting. J Invest Dermatol. 2013;133:1444–1449. doi: 10.1038/jid.2013.20. [DOI] [PubMed] [Google Scholar]
- Uludag H. Bisphosphonates as a foundation of drug delivery to bone. Curr Pharm Des. 2002;8:1929–1944. doi: 10.2174/1381612023393585. [DOI] [PubMed] [Google Scholar]
- Verbraak FD. Antivascular endothelial growth factor treatment in pseudoxanthoma elasticum patients. Dev Ophthalmol. 2010;46:96–106. doi: 10.1159/000320012. [DOI] [PubMed] [Google Scholar]
- Zarbock R, Hendig D, Szliska C, et al. Vascular endothelial growth factor gene polymorphisms as prognostic markers for ocular manifestations in pseudoxanthoma elasticum. Hum Mol Genet. 2009;18:3344–3351. doi: 10.1093/hmg/ddp259. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Jiang Q, Takahagi S, et al. Premature termination codon read-through in the ABCC6 gene: Potential treatment for pseudoxanthoma elasticum. J Invest Dermatol. 2013;133:2672–2677. doi: 10.1038/jid.2013.234. [DOI] [PMC free article] [PubMed] [Google Scholar]